One-pot synthesis of Bi24O31Br10/Bi4V2O11 heterostructures and their photocatalytic properties
ZhangSheng Liu*,
JiNan Niu,
PeiZhong Feng,
YanWei Sui and
YaBo Zhu
School of Material Science and Engineering, China University of Mining and Technology, XuZhou 221116, China. E-mail: lzsliu2008@hotmail.com; Fax: +86-516-83591870; Tel: +86-516-83591979
First published on 12th August 2014
Abstract
Bi24O31Br10/Bi4V2O11 heterojunction photocatalysts were successfully prepared by a facile, one-pot solvothermal method. In the obtained heterojunctions, Bi4V2O11 nanoparticles were uniformly immobilized on or inlaid onto the surfaces of the Bi24O31Br10 nanosheets. They exhibited superior visible light photocatalytic activity towards the degradation of rhodamine B (RhB). Among them, the 25% Bi4V2O11 sample possessed the highest photocatalytic activity, the degradation rate of which was 4 and 6 times as fast as that of bare Bi24O31Br10 and Bi4V2O11, respectively. The enhanced photocatalytic activity could be attributed to the effective separation and transfer of photogenerated carriers. The trapping experiments confirmed that the photogenerated holes and ˙O2− radicals were the main active species responsible for the photodegradation of RhB.
1. Introduction
In the past few decades, semiconductor photocatalysts have attracted substantial attention due to their potential applications in environmental remediation.1,2 TiO2 was once believed to be a powerful photocatalyst owing to its low cost, strong oxidizing power, non-toxic nature and environmentally friendly features.3 However, TiO2 can only be activated by UV light due to its wide band gap, which accounts for less than 5% of solar energy compared with 43% for the visible light.4 In order to use the solar energy more effectively, many visible light driven photocatalysts have been developed, such as Bi2WO6,5,6 BiVO4,7 NaTaO3,8 YFeO3,9 BiOX (X = Cl, Br and I),10–12 Bi24Al2O39,13 Bi12TiO20 (ref. 14) and Bi2MoO6 (ref. 15).Bi24O31Br10, as one of the oxygen-rich bismuth oxyhalides, has been known to researchers.16,17 It has been reported that Bi24O31Br10 possesses a narrower energy band gap compared with the original BiOBr, which enables it to absorb more visible light.18 Xiao et al. prepared hierarchical Bi24O31Br10 nanoflakes by a microwave heating route and investigated its photocatalytic activity. The results revealed that, in comparison with BiOBr, Bi24O31Br10 possesses higher photocatalytic efficiency.19 Nevertheless, the photocatalytic activity of Bi24O31Br10 is still far from efficient for practical application and requires further improvement. Construction of Bi24O31Br10-contained heterostructures may be a promising method for developing highly efficient photocatalysts. Up to now, Bi24O31Br10/BiOBr heterostructure photocatalysts have been developed by a controlled calcination of BiOBr. Similar Bi24O31Cl10/BiOCl heterostructure photocatalysts have also been reported, both of which display enhanced photocatalytic activity.20,21 Recently, another Bi-contained photocatalyst, Bi4V2O11, has been widely studied because of its peculiar structural and optical properties.22,23 Based on the estimated energy band positions, we speculate that the coupling of Bi24O31Br10 and Bi4V2O11 can form type II semiconductor heterostructures, which helps the separation and transfer of photogenerated electron–hole pairs, favoring the enhancement of the photocatalytic activity.
In this paper, the novel Bi24O31Br10/Bi4V2O11 heterostructures were constructed and prepared via a facile, one-pot solvothermal method for the first time. The structural and optical properties of the products were characterized by XRD, FESEM, TEM, XRF, XPS, FT-IR, BET, UV-Vis DRS, EIS and PL. The photocatalytic activities were evaluated by photocatalytic degradation of RhB under visible light irradiation. The results show that Bi24O31Br10/Bi4V2O11 heterostructures exhibit markedly enhanced photocatalytic performance, whose mechanism is investigated and discussed in detail.
2. Experimental
2.1. Preparation of photocatalysts
All chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd. (China), and they were used as received without further purification. Bi24O31Br10/Bi4V2O11 heterostructures were synthesized via a facile one-pot solvothermal method. In a typical process, 2 mmol of Bi(NO3)3·5H2O was first dissolved into 20 mL of ethylene glycol (EG). Second, another 15 mL EG solution containing appropriate stoichiometric amount of cetyltriethylammonium bromide (CTAB) and NH4VO3 was added into the above solution and stirred until a transparent solution was obtained. Third, certain volume of 2 M NaOH solution was added dropwise until the pH value of the solution was 8.0. The resultant precursor solution was transferred into a 50 mL Teflon-lined stainless steel autoclave. Finally, the autoclave was sealed and maintained at 160 °C for 16 h and allowed to gradually cool to room temperature. The precipitate was washed three times with absolute ethanol and distilled water, respectively, and dried at 80 °C in air. Based on the abovementioned process, Bi24O31Br10/Bi4V2O11 heterostructures with the Bi4V2O11 contents of 12.5 at%, 25 at% and 37.5 at% were obtained, which were named as 12.5% Bi4V2O11, 25% Bi4V2O11 and 37.5% Bi4V2O11, respectively. In addition, pure Bi24O31Br10 and Bi4V2O11 were also prepared for comparison.2.2. Characterization
The composition and structure of as-prepared samples were examined by means of X-ray diffraction (XRD, Bruker D8 Advance with Cu Kα radiation at 40 kV and 30 mA). The morphology was investigated by using a field emission scanning electron microscope (SEM, Hitachi S-4800) equipped with an energy dispersive X-ray spectrometer (EDS) and a transmission electron microscope (TEM, Philips, Tecnai 12). X-ray fluorescence (XRF) spectroscopy analysis was conducted on a Bruker S8 Tiger instrument. FT-IR spectra were recorded by using a Vertex70 FTIR spectrometer. The surface properties of the samples were examined by X-ray photoelectron spectroscopy (XPS) with Al Kα X-ray (hν = 1486.6 eV) irradiation operating at 150 W (XPS: Thermo ESCALAB250, USA). The optical property was analyzed by both UV-vis diffuse reflectance spectra (DRS, Varian Cary 300) and photoluminescence spectra (PL, Varian Cary-Eclipse 500). Electrochemical impedance spectroscopy (EIS) was performed on an electrochemical workstation (CHI 660B Chenhua Instrument Company, Shanghai, China).2.3. Photocatalytic and electrochemical performances
The photocatalytic activity of as-prepared samples was determined by the degradation of Rhodamine B (RhB) under visible light irradiation. The visible-light source was a 150 W tungsten-halogen lamp (Beijing Institute of Opto-Electronic Technology, light intensity = 200 mW cm−2). The short-wavelength components (λ < 400 nm) of the light were cut off by using a cutoff glass filter. Experiments were carried out at 20 ± 3 °C as follows: 0.1 g of photocatalyst was added into 100 mL of 15 mg L−1 RhB solution. The distance between the bottom of the lamp and the top of the solution was 10 cm. Before irradiation, the suspension was stirred for 20 min in darkness to reach the adsorption–desorption equilibrium. At given irradiation time intervals, approximate suspensions of 3 mL were taken and centrifuged to remove the catalyst particles. The concentration of remnant RhB was determined by UV-vis spectroscopy at its characteristic wavelength of 550 nm.EIS measurements were performed on a CHI 660D electrochemical workstation (Shanghai Chenhua, China) with a standard three-electrode configuration. The counter electrode was a platinum wire. The reference electrode was a saturated Ag/AgCl electrode. The working electrodes were prepared by dip-coating process. To accomplish this, 20 mg of photocatalyst was suspended in 5 mL ethanol to produce slurry, which was dip-coated onto a 1.5 × 1.5 cm fluorine-tin oxide (FTO) glass electrode. After the films were dried under ambient conditions, they were sintered at 350 °C for 2 h. The electrolyte was 0.5 M Na2SO4 aqueous solution. EIS were carried out at the open circuit potential, and the amplitude of the sinusoidal wave was 10 mV.
3. Results and discussion
3.1. Characterization
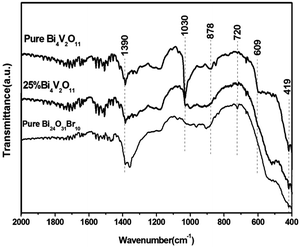
The crystal structure of the as-prepared samples was investigated by means of X-ray powder diffraction (XRD). As shown in Fig. 1, the diffraction peaks of pure Bi24O31Br10 and Bi4V2O11 samples are in good agreement with the monoclinic phase Bi24O31Br10 (JCPDS card no. 75-0888) and the orthogonal phase Bi4V2O11 (JCPDS card no. 42-0135), respectively. Considering that the characteristic peaks of Bi4V2O11 coincide with those of Bi24O31Br10 to a larger extent, no special diffraction peaks for Bi4V2O11 can be found. Nonetheless, the diffraction peaks of Bi24O31Br10 (2θ = 24.1°, 42.8° and 50.4°) are found to be weaker in intensity with increasing Bi4V2O11 content. When the Bi4V2O11 content is 37.5 at%, some peaks (2θ = 24.1° and 42.8°) even disappear completely. The results may be attributed to the crystallinity of Bi4V2O11 in Bi24O31Br10/Bi4V2O11 heterostructures.FT-IR spectra of the Bi24O31Br10, Bi4V2O11 and 25% Bi4V2O11 samples were measured and shown in Fig. 2. The absorption peak existing at 1390 cm−1 is attributed to the bending vibrations of N–O, which may come from the raw material. The absorption peak at 419 cm−1 is assigned to the Bi–O band, and the peak at 720 cm−1 is related to the asymmetry and symmetric stretching vibration peaks of the Bi–Br band in the Bi24O31Br10 structure.24,25 The peaks at 609 cm−1 and 1030 cm−1 can be assigned to the vibrations of V–O–V and VO band, respectively. The absorption at about 878 cm−1 is believed to be associated with the coupled vibration between V–O–V and VO.26,27 It can be seen from Fig. 2 that the 25% Bi4V2O11 sample possesses similar FT-IR spectra to that of pure Bi24O31Br10. The difference is that the peak at 1030 cm−1 can also be found in the spectrum of 25% Bi4V2O11 sample, which indicates the existence of Bi4V2O11.
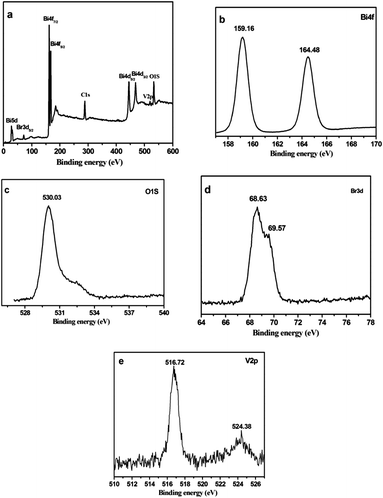
X-ray photoelectron spectroscopy (XPS) was carried out on the 25 at% Bi4V2O11 sample to determine the surface compositions and chemical states of the elements. The shift of the peak position on the charge effect was calibrated by using the binding energy of C1s at 284.78 eV. The survey XPS spectra of 25 at% Bi4V2O11 sample are shown in Fig. 3a. The V2p peak around 516.0 eV can be detected in 25 at% Bi4V2O11. Fig. 3b–e show high-resolution XPS spectra of the primary elements. Two peaks at 159.16 eV and 164.48 eV (Fig. 3b) are assigned to Bi4f7/2 and Bi4f5/2, respectively, which are assigned to Bi3+ in the composites.28 The asymmetric XPS peak of O1s (Fig. 3c) indicates that oxygen species are present in the form of lattice oxygen and hydroxyl groups adhered onto the surface.29 The Br3d5/2 and Br3d3/2 peaks are associated with the binding energies at 68.63 and 69.57 eV (Fig. 3d), respectively. The binding energies of V2p3/2 and V2p1/2 are 516.72 and 524.38 eV (Fig. 3e), which can be characteristic of the V species in Bi4V2O11. Therefore, it may be concluded that Bi4V2O11/Bi24O31Br10 heterostructure photocatalysts have been successfully synthesized.
The morphologies of the as-prepared samples were revealed by SEM, as shown in Fig. 4. It can be seen that pure Bi24O31Br10 has an irregular hierarchical structure, which is further assembled by nanosheets with a thickness of ∼15 nm (Fig. 4a). On the contrary, Bi4V2O11 consists of a number of monodispersed nanoparticles with sizes of about dozens of nanometers (Fig. 4e). After the coupling of Bi24O31Br10 and Bi4V2O11, both 12.5 at% Bi4V2O11 and 25 at% Bi4V2O11 display a degraded hierarchical structure, in which Bi4V2O11 nanoparticles are found to firmly combine with Bi24O31Br10 nanosheets (Fig. 4b and c). However, the 37.5 at% Bi4V2O11 sample shows the conglomeration of nanoparticles, and there are no Bi24O31Br10 nanosheets found in it (Fig. 4e), indicating that excess Bi4V2O11 hinders the formation of Bi24O31Br10 nanosheets. In addition, the EDS pattern (Fig. 4f) shows that the 25 at% Bi4V2O11 sample contains the elements O, V, Br and Bi. This indirectly proves that the prepared sample is the composite of Bi24O31Br10 and Bi4V2O11. In addition, the elemental analysis of 25% Bi4V2O11 sample was carried out by XRF in order to estimate the quantity of Bi4V2O11. The atomic fractions of V, Br and Bi are 1.76%, 20.54% and 77.70%, respectively. It is found that the real content of Bi4V2O11 (29.9%) is slightly higher, which can be attributed to a preferential precipitation of Bi4V2O11.
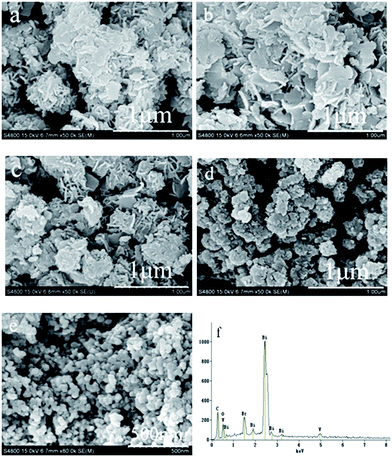 | ||
| Fig. 4 SEM images of as-prepared samples: (a) Bi24O31Br10, (b) 12.5 at% Bi4V2O11, (c) 25 at% Bi4V2O11, (d) 37.5 at% Bi4V2O11, (e) Bi4V2O11 and (f) EDS pattern of 25 at% Bi4V2O11. | ||
The Bi24O31Br10/Bi4V2O11 heterostructures were further characterized by TEM and HRTEM. Fig. 5a shows the TEM image of the 25 at% Bi4V2O11 sample. The grey region with a flake shape is a Bi24O31Br10 nanosheet, whereas the dark region resulted from the Bi24O31Br10 nanoparticles, which is consistent with the SEM result. To further investigate the structural information, the corresponding HRTEM image is shown in Fig. 5b. The (117) plane of Bi24O31Br10 and (200) plane of Bi4V2O11 can be well identified based on the accurate measurement of lattice fringes, indicating that Bi24O31Br10 and Bi4V2O11 have been completely coupled to form Bi24O31Br10/Bi4V2O11 heterostructure.
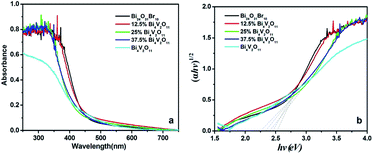
3.2. Optical property
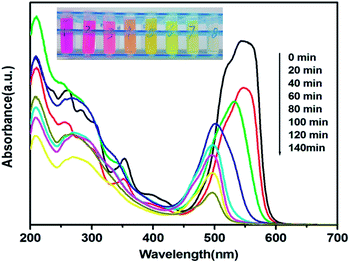
To further investigate the effects of Bi4V2O11/Bi24O31Br10 heterostructures on photocatalytic activity, the photocatalytic degradation experiments of RhB were carried out with Bi24O31Br10, 12.5% Bi4V2O11, 25% Bi4V2O11, 37.5% Bi4V2O11 and Bi4V2O11 as the photocatalysts. The experimental results are shown in Fig. 8a. It can be seen that no RhB degradation can be detected in the absence of a catalyst, suggesting that photolysis of RhB can be ignored under visible light. After 120 min of visible light irradiation, RhB with different photocatalysts is degraded in varying degrees. The degradation rates are 76.6%, 98.3%, 99.4%, 91.0% and 63.2% for Bi24O31Br10, 12.5% Bi4V2O11, 25% Bi4V2O11, 37.5% Bi4V2O11 and Bi4V2O11, respectively. Clearly, Bi24O31Br10/Bi4V2O11 heterojunction composites possess higher photocatalytic activity than bare Bi24O31Br10 and Bi4V2O11. To quantify the photocatalytic ability of these samples, the apparent pseudo-first-order model, ln(C0/C) = kt, is used to describe the photocatalytic reaction, where t is the irradiation time and k is the rate constant. As shown in Fig. 8b, ln(C/C0) exhibits a good linear relationship with the irradiation time, indicating that the photocatalytic reaction belongs to the pseudo-first-order reaction. The reaction rate constants are also concluded in Fig. 8b. This suggests that the Bi4V2O11 content has a significant effect on the photocatalytic performance of Bi24O31Br10/Bi4V2O11 heterojunctions. The 25% Bi4V2O11 sample displays an optimal photocatalytic performance, whose rate constant is 4 and 6 times as fast as that of pure Bi24O31Br10 and Bi4V2O11, respectively. Therefore, it can be concluded that the coupling of Bi4V2O11 and Bi24O31Br10 is highly beneficial for improving photocatalytic activity.
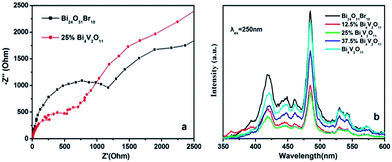 | ||
| Fig. 9 (a) Electrochemical impedance spectroscopy of Bi24O31Br10 and 25% Bi4V2O11; and (b) PL spectra of the as-prepared samples. | ||
Based on the abovementioned results, it is evident that the enhanced activity of Bi24O31Br10/Bi4V2O11 heterojunctions can be attributed to the high efficiency of charge separation and electron transfer processes. In order to understand the photoinduced charge transfer and separation process in detail, the potentials of the conduction band (CB) and valence band (VB) edges of Bi4V2O11 and Bi24O31Br10 were evaluated based on the Mulliken electronegativity theory,32 whose equations are listed as follows:
| EVB = X − E0 + 0.5Eg | (1) |
| ECB = EVB − Eg | (2) |
4. Conclusions
Bi24O31Br10/Bi4V2O11 heterojunctions have been successfully prepared by a facile, one-pot solvothermal method. They have been investigated to be highly active photocatalysts for the photodegradation of RhB under visible light irradiation. Among them, the 25% Bi4V2O11 sample displays the highest degradation efficiency, the reaction rate of which is about 4 and 6 times as fast as that of Bi24O31Br10 and Bi4V2O11, respectively. Based on the EIS and PL results, the enhanced photocatalytic activity is attributed to the effective separation and transfer of the photogenerated carriers. In addition, photogenerated holes and ˙O2− radicals are believed to be the main active species responsible for photocatalytic degradation.Acknowledgements
This work was supported by the National Natural Science Fund (51304198). We would like to thank the Advanced Analysis and Calculation Center of CUMT for their support.Notes and references
- A. Kubacka, M. Fernández García and G. Colón, Chem. Rev., 2012, 112, 1555–1614 CrossRef CAS PubMed.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- S. A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef PubMed.
- Z. Jiang, F. Yang, N. Luo, B. T. T. Chu, D. Sun, H. Shi, T. Xiao and P. P. Edwards, Chem. Commun., 2008, 6372–6374 RSC.
- S. C Zhang, C. Zhang, Y. Man and Y. F. Zhu, J. Solid State Chem., 2006, 179, 62–69 CrossRef PubMed.
- Z. J. Zhang, W. Z. Wang, J. Xu, M. Shang, J. Ren and S. M. Sun, Catal. Commun., 2011, 13, 31–34 CrossRef CAS PubMed.
- S. Eda, M. Fujishima and H. Tada, Appl. Catal., B, 2012, 125, 288–293 CrossRef CAS PubMed.
- D. R. Liu, Y. S. Jiang and G. M. Gao, Chemosphere, 2011, 83, 1546–1552 CrossRef CAS PubMed.
- Y. W. Zhang, J. X. Yang, J. F. Xu, Q. Y. Gao and Z. L. Hong, Mater. Lett., 2012, 81, 1–4 CrossRef CAS PubMed.
- J. Y. Xiong, G. Cheng, G. F. Li, F. Qin and R. Chen, RSC Adv., 2011, 1, 15421553 RSC.
- M. Shang, W. Z. Wang and L. Zhang, J. Hazard. Mater., 2009, 167, 803–809 CrossRef CAS PubMed.
- J. X. Xia, S. Yin, H. M. Li, H. Xu, L. Xu and Q. Zhang, Colloids Surf., A, 2011, 387, 23–28 CrossRef CAS PubMed.
- Z. Wan, G. K. Zhang, J. T. Wang and Y. L. Zhang, RSC Adv., 2013, 3, 19617–19623 RSC.
- J. G. Hou, Y. F. Qu, D. Krsmanovic, C. Ducati, D. Eder and R. V. Kumar, Chem. Commun., 2009, 3937–3939 RSC.
- G. H. Tian, Y. J. Chen, W. Zhou, K. Pan, Y. Z. Dong, C. G. Tian and H. G. Fu, J. Mater. Chem., 2011, 21, 887–892 RSC.
- U. Eggenweiler, E. Keller and V. Kramer, Acta Crystallogr., Sect. B: Struct. Sci., 2000, 56, 431–437 Search PubMed.
- J. W. Wang and Y. D. Li, Chem. Commun., 2003, 2320–2321 RSC.
- X. Xiao, C. Liu, R. P. Hu, X. X. Zuo, J. M. Nan, L. S. Li and L. S. Wang, J. Mater. Chem., 2012, 22, 22840–22843 RSC.
- X. Xiao, R. P. Hu, C. Liu, C. L. Xing, X. X. Zuo, J. M. Nan and L. S. Wang, Chem. Eng. J., 2013, 225, 790–797 CrossRef CAS PubMed.
- C. Yu, W. Zhou, J. Yu, F. Cao and X. Li, Chin. J. Chem., 2012, 30, 721–726 CrossRef CAS PubMed.
- F. T. Li, Q. Wang, X. J. Wang, B. Li, Y. J. Hao, R. H. Liu and D. S. Zhao, Appl. Catal., B, 2014, 150–151, 574–584 CrossRef CAS PubMed.
- V. Thakral and S. Uma, Mater. Res. Bull., 2010, 45, 1250–1254 CrossRef PubMed.
- X. F. Chen, J. B. Liu, H. Wang, Y. L. Ding, Y. X. Sun and H. Yan, J. Phys. Chem. B, 2013, 1, 877–883 CAS.
- V. P. Olstoy and E. V. Telstobrov, Solid State Ionics, 2002, 151, 165–169 CrossRef.
- G. Cheng, J. Y. Xiong and F. J. Stadler, New J. Chem., 2013, 37, 3207–3213 RSC.
- C. Wen, Q. M. Li and F. P. Jun, J. Mater. Sci., 2004, 39, 2625–2627 CrossRef.
- F. Prinetto and G. Ghiotti, J. Phys. Chem. B, 1998, 102, 10316–10325 CrossRef CAS.
- L. Chen, Q. Zhang, R. Huang, S. F. Yin, S. L. Luo and C. T. Au, Dalton Trans., 2012, 41, 9513–9518 RSC.
- X. Zhang, F. Jia and L. Zhang, J. Phys. Chem. C, 2008, 112, 747–753 CAS.
- T. X. Wu, H. Hidaka and N. Serpone, J. Phys. Chem. B, 1998, 102, 5845–5851 CrossRef CAS.
- X. F. Cao, L. Zhang, X. T. Chen and Z. L. Xue, CrystEngComm, 2011, 13, 306–311 RSC.
- X. P. Lin, J. C. Xing, W. D. Wang, Z. C. Shan, F. F. Xu and F. Q. Huang, J. Phys. Chem. C, 2007, 111, 18288–18293 CAS.
- X. Zong, H. Yan, G. Wu, G. Ma, F. Wen and L. Wang, J. Am. Chem. Soc., 2008, 130, 7176–7177 CrossRef CAS PubMed.
- X. C. Zhang, T. Y. Guo, X. W. Wang, Y. W. Wang, C. M. Fan and H. Zhang, Appl. Catal., B, 2014, 150–151, 486–495 CrossRef CAS PubMed.
- L. Q. Ye, J. Y. Liu, Z. Jiang, T. Y. Peng and L. Zan, Appl. Catal., B, 2013, 142–143, 1–7 CAS.
- A. Fujishima and X. T. Zhang, C. R. Chim., 2006, 9, 750–760 CrossRef CAS PubMed.
- S. Kim and W. Choi, Environ. Sci. Technol., 2002, 36, 2019–2025 CrossRef CAS.
- Y. Fu, C. Chang, P. Chen, X. L. Chu and L. Y. Zhu, J. Hazard. Mater., 2013, 254–255, 185–192 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |