Stereoselective synthesis of functionalized perhydropyrrolo[1,2-b]isoxazoles based on (3 + 2)-annulation of donor–acceptor cyclopropanes and isoxazolines
Konstantin V.
Potapov
a,
Maxim A.
Novikov
 a,
Yaroslav V.
Kozmenko
ab,
Pavel N.
Solyev
a,
Yaroslav V.
Kozmenko
ab,
Pavel N.
Solyev
 b,
Alexander D.
Volodin
c,
Alexander A.
Korlyukov
b,
Alexander D.
Volodin
c,
Alexander A.
Korlyukov
 c,
Roman A.
Novikov
c,
Roman A.
Novikov
 *a and
Yury V.
Tomilov
*a
*a and
Yury V.
Tomilov
*a
aN.D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47 Leninsky prosp., 119991 Moscow, Russia. E-mail: novikovfff@bk.ru; tom@ioc.ac.ru
bEngelhardt Institute of Molecular Biology of the Russian Academy of Sciences, 32 Vavilov St., Moscow 119991, Russia
cA. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 28 Vavilov St., Moscow, 119334, Russian Federation
First published on 27th November 2025
Abstract
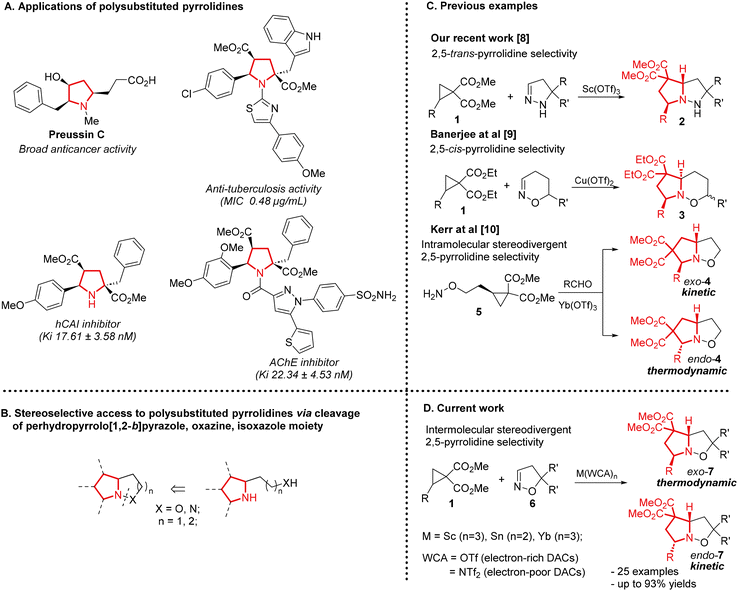
A stereoselective route to access substituted pyrrolidine cores via Lewis acid catalyzed (3 + 2)-annulation of donor–acceptor cyclopropanes (DACs) and isoxazolines has been developed. Exclusive cis-2,5-stereoselectivity was governed by kinetically controlled conditions using Sn(OTf)2 as the catalyst, while excellent trans-2,5-stereoselectivity was achieved by thermodynamically controlled conditions using Sc(OTf)3 as the catalyst. For DACs bearing electron-poor substituents, Yb(NTf2)3 proved to be the most efficient catalyst due to its higher Lewis acidity compared to triflates. The isoxazoline (3 + 2)-annulation reaction was also extended to bicyclo[1.1.0]butanes (BCBs), providing easy access to the 2-azabicyclo[2.1.1]hexane core, which may be considered as a promising 3D-bioisosteric replacement for pyrrole and pyrrolidine motifs.
The stereoselective synthesis of complex functionalized structures from readily available compounds in a minimal number of steps remains one of the pressing challenges in the development of modern synthetic methodologies.1 Over the past decades, cycloaddition and annulation reactions of donor–acceptor cyclopropanes (DACs) with various substrates have demonstrated broad potential for controlling the chemo-, regio-, and stereoselectivity of these processes through subtle variation of reaction conditions.2
DAC cycloaddition reactions are widely used to construct various substituted five- and six-membered heterocycles.3 Among the various heterocycles synthesized through DAC cycloaddition reactions, pyrrolidine derivatives are of particular interest since the substituted pyrrolidine moiety is a key structural unit in a number of biologically active and naturally occurring compounds that exhibit diverse types of activities (Scheme 1A).4
Under the action of various Lewis acids, DACs undergo cycloaddition with acyclic imines with rather high efficiency to afford, in most cases, substituted pyrrolidines with high diastereoselectivity.5 The only, yet significant, limitation is the requirement to use imines derived exclusively from aromatic aldehydes, which substantially reduces the synthetic significance of this methodology. This limitation does not apply to reactions with cyclic imines.6 Accordingly, an alternative synthetic approach to access substituted pyrrolidines may involve DAC cycloaddition with pyrazolines, oxazolines, and related C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–X-containing heterocycles to yield pyrrolidine-fused structures in which the N–X bond can be readily cleaved under reductive conditions to give functionalized pyrrolidine moieties (Scheme 1B).7 However, the available data on such DAC cycloaddition reactions are highly limited.
N–X-containing heterocycles to yield pyrrolidine-fused structures in which the N–X bond can be readily cleaved under reductive conditions to give functionalized pyrrolidine moieties (Scheme 1B).7 However, the available data on such DAC cycloaddition reactions are highly limited.
In the previous work, we showed that the reaction of 2-arylcyclopropane-1,1-dicarboxylates 1 with pyrazolines is efficiently catalyzed by Sc(OTf)3 to give 1,2-diazabicyclo[3.3.0]octanes 2 as single diastereomers with trans-selectivity of the 2,5-substituents at the pyrrolidine moiety that is formed (Scheme 1C).8
A similar [3 + 2]-cycloaddition of DAC 1 to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of unsaturated six-membered nitrogen-containing heterocycles was reported by Banerjee et al.9 Its distinguishing feature is the opposite cis-selectivity of the 2,5-substituents at the pyrrolidine moiety formed (Scheme 1C).
N bond of unsaturated six-membered nitrogen-containing heterocycles was reported by Banerjee et al.9 Its distinguishing feature is the opposite cis-selectivity of the 2,5-substituents at the pyrrolidine moiety formed (Scheme 1C).
An elegant stereodivergent method for the intramolecular construction of a hexahydropyrroloisoxazole derivative via opening of a three-membered ring was demonstrated by Kerr et al.10 The bicyclic product 4 was obtained through intramolecular [3 + 2]-cycloaddition of a zwitterionic intermediate, which is formed by the reaction of cyclopropylalkoxylamine 5 with aldehydes, to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the oxime. In this case, the cis/trans isomerism of cyclization products 4 was determined by the order of mixing of the aldehyde and the catalyst with cyclopropyl derivative 5; however, in all cases, hexahydropyrroloisoxazoles 4 were obtained with high diastereoselectivity. The heterocycle 4 formed in this process contained both ester groups at the β-position to the nitrogen atom (Scheme 1C).
N bond of the oxime. In this case, the cis/trans isomerism of cyclization products 4 was determined by the order of mixing of the aldehyde and the catalyst with cyclopropyl derivative 5; however, in all cases, hexahydropyrroloisoxazoles 4 were obtained with high diastereoselectivity. The heterocycle 4 formed in this process contained both ester groups at the β-position to the nitrogen atom (Scheme 1C).
In this work, we propose a stereoselective method for constructing the pyrrolidine moiety via [3 + 2]-cycloaddition of DACs with isoxazolines catalyzed by Lewis acids. In this case, stereoselectivity is regulated by kinetic or thermodynamic control, depending on the choice of the metal catalyst (Sn or Sc). Directed regulation of the Lewis acidity of the catalyst through the selection of a weakly coordinating counter-ion (WCA) NTf2 instead of OTf allowed us to successfully use less-reactive DACs bearing strongly electron-acceptor aromatic substituents in this process (Scheme 1D).
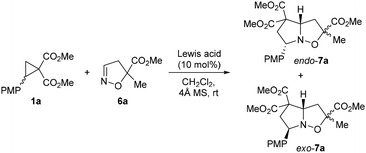
In the first step, 2-(4-methoxyphenyl)cyclopropane-1,1-dicarboxylate 1a and isoxazoline 6a were chosen as the model substrates, while 10 mol% of scandium, gallium, indium, ytterbium, copper, and tin triflates were chosen as the Lewis acids. All the reactions were carried out in dichloromethane at 25 °C (Table 1).
| Entry | Lewis acid (10 mol%) | t, h | Yieldsb (%) | |
|---|---|---|---|---|
| endo-7a | exo-7a | |||
| a Reactions were carried out at rt in CH2Cl2 with 1 equiv. of 1a and 6a, Lewis acid (10 mol%), and Ar atmosphere. b NMR yields (1,4-dinitrobenzene as the internal standard). c Isolated yields. | ||||
| 1 | Sc(OTf)3 | 2 | 7c | 91c |
| 2 | Sc(OTf)3 | 12 | Trace | 93 |
| 3 | Yb(OTf)3 | 2 | Trace | 63c |
| 4 | In(OTf)3 | 12 | 21 | 26 |
| 5 | Ga(OTf)3 | 12 | 18 | 12 |
| 6 | Cu(OTf)2 | 12 | 33 | 34 |
| 7 | Cu(OTf)2 | 72 | 32 | 39 |
| 8 | Sn(OTf)2 | 12 | 89 | – |
It was found that the same expected products of formal (3 + 2)-annulation – four diastereomers of substituted 1-aza-2-oxabicyclo[3.3.0]octane 7a – were obtained in all cases. The yields and isomer ratios varied significantly, depending on the nature of the catalysts used. The stereochemical outcome differed primarily in the endo- or exo-orientation of the para-methoxyphenyl substituent in the bicyclo[3.3.0]octane moiety (Table 1), whereas the ratio of the diastereomers (referred to here as anti- and syn-isomers for clarity) at the C(3) atom remained ca. 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for endo-7a and 1.2
1 for endo-7a and 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for exo-7a.
1 for exo-7a.
Nearly complete conversion of the initial cyclopropane 1a was observed in all cases; however, scandium and tin triflates exhibited the highest efficiency in this process, while the other catalysts predominantly led to side reactions of cyclopropane 1a.
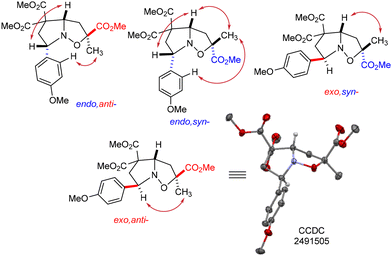
The stereochemistry of all the diastereomers obtained in the reaction was determined by X-ray crystallography and NMR spectroscopy. Crystallographic data obtained for one of the crystals of the exo,anti-7a isomer, along with the assignment of its signals in the 1H and 13C NMR spectra, served as the basis for the assignment of the remaining isomers using 2D {1H,1H}-NOESY NMR experiments (Fig. 1).
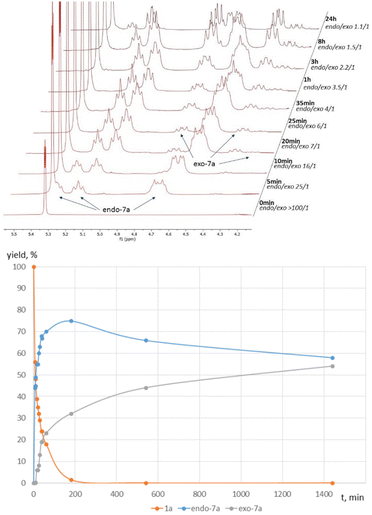
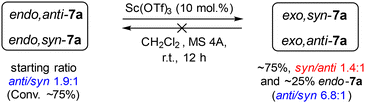
Next, after information on the stereochemistry of the products was obtained, the mechanism of the formation of exo- and endo-diastereomers 7a was clarified by NMR monitoring of this reaction in the presence of Sc(OTf)3. It was found that significant conversion of the initial cyclopropane 1a occurred already within 1 h, with the initial cycloaddition products being the pair of endo-diastereomers 7a, which subsequently underwent slow conversion to the corresponding pair of exo-diastereomers (Fig. 2). The isomerization rate of the syn-diastereomer was higher than that of the anti-isomer. Apparently, endo-7a forms more rapidly under the (3 + 2)-annulation conditions; however, due to the presence of a strongly polarized C–C bond in 7a, the annulation is reversible, which results in the gradual isomerization of endo-7a into the more thermodynamically stable exo-isomers.
To confirm the possibility of the cleavage of the C(5)–C(6) bond in the resulting bicyclic endo-7a adducts and their irreversible isomerization to more thermodynamically stable exo-7a isomers, a series of control experiments were carried out. For this purpose, both pairs of isomeric products, endo- and exo-7a, were preliminarily isolated in relatively pure form. It was found that both syn- and anti-isomers of endo-7a underwent substantial isomerization to exo-7a within 12 h in the presence of Sc(OTf)3. In contrast, the reverse isomerization of exo-7a to endo-7a was not observed in the presence of Sc(OTf)3 or Sn(OTf)2 (Scheme 2), which supports our hypothesis.
It is interesting to note that a change in the configuration of the aryl substituent leads to a change in the orientation of the Me and CO2Me substituents relative to the bicyclic moiety. As a result, the seemingly more favorable position of the anti-CO2Me group is converted into the syn-position. Consequently, the isomerization rate of the endo,anti-7a isomer appears to be lower than that of the endo,syn-isomer.
It was also found that the C(5)–C(6) bond could be cleaved even under thermal conditions in the absence of any added Lewis acid, but only at significantly higher temperatures. In fact, endo-7a undergoes isomerization by 11% within 2 h at 150 °C, while the content of exo-7a isomers reaches 60% after 4 h at 170° C. As in the catalytic variant, the isomerization rate of the syn-isomer was higher than that of the anti-isomer. The reverse transformation of exo-7a into endo-7a was practically not observed.
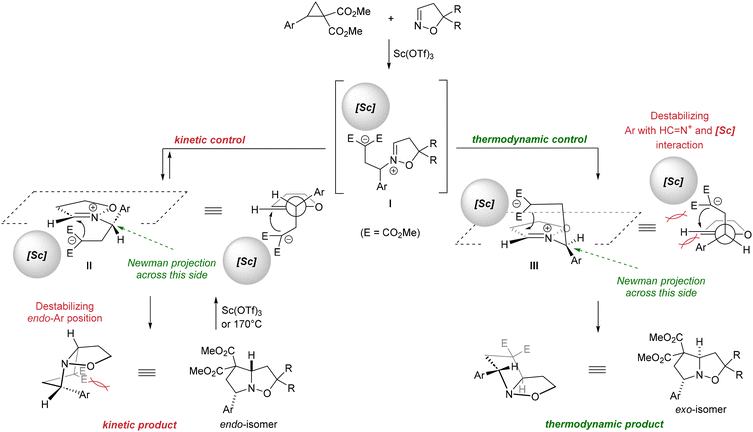
The regularities described above clearly indicate that the endo-7a diastereomer is formed as the kinetically controlled product, while exo-7a is the thermodynamically controlled product. The proposed mechanism involves activation of the three-membered ring 1a by the Lewis acid through the carboxylate groups, followed by a nucleophilic attack by the nitrogen atom of isoxazoline 6 to form intermediate I. The subsequent attack of the malonyl moiety at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond creates the target bicyclo[3.3.0]octane skeleton. The stereoselectivity of the formation of the endo-7a kinetic product is presumably controlled by the more favorable conformation of intermediate II for the nucleophilic attack at the C
N bond creates the target bicyclo[3.3.0]octane skeleton. The stereoselectivity of the formation of the endo-7a kinetic product is presumably controlled by the more favorable conformation of intermediate II for the nucleophilic attack at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond compared to that of conformation III (Scheme 3). In turn, the presence of two geminal ester groups, additionally coordinated by the Lewis acid on the one hand and by the donor O–N moiety on the other hand, leads to strong polarization of the C(5)–C(6) bond in endo-7a and, consequently, to its cleavage and regeneration of intermediate I. This, in turn, makes it possible to shift the equilibrium toward the thermodynamically more favorable exo-7a isomers with increasing reaction time.
N bond compared to that of conformation III (Scheme 3). In turn, the presence of two geminal ester groups, additionally coordinated by the Lewis acid on the one hand and by the donor O–N moiety on the other hand, leads to strong polarization of the C(5)–C(6) bond in endo-7a and, consequently, to its cleavage and regeneration of intermediate I. This, in turn, makes it possible to shift the equilibrium toward the thermodynamically more favorable exo-7a isomers with increasing reaction time.
 | ||
| Scheme 3 Plausible stereochemical model of the reaction (the Lewis acid on the malonate fragment of ACDC and the substituent of the isoxazoline moiety are omitted for clarity). | ||
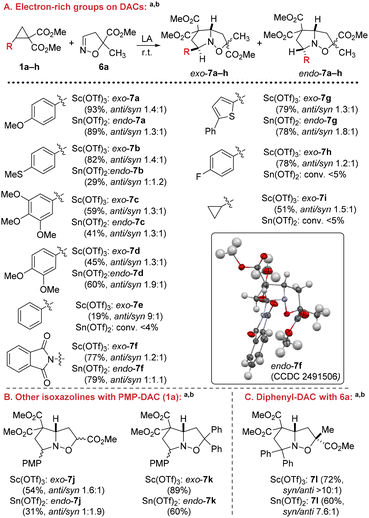
Next, we carried out a series of [3 + 2]-cycloaddition reactions of isoxazoline 6a with donor–acceptor cyclopropanes 1a–i bearing various aromatic substituents in the DAC moiety. One of the drawbacks of the [3 + 2] cycloaddition reactions was the high sensitivity of the process to the electronic effects of substituents in the donor part of the DAC. In fact, when Sc(OTf)3 or Sn(OTf)2 was used, only cyclopropanes containing strong electron-donor substituents efficiently underwent the target reaction, giving the desired products in high yields as either exo- or endo-isomers, with the configuration of the main center depending on the Lewis acid used (Scheme 4A).
It was also shown that the rather highly reactive PMP-DAC 1a successfully reacted with other isoxazolines, in particular, 5-methoxycarbonyl- and 5,5-diphenylisoxazolines 6b and 6c, exhibiting the same regularities as in the case of isoxazoline 6a (Scheme 4B).
Incorporation of a second phenyl substituent into the donor part of the DAC (1l) does not significantly affect the efficiency of the process in the presence of either Sc(OTf)3 or Sn(OTf). However, it eliminates the formation of a mixture of exo- and endo-isomers, and isomer 7l with the syn-position of the methoxycarbonyl group at the C(3) atom relative to the bicyclic system becomes predominant in this case (Scheme 4C).
At the same time, even DACs lacking electron-acceptor groups in the aryl moiety, such as Ph, undergo annulation only with great difficulty. For example, the conversion of 1e in the presence of Sc(OTf)3 at room temperature was as low as 19% after 20 h (Table 2, entry 1). A somewhat better result was obtained at 40 °C; however, the conversion of cyclopropane was also below 40% in this case (Table 2, entry 2).
| Entry | Lewis acid | T, °C (t, h) | Conv. 1e, % | Yields, % (dr) | |
|---|---|---|---|---|---|
| exo-7e (anti/syn) | endo-7e (anti/syn) | ||||
| 1 | Sc(OTf)3 | 20 (20) | 19 | 17 | 2 |
| 2 | Sc(OTf)3 | 40 (8) | 38 | 36 (1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
2 |
| 3 | Yb(NTf2)3 | 20 (20) | 100 | 62 (1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
19 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2) 1.2) |
| 4 | Sc(NTf2)3 | 20 (20) | 100 | 59 (1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
20 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
| 5 | Sn(NTf2)2 | 20 (20) | <10 | 4 | 4 |
| 6 | Yb(NTf2)3 | 40 (6) | 100 | 65 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
15 (syn) |
| 7 | Yb(NTf2)3 | 80 (6) | 100 | 63 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
8 (syn) |
This drawback was overcome by the use of triflimides, which are more reactive Lewis superacids.11 Scandium, ytterbium, and tin(II) triflimides were synthesized using the reported procedures.12
As expected, replacement of the catalyst with the more electrophilic Lewis acids Yb(NTf2)3 and Sc(NTf2)3 resulted in the acceleration of the (3 + 2)-annulation of non-activated DACs with isoxazolines. For example, cyclopropane 1e reacted with isoxazoline 6a even at room temperature with complete conversion, affording the target product 7e in yields up to 80% (Table 2, entries 3 and 4). Sn(NTf2)2 exhibited low activity, which did not allow endo-7e to become the predominant reaction product (Table 2, entry 5).
However, unlike the DACs with electron-donor aryl substituents, this reaction could not be completely shifted toward the thermodynamically stable exo-7e isomers by increasing the reaction temperature (Table 2, entries 6 and 7), which may be caused by catalyst deactivation.
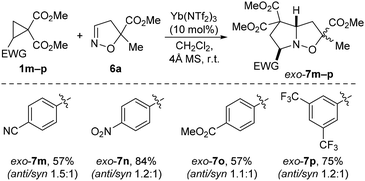
Next, using the developed triflimide methodology, a series of [3 + 2] cycloaddition reactions were carried out with low-activity DACs bearing various electron-acceptor groups in the aromatic moiety, which reduce the reactivity of cyclopropane. It was found that even in the case of DACs such as the 4-cyano- and 4-nitrophenyl derivatives, ytterbium triflimide demonstrated high efficiency in reactions with isoxazoline 6a, affording the formal [3 + 2] cycloaddition products 7m–p in 57–84% yields with a high degree of exo-orientation of the aryl substituent (Scheme 5).
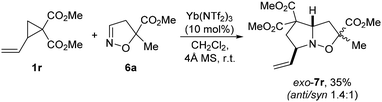
The reaction of isoxazoline 6a catalyzed by Yb(NTf2)3 occurs with 2-vinylcyclopropane-1,1-dicarboxylate 1r as well. Although the yield was not high, the corresponding bicyclic adduct 7r was also isolated as two anti- and syn-isomers with exo-orientation of the vinyl substituent (Scheme 6).
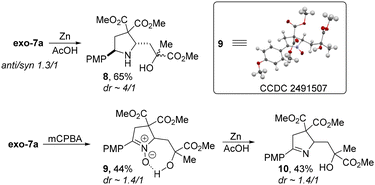
The possibility of selective reduction of the N–O bond in the exo-7a adduct was also demonstrated. Direct reduction with Zn in AcOH yielded the pyrrolidine derivative 8 with the substituents at positions 2 and 5 exclusively in the trans-configuration (Scheme 7). At the same time, conversion of 7a into N-oxide 9 prior to the reduction made it possible to obtain the dihydropyrrole derivative 10 that also finds use as a key structural fragment in a number of biologically active compounds.
Bicyclo[1.1.0]butanes (BCBs) have found extremely broad application in various cycloaddition reactions in recent years, providing easy access to a range of bicyclo[n.1.1]alkanes, which are currently being actively studied as promising 3D bioisosteres of diverse arenes and heteroarenes.13
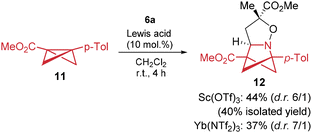
BCBs bearing aryl donors and carbonyl acceptors can be regarded as extremely strained variants of well-known donor–acceptor cyclopropanes (DACs). Therefore, we demonstrated that isoxazolines can also be successfully involved in formal [3 + 3]-cycloaddition with 1-aryl-3-EWG-substituted BCBs in the presence of catalytic amounts of Sc(OTf)3 or Yb(NTf2)3 (Scheme 8). It should be noted that the optimized yields of ca. 40% of compound 12 (see the SI for details) are due to the high propensity of BCBs to undergo oligomerization, a behavior typical of many reactions involving BCBs.
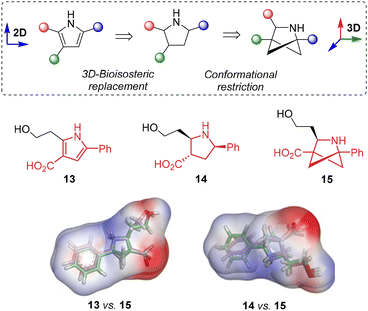
The 2-azabicyclo[2.1.1]hexane moiety obtained in this reaction can be considered as a 3D bioisosteric replacement both for the planar aromatic pyrrole moiety and for the conformationally non-rigid saturated pyrrolidine moiety. To demonstrate this possibility, simplified model structures 13–15 were used as examples and their geometric and electronic parameters were compared (Table 3, Fig. 3). As can be seen from Table 3, substitution of either the pyrrole moiety in 13 or the pyrrolidine moiety in 14 with a 2-azabicyclo[2.1.1]hexane moiety (structure 15) results in only minor changes in the molecular geometry, the molecular surface area (ASA), and the topological polar surface area (TPSA). At the same time, parameters such as lipophilicity, solubility in aqueous media, and skin permeability increase, indicating the strong potential of such 3D bioisosteric replacement for drug design.
Conclusions
In this work, we propose a stereoselective intermolecular approach for the construction of the pyrrolidine moiety via [3 + 2] cycloaddition of DACs with isoxazolines catalyzed by Lewis acids. Stereoselectivity is controlled either kinetically or thermodynamically, which is achieved by employing Sn or Sc triflates. In addition, directed regulation of the Lewis acidity of the catalyst by selecting a weakly coordinating anion (WCA) NTf2 instead of OTf enables successful involvement of low-reactivity DACs bearing electron-acceptor substituents at the aromatic ring. The reaction of isoxazoline [3 + 2] cycloaddition was further extended to bicyclo[1.1.0]butanes (BCBs), providing easy access to the 2-azabicyclo[2.1.1]hexane core, which can be considered as a promising 3D-bioisosteric replacement of pyrrole and pyrrolidine motifs.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental procedures, characterization data, NMR spectra of compounds and X-ray crystallographic data Supplementary information is available. See DOI: https://doi.org/10.1039/d5ob01604e.CCDC 2491505–2491507: Experimental Crystal Structure Determination, 2025, https://dx.doi.org/10.5517/ccdc.csd.cc2pmm43, https://dx.doi.org/10.5517/ccdc.csd.cc2pmm54, https://dx.doi.org/10.5517/ccdc.csd.cc2pmm65, contain the supplementary crystallographic data for this paper.18a–c
Acknowledgements
This work was funded by the Russian Science Foundation (RSF) under the research project no. 25-13-00574. This work was performed using the equipment in the Shared Research Center (Department of Structural Studies) of the N. D. Zelinsky Institute of Organic Chemistry, RAS, Moscow. A. A. Korlyukov and A. D. Volodin are grateful to the Ministry of Science and Higher Education of the Russian Federation (Contract No. 075-00276-25-00) for providing access to the equipment of the Center for Collective Use of INEOS RAS in X-ray crystallographic studies.References
- (a) I. P. Beletskaya, C. Nájera and M. Yus, Stereodivergent Catalysis, Chem. Rev., 2018, 118, 5080–5200 CrossRef CAS PubMed; (b) X. Huo, G. Li, X. Wang and W. Zhang, Bimetallic Catalysis in Stereodivergent Synthesis, Angew. Chem., Int. Ed., 2022, 61, e202210086 CrossRef CAS; (c) A. Kumari, A. Jain and N. K. Rana, A Review on Solvent-Controlled Stereodivergent Catalysis, Tetrahedron, 2024, 150, 133754 CrossRef CAS; (d) H. Sun, Y. Ma, G. Xiao and D. Kong, Stereodivergent Dual Catalysis in Organic Synthesis, Trends Chem., 2024, 6, 684–701 CrossRef CAS; (e) P. Li, S. Sun, L. Wei, W. Zi, C.-J. Wang and W. Zhang, Stereodivergent Synthesis of Multi-Stereocentric Compounds by Synergistic Dual Catalysis, CCS Chem., 2025, 1–63 Search PubMed.
- (a) T. F. Schneider, J. Kaschel and D. B. Werz, A New Golden Age for Donor–Acceptor Cyclopropanes, Angew. Chem., Int. Ed., 2014, 53, 5504–5523 CrossRef CAS PubMed; (b) H. K. Grover, M. R. Emmett and M. A. Kerr, Carbocycles from Donor–Acceptor Cyclopropanes, Org. Biomol. Chem., 2015, 13, 655–671 RSC; (c) P. G. Sergeev, R. A. Novikov and Yu. V. Tomilov, Cyclization Reactions of Cyclopropane Derivatives with Conjugated Carbo- and Heterosystems, Russ. Chem. Rev., 2024, 93, RCR5111 CrossRef; (d) V. Pirenne, B. Muriel and J. Waser, Catalytic Enantioselective Ring-Opening Reactions of Cyclopropanes, Chem. Rev., 2021, 121, 227–263 CrossRef CAS PubMed.
- (a) A. Deepthi, C. B. Meenakshy and M. Mohan, Synthesis of Heterocycles from Donor-Acceptor Cyclopropanes: A Five-Year Recap, Synthesis, 2023, 3875–3894 CrossRef CAS; (b) A. Ghosh, R. Dey and P. Banerjee, Relieving the Stress Together: Annulation of Two Different Strained Rings towards the Formation of Biologically Significant Heterocyclic Scaffolds, Chem. Commun., 2021, 57, 5359–5373 RSC; (c) M. Bao and M. P. Doyle, Asymmetric [3 + n]–Cycloaddition Reactions of Donor–Acceptor Cyclopropanes, ChemCatChem, 2023, 15, e202301090 CrossRef CAS; (d) A. U. Augustin and D. B. Werz, Exploiting Heavier Organochalcogen Compounds in Donor–Acceptor Cyclopropane Chemistry, Acc. Chem. Res., 2021, 54, 1528–1541 CrossRef CAS PubMed; (e) F. Doraghi, S. Karimian, O. H. Qareaghaj, M. J. Karimi, B. Larijani and M. Mahdavi, Recent Advances in Ring-Opening Reactions of 2-Substituted Donor-Acceptor Cyclopropanes under Metal Catalysis, J. Organomet. Chem., 2024, 1005, 122963 CrossRef CAS.
- (a) W. Zhou, B. Yang, Y. Zou, K. Rahman, X. Cao, Y. Lei, R. Lai, Z. F. Fu, X. Chen and G. Cao, Screening of Compounds for Anti-Tuberculosis Activity, and in Vitro and in Vivo Evaluation of Potential Candidates, Front. Microbiol., 2021, 12, 658637 CrossRef PubMed; (b) S. Poyraz, H. A. Döndaş, N. Y. Döndaş and J. M. Sansano, Recent Insights about Pyrrolidine Core Skeletons in Pharmacology, Front. Pharmacol., 2023, 14, 1239658 CrossRef CAS PubMed; (c) R. Seabra, F. Malhão, A. Correia, C. Costa, A. Kijjoa and E. Rocha, Effects and Mechanisms of Action of Preussin, a Marine Fungal Metabolite, against the Triple-Negative Breast Cancer Cell Line, MDA-MB-231, in 2D and 3D Cultures, Mar. Drugs, 2023, 21, 166 CrossRef CAS PubMed; (d) S. Poyraz, H. A. Döndaş, C. Yamali, S. Belveren, Y. Demir, S. Aydınoglu, N. Y. Döndaş, T. Taskin-Tok, S. Taş, M. Ülger and J. M. Sansano, Design, Synthesis, Biological Evaluation and Docking Analysis of Pyrrolidine-Benzenesulfonamides as Carbonic Anhydrase or Acetylcholinesterase Inhibitors and Antimicrobial Agents, J. Biomol. Struct. Dyn., 2024, 42, 3441–3458 CrossRef CAS PubMed.
- (a) Y.-B. Kang, Y. Tang and X.-L. Sun, Scandium Triflate Catalyzed Cycloaddition of Imines with 1,1-Cyclopropanediesters: Efficient and Diastereoselective Synthesis of Multisubstituted Pyrrolidines, Org. Biomol. Chem., 2006, 4, 299–301 RSC; (b) A. T. Parsons, A. G. Smith, A. J. Neel and J. S. Johnson, Dynamic Kinetic Asymmetric Synthesis of Substituted Pyrrolidines from Racemic Cyclopropanes and Aldimines: Reaction Development and Mechanistic Insights, J. Am. Chem. Soc., 2010, 132, 9688–9692 CrossRef CAS PubMed; (c) P. Su, H. Li, W. Chen, G. Luo, G. Yang and Z. Chai, Lewis Acid Catalyzed [3 + 2] Annulations of γ–Butyrolactam–Fused Donor–Acceptor Cyclopropanes with Aromatic Aldehydes and Aldimines, Eur. J. Org. Chem., 2020, 5380–5387 CrossRef CAS; (d) M. Bao and M. P. Doyle, Stereoretentive Catalytic [3 + 2]/[3 + 3]-Cycloaddition of Nonracemic Donor–Acceptor Cyclopropanes: Synthesis of Substituted Pyrrolidines and 1,2-Oxazinanes, Org. Lett., 2023, 25, 3029–3033 CrossRef CAS PubMed; (e) S. M. Antropov, S. A. Tokmacheva, I. I. Levina, O. A. Ivanova and I. V. Trushkov, Synthesis of Bridged Bicyclic Systems Peri–Annulated to the Indole Ring: Tropane–Fused Indoles, Adv. Synth. Catal., 2024, 366, 2784–2790 CrossRef CAS; (f) S. M. Antropov, S. A. Tokmacheva, I. I. Levina and I. V. Trushkov, Synthesis of Bridged Azaheterocycles Peri-Annulated to the Indole Core, Russ. Chem. Bull., 2025, 74, 102–109 CrossRef CAS.
- (a) J. E. Curiel Tejeda, L. C. Irwin and M. A. Kerr, Annulation Reactions of Donor–Acceptor Cyclopropanes with (1-Azidovinyl)Benzene and 3-Phenyl-2H-Azirine, Org. Lett., 2016, 18, 4738–4741 CrossRef CAS PubMed; (b) J.-A. Xiao, J. Li, P.-J. Xia, Z.-F. Zhou, Z.-X. Deng, H.-Y. Xiang, X.-Q. Chen and H. Yang, Diastereoselective Intramolecular [3 + 2]-Annulation of Donor–Acceptor Cyclopropane with Imine-Assembling Hexahydropyrrolo[3,2-c]Quinolinone Scaffolds, J. Org. Chem., 2016, 81, 11185–11194 CrossRef CAS PubMed; (c) J. Li, J.-A. Xiao, S.-J. Zhao, H.-Y. Xiang and H. Yang, Facile Construction of Pyrrolo[1,2-a]Indolenine Scaffold via Dia-stereoselective [3 + 2] Annulation of Donor–Acceptor Cyclopropane with Indolenine, Synthesis, 2017, 4292–4298 CAS; (d) H. S. Dutta, A. Ahmad, A. A. Khan, M. Kumar, Raziullah, J. Vaishnav, M. Gangwar, R. S. Ampapathi and D. Koley, Diastereoselective [3 + 2] Cycloaddition of Quinoxalin-2(1 H)-Ones with Donor–Acceptor Cyclopropanes: Efficient Synthesis of Tetrahydro Pyrrolo[1,2- a]Quinoxalin-4(5 H)-Ones, J. Org. Chem., 2021, 86, 16558–16572 CrossRef CAS PubMed; (e) X.-Y. Wang, X.-B. Wang, Y. Tian, C. Peng, M.-S. Xie and H.-M. Guo, Cobalt-Catalyzed Asymmetric Dearomative [3 + 2] Annulation of Quinolines, Isoquinolines, and Pyridines, ACS Catal., 2023, 13, 11528–11540 CrossRef CAS.
- (a) S. J. Gharpure, S. A. Hajam and D. S. Vishwakarma, TMSOTf–Mediated Aminocyclization/[1,3]–Sulfonyl Migration of N –Homopropargyl Hydroxylamines for the Stereoselective Synthesis of 3-Sulfonyl Cyclic Nitrones, Adv. Synth. Catal., 2024, 366, 5044–5049 CrossRef CAS; (b) Y.-X. Li, J.-Z. Wang, Y. Shimadate, M. Kise, A. Kato, Y.-M. Jia, G. W. J. Fleet and C.-Y. Yu, Diastereoselective Synthesis, Glycosidase Inhibition, and Docking Study of C-7-Fluorinated Casuarine and Australine Derivatives, J. Org. Chem., 2022, 87, 7291–7307 CrossRef CAS PubMed; (c) A. Ranzenigo, C. Mercurio, M. Karrenbrock, F. M. Cordero, G. Cardini, M. Pagliai and A. Brandi, Regioselective Deuteration of a 3,4-Dialkoxypyrroline N –Oxide and Synthesis of 8a– d –Indolizidines, Eur. J. Org. Chem., 2020, 3423–3429 CrossRef CAS; (d) K. Nagasawa and J. Shimokawa, Identification of Target Protein for Batzelladines as CD4, Heterocycles, 2019, 99, 521 CrossRef PubMed; (e) C. Matassini, G. D'Adamio, C. Vanni, A. Goti and F. Cardona, Studies for the Multimerization of DAB–1-Based Iminosugars through Iteration of the Nitrone Cycloaddition/Ring–Opening/Allylation Sequence, Eur. J. Org. Chem., 2019, 4897–4905 CrossRef CAS; (f) E. L. Kui, A. Kanazawa, J. Behr and S. Py, Ring–Junction–Substituted Polyhydroxylated Pyrrolizidines and Indolizidines from Ketonitrone Cycloadditions, Eur. J. Org. Chem., 2018, 2178–2192 Search PubMed; (g) M. Pieczykolan, B. Furman and M. Chmielewski, Formal Synthesis of Thienamycin, J. Antibiot., 2017, 70, 781–787 CrossRef CAS PubMed; (h) B. Cheng, Y. Hirokami, Y.-X. Li, A. Kato, Y.-M. Jia and C.-Y. Yu, Synthesis and Glycosidase Inhibition of C-7 Modified Casuarine Derivatives, Chin. Chem. Lett., 2017, 28, 1701–1704 CrossRef CAS; (i) Y.-X. Li, K. Kinami, Y. Hirokami, A. Kato, J.-K. Su, Y.-M. Jia, G. W. J. Fleet and C.-Y. Yu, Gem-Difluoromethylated and Trifluoromethylated Derivatives of DMDP-Related Iminosugars: Synthesis and Glycosidase Inhibition, Org. Biomol. Chem., 2016, 14, 2249–2263 RSC.
- (a) Y. V. Tomilov, R. A. Novikov and O. M. Nefedov, Lewis Acid Catalyzed Reactions of Donor–Acceptor Cyclopropanes with 1- and 2-Pyrazolines: Formation of Substituted 2-Pyrazolines and 1,2-Diazabicyclo[3.3.0]Octanes, Tetrahedron, 2010, 66, 9151–9158 CrossRef CAS; (b) R. A. Novikov, Yu. V. Tomilov and O. M. Nefedov, Formation of the Double Addition Products of Donor-Acceptor Cyclopropanes with 2-Pyrazolines in the Presence of Lewis Acids, Russ. Chem. Bull., 2012, 61, 1917–1924 CrossRef CAS; (c) R. A. Novikov, E. V. Shulishov and Y. V. Tomilov, Unusual C-Alkylation of Pyrazolines with 2-(Het)Arylcyclopropane-1,1-Dicarboxylates in the Presence of GaCl3, Mendeleev Commun., 2012, 22, 87–89 CrossRef CAS.
- (a) P. Kumar, R. Kumar and P. Banerjee, Accessing Dihydro-1,2-Oxazine via Cloke–Wilson-Type Annulation of Cyclopropyl Carbonyls: Application toward the Diastereoselective Synthesis of Pyrrolo[1,2-b,][1,2]Oxazine, J. Org. Chem., 2020, 85, 6535–6550 CrossRef CAS PubMed; (b) R. Dey, P. Kumar and P. Banerjee, Lewis Acid Catalyzed Annulation of Cyclopropane Carbaldehydes and Aryl Hydrazines: Construction of Tetrahydropyridazines and Application Toward a One-Pot Synthesis of Hexahydropyrrolo[1,2-b]Pyridazines, J. Org. Chem., 2018, 83, 5438–5449 CrossRef CAS PubMed; (c) A. A. Akaev, S. I. Bezzubov, V. G. Desyatkin, N. S. Vorobyeva, A. G. Majouga, M. Ya. Melnikov and E. M. Budynina, Stereocontrolled [3 + 2] Cycloaddition of Donor–Acceptor Cyclopropanes to Iminooxindoles: Access to Spiro[Oxindole-3,2′-Pyrrolidines], J. Org. Chem., 2019, 84, 3340–3356 CrossRef CAS PubMed.
- (a) S. K. Jackson, A. Karadeolian, A. B. Driega and M. A. Kerr, Stereodivergent Methodology for the Synthesis of Complex Pyrrolidines, J. Am. Chem. Soc., 2008, 130, 4196–4201 CrossRef CAS PubMed; (b) L. C. Irwin, M. A. Allen, M. R. Vriesen and M. A. Kerr, Annulation of Oxime–Ether Tethered Donor–Acceptor Cyclopropanes, Chem. – Eur. J., 2020, 26, 171–175 CrossRef CAS PubMed; (c) A. B. Leduc and M. A. Kerr, Total Synthesis of (−)–Allosecurinine, Angew. Chem., Int. Ed., 2008, 47, 7945–7948 CrossRef CAS PubMed.
- J. Gal, C. Iacobucci, I. Monfardini, L. Massi, E. Duñach and S. Olivero, Metal Triflates and Triflimides as Lewis “Superacids”: Preparation, Synthetic Application and Affinity Tests by Mass Spectrometry, J. Phys. Org. Chem., 2013, 26, 87–97 CrossRef CAS.
- M. J. Earle, U. Hakala, B. J. McAuley, M. Nieuwenhuyzen, A. Ramani and K. R. Seddon, Metal Bis{(Trifluoromethyl)Sulfonyl}amide Complexes: Highly Efficient Friedel–Crafts Acylation Catalysts, Chem. Commun., 2004, 1368–1369 RSC.
- (a) C. B. Kelly, J. A. Milligan, L. J. Tilley and T. M. Sodano, Bicyclobutanes: From Curiosities to Versatile Reagents and Covalent Warheads, Chem. Sci., 2022, 13, 11721–11737 RSC; (b) M. Golfmann and J. C. L. Walker, Bicyclobutanes as Unusual Building Blocks for Complexity Generation in Organic Synthesis, Commun. Chem., 2023, 6, 9 CrossRef CAS PubMed; (c) Y. Koo, J. Jeong and S. Hong, Recent Advances in Catalytic Asymmetric Transformations of Bicyclobutane: A Versatile Building Block for Enantiopure Bioisosteric Molecules, ACS Catal., 2025, 8078–8093 CrossRef.
- A. Daina, O. Michielin and V. Zoete, iLOGP: A Simple, Robust, and Efficient Description of n -Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach, J. Chem. Inf. Model., 2014, 54, 3284–3301 CrossRef CAS PubMed.
- J. Ali, P. Camilleri, M. B. Brown, A. J. Hutt and S. B. Kirton, Revisiting the General Solubility Equation: In Silico Prediction of Aqueous Solubility Incorporating the Effect of Topographical Polar Surface Area, J. Chem. Inf. Model., 2012, 52, 420–428 CrossRef CAS.
- R. O. Potts and R. H. Guy, Predicting Skin Permeability, Pharm. Res., 1992, 9, 663–669 CrossRef CAS PubMed.
- Y. C. Martin, A Bioavailability Score, J. Med. Chem., 2005, 48, 3164–3170 CrossRef CAS PubMed.
- (a) CCDC 2491505: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pmm43; (b) CCDC 2491506: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pmm54; (c) CCDC 2491507: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pmm65.
| This journal is © The Royal Society of Chemistry 2026 |