A telescoped diastereoselective synthesis of phthalimido-substituted spiro-oxazoline oxindoles via an aziridine expansion strategy
Anna A.
Nikolaeva
,
Ilya P.
Filippov
 ,
Oleg E.
Polekh
,
Alena S.
Pankova
,
Oleg E.
Polekh
,
Alena S.
Pankova
 and
Nikolai V.
Rostovskii
and
Nikolai V.
Rostovskii
 *
*
St. Petersburg State University, Institute of Chemistry, 7/9 Universitetskaya Emb., Saint Petersburg 199034, Russia. E-mail: n.rostovskiy@spbu.ru
First published on 28th November 2025
Abstract
A diastereoselective efficient synthesis of 5′H-spiro[indoline-3,2′-oxazol]-2-ones bearing a pharmacophore phthalimide fragment was developed. The approach is based on the thermal ring opening of spiro-aroyl-N-phthalimidoaziridine oxindoles, which are readily accessible via aminoaziridination of methylideneoxindoles. The formation of the final spiro compounds proceeds through the generation of azomethine ylides, followed by a selective 1,5-electrocyclization involving the aroyl carbonyl group and a formal 1,3-migration of the phthalimide substituent. The mechanism was confirmed by DFT calculations.
Introduction
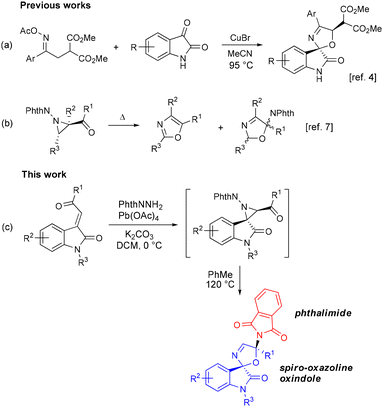
Spirocyclic scaffolds are found in various approved drugs, drug candidates and natural products.1 Among them, the spirooxindole heterocyclic scaffold occupies a privileged position.2 This is due to its rigidity and unique 3D-structure, including a hydrogen bond donor–acceptor amide fragment and a hydrophobic aromatic part. Numerous synthetic approaches have been developed to access spirooxindoles bearing different carbo- or heterocyclic units.3 Majority of the latter contain a saturated five-membered ring with one heteroatom, such as pyrrolidine or tetrahydrofuran.2a Compounds with an unsaturated ring with more than one heteroatom, for example pyrazoline or isoxazoline, are rarer and mainly accessible via 1,3-dipolar cycloaddition reactions.3d In 2021, the synthesis of a new class of spirooxindoles, namely 5′H-spiro[indoline-3,2′-oxazol]-2-ones, was first reported.4 It is based on a copper-catalyzed regioselective (3 + 2) annulation of malonate-tethered acyl oximes with isatins (Scheme 1a).Given the importance of spirooxindole derivatives and in continuation of our efforts toward the synthesis of 3-oxazolines,5 in this work, we aimed to develop a novel approach to access the 5′H-spiro[indoline-3,2′-oxazol]-2-one scaffold. Aziridines, which are strained three-membered heterocycles, have proven themselves as convenient substrates for the synthesis of more complex heterocycles.6 For example, it is known that 2,5-dihydrooxazoles with a phthalimide group (PhthN) are formed along with the corresponding oxazoles upon the thermolysis of certain N-phthalimidoaziridines (Scheme 1b), such as 2-benzoyl-3-styrylaziridine7a and thienyl-substituted diacetylaziridines.7b In this case, the formation of 2,5-dihydrooxazoles is accompanied by a shift of the phthalimide fragment from the nitrogen atom to the carbon atom. We envisioned that the desired spiro-2,5-dihydrooxazole oxindoles could be constructed via thermal transformation of N-phthalimidoaziridines obtained from the corresponding acylmethylideneoxindoles (Scheme 1c). To our delight, this assumption worked, and the target products were formed as single diastereomers. The advantages of this approach are the synthetic availability of substrates, the use of a telescoped methodology and an opportunity of introducing an additional pharmacophore – the phthalimide fragment. Notably, phthalimide derivatives exhibit anti-inflammatory, anti-Alzheimer, antiepileptic, antiplatelet, anticancer, antibacterial, antifungal, antiparasitic, antiviral, and antidiabetic properties, among others.8
It should be noted that spiro-aziridine oxindoles with a phthalimide substituent at the aziridine nitrogen have not been described in the literature.9 Only a range of spiro-aziridine oxindoles bearing carbon10a or sulfur10b substituents at the aziridine nitrogen have been reported. To the best of our knowledge, no transformations of such aziridines into oxazole derivatives have been reported.
Results and discussion
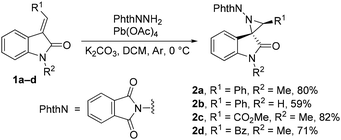
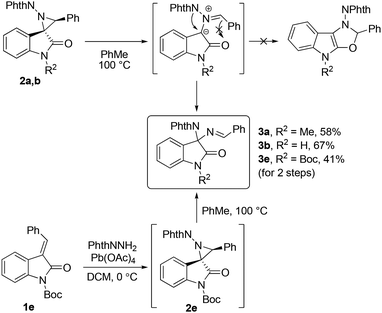
Firstly, a series of variously substituted spirocyclic aziridines 2a–d were obtained in good yields as single diastereomers from the corresponding (E)-methylideneoxindoles 1a–d using N-aminophthalimide and lead tetraacetate in dichloromethane (DCM) (Scheme 2).11 The structures of aziridines 2a–d were determined using nuclear magnetic resonance (NMR) spectra and high-resolution mass spectra (HRMS).Since N-phthalimidoaziridines spiro-fused with oxindoles are unknown, we examined the behaviour of the obtained aziridines 2 upon heating. For these compounds, the cleavage of the aziridine C–C bond to form an azomethine ylide followed by its cyclization involving the adjacent C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond to a fused oxazole derivative can be anticipated, similar to the behavior reported for N-phthalimidoaziridines spiro-fused with indane-1,3-dione.12 Heating of phenyl-substituted aziridines 2a and b resulted in the formation of previously unknown diaminooxindole derivatives 3a and b, which are formed as a result of a 1,2-migration of a phthalimide substituent, likely in the azomethine ylide (Scheme 3). A similar reaction was observed for the in situ generated phenylaziridine 2e with a Boc substituent at the oxindole nitrogen. The structures of compounds 3a, b, and e were determined on the basis of 2D NMR spectra and HRMS. The cyclization of the ylide to the fused oxazole derivative does not occur. The isomerization into the imine was also the major process during the heating of aziridine 2b with an excess of dimethyl acetylenedicarboxylate or dimethyl maleate, and potential 1,3-dipolar cycloaddition was not observed. Unfortunately, aziridine 2c with an ester group underwent non-selective decomposition upon heating in the temperature range of 60–140 °C. The addition of BF3·OEt2 or copper(II) complexes did not lead to any selective transformations.
O bond to a fused oxazole derivative can be anticipated, similar to the behavior reported for N-phthalimidoaziridines spiro-fused with indane-1,3-dione.12 Heating of phenyl-substituted aziridines 2a and b resulted in the formation of previously unknown diaminooxindole derivatives 3a and b, which are formed as a result of a 1,2-migration of a phthalimide substituent, likely in the azomethine ylide (Scheme 3). A similar reaction was observed for the in situ generated phenylaziridine 2e with a Boc substituent at the oxindole nitrogen. The structures of compounds 3a, b, and e were determined on the basis of 2D NMR spectra and HRMS. The cyclization of the ylide to the fused oxazole derivative does not occur. The isomerization into the imine was also the major process during the heating of aziridine 2b with an excess of dimethyl acetylenedicarboxylate or dimethyl maleate, and potential 1,3-dipolar cycloaddition was not observed. Unfortunately, aziridine 2c with an ester group underwent non-selective decomposition upon heating in the temperature range of 60–140 °C. The addition of BF3·OEt2 or copper(II) complexes did not lead to any selective transformations.
 | ||
| Scheme 3 Thermolysis of phenyl-substituted aziridines 2: synthesis of diaminooxindoles 3. Isolated yields are given. | ||
Heating of benzoylaziridine 2d at 100 °C for 4 h led to the target aziridine ring expansion product with a formal 1,3-migration of the phthalimide substituent, spirocyclic 3-oxazoline 4a, which was isolated by column chromatography as a single diastereomer in 67% yield (Scheme 4). The second diastereomer 4a′ was detected in trace amounts in the reaction mixture by 1H NMR spectroscopy. The structure of product 4a was determined using 2D NMR spectra and HRMS and confirmed by the X-ray diffraction analysis. The phthalimide substituent in the product is trans-oriented to the amide part of the oxindole. In this case, cyclization involving the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the amide group again does not occur.
O bond of the amide group again does not occur.
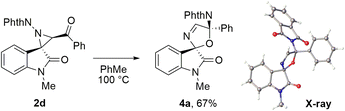 | ||
| Scheme 4 Thermolysis of benzoyl-substituted aziridine 2d: synthesis of spiro-2,5-dihydrooxazole oxindole 4a. Isolated yield is given. | ||
A brief optimization of the conditions for the synthesis of spirocyclic dihydrooxazole 4a showed that heating in toluene at 120 °C is optimal (yield 75%, reaction time 45 min). It is also possible to carry out both steps of the one-pot reaction in DCM without any loss of efficiency (the second step is conducted in an Ace pressure tube at 120 °C).
To study the reaction scope, a series of (aroylmethylidene)oxindoles (1f–p) were synthesized by the Wittig reaction from isatins and acylphosphonium ylides. All methylideneoxindoles were formed selectively as E-isomers. Further syntheses of spiro-2,5-dihydrooxazole oxindoles 4 were carried out as a telecoped process without isolation of intermediate aziridines 2 (in all cases, they were detected by TLC). It was shown (Scheme 5) that the method is tolerant toward a variety of substituents on the aroyl group. The product yield decreased when a strong electron-withdrawing substituent, nitro group, was introduced in the benzoyl fragment. Biphenyl, 2-naphthyl, and 2-thienyl substituents were successfully incorporated into the oxazoline moiety of products 4e, 4f, and 4g, respectively. In the latter case, the yield was lower. The spiro compound 4h without a substituent at the nitrogen atom of the oxindole was obtained, albeit in a low yield (side products were formed at the second step). Variation of substituents on the aromatic part of the oxindole fragment was examined. The 7-fluoro- and 5-nitro derivatives 4i and 4j were synthesized in good yields. Spirooxazoline 4k with a methoxy group at C5 of the oxindole was not obtained. Attempts to prepare compounds 4l and 4m by heating the acetylaziridine and the aziridine bearing a Boc group at the oxindole nitrogen under the same conditions led to non-selective decomposition. Probably, in these cases, the cyclization to spirooxazoline becomes unfavorable and the intermediate azomethine ylide undergoes decomposition.
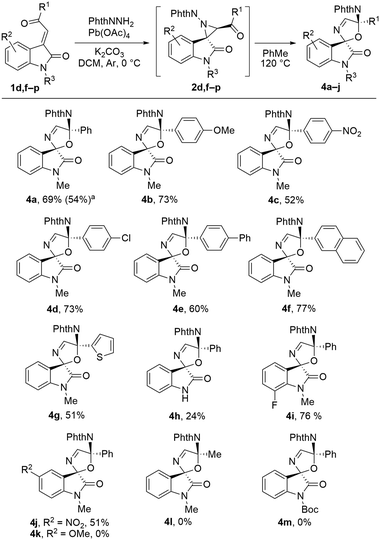 | ||
Scheme 5 Scope of spiro-2,5-dihydrooxazole oxindoles 4. Isolated yields for 2 steps are given. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Synthesis at the 2 mmol scale. Synthesis at the 2 mmol scale. | ||
The reaction of (Z)-methylideneoxindole 1d′ for preparing another oxazoline diastereomer 4a′ using the same procedure failed. A complex mixture of products was formed, containing, according to the 1H NMR spectrum, small amounts of diastereomeric oxazolines 4a and 4a′. We also tested other aziridinating reagents (3-amino-2-methylquinazolin-4(3H)-one and 2-amino-1H-benzo[de]isoquinolinone-1,3(2H)-dione) in place of N-aminophthalimide under the same conditions, but methylideneoxindole 1d did not react with them.
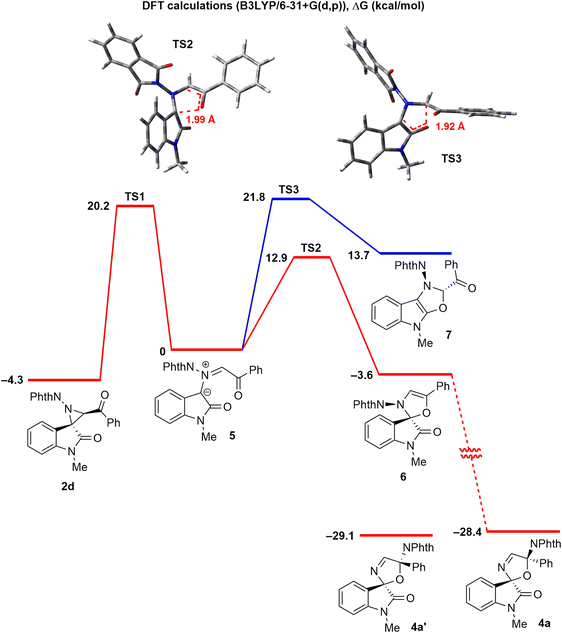
To investigate the mechanism of formation of spirooxazolines 4 in greater detail, density functional theory (DFT) calculations (B3LYP/6-31+g(d,p), polarizable continuum model (PCM) for toluene, 373 K) were performed for the transformations of N-phthalimidoaziridine 2d (Scheme 6). It was found that 2d undergoes a conrotatory electrocyclic ring opening to form ylide 5 with a barrier of 24.5 kcal mol−1 (TS1); ylide 5 is less stable than the starting aziridine by 4.3 kcal mol−1. Further 1,5-electrocyclization involving a benzoyl group proceeds stereoselectively to give spiro-4-oxazoline 6 (red line), in which the phthalimide substituent is cis-oriented to the aromatic part of the oxindole, with a relatively low barrier (TS2, 12.9 kcal mol−1). It is noteworthy that this reaction is reversible since the ring opening of oxazoline 6 back to ylide 5 also has quite a low barrier (16.5 kcal mol−1). Finally, after formal 1,3-shift of a phthalimide substituent, the thermodynamically more favourable isomeric spiro-3-oxazoline 4a is formed. Likely, this is a stepwise reaction in which the phthalimide anion initially leaves and then attaches to the carbon atom. The observed high diastereoselectivity of the oxazoline formation is probably related to the specific stereochemistry of intermediate 6. The 1,3-migration of the phthalimide substituent takes place on the same side of the oxazoline ring. Our calculations also showed that diastereomer 4a′ is more stable than 4a by 0.7 kcal mol−1. So, the high diastereoselectivity of oxazoline formation cannot be accounted for by thermodynamic factors.
An alternative cyclization pathway of ylide 5, namely 1,5-electrocyclization onto the amide fragment (Scheme 6, blue line), according to the calculations, proceeds stereoselectively to afford the trans-isomer of the fused dihydrooxazole 7, but it has a much higher barrier (TS3, 21.8 kcal mol−1) than the cyclization leading to the spiro compound 6. The reason for this is the low relative thermodynamic stability of compound 7 compared to ylide 5, which is attributed to the instability of the 4-oxazoline moiety,13 which is further increased in the 5–5 fused ring system. Indeed, while 1,3-dipolar cycloadditions of azomethine ylides derived from isatin are well known, 1,5-electrocyclization to give a fused 4-oxazoline has not been reported.14
It should be noted that, despite extensive knowledge of 1,5-electrocyclizations,15 such cyclizations of acylazomethine ylides have only been sparsely studied and are rarely discussed in the literature.5,16 In the transition states TS2 and TS3, the dihedral angle indicated in Scheme 6 is close to 180°. That is, lone pair electrons of the oxygen atom are involved in the bonding.17 Thus, both 1,5-electrocyclizations of ylide 5, leading to structures 6 and 7, can be referred to as pseudopericyclic reactions.
Finally, we demonstrated that the phthalimide fragment in spiro compounds 4 can be transformed. Thus, the reaction of compound 4a with sodium cyanoborohydride in a DCM–MeOH system led to the amino-substituted spiro-2,5-dihydrooxazole oxindole 8 in good yield (Scheme 7). The structure of compound 8 was determined using NMR spectra and HRMS. Obviously, this compound is formed by the methanolysis of the phthalimide fragment in 4a.
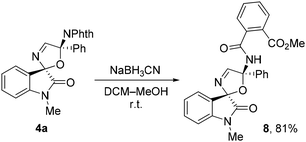 | ||
| Scheme 7 Synthesis of compound 8via transformation of the phthalimide group. Isolated yield is given. | ||
Conclusions
Previously unknown N-phthalimidoaziridines spiro-fused with oxindole were obtained by oxidative aminoaziridination of 3-methylideneoxindoles. Thermolysis of phenyl-substituted aziridines proceeded with a 1,2-migration of the phthalimide substituent to give 3-phthalimido-3-(benzylideneamino)oxindoles in good yields. In contrast, thermolysis of aroyl-substituted aziridines led to a novel diastereoselective and efficient synthesis of spiro-2,5-dihydrooxazole oxindoles. The formation of these compounds involves selective 1,5-electrocyclization of the azomethine ylide with participation of the aroyl carbonyl group and formal 1,3-migration of the phthalimide substituent. According to DFT calculations, cyclization of the azomethine ylide onto the aroyl group is kinetically and thermodynamically favoured compared to the cyclization on the amide carbonyl. The features of the developed approach toward spiro-2,5-dihydrooxazole oxindoles are the synthetic availability of substrates, the use of a telescoped methodology and the opportunity of introducing an additional pharmacophore – the phthalimide fragment.Experimental
General procedure for the synthesis of aziridines 2
Methylideneoxindole 1 (1 mmol), N-aminophthalimide (243 mg, 1.5 mmol) and anhydrous K2CO3 (1.11 g, 8 mmol) were placed in a round-bottom flask and anhydrous DCM (20 mL) was added. The flask was purged with argon, sealed with a septum and a solution of Pb(OAc)4 (665 mg, 1.5 mmol) in anhydrous DCM (7 mL) was added using a syringe at 0 °C within 15 min. After the reaction was completed (monitored by TLC), the resulting mixture was passed through a Celite plug, the solvent was removed in vacuo, and the residue was purified by column chromatography on silica gel to give aziridines 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
White solid, mp 156–157 °C.
1H NMR (400 MHz, CDCl3) δ 7.94–7.66 (m, 4H), 7.60–7.53 (m, 2H), 7.45–7.37 (m, 3H), 7.33 (td, J = 7.6, 1.3 Hz, 1H), 6.97 (d, J = 7.7 Hz, 1H), 6.86 (td, J = 7.7, 1.0 Hz, 1H), 6.39 (dd, J = 7.6, 1.3 Hz, 1H), 5.00 (s, 1H), 3.32 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 169.0, 164.6, 144.8, 134.0, 131.9, 130.6 (br), 130.0 (br), 129.3, 128.5, 128.4, 128.4, 123.6, 123.2, 122.4, 122.4, 108.4, 57.1, 52.1, 26.8.
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C24H17N3NaO3+ 418.1162; found 418.1161.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.
10.
Beige solid, mp 123–125 °C (dec.)
1H NMR (400 MHz, CDCl3) δ 7.84–7.69 (m, 5H), 7.55–7.54 (m, 2H), 7.43–7.36 (m, 3H), 7.26–7.19 (t, J = 7.7 Hz, 1H), 6.89 (d, J = 7.8 Hz, 1H), 6.80 (t, J = 7.6 Hz, 1H), 6.35 (d, J = 7.5 Hz, 1H), 4.95 (s, 1H).
13C{1H} NMR (100 MHz, CDCl3) δ 170.6, 141.9, 134.2, 131.9, 129.4, 128.7, 128.63, 128.60, 124.2, 123.4, 122.9, 122.6, 110.3, 57.6, 52.4.
HRMS (ESI/Q-TOF) m/z: [M + H]+ calcd for C23H16N3O3+ 382.1186; found 382.1189.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
White solid, mp 153–155 °C.
1H NMR (400 MHz, CDCl3) δ 7.88–7.69 (m, 4H), 7.53–7.39 (m, 2H), 7.15 (td, J = 7.7, 1.1 Hz, 1H), 6.99 (d, J = 7.7 Hz, 1H), 4.44 (s, 1H), 3.90 (s, 3H), 3.28 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 167.4, 165.0, 163.9 (br), 145.0, 134.1, 130.2, 130.0 (br), 124.1, 123.4, 123.2, 121.3, 108.8, 52.9, 52.7, 50.7, 26.9.
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C20H15N3NaO5+ 400.0904; found 400.0905.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
White solid, mp 146–149 °C.
1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 7.0 Hz, 2H), 7.95–7.69 (m, 4H), 7.65–7.58 (m, 1H), 7.49 (t, J = 7.8 Hz, 2H), 7.38 (td, J = 7.9, 1.3 Hz, 1H), 7.28–7.25 (m, 1H), 7.06 (td, J = 7.6, 1.0 Hz, 1H), 6.96 (d, J = 7.9 Hz, 1H), 5.25 (s, 1H), 3.32 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 189.4, 167.7, 164.2, 144.8, 135.8, 134.2, 134.2, 130.3 (br), 130.1, 129.7 (br), 128.9, 128.7, 124.2, 123.4, 123.2, 121.3, 108.8, 56.3, 52.0, 27.0.
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C25H17N3NaO4+ 446.1111; found 446.1110.
General procedure for the synthesis of diaminooxindoles 3
A solution of aziridines 2a, 2b, and 2e (0.15 mmol) in anhydrous toluene (2 mL) in a screw-cap tube was stirred at 100 °C (oil bath temperature) for 1.5 h. After the reaction was completed (monitored by TLC), the solvent was removed in vacuo, and the residue was purified by column chromatography on silica gel to give diaminooxindoles 3.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
3.
Pale-yellow solid, mp 186–189 °C.
1H NMR (400 MHz, CDCl3) δ 8.25 (s, 1H), 7.85–7.78 (m, 4H), 7.73 (dd, J = 5.5, 3.1 Hz, 2H), 7.52 (dd, J = 7.4, 1.3 Hz, 1H), 7.48–7.38 (m, 4H), 7.14 (td, J = 7.6, 1.0 Hz, 1H), 6.98 (d, J = 7.9 Hz, 1H), 3.36 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 170.3, 167.0, 162.8, 143.9, 135.2, 134.2, 131.9, 131.6, 130.8, 129.2, 128.5, 126.4, 125.7, 123.3 (2C), 108.9, 79.4, 26.8.
HRMS (ESI/Q-TOF) m/z: [M + H]+ calcd for C24H19N3O3+ 396.1348; found 396.1345.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.
7.
Pale-beige solid, mp 179–180 °C (dec.).
1H NMR (400 MHz, CDCl3) δ 8.20 (s, 1H), 7.89 (s, 1H), 7.89–7.81 (m, 4H), 7.73–7.71 (m, 2H), 7.47–7.26 (m, 5H), 7.09 (t, J = 7.8 Hz, 1H), 6.97 (d, J = 7.8 Hz, 1H).
13C{1H} NMR (100 MHz, CDCl3) δ 171.5, 167.1, 162.9, 140.9, 135.4, 134.4, 132.1, 131.9, 131.0, 129.4, 128.7, 126.7, 126.3, 123.6, 110.9, 79.8.
HRMS (ESI/Q-TOF) m/z: [M + H]+ calcd for C23H16N3O3+ 382.1186; found 382.1188.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30.
30.
Pale-beige solid, mp 136–137 °C (dec.).
1H NMR (400 MHz, CDCl3) δ 8.17 (s, 1H), 8.03 (d, J = 8.3 Hz, 1H), 7.82–7.79 (m, 4H), 7.74–7.70 (m, 2H), 7.51–7.38 (m, 5H), 7.22 (t, J = 7.5 Hz, 1H), 1.66 (s, 9H).
13C{1H} NMR (100 MHz, CDCl3) δ 167.9, 167.0, 163.7, 149.2, 140.2, 135.3, 134.5, 132.0, 131.97, 131.1, 129.5, 128.7, 125.7, 125.6, 125.3, 123.6, 116.1, 85.0, 79.5, 28.2.
HRMS (ESI/Q-TOF) m/z: [M + H]+ calcd for C28H24N3O5+ 482.1711; found 482.1723.
General procedure for the synthesis of spiro-2,5-dihydrooxazole oxindoles 4
Methylideneoxindoles 1d,f–p (0.3 mmol), N-aminophthalimide (72.9 mg, 0.45 mmol) and anhydrous K2CO3 (334 mg, 2.4 mmol) were placed in a round-bottom flask and anhydrous DCM (6 mL) was added. The flask was purged with argon, sealed with a septum and a solution of Pb(OAc)4 (199.4 mg, 0.45 mmol) in anhydrous DCM (2 mL) was added using a syringe at 0 °C within 15 min. After the reaction was completed (monitored by TLC), the resulting mixture was passed through a Celite plug and the solvent was removed in vacuo. The residual crude aziridines 2d and 2f–p was dissolved in anhydrous toluene (6 mL), placed in a screw-cap tube and stirred at 120 °C (oil bath temperature) for 35–120 min. The reaction progress was monitored by TLC. After completion of the reaction, the solvent was removed in vacuo, and the residue was purified by column chromatography on silica gel to give spiro compounds 4. The details for each synthesis are indicated below.Synthesis from methylideneoxindole 1d. Compound 4a (85 mg, yield 67%) was obtained from methylideneoxindole 1d (78.9 mg, 0.3 mmol) according to the general procedure [2nd step: 50 min; eluent for chromatography: EtOAc–hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.75].
1.75].
2 mmol scale synthesis. Compound 4a (455 mg, yield 54%) was obtained from methylideneoxindole 1d (526 mg, 2 mmol) according to the general procedure [2nd step: 50 min; eluent for chromatography: EtOAc–hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.75], with a proportional increase in quantities of the reagents.
1.75], with a proportional increase in quantities of the reagents.
Synthesis from aziridine 2d. A solution of aziridine 2d (60 mg, 0.14 mmol) in anhydrous toluene (2 mL) was stirred at 100 °C for 4 h. After the reaction was completed (controlled by TLC), the solvent was removed in vacuo, and the residue was purified by column chromatography on silica gel (eluent: EtOAc–hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.75) to give product 4a (40.2 mg, yield 67%).
1.75) to give product 4a (40.2 mg, yield 67%).
Pale-yellow solid, mp 240–241 °C.
1H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 7.93 (dd, J = 5.5, 3.1 Hz, 2H), 7.85–7.77 (m, 4H), 7.47 (t, J = 7.5 Hz, 2H), 7.42–7.32 (m, 2H), 6.97–6.85 (m, 3H), 3.29 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 170.6, 166.8, 162.2, 143.8, 135.8, 134.8, 131.5, 131.4, 129.0, 128.9, 125.9, 125.1, 124.3, 124.0, 123.4, 108.8, 108.4, 100.4, 26.5.
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C25H17N3NaO4+ 446.1111; found 446.1110.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2].
2].
Orange solid, mp 186–189 °C.
1H NMR (400 MHz, CDCl3) δ 8.39 (s, 1H), 7.93 (dd, J = 5.5, 3.0 Hz, 2H), 7.83 (dd, J = 5.5, 3.0 Hz, 2H), 7.71 (d, J = 8.9 Hz, 2H), 7.34 (td, J = 7.7, 1.5 Hz, 1H), 6.98 (d, J = 8.9 Hz, 2H), 6.96–6.91 (m, 1H), 6.91–6.84 (m, 2H), 3.83 (s, 3H), 3.29 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 170.7, 166.9, 162.3, 160.1, 143.9, 134.8, 131.6, 131.4, 127.9, 127.3, 125.3, 124.3, 124.0, 123.4, 114.3, 108.8, 108.2, 100.5, 55.3, 26.5.
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C26H19N3NaO5+ 476.1217; found 476.1214.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2].
2].
Orange solid, mp 251–252 °C (dec.).
1H NMR (400 MHz, CDCl3) δ 8.39 (s, 1H), 8.33 (d, J = 9.0 Hz, 2H), 8.01 (d, J = 9.0 Hz, 2H), 7.95 (dd, J = 5.5, 3.0 Hz, 2H), 7.87 (dd, J = 5.5, 3.0 Hz, 2H), 7.36–7.41 (m, 1H), 7.01–6.97 (m, 1H), 6.95–6.90 (m, 2H), 3.31 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 170.3, 166.7, 160.8, 148.2, 143.8, 142.8, 135.2, 131.8, 131.3, 127.3, 124.6, 124.4, 124.2, 124.1, 123.6, 109.0 (2C), 99.4, 26.6.
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C25H16N4NaO6+ 491.0962; found 491.0958.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2].
2].
Pale-yellow solid, mp 224–226 °C.
1H NMR (400 MHz, CDCl3) δ 8.38 (s, 1H), 7.94 (dd, J = 5.5, 3.0 Hz, 2H), 7.84 (dd, J = 5.5, 3.0 Hz, 2H), 7.74 (d, J = 8.7 Hz, 2H), 7.44 (d, J = 8.7 Hz, 2H), 7.36 (td, J = 7.7, 1.4 Hz, 1H), 7.01–6.92 (m, 1H), 6.91–6.85 (m, 2H), 3.29 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 170.5, 166.8, 161.7, 143.8, 135.1, 134.9, 134.5, 131.5, 131.4, 129.1, 127.5, 124.9, 124.3, 124.1, 123.5, 108.9, 108.5, 99.9, 26.5.
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C25H1635ClN3NaO4+ 480.0722; found 480.0719.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5].
1.5].
Pale-orange solid, mp 253–254 °C (dec.).
1H NMR (400 MHz, CDCl3) δ 8.45 (s, 1H), 7.95 (dd, J = 5.5, 3.1 Hz, 2H), 7.88–7.83 (m, 4H), 7.69 (d, J = 8.4 Hz, 2H), 7.63–7.59 (m, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.40–7.32 (m, 2H), 7.01–6.87 (m, 3H), 3.31 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 170.6, 166.9, 162.1, 143.8, 141.9, 140.5, 134.9, 134.8, 131.5, 131.5, 128.7, 127.7, 127.5, 127.2, 126.4, 125.1, 124.3, 124.0, 123.4, 108.8, 108.5, 100.4, 26.5.
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C31H21N3NaO4+ 522.1424; found 522.1422.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3].
3].
White solid, mp 256–257 °C.
1H NMR (400 MHz, CDCl3) δ 8.50 (s, 1H), 8.33 (d, J = 2.0 Hz, 1H), 7.97–7.90 (m, 4H), 7.89–7.81 (m, 4H), 7.55–7.48 (m, 2H), 7.36 (td, J = 7.3, 2.0 Hz, 1H), 7.00–6.92 (m, 2H), 6.89 (d, J = 7.8 Hz, 1H), 3.32 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 170.6, 166.9, 162.1, 143.9, 134.8, 133.4, 133.21, 133.15, 131.5, 131.4, 128.8, 128.7, 127.6, 126.7, 126.4, 125.9, 125.1, 124.3, 124.0, 123.4, 122.9, 108.8, 108.5, 100.6, 26.5.
HRMS (ESI/Q-TOF) m/z: [M + H]+ calcd for C29H20N3O4+ 474.1448; found 474.1454.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3].
3].
Pale-orange solid, mp 214–216 °C (dec.).
1H NMR (400 MHz, CDCl3) δ 8.46 (s, 1H), 7.95 (dd, J = 5.5, 3.1 Hz, 2H), 7.84 (dd, J = 5.5, 3.1 Hz, 2H), 7.56 (dd, J = 3.7, 1.3 Hz, 1H), 7.42–7.32 (m, 2H), 7.08 (dd, J = 5.0, 3.7 Hz, 1H), 6.94 (t, J = 7.6 Hz, 1H), 6.86 (m, 2H), 3.28 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 170.2, 166.4, 161.2, 143.9, 139.4, 134.9, 131.5, 131.4, 127.8, 127.1, 127.0, 124.8, 124.2, 124.1, 123.3, 108.8, 108.2, 98.3, 26.5.
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C23H15N3NaO4S+ 452.0675; found 452.0674.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2].
2].
Orange solid, mp 136–139 °C (dec.).
1H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 7.94 (dd, J = 5.5, 3.0 Hz, 2H), 7.83 (dd, J = 5.5, 3.0 Hz, 2H), 7.81–7.74 (m, 2H), 7.57–7.43 (m, 3H), 7.43–7.36 (m, 1H), 7.28 (m, 1H), 6.98–6.84 (m, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 172.2, 166.9, 162.3, 140.9, 135.8, 134.8, 131.5, 131.4, 129.1, 128.8, 125.9, 125.5, 124.7, 124.0, 123.4, 110.8, 108.6, 100.4.
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C24H15N3NaO4+ 432.0955; found 432.0954.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2].
2].
White solid, mp 238–239 °C.
1H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 7.93 (dd, J = 5.4, 3.0 Hz, 2H), 7.83 (dd, J = 5.4, 3.0 Hz, 2H), 7.78 (d, J = 7.6 Hz, 2H), 7.47 (dd, J = 8.5, 6.8 Hz, 2H), 7.43–7.36 (m, 1H), 7.08 (dd, J = 11.3, 8.4 Hz, 1H), 6.88 (td, J = 8.0, 4.2 Hz, 1H), 6.72–6.68 (m, 1H), 3.50 (d, J = 2.8 Hz, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 170.3, 166.8, 162.5, 147.8 (d, J = 245.3 Hz), 135.6, 134.9, 131.4, 130.4 (d, J = 8.9 Hz), 129.1, 128.9, 127.8 (d, J = 2.8 Hz), 125.9, 124.1 (d, J = 6.4 Hz), 124.0, 120.2 (d, J = 3.4 Hz), 119.4 (d, J = 19.4 Hz), 108.0 (d, J = 2.8 Hz), 100.6, 29.1 (d, J = 5.7 Hz).
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C25H16FN3NaO4+ 464.1017; found 464.1013.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2].
2].
Pale-orange solid, mp 227–230 °C.
1H NMR (400 MHz, CDCl3) δ 8.48 (s, 1H), 8.32 (dd, J = 8.6, 2.3 Hz, 1H), 7.97 (dd, J = 5.5, 3.1 Hz, 2H), 7.85 (dd, J = 5.5, 3.1 Hz, 2H), 7.80–7.70 (m, 3H), 7.49 (t, J = 7.6 Hz, 2H), 7.41–7.45 (m, 1H), 6.98 (d, J = 8.6 Hz, 1H), 3.37 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 170.7, 167.0, 163.6, 149.2, 143.8, 135.1, 135.0, 131.3, 129.3, 129.0, 128.2, 126.0, 125.8, 124.1, 120.4, 108.7, 106.8, 100.8, 27.0.
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C25H16N4NaO6+ 491.0962; found 491.0958.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 2 mL) in a round-bottom flask, NaBH3CN (94.5 mg, 1.5 mmol) was added portion-wise. The reaction vessel was equipped with a bubbler and the mixture was stirred for 12 h at room temperature. After the reaction was completed (monitored by TLC), the solvent was removed in vacuo, and water was added to the residue. The precipitate formed was filtered, dissolved in DCM, and the solution was dried over anhydrous Na2SO4. The solvent was removed in vacuo, and the crude product was purified by column chromatography on silica gel to give compound 8 (55 mg, yield 81%).
3, 2 mL) in a round-bottom flask, NaBH3CN (94.5 mg, 1.5 mmol) was added portion-wise. The reaction vessel was equipped with a bubbler and the mixture was stirred for 12 h at room temperature. After the reaction was completed (monitored by TLC), the solvent was removed in vacuo, and water was added to the residue. The precipitate formed was filtered, dissolved in DCM, and the solution was dried over anhydrous Na2SO4. The solvent was removed in vacuo, and the crude product was purified by column chromatography on silica gel to give compound 8 (55 mg, yield 81%).
White solid, mp 220–223 °C.
1H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 7.99 (d, J = 7.7 Hz, 1H), 7.86–7.82 (m, 3H), 7.62–7.40 (m, 7H), 7.12 (t, J = 7.6 Hz, 1H), 6.89 (d, J = 7.9 Hz, 1H), 6.60 (s, 1H), 3.93 (s, 3H), 3.26 (s, 3H).
13C{1H} NMR (100 MHz, CDCl3) δ 171.2, 168.9, 166.5, 163.8, 143.8, 138.4, 137.3, 132.4, 131.3, 130.4, 130.1, 129.3, 128.8, 128.7, 127.2, 126.33, 126.28, 126.0, 123.7, 108.4, 107.4, 100.2, 52.7, 26.5.
HRMS (ESI/Q-TOF) m/z: [M + Na]+ calcd for C26H21N3NaO5+ 478.1373; found 478.1372.
Author contributions
A. A. N., I. P. F., O. E. P., and A. S. P. conducted all the experiments and characterized the novel compounds. N. V. R. together with I. P. F. conceived the project and wrote the manuscript. N. V. R. performed the DFT calculations. A. S. P. contributed to the editing of the manuscript. All authors contributed to discussions.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data supporting this article are included as part of the supplementary information (SI). Supplementary information: synthesis and characterization of compounds 1, calculation details, X-ray data for compound 4a, and 1H, 13C and 2D NMR spectra. See DOI: https://doi.org/10.1039/d5ob01645b.CCDC 2495681 contains the supplementary crystallographic data for this paper.18
Acknowledgements
We gratefully acknowledge the financial support from the Russian Science Foundation, grant 22-73-10184-P. This research used the facilities of the Magnetic Resonance Research Centre, Chemical Analysis and Materials Research Centre, Cryogenic Centre, and Centre for X-ray Diffraction Studies of the Research Park of St Petersburg State University.References
- (a) K. Hiesinger, D. Dar'in, E. Proschak and M. Krasavin, Spirocyclic scaffolds in medicinal chemistry, J. Med. Chem., 2021, 64, 150–183 CrossRef CAS PubMed; (b) Y. Zheng, C. M. Tice and S. B. Singh, The use of spirocyclic scaffolds in drug discovery, Bioorg. Med. Chem. Lett., 2014, 24, 3673–3682 CrossRef CAS PubMed; (c) E. Chupakhin, O. Babich, A. Prosekov, L. Asyakina and M. Krasavin, Spirocyclic motifs in natural products, Molecules, 2019, 24, 4165 CrossRef CAS PubMed; (d) V. N. Charushin, E. V. Verbitskiy, O. N. Chupakhin, D. V. Vorobyeva, P. S. Gribanov, S. N. Osipov, A. V. Ivanov, S. V. Martynovskaya, E. F. Sagitova, V. D. Dyachenko, I. V. Dyachenko, S. G. Krivokolysko, V. V. Dotsenko, A. V. Aksenov, D. A. Aksenov, N. A. Aksenov, A. A. Larin, L. L. Fershtat, V. M. Muzalevskiy, V. G. Nenajdenko, A. V. Gulevskaya, A. F. Pozharskii, E. A. Filatova, K. V. Belyaeva, B. A. Trofimov, I. A. Balova, N. A. Danilkina, A. I. Govdi, A. S. Tikhomirov, A. E. Shchekotikhin, M. S. Novikov, N. V. Rostovskii, A. F. Khlebnikov, Yu. N. Klimochkin, M. V. Leonova, I. M. Tkachenko, V. A. Mamedov, V. L. Mamedova, N. A. Zhukova, V. E. Semenov, O. G. Sinyashin, O. V. Borshchev, Yu. N. Luponosov, S. A. Ponomarenko, A. S. Fisyuk, A. S. Kostyuchenko, V. G. Ilkin, T. V. Beryozkina, V. A. Bakulev, A. S. Gazizov, A. A. Zagidullin, A. A. Karasik, M. E. Kukushkin, E. K. Beloglazkina, N. E. Golantsov, A. A. Festa, L. G. Voskresensky, V. S. Moshkin, E. M. Buev, V. Ya. Sosnovskikh, I. A. Mironova, P. S. Postnikov, V. V. Zhdankin, M. S. Yusubov, I. A. Yaremenko, V. A. Vil, I. B. Krylov, A. O. Terent'ev, Yu. G. Gorbunova, A. G. Martynov, A. Yu. Tsivadze, P. A. Stuzhin, S. S. Ivanova, O. I. Koifman, O. N. Burov, M. E. Kletskii, S. V. Kurbatov, O. I. Yarovaya, K. P. Volcho, N. F. Salakhutdinov, M. A. Panova, Ya. V. Burgart, V. I. Saloutin, A. R. Sitdikova, E. S. Shchegravina and A. Yu. Fedorov, The chemistry of heterocycles in the 21st century, Russ. Chem. Rev., 2024, 93, RCR5125 CrossRef.
- (a) A. J. Boddy and J. A. Bull, Stereoselective synthesis and applications of spirocyclic oxindoles, Org. Chem. Front., 2021, 8, 1026–1084 RSC; (b) S. S. Panda, A. S. Girgis, M. N. Aziz and M. S. Bekheit, Spirooxindole: A Versatile Biologically Active Heterocyclic Scaffold, Molecules, 2023, 28, 618 CrossRef CAS PubMed; (c) B. Yu, D.-Q. Yu and H.-M. Liu, Spirooxindoles: Promising scaffolds for anticancer agents, Eur. J. Med. Chem., 2015, 97, 673–698 CrossRef CAS PubMed.
- For reviews, see: (a) J. Bariwal, L. G. Voskressensky and E. V. Van der Eycken, Recent advances in spirocyclization of indole derivatives, Chem. Soc. Rev., 2018, 47, 3831–3848 RSC; (b) G.-J. Mei and F. Shi, Catalytic asymmetric synthesis of spirooxindoles: recent developments, Chem. Commun., 2018, 54, 6607–6621 RSC; (c) N. R. Ball-Jones, J. J. Badillo and A. K. Franz, Strategies for the enantioselective synthesis of spirooxindoles, Org. Biomol. Chem., 2012, 10, 5165–5181 RSC; (d) G. Molteni and A. Silvani, Spiro-2-oxindoles via 1,3-dipolar cycloadditions. A decade update, Eur. J. Org. Chem., 2021, 1653–1675 CrossRef CAS. For recent examples, see: (e) A. Gogoi, S. Mukhopadhyay, R. Chouhan and S. K. Das, Synthesis of benzimidazole-fused 1,4-benzoxazepines and benzosultams spiro-connected to a 2-oxindole core via a tandem epoxide-opening/SNAr approach, Org. Biomol. Chem., 2024, 22, 353–363 RSC; (f) D. D. Karcev, M. M. Efremova, A. P. Molchanov, N. V. Rostovskii, M. A. Kryukova, A. S. Bunev and D. A. Khochenkov, Selective and Reversible 1,3-Dipolar Cycloaddition of 2-(2-Oxoindoline-3-ylidene)acetates with Nitrones in the Synthesis of Functionalized Spiroisoxazolidines, Int. J. Mol. Sci., 2022, 23, 12639 CrossRef CAS PubMed; (g) Q. Liu, I. Yoshikawa, K. Okuyama and K. Kudo, Asymmetric synthesis of spiro[oxindole-3,2′-pyrrolidine]s through organocatalytic 1,3-dipolar cycloaddition via cycloreversion of precursor isatinimine homodimers, Org. Biomol. Chem., 2025, 23, 1098–1103 RSC; (h) M. S. Islam, A. M. Al-Majid, E. N. Sholkamy, A. Barakat, M. Viale, P. Menichini, A. Speciale, F. Loiacono, M. Azam, V. P. Verma, S. Yousuf and M. Teleb, Optimized spirooxindole-pyrazole hybrids targeting the p53-MDM2 interplay induce apoptosis and synergize with doxorubicin in A549 cells, Sci. Rep., 2023, 13, 7441 CrossRef CAS PubMed; (i) H. Yuan, Y.-Z. Wu, Y.-H. Fang, C.-H. Chen, C. Liang and D.-L. Mo, Synthesis of Spirooxindole-1,2-oxazinan-5-ones through 2,2,2-Trifluoroethanol Promoted [3 + 3] Cycloaddition of N-Vinyl Oxindole Nitrones and Oxyallyl Cations, J. Org. Chem., 2023, 88, 16155–16166 CrossRef CAS PubMed; (j) H. Yuan, D.-L. Lu, C. Liang and D.-L. Mo, Synthesis of Spirooxindole-Benzo[d]oxazoles and Dihydrobenzofurans through Cycloaddition and Rearrangement of N-Vinyl Nitrones and Arynes, Adv. Synth. Catal., 2022, 364, 1409–1414 CrossRef CAS.
- J. Duan, Y. Mao, A. Xian, B. Rong, G. Xu, Z. Li, L. Zhao, N. Zhu and K. Guo, Copper-catalyzed regioselective [3+2] annulation of malonate-tethered acyl oximes with isatins, Chem. Commun., 2021, 57, 3379–3382 RSC.
- I. P. Filippov, M. S. Novikov and N. V. Rostovskii, One-pot modular synthesis of 3-oxazolines from 2H-azirines, diazo compounds, and m-CPBA enabled by 2-azadiene–oxene (4 + 1) cycloaddition, Org. Chem. Front., 2025, 12, 5811–5817 RSC.
- (a) H. J. Dequina, C. L. Jones and J. M. Schomaker, Recent updates and future perspectives in aziridine synthesis and reactivity, Chem, 2023, 9, 1658–1701 CrossRef CAS PubMed; (b) D. R. Mishra and N. P. Mishra, Recent breakthroughs in ring-opening annulation reactions of aziridines, Org. Biomol. Chem., 2025, 23, 2967–2996 RSC; (c) H. Byeon, H.-J. Ha and J. W. Yang, Recent advances in the application of contiguously functionalized aziridines for the synthesis of pharmaceutical precursors via regioselective ring-opening reactions, Mol. Catal., 2025, 576, 114943 CAS; (d) A. S. Pankova and M. A. Kuznetsov, Recent Advances in the Chemistry of 2-Acylaziridines, Synthesis, 2017, 49, 5093–5104 CrossRef CAS.
- (a) M. A. Kuznetsov and V. V. Voronin, Thermal Transformations of Alk-1-enyl-N-phthalimidoaziridines, Russ. J. Org. Chem., 2013, 49, 83–94 CrossRef CAS; (b) A. S. Pankova, Two-Step Construction of Thiophene–Oxazole Dyads with Fluorescent Properties by the Ring Expansion of Aziridines, J. Org. Chem., 2022, 87, 11121–11130 CrossRef CAS PubMed.
- G. F. S. Fernandes, J. R. Lopes, J. L. Dos Santos and C. B. Scarim, Phthalimide as a versatile pharmacophore scaffold: Unlocking its diverse biological activities, Drug Dev. Res., 2023, 84, 1346–1375 CrossRef CAS PubMed.
- A. P. Sakla, P. Kansala and N. Shankaraiah, Syntheses and reactivity of spiro-epoxy/aziridine oxindole cores: developments in the past decade, Org. Biomol. Chem., 2020, 18, 8572–8596 RSC.
- (a) Y. Qu, Z. Liu, Y. Zhou, X. Feng and X. Liu, Asymmetric Catalytic Aziridination to Synthesize Spiro-aziridine Oxindoles, Chem. – Eur. J., 2025, 31, e202500302 CrossRef CAS PubMed; (b) S. Bhandari, N. Kulkarni, A. P. Sakla and N. Shankaraiah, Lewis-acid catalyzed dehydrative [3+2] cycloaddition reaction: A facile synthetic approach to spiro-benzoindoline oxindoles, Tetrahedron Lett., 2020, 61, 152007 CrossRef CAS.
- M. A. Kuznetsov, L. M. Kuznetsova and A. S. Pankova, Oxidative aminoaziridination: past, present, and future, Tetrahedron Lett., 2016, 57, 3575–3585 CrossRef CAS.
- A. S. Pankova and M. A. Kuznetsov, Synthesis and thermal transformations of spiro-fused N-phthalimidoaziridines, Tetrahedron Lett., 2014, 55, 2499–2503 CrossRef CAS.
- E. Vedejs and J. W. Grissom, A comparison of 4-oxazoline and 2-acylaziridine routes to azomethine ylides, J. Org. Chem., 1988, 53, 1882–1887 CrossRef CAS.
- F. R. Miankooshki, M. Bayat, S. Nasri and N. H. Samet, 1,3-Dipolar cycloaddition reactions of isatin-derived azomethine ylides for the synthesis of spirooxindole and indole-derived scaffolds: recent developments, Mol. Divers., 2023, 27, 2365–2397 CrossRef CAS PubMed.
- (a) Z. Huang, W. Liu and W.-Xi. Zhang, Overview of 6π 1,5-Electrocyclization over the Past 40 Years, Chin. J. Chem., 2023, 41, 725–740 CrossRef CAS; (b) R. Huisgen, 1,5,-Electrocyclizations - An Important Principle of Heterocyclic Chemistry, Angew. Chem., Int. Ed. Engl., 1980, 19, 947–973 CrossRef.
- (a) N. V. Rostovskii, M. S. Novikov, A. F. Khlebnikov, G. L. Starova and M. S. Avdontseva, Azirinium ylides from α-diazoketones and 2H-azirines on the route to 2H-1,4-oxazines: three-membered ring opening vs 1,5-cyclization, Beilstein J. Org. Chem., 2015, 11, 302–312 CrossRef CAS PubMed; (b) J. E. Baldwin, R. G. Pudussery, A. K. Qureshi and B. Sklarz, Valence rearrangement of hetero systems. The 4-isoxazolines, J. Am. Chem. Soc., 1968, 90, 5325–5326 CrossRef CAS.
- (a) J. A. Ross, R. P. Seiders and D. M. Lemal, An Extraordinary Facile Sulfoxide Automerization, J. Am. Chem. Soc., 1976, 98, 4325–4327 CrossRef CAS; (b) D. M. Birney, Theory, Experiment and Unusual Features of Potential Energy Surfaces of Pericyclic and Pseudopericyclic Reactions with Sequential Transition Structures, Curr. Org. Chem., 2010, 14, 1658–1668 CrossRef CAS.
- CCDC 2495681: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pryv8.
| This journal is © The Royal Society of Chemistry 2026 |