Deoxygenative functionalization of trifluoromethyl ketones
Shuhei
Shimoyama
,
Miki B.
Kurosawa
and
Junichiro
Yamaguchi
 *
*
Department of Applied Chemistry, Waseda University, 513 Wasedatsurumakicho, Shinjuku, Tokyo 162-0041, Japan. E-mail: junyamaguchi@waseda.jp
First published on 2nd December 2025
Abstract
A transition-metal-free deoxygenative functionalization of trifluoromethyl ketones via a Pudovik addition/phospha-Brook rearrangement sequence followed by benzylic substitution has been developed. The reaction proceeds under mild conditions to afford a variety of benzylic trifluoromethyl compounds in good yields. The method tolerates both electron-rich and halogen-substituted aromatic substrates, and accommodates diverse nucleophiles including amides, thiols, alkenes, and other heteroatom-based reagents. Mechanistic studies indicate that a benzyl phosphinate intermediate is formed through the phospha-Brook rearrangement and plays a pivotal role in facilitating the substitution step. This transformation offers a concise and efficient route to benzylic CF3 scaffolds from readily available trifluoromethyl ketones.
Introduction
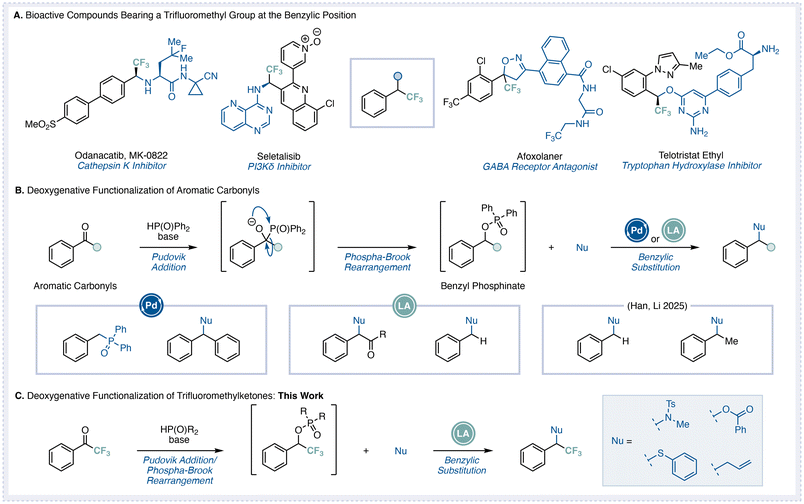
Fluorine atoms incorporated into organic molecules are known to enhance metabolic stability and lipophilicity, and thus have been widely utilized in the pharmaceutical and agrochemical fields.1 Among fluorinated motifs, bioactive compounds bearing a trifluoromethyl group at the benzylic position are particularly prevalent. Representative examples include Odanacatib (a cathepsin K inhibitor), Seletalisib (a PI3K inhibitor), Afoxolaner (a GABA receptor antagonist), and Telotristat ethyl (a tryptophan hydroxylase inhibitor), all of which are marketed pharmaceuticals featuring this structural motif (Fig. 1A).2 Despite their importance, the development of efficient synthetic methods to access such benzylic trifluoromethyl frameworks therefore remains a significant challenge.3 One representative strategy involves the deoxygenative transformation of readily available trifluoromethyl ketones.4,5 However, previously reported deoxygenative transformations of trifluoromethyl ketones generally require multiple reaction steps, limiting their practicality. Although reductive amination of trifluoromethyl ketones can be accomplished in a single step or through a one-pot process,4 the introduction of other functional groups typically requires prior conversion into more reactive intermediates.5 Moreover, many of these transformations rely on transition-metal catalysts, which can compromise functional-group tolerance and operational simplicity.Recently, several research groups, including ours, have reported the development of deoxygenative transformation reactions of aromatic carbonyl compounds that proceed through a Pudovik addition followed by a phospha-Brook rearrangement and subsequent nucleophilic substitution in a one-pot process.6–9 When aromatic carbonyl compounds are treated with phosphine oxides under basic conditions, an initial Pudovik addition occurs to form a P–C bond, which is subsequently rearranged via the phospha-Brook process to generate the corresponding benzyl phosphinates. Subsequent substitution reactions involving the elimination of the phosphinate moiety enable one-step deoxygenative functionalizations of aromatic carbonyl compounds (Fig. 1B). We have previously reported Pd-catalyzed deoxygenative conversions of esters and diaryl ketones,7 as well as TMSOTf-mediated transformations of aromatic dicarbonyl compounds and aldehydes under Lewis acidic conditions.8 More recently, Han and Li independently disclosed a metal-free deoxygenative transformation of aromatic carbonyl compounds.9
Based on these precedents, we envisioned that electrophilic trifluoromethyl ketones could serve as suitable substrates for a phospha-Brook rearrangement-driven deoxygenative functionalization (Fig. 1C). In our previous studies, we found that treatment of trifluoromethyl ketones with base such as K2CO3 often led to undesired β-fluoride elimination during the addition step.10 Therefore, the identification of reaction conditions that enable smooth formation of the corresponding phosphinate intermediates is crucial.11 Furthermore, because the resulting phosphinates retain a CF3 substituent, the subsequent SN1-type substitution step may be hampered by the reduced stability of the benzylic cation. We thus anticipated that careful control of both the electronic nature of the substrates and the reactivity of the phosphine oxide would be required to achieve efficient conversion. Thus, controlling both the elimination and substitution processes is key to achieving an efficient deoxygenative transformation. In the present study, we have developed a transition-metal-free deoxygenative transformation of trifluoromethyl ketones via a Pudovik addition/phospha-Brook rearrangement and subsequent nucleophilic substitution sequence. The reaction tolerates a wide range of nucleophiles, including nitrogen-, oxygen-, sulfur-, and carbon-based species, offering a concise and efficient platform for the synthesis of benzylic trifluoromethyl derivatives.
Results and discussion
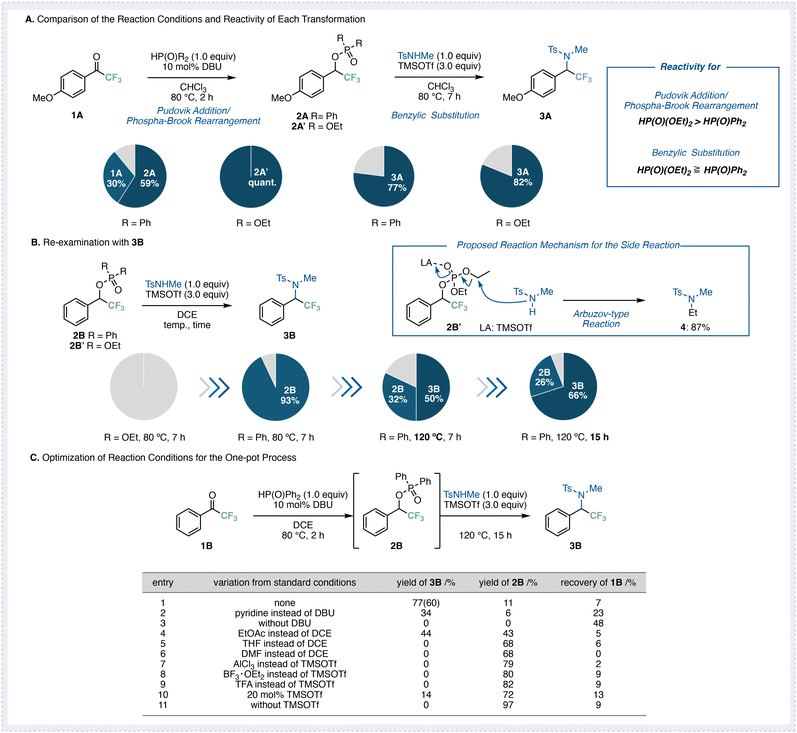
Since this transformation involves two consecutive steps, namely, a Pudovik addition/phospha-Brook rearrangement followed by a benzylic substitution, we first examined each step individually (Fig. 2A). Given the high electrophilicity of trifluoromethyl ketones, we employed reaction conditions similar to those previously reported for related dicarbonyl systems.8 Thus, commercially available 4-methoxyphenyl trifluoromethyl ketone (1A) was treated with diphenylphosphine oxide (1.0 equiv.) and DBU (10 mol%) in CHCl3 at 80 °C for 2 h. Under these conditions, the Pudovik addition/phospha-Brook rearrangement proceeded smoothly to afford the corresponding phosphinate 2A in 59% yield, along with 30% recovery of the starting material 1A. When diphenylphosphine oxide was replaced with diethyl phosphite, the reaction proceeded more efficiently to afford the adduct 2A′ in quantitative yield.Next, the isolated intermediates 2A and 2A′ were subjected to the benzylic substitution step using N-methyl-p-toluenesulfonamide (1.0 equiv.) as the nucleophile in the presence of TMSOTf (3.0 equiv.) in CHCl3 at 80 °C for 7 h. These results indicate that diethyl phosphite exhibits higher reactivity than diphenylphosphine oxide in the Pudovik addition/phospha-Brook rearrangement step, while both reagents show comparable, though slightly distinct, reactivity in the subsequent substitution step, with the diethyl phosphite proving somewhat superior overall.
We then examined the reaction of simple phenyl trifluoromethyl ketone (1B) instead of 4-methoxyphenyl trifluoromethyl ketone (1A) (Fig. 2B). Under the same substitution conditions using the phosphinate 2B′ derived from 1B, no desired deoxygenative product was obtained. In contrast, the Pudovik addition/phospha-Brook rearrangement from 1B alone proceeded quantitatively to give the corresponding phosphinate intermediate, suggesting that the difficulty lay in the benzylic substitution step. Further analysis revealed the formation of N-ethyl-N-methyl-p-toluenesulfonamide (4) in good yield as a side product, which was attributed to a Michaelis–Arbuzov-type reaction between the intermediate 2B′ and the nucleophile. To suppress this undesired pathway, we replaced diethyl phosphite with diphenylphosphine oxide, which is not prone to nucleophilic attack, and examined the reaction. When diphenylphosphine oxide was used, the intermediate 2B was obtained in high yield at 80 °C. However, elevating the temperature to 120 °C afforded the desired product 3B in 50% yield. Because unreacted 2B was still present, extending the reaction time to 15 h improved the yield of 3B to 66%. These findings demonstrate that the use of diphenylphosphine oxide effectively suppresses side reactions leading to 2B and promotes selective benzylic substitution. Moreover, by prolonging the reaction time and increasing the temperature, the phosphinate substitution could also be achieved for substrate 1B, which possesses a less stabilized benzylic cation compared to 1A.
Having optimized the two individual steps, we next attempted to combine them into a one-pot process (Fig. 2C). Under the optimized conditions, the desired deoxygenative adduct 3B was obtained in 60% isolated yield (entry 1). We then examined the effects of the base, solvent, and acid on the reaction efficiency. When DBU was replaced with pyridine, the first step proceeded less efficiently, resulting in a decreased yield of 3B (entry 2). In the absence of DBU, neither 2B nor 3B was obtained, and only the product derived from the Pudovik addition of 1B was observed (entry 3), indicating that a base is essential for the phospha-Brook rearrangement.
Changing the solvent to EtOAc slowed the second step, leading to partial recovery of 2B. In THF or DMF, only the intermediate 2B was detected (entries 5 and 6), suggesting that in coordinating polar solvents, the Lewis acid has difficulty interacting with the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the phosphinate. Among the acids tested, TMSOTf exhibited the best performance (entries 7–9), whereas reducing its amount to a catalytic level significantly decreased the yield (entry 10). In the absence of TMSOTf, only the phosphinate 2B was obtained in high yield (entry 11).
O group of the phosphinate. Among the acids tested, TMSOTf exhibited the best performance (entries 7–9), whereas reducing its amount to a catalytic level significantly decreased the yield (entry 10). In the absence of TMSOTf, only the phosphinate 2B was obtained in high yield (entry 11).
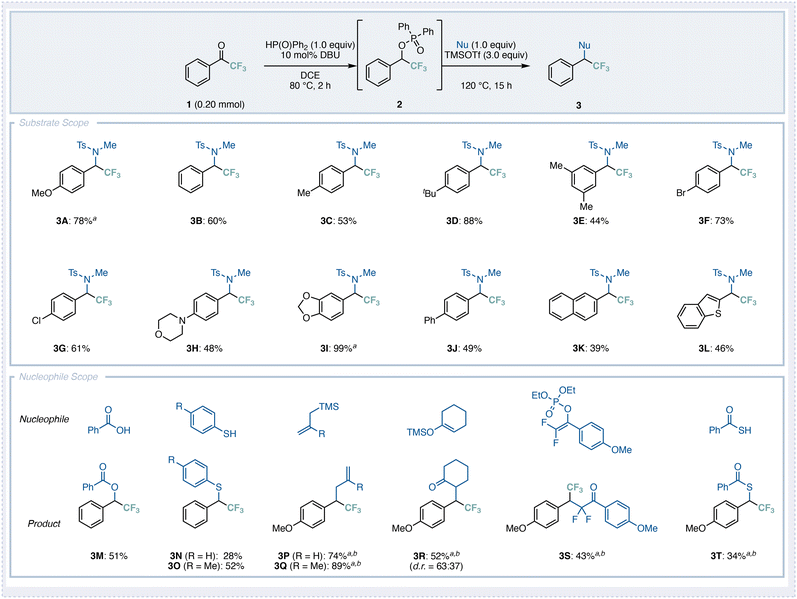
We next investigated the substrate scope of this deoxygenative transformation (Fig. 3). The representative trifluoromethyl ketones 3A and 3B reacted smoothly with N-methyl-p-toluenesulfonamide to afford the corresponding products in good yields. Substrates bearing alkyl substituents such as methyl, tert-butyl, or dimethyl groups on the aromatic ring were well tolerated, affording the corresponding products 3C–3E in good to moderate yields. Because the present reaction proceeds without the use of transition-metal catalysts, halogenated substrates such as bromo (3F) and chloro (3G) derivatives also participated successfully, as did a substrate containing a morpholino group (3H). For substrates such as 3B and 3H, which contain electron-donating substituents at the para-position of the aromatic ring, incomplete conversion of the starting material 1 was observed, suggesting that the first step (from 1 to 2) proceeded more slowly under the standard conditions. In contrast, for substrates 3F and 3G, the benzylic substitution step (the second stage) was partially inhibited, likely due to the reduced ability of the benzylic cation intermediate to be stabilized via the SN1-type mechanism; as a result, 20–30% of the phosphinate intermediate 2 remained unreacted.
In the case of more electron-deficient trifluoromethyl ketones bearing electron-withdrawing groups such as CO2Me or acetyl, the phospha-Brook rearrangement proceeded smoothly, but the subsequent benzylic substitution did not occur at all (see the SI for details). In contrast, the dioxole derivative underwent the reaction almost quantitatively to give 3I. Furthermore, biphenyl (3J), naphthalene (3K), and benzothiophene (3L) derivatives were also applicable, affording the corresponding deoxygenated products in moderate yields.
We next explored the scope of nucleophiles using trifluoromethyl ketones 1B and 1A as representative substrates. When benzoic acid or benzenethiol was employed, the corresponding deoxygenated products 3M and 3N were obtained in moderate to low yields. We speculated that the relatively low nucleophilicity of these reagents accounted for the diminished efficiency. Indeed, when p-toluenethiol, bearing an electron-donating methyl group, was used, the desired product 3O was obtained in moderate yield. In contrast, TMS-substituted alkenes reacted smoothly under the standard conditions to afford the corresponding products 3P and 3Q in high yields. The use of a silyl enol ether also provided the desired product 3R in moderate yield. When a difluoroenol phosphinate was employed, the corresponding difluorinated compound 3S was obtained in moderate yield. Finally, when a thiocarboxylic acid was used as a nucleophile to enhance reactivity, the corresponding product 3T was formed in 34% yield.
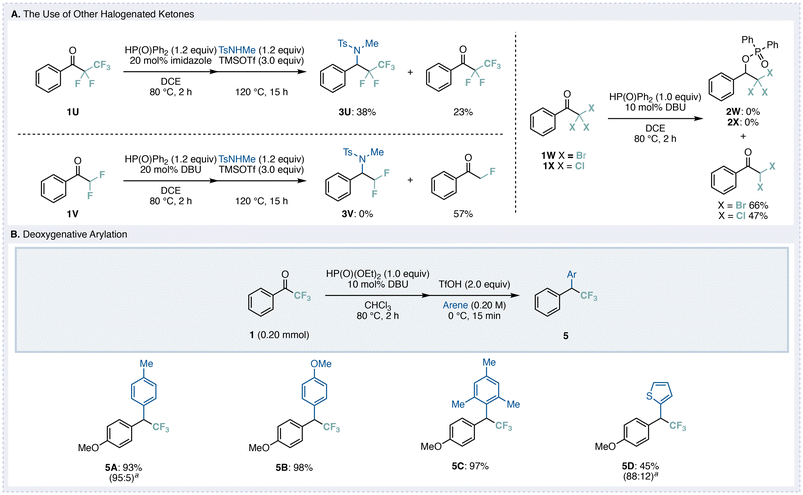
We next applied this method to other fluorinated or halogenated ketones (Fig. 4A). When pentafluoroethyl ketone 1U was used, the desired deoxygenative addition product 3U was obtained in 38% yield upon replacing DBU with imidazole. The low yield was attributed to partial recovery of the starting material 1U (14%) and the formation of a side product (23%) derived from defluorination at the α-position of the ketone. Other substrates such as difluoromethyl ketone, tribromomethyl ketone, and trichloromethyl ketone were also examined; however, only dehalogenation occurred, and the desired deoxygenative adducts were not obtained. These results suggest that, under the reaction conditions, a Perkow-type reduction pathway predominates, leading to dehalogenation rather than the intended deoxygenation.
We also investigated a deoxygenative arylation process (Fig. 4B). After preparing the phosphinate intermediate, various arenes were added in the presence of TfOH at 0 °C under solvent-controlled conditions.12 The reaction proceeded via a Friedel–Crafts electrophilic substitution, affording the corresponding adducts 5A–5D when toluene, anisole, mesitylene, or thiophene was used as the arene component.
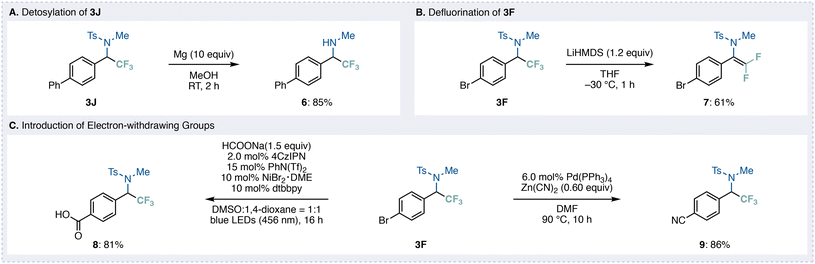
To further highlight the synthetic utility of the benzylic trifluoromethyl products obtained through this deoxygenative transformation, several downstream derivatizations were examined (Fig. 5). First, removal of the tosyl group of 3J was achieved smoothly by treatment with magnesium in methanol, affording the corresponding primary amine 6 in good yield (Fig. 5A).
In addition, exposure of 3F to a strong base (LHMDS) promoted efficient β-fluoride elimination to deliver the difluorinated compound 7 in 61% yield (Fig. 5B).13 This result highlights the potential of the CF3 group to undergo further selective defluorination under basic conditions, enabling access to structurally distinct fluorinated motifs. Furthermore, we explored the reactivity of benzylic trifluoromethyl product 3F under C–C bond-forming conditions (Fig. 5C). Dual photoredox/Ni catalysis in the presence of sodium formate enabled reductive carboxylation to furnish carboxylic acid 8 in good yield.14 In contrast, Pd-catalyzed cyanation using zinc cyanide provided the corresponding nitrile 9 in high yield.15 Because these strongly electron-withdrawing substituents significantly reduce the reactivity of the parent trifluoromethyl ketones toward direct deoxygenative functionalization, these two-step sequences represent valuable complementary pathways to access challenging derivatives.
Conclusions
In summary, we have developed a transition-metal-free deoxygenative functionalization of trifluoromethyl ketones via a Pudovik addition/phospha-Brook rearrangement and nucleophilic substitution sequence. This transformation proceeds smoothly under mild conditions and enables one-pot access to diverse benzylic trifluoromethyl derivatives. The method tolerates various nucleophiles, including nitrogen-, oxygen-, sulfur-, and carbon-based species, thereby offering a versatile and efficient platform for molecular diversification. Mechanistic investigations revealed that controlling both the β-fluoride elimination in the addition step and the SN1-type benzylic substitution is crucial for achieving high efficiency. Furthermore, the reaction could be extended to Friedel–Crafts-type arylations, highlighting its broad applicability. This operationally simple and conceptually distinct strategy provides a new avenue for the construction of valuable CF3-containing frameworks from readily available ketones.Author contributions
J. Y. directed the projects and designed the experiments. M. K. discovered the reaction, and S. S. mainly performed the experiments. S. S. and J. Y. contributed to data analysis. J. Y. wrote the manuscript with feedback from the other authors.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: for experimental procedures, spectroscopic data for compounds including 1H and 13C NMR spectra. See DOI: https://doi.org/10.1039/d5ob01657f.Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP21H05213 (Digi-TOS) and JP25K01775 (to J. Y.). This work was partly supported by JST CREST Grant Number JPMJCR24T3 (to J. Y.). Materials Characterization Central Laboratory in Waseda University is acknowledged for the support of NMR and HRMS measurement.References
- (a) D. O'Hagan, Chem. Soc. Rev., 2007, 37, 308–319 RSC; (b) R. Berger, G. Resnati, P. Metrangolo, E. Weber and J. Hulliger, Chem. Soc. Rev., 2011, 40, 3496–3508 RSC; (c) Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422–518 CrossRef CAS PubMed; (d) J. Han, L. Kiss, H. Mei, A. M. Remete, M. Ponikvar-Svet, D. M. Sedgwick, R. Roman, S. Fustero, H. Moriwaki and V. A. Soloshonok, Chem. Rev., 2021, 121, 4678–4742 CrossRef CAS PubMed; (e) D. O'Hagan and R. J. Young, Med. Chem. Res., 2023, 32, 1231–1234 CrossRef.
- (a) J. Y. Gauthier, N. Chauret, W. Cromlish, S. Desmarais, L. T. Duong, J.-P. Falgueyret, D. B. Kimmel, S. Lamontagne, S. Léger, T. LeRiche, C. S. Li, F. Massé, D. J. McKay, D. A. Nicoll-Griffith, R. M. Oballa, J. T. Palmer, M. D. Percival, D. Riendeau, J. Robichaud, G. A. Rodan, S. B. Rodan, C. Seto, M. Thérien, V.-L. Truong, M. C. Venuti, G. Wesolowski, R. N. Young, R. Zamboni and W. C. Black, Bioorg. Med. Chem. Lett., 2008, 18, 923–928 CrossRef CAS PubMed; (b) R. A. Allen, D. C. Brookings, M. J. Powell, J. Delgado, L. K. Shuttleworth, M. Merriman, I. J. Fahy, R. Tewari, J. P. Silva, L. J. Healy, G. C. G. Davies, B. Twomey, R. M. Cutler, A. Kotian, A. Crosby, G. McCluskey, G. F. Watt and A. Payne, J. Pharmacol. Exp. Ther., 2017, 361, 429–440 CrossRef CAS PubMed; (c) W. L. Shoop, E. J. Hartline, B. R. Gould, M. E. Waddell, R. G. McDowell, J. B. Kinney, G. P. Lahm, J. K. Long, M. Xu, T. Wagerle, G. S. Jones, R. F. Dietrich, D. Cordova, M. E. Schroeder, D. F. Rhoades, E. A. Benner and P. N. Confalone, Vet. Parasitol., 2014, 201, 179–189 CrossRef CAS PubMed; (d) M. H. Kulke, T. O'Dorisio, A. Phan, E. Bergsland, L. Law, P. Banks, J. Freiman, K. Frazier, J. Jackson, J. C. Yao, L. Kvols, P. Lapuerta, B. Zambrowicz, D. Fleming and A. Sands, Endocr.-Relat. Cancer, 2014, 21, 705–714 CrossRef CAS PubMed.
- R. Shaw, N. Sihag, H. Bhartiya and M. R. Yadav, Org. Chem. Front., 2023, 11, 954–1014 RSC.
- (a) A. K. Holzer, K. Hiebler, F. G. Mutti, R. C. Simon, L. Lauterbach, O. Lenz and W. Kroutil, Org. Lett., 2015, 17, 2431–2433 CrossRef CAS PubMed; (b) W. Cai, D. Cai, H. Liang, X. Ren and B. Zhao, J. Org. Chem., 2023, 88, 7849–7857 CrossRef CAS PubMed; (c) Y.-Z. Wang, L. Hu, S.-T. Bai and X. Zhang, Org. Lett., 2023, 25, 5033–5037 CrossRef CAS PubMed.
- (a) L. Li, P. Sivaguru, D. Wei, M. Liu, Q. Zhu, S. Dong, E. Casali, N. Li, G. Zanoni and X. Bi, Nat. Commun., 2024, 15, 1951 CrossRef CAS PubMed; (b) L. Li, Y. Ning, H. Chen, Y. Ning, P. Sivaguru, P. Liao, Q. Zhu, Y. Ji, G. de Ruiter and X. Bi, Angew. Chem., Int. Ed., 2024, 63, e202313807 CrossRef CAS PubMed; (c) S. Liu, Y. Yang, Q. Song, Z. Liu, Y. Lu, Z. Wang, P. Sivaguru and X. Bi, Nat. Chem., 2024, 16, 988–997 CrossRef CAS PubMed; (d) C. He, W. Song, D. Wei, W. Zhao, Q. Yu, J. Tang, Y. Ning, K. Murali, P. Sivaguru, G. De Ruiter and X. Bi, Angew. Chem., Int. Ed., 2024, 63, e202408220 CrossRef CAS PubMed; (e) S. Liu, Y. Yang, Q. Song, Z. Liu, P. Sivaguru, Y. Zhang, G. De Ruiter, E. A. Anderson and X. Bi, Nat. Commun., 2024, 15, 9998 CrossRef CAS PubMed; (f) X. Zhang, Q. Song, S. Liu, P. Sivaguru, Z. Liu, Y. Yang, Y. Ning, E. A. Anderson, G. De Ruiter and X. Bi, Nat. Chem., 2025, 17, 215–225 CrossRef CAS PubMed; (g) T. Okano, K. Ito, T. Ueda and H. Muramatsu, J. Fluorine Chem., 1986, 32, 377–388 CrossRef CAS; (h) A. A. Zemtsov, S. S. Lunkov, V. V. Levin and A. D. Dilman, Eur. J. Org. Chem., 2021, 1007–1010 CrossRef CAS; (i) T. Hagiwara, K. Tanaka and T. Fuchikami, Tetrahedron Lett., 1996, 37, 8187–8190 CrossRef CAS.
- (a) J. Wang, J. Xiao, Z.-L. Tang, D.-H. Lan and L.-B. Han, J. Org. Chem., 2024, 89, 5109–5117 CrossRef CAS PubMed; (b) X. Wu, S. Lu, W. Zhong and T. Xu, Nat. Commun., 2025, 16, 3628 CrossRef CAS PubMed; (c) X. Wu, A. Alimu, W. Zhong and T. Xu, Org. Lett., 2025, 27, 6025–6030 CrossRef CAS PubMed.
- (a) M. B. Kurosawa, R. Isshiki, K. Muto and J. Yamaguchi, J. Am. Chem. Soc., 2020, 142, 7386–7392 CrossRef CAS PubMed; (b) M. B. Kurosawa, K. Kato, K. Muto and J. Yamaguchi, Chem. Sci., 2022, 13, 10743–10751 RSC; (c) M. Sakihara, M. B. Kurosawa, M. Watanabe, S. Shimoyama and J. Yamaguchi, J. Org. Chem., 2024, 89, 8157–8167 CrossRef CAS PubMed.
- M. Sakihara, S. Shimoyama, M. B. Kurosawa and J. Yamaguchi, Bull. Chem. Soc. Jpn., 2024, 97, uoae078 CrossRef CAS.
- F. Liu, J. Dong, R. Cheng, S.-F. Yin, L. Chen, L. Su, R. Qiu, Y. Zhou, L.-B. Han and C.-J. Li, Sci. Adv., 2025, 11, eads4626 CrossRef CAS PubMed.
- M. B. Kurosawa, S. Shimoyama, H. Tanaka and J. Yamaguchi, Chem. Sci., 2025, 16, 13390–13400 RSC.
- (a) A. Yu. Aksinenko, V. B. Sokolov, T. V. Goreva and G. F. Makhaeva, Russ. Chem. Bull., 2010, 59, 102–106 CrossRef CAS; (b) M. Kuroboshi, T. Ishihara and T. Ando, J. Fluorine Chem., 1988, 39, 293–298 CrossRef CAS; (c) L. E. Kaïm, L. Gaultier, L. Grimaud and A. D. Santos, Synlett, 2005, 2335–2336 CrossRef; (d) N. Li, Q. Wu, Y. Huang, J. Li and E. Shi, Org. Lett., 2025, 27, 4632–4637 CrossRef CAS PubMed; (e) Q. Li, Y.-M. Sun, L. Yao, S.-Y. Ji, H.-X. Zheng, J.-H. Wen, Q. Xu and C.-Q. Zhao, New J. Chem., 2024, 48, 18520–18525 RSC.
- (a) A. Kondoh, R. Ozawa, T. Aoki and M. Terada, Org. Biomol. Chem., 2017, 15, 7277–7281 RSC; (b) A. Kondoh, N. Tasato, T. Aoki and M. Terada, Org. Lett., 2020, 22, 5170–5175 CrossRef CAS PubMed; (c) V. D. Vuković, E. Richmond, E. Wolf and J. Moran, Angew. Chem., Int. Ed., 2017, 56, 3085–3089 CrossRef PubMed.
- R. Anilkumar and D. J. Burton, Tetrahedron Lett., 2003, 44, 6661–6664 CrossRef CAS.
- G. C. Smith, D. H. Zhang, W. Zhang, A. H. Soliven and W. M. Wuest, J. Org. Chem., 2023, 88, 9565–9568 CrossRef CAS PubMed.
- D. M. Tschaen, R. Desmond, A. O. King, M. C. Fortin, B. Pipik, S. King and T. R. Verhoeven, Synth. Commun., 1994, 24, 887–890 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHCl3 (1.0 mL) was used as the solvent and reaction conducted 80 °C, 7 h in step 2.
CHCl3 (1.0 mL) was used as the solvent and reaction conducted 80 °C, 7 h in step 2.