Open Access Article
Open Access ArticleCu-Catalyzed ligand-free synthesis of rosuvastatin based novel indole derivatives as potential anticancer agents†
K. Shiva
Kumar
*a,
Bandari
Rajesham
a,
Meesa Siddi
Ramulu
a,
Boyapally
Bhaskar
a,
Surjya Narayan
Dash
b,
Mohd Ashraf
Ashfaq
c,
Raju
Nagarapu
c,
Aleem Ahmed
Khan
c,
Sanna
Lehtonen
 b and
Manojit
Pal
*d
b and
Manojit
Pal
*d
aDepartment of Chemistry, Osmania University, Hyderabad-500 007, India. E-mail: shivakumarkota@yahoo.co.in; Tel: +91 40 27682337
bDepartment of Pathology, University of Helsinki, 00290, Helsinki, Finland
cCentral Laboratory for Stem Cell Research and Translational Medicine, CLRD Deccan Colleges of Medical Sciences, Kanchanbagh, Hyderabad-500 058, India
dDr Reddy's Institute of Life Sciences, Hyderabad Central University, Campus, Gachibowli, Hyderabad-500 046, India. E-mail: manojitpal@rediffmail.com; Tel: +91 40 6657 1500
First published on 17th October 2016
Abstract
Rosuvastatin based novel indole derivatives designed as potential anti-cancer agents were synthesized via a newly developed ligand-free, simple, straightforward and inexpensive one-pot method. The methodology involved a Cu-catalyzed coupling-cyclization of a rosuvastatin based alkyne with o-iodoanilides in the presence of CuI and K2CO3 in PEG-400. Three of the synthesized compounds showed promising anti-proliferative activities against cancer cell lines and an increase of p21 mRNA expression and apoptotic effects in zebrafish embryos/larvae.
The inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase e.g. statins are a well known therapy for the treatment of cardiovascular diseases especially hypercholesterolemia.1 They have also attracted enormous interest as potential anticancer agents2 because of preclinical evidence on their antiproliferative, proapoptotic, anti-invasive and radiosensitizing properties. Indeed, their inhibition of HMG-CoA reduces mevalonate synthesis thereby decreasing the downstream products of the mevalonate pathway, which in turn reduces protein farnesylation and geranylgeranylation. This causes decrease in (i) the expression of matrix metalloproteinase-9 and E-selectin (implicated in tumor cell metastasis), (ii) angiogenesis via inhibition of TNF-α thereby limiting tumor growth and (iii) translocation of Ras and Rho proteins to the cell membrane thereby decreasing tumor cell proliferation and migration. Studies have shown that statin's use is associated with a decreased risk of cancer-specific mortality in breast, colorectal and prostate cancer3 and has an excellent long term safety.4
Rosuvastatin (A, Fig. 1), a member of the statin family, has been reported to show anti-proliferative as well as apoptotic effects when tested against papillary thyroid5 and breast cancer cell lines.6 All these reports prompted us to explore rosuvastatin as a starting point for the identification of new anti-cancer agents. While the side chain of rosuvastatin is considered as the pharmacophore for its lipid lowering activities the heteroaryl part appeared to be an interesting scaffold for the design of new anticancer agents. Indeed, 4-aryl substituted pyrimidin-2-amine derivatives have been explored as anticancer agents (selective adenosine A1 receptor antagonists) earlier.7 In view of anticancer properties of compounds8 containing 2-substituted indole framework we replaced the side chain of A by this moiety to design a new template B for the generation of small molecules as potential anticancer agents (Fig. 1). We therefore required a direct, efficient and inexpensive synthetic method for accessing a focused library of molecules based on B for pharmacological screen.
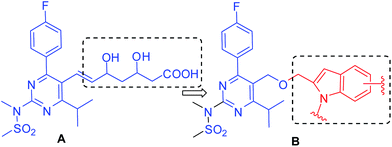 | ||
| Fig. 1 Design of rosuvastatin-indole based new template for the identification of potential anticancer agents. | ||
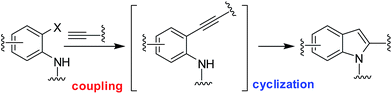
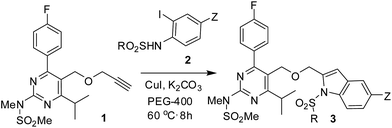
Among the various methods reported for the synthesis of indoles,9 intramolecular cyclization of 2-alkynylanilid(n)es catalyzed by a range of transition metal catalysts10 including copper, molybdenum, iridium, mercury, gold, platinum, and rhodium has been explored extensively. Indeed, because of their economic advantages and potential uses in large-scale reactions the copper catalysts have attracted particular attention for this purpose.11 Apart from their uses in the cyclization of 2-alkynylanilid(n)es copper catalysts have also been explored in the one-pot coupling-cyclization process [i.e. in situ generation of 2-alkynylanilid(n)es followed by cyclization in the same pot] (Scheme 1) leading to indole derivatives.12 However, these methods involved the use of expensive or complex ligands such as PPh3 or [Cu(phen)(PPh3)2]NO3 or L-proline etc. and a hazardous organic solvent such as toluene or DMF. In our effort we have reported the synthesis of indole derivatives via the coupling-cyclization strategy (Scheme 1) using Pd/C–CuI–PPh3 as a catalyst system.13 Though appeared to be effective and useful these methods however involve the use of a bimetallic catalyst system and an expensive phosphine ligand. Recently, we have demonstrated that the coupling-cyclization strategy can also be performed using a Cu-salt as the single and only catalyst in PEG-400.14 The methodology is free from the use of expensive and toxic palladium catalysts, ligands and harmful organic solvents and has been explored for the synthesis of isocoumarins and isoquinolin-1(2H)-ones earlier. In further continuation of this work we now report a straightforward and direct synthesis of rosuvastatin based novel indole analogues (3) via the coupling-cyclization of terminal alkynes (1) with various o-iodoanilides (2) in the presence of CuI and K2CO3 in PEG-400 (Scheme 2). The synthesized indoles were evaluated for their cytotoxic/pro-apoptotic effects in vitro and in vivo the preliminary results of which are presented.
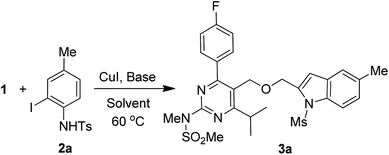
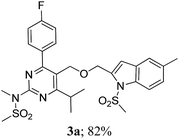
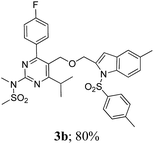
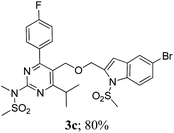
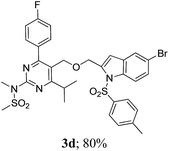
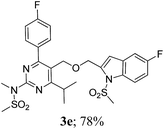
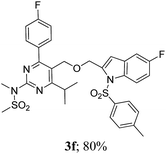
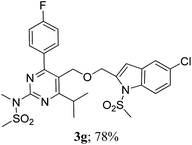
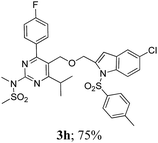
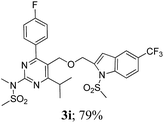
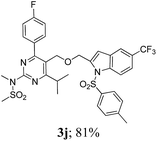
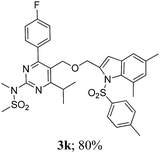
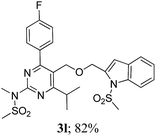
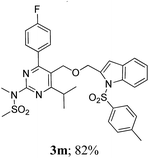
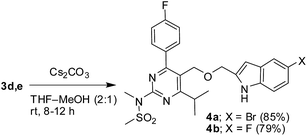
The key starting alkyne 1 was prepared via the reaction of a rosuvastatin intermediate15a with propargyl bromide (see ESI†). Subsequently, the reaction of 1 with N-(2-iodo-4-methylphenyl)methanesulfonamide (2a) was carried out under various conditions. Initially, the reaction was performed in PEG-400 in the presence of 15 mol% CuI and K2CO3 (entry 1, Table 1) when the desired indole 3a was obtained in 50% yield. The increase of catalyst loading from 15 to 20 mol% improved the product yield to 82% (entry 2, Table 1) though a further increase (i.e. to 30 mol%) did not improve the yield (entry 3, Table 1). Performing the reaction for a longer time or at elevated temperature (entry 4 and 5, Table 1) also did not improve the yield. The use of water in place of PEG-400 (entry 6, Table 1) or other bases e.g. Cs2CO3 and K3PO4 (entry 7 and 8, Table 1) was examined but found to be less effective. The lowering of reaction temperature was also found to be less effective (entry 9, Table 1). Thus, the combination of CuI–K2CO3 in PEG-400 was identified as the best reaction condition (entry 2, Table 1) for the synthesis of 3a and therefore was used for the preparation of other analogues. We then prepared a range of desired indoles (3) (Table 2) via reacting the alkyne (1) with various o-iodosulphanilides (2) under the optimized reaction conditions.15b The reaction proceeded well in all these cases irrespective of presence of groups such as Me, F, Cl, Br and CF3 on the anilide ring affording the desired indoles 3 in good to acceptable yields. All the products were characterized by spectral data. The presence of indole ring in compound 3 was confirmed by the appearance of a singlet in the range ∼6.6–6.8 δ due to the C-3 indole proton in the corresponding 1H NMR spectra. Further, the appearance of two singlets near ∼4.9 and 4.5 δ indicated the presence of –CH2–O–CH2– moiety in compound 3. To expand the scope of this Cu-catalyzed methodology further, a selective desulfonylation of compound 3d and 3e were performed using Cs2CO3 in THF–MeOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the indole derivative 4a and 4b, respectively (Scheme 3).
1) to afford the indole derivative 4a and 4b, respectively (Scheme 3).
| Entry | CuI (mol%) | Base | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reactions were carried out using alkyne 1 (1 equiv.), 2a (1 equiv.), base (2.0 mmol), and CuI in a solvent (5.0 mL) under nitrogen. b Isolated yield. c The reaction was performed at 80 °C. d The reaction was performed at 25 °C. | |||||
| 1 | 15 | K2CO3 | PEG-400 | 8 | 50 |
| 2 | 20 | K2CO3 | PEG-400 | 8 | 82 |
| 3 | 30 | K2CO3 | PEG-400 | 8 | 60 |
| 4 | 20 | K2CO3 | PEG-400 | 12 | 80 |
| 5 | 30 | K2CO3 | PEG-400 | 6 | 72c |
| 6 | 20 | K2CO3 | H2O | 8 | 30 |
| 7 | 20 | K3PO4 | PEG-400 | 12 | 65 |
| 8 | 20 | CsCO3 | PEG-400 | 12 | 72 |
| 9 | 20 | K2CO3 | PEG-400 | 8 | 55d |
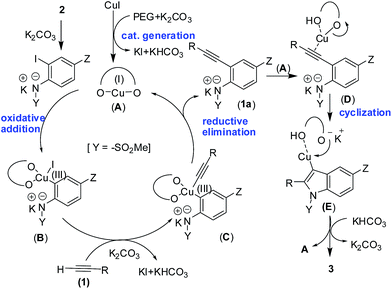
A working mechanism is proposed (Scheme 4) for the present Pd/ligand-free Cu-catalyzed coupling-cyclization16a,b method leading to indoles. Since the solvent PEG-400 can play the role of reaction medium as well as a ligand hence a Cu(I) complex (A) is formed via the interaction of CuI with PEG.16b,c The complex A then on oxidative addition with o-iodoanilide forms the arene–Cu(III) species B. The interaction of alkyne 1 with B in the presence of K2CO3 affords the arene–Cu(III)–alkyne species C, which on reductive elimination furnished the o-alkynyl anilide intermediate 1a.16d The intermolecular cyclization of 1a in the presence of A affords the desired indole 3 with the regeneration of active Cu(I) catalyst A.
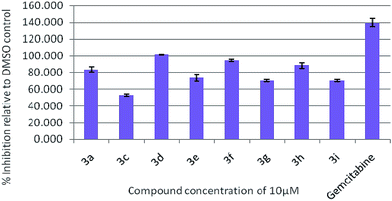
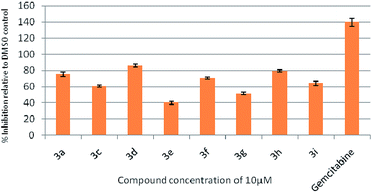
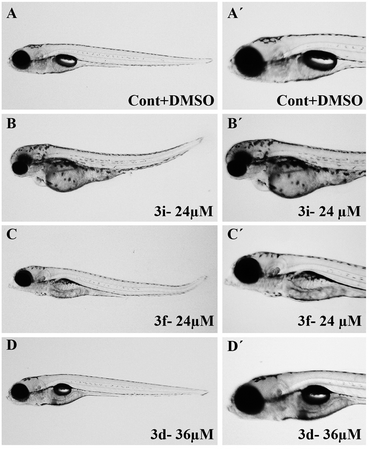
To assess their anticancer properties some of the compounds synthesized were tested in vitro at 10 μM against A549 (human lung carcinoma cells) and TZM-BL (human cervical carcinoma cells) cell lines using a sulphorhodamine B (SRB) assay with gemcitabine as a reference compound. In general, compound 3a, 3d, 3f, 3h and 3i showed marginally better activities than 3c, 3e, and 3g against both the cell lines (Fig. 2 and 3). Having found them active in cell based assay we then tested the best active compounds i.e.3i, 3f and 3d for their cytotoxic effect in the zebrafish embryos and larvae at different doses (Tables 3 and 4).17 It was observed that the embryos treated with 24 μM of 3i and 3f (n = 20 each) showed phenotypic changes (with short eye, pericardial and yolk sac edema, defective upper and lower jaw) whereas embryos treated with compound 3d (and the control embryos treated with 0.1% DMSO) did not show any phenotypic changes at this dose (Table 3 and Fig. 4). However, an increase of the concentration of 3d to 36 μM led to mild phenotypic changes (Table 3 and Fig. 4). Notably, the observed phenotypic changes in embryos were due to the possible cytotoxic effects of test compounds. Nevertheless, the survival rate of embryos at 24 μM was found to be 20% for compound 3i, 60% for 3f and 90% for 3d. Thus the NOAEL (No Observed Adverse Effect Level) of these compounds appeared to be <12 μM for 3i, <12 μM for 3f and <24 μM for 3d (n = 20 each). To assess their effect at the stage when the zebrafish larvae become active and start swimming, the same concentrations of 3i, 3f and 3d were used to treat larvae from 4 till 7 dpf (Table 4). Notably, no severe phenotypic changes were observed (Fig. 5). The mild phenotypic changes detected included pericardial and yolk sac edema at 24 μM for 3i and 3f (n = 20 each) and 36 μM for 3d (Table 4). Even though liver toxicity was observed in all cases the survival rate was ∼90% for all compounds. The NOAEL of these compounds appeared to be <12 μM for 3i, and 3f and <24 μM for 3d (n = 20 each).
| Phenotypical changes | DMSO | Concentrations (μM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3i | 3f | 3d | ||||||||||||
| 0.1% | 3 | 6 | 12 | 24 | 3 | 6 | 12 | 24 | 3 | 6 | 12 | 24 | 36 | |
| a The graded levels of phenotypical changes: (—) nil; (x) mildly toxic; (xx) medium toxic; (xxx) highly toxic. dpf: days post fertilization. NOAEL: No Observed Adverse Effect Level. MTC: maximum tolerated concentration. | ||||||||||||||
| Body shape | — | — | — | — | x | — | — | — | x | — | — | — | — | — |
| Head | — | — | — | xx | xxx | — | — | — | x | — | — | — | — | — |
| Eye | — | — | — | xx | xxx | — | — | — | x | — | — | — | — | — |
| Intestine | — | — | — | xx | xxx | — | — | — | x | — | — | — | — | x |
| Liver | — | — | — | xx | xxx | — | — | — | x | — | — | — | x | xx |
| Heart | — | — | — | x | xx | — | — | — | x | — | — | — | — | x |
| Jaw | — | — | — | xx | xx | — | — | — | — | — | — | — | — | x |
| NOAEL (μM) | — | <12 | <12 | <24 | ||||||||||
| MTC (μM) | 24 | 24 | 36 | |||||||||||
| Phenotypical changes | DMSO | Concentrations (μM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3i | 3f | 3d | ||||||||||||
| 0.1% | 3 | 6 | 12 | 24 | 3 | 6 | 12 | 24 | 3 | 6 | 12 | 24 | 36 | |
| a The graded levels of phenotypical changes: (—) nil; (x) mildly toxic; (xx) medium toxic; (xxx) highly toxic. dpf: days post fertilization. NOAEL: No Observed Adverse Effect Level. MTC: maximum tolerated concentration. | ||||||||||||||
| Body shape | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Head | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Eye | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Intestine | — | — | — | — | x | — | — | — | — | — | — | — | — | — |
| Liver | x | — | — | xx | xx | — | — | — | xx | — | — | — | x | xxx |
| Heart | x | — | — | — | xx | — | — | — | xx | — | — | — | — | x |
| Jaw | — | — | — | — | xx | — | — | — | xx | — | — | — | — | x |
| NOAEL (μM) | — | <12 | <12 | <24 | ||||||||||
| MTC (μM) | 24 | 24 | 36 | |||||||||||
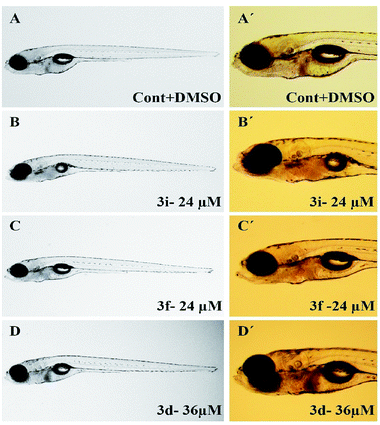 | ||
| Fig. 5 Representative images of zebrafish larvae (7 dpf) treated between 4 and 7 dpf with test compounds at MTC. | ||
In order to gain further insight regarding apoptotic effects18 of these compounds we examined their possible role in the increase of p21 mRNA expression level in zebrafish. P21 is a direct target of p53.19 Indeed, p53 loss is responsible for the development of majority of cancers in human and in zebrafish, p53 similarly acts as a tumor suppressor and key mediator of apoptosis.20 Thus, we evaluated21 the p21 mRNA level in zebrafish larvae treated with the test compounds both at NOEAL and MTC for 48 h (from 4 dpf to 6 dpf). Interestingly, a significant 4–5 fold increase in p21 expression level (*** p < 0.0001) was observed at NOEAL that was doubled at MTC in all cases (Fig. 6). These observations indicated that all these compounds considerably activated the apoptotic pathway. Notably, compounds at the lower concentration also activated the apoptotic pathway (where no obvious phenotypes were observed) and initiated cell death that is beneficial particularly for the protection from cancer. Nevertheless, the MTC appeared to be the cytotoxic dose for these compounds. Overall, the present class of compounds that showed growth inhibition of two cancer cell lines, and apoptotic effects in zebrafish embryos and larvae as visualized by an increase of p21 mRNA expression deserves further study as potential anticancer agents.
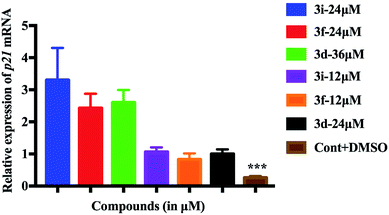 | ||
| Fig. 6 Activation of the apoptotic pathway in zebrafish larvae as visualized by significantly increased p21 expression after exposure to NOAEL and MTC of compounds. | ||
In conclusion, a library of indole based novel small molecules derived from rosuvastatin was designed as potential anti-cancer agents. These compounds were synthesized using a ligand-free Cu-catalyzed coupling-cyclization method that involved the reaction of a rosuvastatin based alkyne with o-iodoanilides in the presence of K2CO3 in PEG-400. The present method afforded a range of desired products in good to acceptable yields. Three of the synthesized compounds e.g.3i, 3f and 3d showed promising anti-proliferative properties when tested against human lung carcinoma and human cervical carcinoma cell lines. They also showed proapoptotic effects in zebrafish embryos/larvae via activation of p53 pathway as indicated by an increase in the expression of p21, a direct target of p53. Overall, our study not only highlights the development of an operationally simple, straightforward and inexpensive one-pot method leading to indoles but also suggests that the described rosuvastatin-indole based framework could be a useful template for the design and discovery of potential anticancer agents.
Acknowledgements
KSK thanks University Grants Commission (UGC), New Delhi, for the award of Assistant Professorship under its FRP and CSIR, India, for the financial support [02(0234)/15/EMR-II]. B. R. and B. B. thanks UGC and CSIR, New Delhi, respectively, for a Junior Research Fellowship. MP thanks CSIR [Grant 02(0127)/13/EMR-II] for support.Notes and references
- C. A. Aguilar-Salinas, H. Barrett and G. Schonfeld, Atherosclerosis, 1993, 141, 203 CrossRef.
- H. K. Takahashi, G. Weitz-Schmidt, H. Iwagaki, T. Yoshino, N. Tanaka and M. Nishibori, J. Leukocyte Biol., 2006, 80, 215 CrossRef CAS PubMed.
- (a) C. R. Cardwell, B. M. Hicks, C. Hughes and L. J. Murray, J. Clin. Oncol., 2014, 32, 3177 CrossRef PubMed; (b) S. F. Nielsen, B. G. Nordestgaard and S. E. Bojesen, N. Engl. J. Med., 2012, 367, 1792 CrossRef CAS PubMedO. Yu, M. Eberg, S. Benayoun, A. Aprikian, G. Batist, S. Suissa and L. Azoulay, J. Clin. Oncol., 2014, 32, 5 CrossRef CAS PubMed.
- M. F. Demierre, Curr. Opin. Oncol., 2006, 18, 180 CrossRef PubMed.
- N. D. Zeybek, N. E. Gulcelik, F. F. Kaymaz, C. Sarisozen, I. Vural, E. Bodur, H. Canpinar, A. Usman and E. Asan, J. Endocrinol., 2011, 210, 105 CrossRef CAS PubMed.
- H. Erbaş, O. Bal and E. Cakır, Balk. Med. J., 2015, 32, 89 CrossRef PubMed.
- L. C. W. Chang, R. F. Spanjersberg, J. K. V. F. D. Künzel, T. M. Krieger, G. V. D. Hout, M. W. Beukers, J. Brussee and A. P. IJzerman, J. Med. Chem., 2004, 47, 6529 CrossRef CAS PubMed.
- U. Jacquemard, N. Dias, A. Lansiaux, C. Bailly, C. Loge, J. M. Robert, O. Lozach, L. Meijer, J. Y. Merour and S. Routier, Bioorg. Med. Chem., 2008, 16, 4932 CrossRef CAS PubMed.
- For selected reviews, see: (a) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed; (b) G. Leni and R. C. Larock, Chem. Rev., 2004, 104, 2285 CrossRef PubMed; (c) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed; (d) H.-J. Knolker and K. R. Reddy, Chem. Rev., 2002, 102, 4303 CrossRef PubMed.
- (a) K. Hiroya, S. Itoh and T. Sakamoto, Tetrahedron, 2005, 61, 10958 CrossRef CAS; (b) K. Hiroya, S. Itoh and T. Sakamoto, J. Org. Chem., 2004, 69, 1126 CrossRef CAS PubMed; (c) S. Cacchi, G. Fabrizi and L. M. Parisi, Org. Lett., 2003, 5, 3843 CrossRef CAS PubMed; (d) K. Hiroya, S. Itoh, M. Ozawa, Y. Kanamori and T. Sakamoto, Tetrahedron Lett., 2002, 43, 1277 CrossRef CAS; (e) J. Ezquerra, C. Pedregal, C. Lamas, J. Barluenga, M. Pérez, M. A. García-Martín and J. M. González, J. Org. Chem., 1996, 61, 5804 CrossRef CAS; (f) I. Ambrogio, A. Arcadi, S. Cacchi, G. Fabrizi and F. Marinelli, Synlett, 2007, 1775 CAS; (g) Y. Zhang, J. P. Donahue and C. J. Li, Org. Lett., 2007, 9, 627 CrossRef CAS PubMed; (h) X. Li, A. R. Chianese, T. Vogel and R. H. Crabtree, Org. Lett., 2005, 7, 5437 CrossRef CAS PubMed; (i) T. Kurisaki, T. Naniwa, H. Yamamoto, H. Imagawa and M. Nishizawa, Tetrahedron Lett., 2007, 48, 1871 CrossRef CAS; (j) F. E. McDonald and A. K. Chatterjee, Tetrahedron Lett., 1997, 38, 7687 CrossRef CAS; (k) T. Shimada, I. Nakamura and Y. Yamamoto, J. Am. Chem. Soc., 2004, 126, 10546 CrossRef CAS PubMed; (l) B. M. Trost and A. McClory, Angew. Chem., Int. Ed., 2007, 46, 2074 CrossRef CAS PubMed.
- For an excellent review, see: S. Cacchi, G. Fabrizi and A. Goggiamani, Org. Biomol. Chem., 2011, 9, 641 CAS.
- (a) F. Liu and D. Ma, J. Org. Chem., 2007, 72, 4844 CrossRef CAS PubMed; (b) S. Cacchi, G. Fabrizi and L. M. Parisi, Org. Lett., 2003, 5, 3843 CrossRef CAS PubMed; (c) S. Cacchi, G. Fabrizi, L. M. Parisi and R. Bernini, Synlett, 2004, 287 CrossRef CAS; (d) G. A. Slough, V. Krchnak, P. Helquist and S. M. Canham, Org. Lett., 2004, 6, 2909 CrossRef CAS PubMed.
- (a) M. Pal, V. Subramanian, V. R. Batchu and I. Dager, Synlett, 2004, 1965 CrossRef CAS; (b) M. Layek, U. Lakshmi, D. Kalita, D. K. Barange, A. Islam, K. Mukkanti and M. Pal, Beilstein J. Org. Chem., 2009, 5, DOI:10.3762/bjoc.5.46; (c) A. Nakhi, B. Prasad, U. Reddy, R. M. Rao, S. Sandra, R. Kapavarapu, D. Rambabu, G. R. Krishna, C. M. Reddy, K. Ravada, P. Misra, J. Iqbal and M. Pal, Med. Chem. Commun., 2011, 2, 1006 RSC; (d) R. M. Rao, U. Reddy, A. Nakhi, N. Mulakayala, M. Alvala, M. K. Arunasree, R. R. Poondra, J. Iqbal and M. Pal, Org. Biomol. Chem., 2011, 9, 3808 RSC; (e) B. Prasad, R. Adepu, S. Sandra, D. Rambabu, G. R. Krishna, C. M. Reddy, G. S. Deora, P. Misra and M. Pal, Chem. Commun., 2012, 48, 10434 RSC.
- (a) R. G. Chary, G. R. Reddy, Y. S. S. Ganesh, K. V. Prasad, S. K. P. Chandra, S. Mukherjee and M. Pal, RSC Adv., 2013, 3, 9641 RSC; (b) R. G. Chary, G. Dhananjaya, K. V. Prasad, S. Vaishaly, Y. S. S. Ganesh, B. Dulla, K. S. Kumar and M. Pal, Chem. Commun., 2014, 50, 6797 RSC.
- (a) N. Joshi, A. S. Khile, Y. B. Kajale and H. H. Kamble, World patent Application No. WO 2008/059519 A2, 2008; (b) While all the reactions were performed using o-iodosulphanilides (2) the use of an o-bromosulphanilide e.g. N-(2-bromo-4-methylphenyl)-4-methylbenzenesulfonamide was also examined. When reacted with the alkyne 1 under the optimized reaction conditions (i.e. entry 2 of Table 1) it afforded the desired compound 3a in low yield (12%) indicating higher reactivity of iodo derivative over bromo analogue under the condition employed.
- (a) V. Declerck, J. Martinez and F. Lamaty, Synlett, 2006, 18, 3029 Search PubMed; (b) J. Mao, J. Guo, F. Fang and S. J. Ji, Tetrahedron, 2008, 64, 3905 CrossRef CAS; (c) N. Yoshikai and E. Nakamura, Chem. Rev., 2012, 112, 2339 CrossRef CAS PubMed; (d) Though Pd-free Cu-catalyzed Sonogashira reaction have been reported earlier (see for example, H. Jiang, H. Fu, R. Qiao, Y. Jiang and Y. Zhao, Synthesis, 2008, 2417 CAS ), studies have indicated that these reactions can proceed even with ppb levels of Pd, see: Z. Gonda, G. L. Tolnai and Z. Novák, Chem.–Eur. J., 2010, 16, 11822 Search PubMed.
- (a) Zebrafish is an excellent model for screening of potential drugs due to the transparency and conserved gene expression applicable for high throughput analysis, see: Y. Gibert, M. C. Trengove and A. C. Ward, Curr. Med. Chem., 2013, 20, 2458 CrossRef CAS PubMed; (b) Zebrafish lines wild-type Turku were maintained and raised at the zebrafish core facility Biomedicum, Helsinki, Finalnd. All animal experiments were performed in compliance with the national ethical guidelines, following the regulations set by the European Union, and were approved by the National Animal Experiment Board. Additionally, informed consent was obtained for any experimentation with human subjects.
- J. F. Kerr, A. H. Wyllie and A. R. Currie, Br. J. Cancer, 1972, 26, 239 CrossRef CAS PubMed.
- U. Langheinrich, E. Hennen, G. Stott and G. Vacun, Curr. Biol., 2002, 12, 2023 CrossRef CAS PubMed.
- N. Y. Storer and L. I. Zon, Cold Spring Harbor Perspect. Biol., 2010, a001123, DOI:10.1101/cshperspect.a001123.
- For our earlier study, see: S. N. Dash, E. Lehtonen, A. A. Wasik, A. Schepis, J. Paavola, P. Panula, W. J. Nelson and S. Lehtonen, J. Cell Sci., 2014, 127, 1476 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data for all new compounds, and copies of spectra. See DOI: 10.1039/c6ra20148b |
| This journal is © The Royal Society of Chemistry 2016 |