Nanodispersions of monoglycerides of punicic acid: a potential nutrient precursor with higher oxidative stability and cytotoxicity
Ying Cao,
Lina Wang,
Mingli He,
Yanhua Zhang and
Huajie Wang*
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007, P. R. China. E-mail: wanghuajie972001@163.com; Fax: +86-373-3328507; Tel: +86-373-3326335
First published on 11th August 2014
Abstract
Punicic acid belongs to one of conjugated linolenic acids isomers (CLnAs) and contains many outstanding functions related to human health such as anti-carcinogenic, anti-diabetes, anti-hyperlipidemia, anti-obesity and anti-atherosclerotic properties; moreover, interest in the punicic acid is growing. Note that since punicic acid is extremely susceptible to oxidation, antioxidants were often added to increase its stability. However, sometimes such method resulted in the great decrease of its antitumor activity. In addition, punicic acid is quite insoluble in water and only a minor fraction of it can be absorbed. Therefore, the present study aims to identify a method, which can maintain the cytotoxicity when there is an increase in the oxidative stability and solubility of punicic acid. In brief, free punicic acid was modified with glycerol and the amphiphilic monoglycerides of punicic acid were obtained, which were further dispersed to nanodispersions. Oxidative stability, cytotoxicity to NIH3T3 cells and digestion in intestinal juice of such nanodispersions under different conditions were studied. It was found that such nanodispersions showed higher oxidative stability, higher solubility in water. Most importantly, its cytotoxicity to NIH3T3 cells was also as strong as that of free punicic acid. Furthermore, since it can be hydrolyzed by pancreatin in intestinal juice, the nanodispersions formed by monoglycerides of punicic acid can be used as nutrient precursor of free punicic acid.
1. Introduction
Punicic acid (9-trans, 11-cis, 13-trans, conjugated linolenic acid) is contained about 72% in pomegranate seed oil and belongs to one of conjugated linolenic acids isomers (CLnA).1 It was found that punicic acid and other CLnA isomers, such as α-eleostearic acid (9-cis, 11-trans, 13-trans-CLnA), catalpic acid (9-trans, 11-trans, 13-cis-CLnA), calendic acid (8-trans, 10-trans, 12-cis-CLnA), contain many outstanding functions related to human health, such as anti-carcinogenic, anti-diabetes, anti-hyperlipidemia, anti-obesity and anti-atherosclerotic properties both in vitro and in vivo.2–5For example, Suzuki et al. reported that α-eleostearic acid and punicic acid showed much higher cytotoxicity.6 Arao et al. reported the hypolipidemic effect of punicic acid in human liver derived HepG2 cells.7 Punicic acid induced obesity and insulin resistance in mice, independent of changes in food intake or energy expenditure.8 Punicic acid can also alleviate hepatic triacylglycerol accumulation in obese, hyperlipidemic OLETF rats.9 Pomegranate seed oil decreases weight gain and type 2 diabetes risk in CD-1 mice.10 Therefore, to date, punicic acid has already gained considerable attention.
However, there are two main problems that need to be addressed in the studies of punicic acid. The first problem is that the highly unsaturated structure and conjugated triene system of punicic acid makes it extremely susceptible to oxidation, light or thermal treatments. For example, we previously found that punicic acid could be completely oxidized after exposure to air for 30 min at 50 °C.11 Therefore, it becomes very difficult for the storage and carriage of punicic acid products.
To solve this problem, many methods were used, including mixing with edible oils and fats, microencapsulation, cyclodextrin inclusion, and addition of antioxidants.12,13 However, the protective effects of the first three methods were not very good and the encapsulation rate was also low. For the method of addition of antioxidants, although the oxidative stability increases greatly, the antitumor activity of conjugated fatty acid decreased sharply sometimes.14 Therefore, it is very important to determine a more appropriate method, which can maintain the biological activity of punicic acid and also increase its oxidative stability at the same time.
The second problem is that punicic acid is quite insoluble in water, and only a minor fraction can be absorbed. Owing to this characteristic, the bioavailability of punicic acid is low and the range of application is also limited.
Recently, many kinds of micro or nano delivery systems, including liposome, solid lipid nanoparticles, noisome, polymeric micelles and inorganic drug carrier, were designed and established. Nutrients or drugs with high instability and high lipid solubility can be carried and protected by such delivery systems. For example, astaxanthin nanodispersions increase the dissolution rate and saturation solubility due to a reduced size and increased surface area.15 Therefore, with this modern nano carrier technology, solubility, stability, and bioavailability of bioactive molecule can be considerably improved. These nano delivery systems were established on the basis of the amphiphilic molecules, such as phospholipid, amphiphilic block copolymer, modified protein, and polysaccharose.
Inspired by the delivery systems and carrier materials, an amphiphilic monoglycerides of punicic acid was synthesized as a precursor, which was then subsequently dispersed to nano scale in water. The physicochemical structures, oxidative stability, micro-morphology, cytotoxicity to NIH3T3 cells and digestibility in vitro of both monoglycerides of punicic acid and nanodispersions were observed in this study.
The present study had two objectives. The first is to increase the oxidative stability of punicic acid by establishing the nanodispersions on the basis of amphiphilic monoglycerides of punicic acid. The second is to estimate the cytotoxicity to NIH3T3 cells of nanodispersions. The possibility of nanodispersions used as a nutrient precursor was also discussed.
2. Material and methods
2.1 Materials
Punicic acid (purity ≥ 90%) was purified from pomegranate seed according to our previous work.16 The mouse embryonic fibroblast cell line NIH3T3 was purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. Pancreatin (USP grade) was purchased from Aladdin. All other solvents and chemicals were analytical grade. TLC plate is 20 × 20 cm (Macherey-Nagel, Duren, Germany).2.2 Synthesis and purification of monoglycerides of punicic acid
Punicic acid acyl chloride was firstly prepared by treating punicic acid with molar equivalent of thionyl chloride in the presence of pyridine under N2 protection. Then, the obtained punicic acid acyl chloride (10 mmol) and molecular sieve (1 g) were added into acetone containing glycerol (50 mmol). After the mixture was kept static at 45 °C for 4 h under N2 protection, the crude product was obtained by a reduced pressure rotary evaporator. After washing with 1 mol l−1 HCL and then distilled water to neutrality, the crude product was purified by TLC using petroleum ether–ethyl ether–methanol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) and stored at −20 °C for future use.
0.5) and stored at −20 °C for future use.
2.3 Preparation of nanodispersions of monoglycerides of punicic acid
20 mg ml−1 of the monoglycerides of punicic acid ethanol solution (0.1 ml) was quickly injected into 1.9 ml of distilled water (or cell nutrient solution) under high speed stirring. Nanodispersions of monoglycerides of punicic acid were prepared by mixing the O/W emulsion in a high-speed disperser (HSD), followed by passing of ultrasonic waves by ultrasonic processor (Biosafer 400UP, 80 W). The temperature throughout the process of ultrasonication was maintained at 10 °C. After ultrasonication for 20 min, the solution was filtrated by 0.22 μm microfiltration membrane.2.4 Physicochemical characterization of monoglycerides of punicic acid
2.5 Oxidation of monoglycerides of punicic acid in air
To evaluate the modification of glycerol to the oxidative stability of punicic acid, we oxidized the monoglycerides of punicic acid in air at 50 °C. The remaining amounts of monoglycerides of punicic acid were analyzed by UV analysis. The free punicic acid with the same molar concentration was used as the control. Briefly, 1.5 mg monoglycerides of punicic acid was dissolved in 6 ml of the water–ethanol solution. 300 μl of such solution (75 μg monoglycerides of punicic acid) were dropped into 20 test tubes. After drying by N2 Thermovap sample concentrator, the samples were oxidized in air at 50 °C. At the given time intervals, 2 ml ethanol was added and was then taken to UV analysis. The UV adsorption of unoxidized punicic acid at 273 nm was measured and the amount of punicic acid was quantified according to the standard curve of pure punicic acid solution. All the experiments were performed in triplicates.182.6 Morphology observation of nanodispersions
Morphologies of monoglycerides of punicic acid nanodispersions were observed by HRTEM (JEOL-100CX, JEOL, Japan). Briefly, droplet of dispersions (5 μl) was dropped onto a perforated carbon-coated Formvar support and observed by HRTEM. In addition, the mean nanodispersions droplet sizes were determined by using the laser light-scattering method with a Zetasizer 2000 (Malvern Instruments, Worcestershire, UK).2.7 Oxidation of nanodispersions in cell nutrient solution
It is already found that the anticancer mechanism of punicic acid is associated with its high instability. Since the monoglycerides of punicic acid was firstly dispersed to nano scales and then cultured with the NIH3T3 cells, it was also necessary to measure the oxidative stability of nanodispersions in cell nutrient solution except in air. In brief, nanodispersions (0.1 mg ml−1 in cell nutrient solution) were dropped into 20 test tubes (1 ml per tube). These test tubes were oxidized under continuous shaking at 37 °C. At the given intervals, one tube was taken and 3 ml acetic ether was added. After centrifugation (4000 rpm), the top acetic ether layer was extracted and subjected to UV analysis. The amount of punicic acid was quantified according to the standard curve of pure punicic acid solution.2.8 Survivability of nanodispersions to the simulated intestinal fluid
Whether used as a nutrient precursor or a potential carrier, it was very important to understand the survivability of nanodispersions to digestive enzyme. The simulated intestinal fluid (SIF) was then prepared and mixed with the nanodispersions. The remaining amount of monoglycerides of punicic acid and the free punicic acid hydrolyzed by enzyme were analyzed by HPLC.In brief, SIF was prepared as follows: monopotassium phosphate (6.8 g) was dissolved in 500 ml water and the pH was adjusted to 6.8 by 0.2 M NaOH. After dissolving the pancreatin (10 g) in moderate water, monopotassium phosphate and pancreatin solution were then mixed and diluted with water to 1000 ml. The nanodispersions of monoglycerides of punicic acid (0.2 mg ml−1 in SIF) were dropped into several test tubes (1 ml per tube). These test tubes were shaken at 37 °C and one test tube was shaken at the given time intervals. 1 ml of 0.5 M HCl and 1 ml acetic ether were then added to terminate the digestion reaction. After centrifugation (3500 rpm), the top acetic ether layer was saved and subjected to HPLC analysis (Agilent-1260 HPLC). The separated monoglycerides of punicic acid and free punicic acid were monitored at 273 nm. A RP-HPLC was performed at 27 °C using a ZORBAX SB-C18 (150 × 2.1 mm i.d., 1.8 μm, Agilent, USA). A gradient system with the mobile phase consisting of A (0.1% formic acid in H2O) and B (methanol) was chosen at a flow rate of 200 μl min−1. The gradient program used was as follows: initial 30% A and 70% B; linear gradient 100% B in 10 min; hold for 10 min, return to initial conditions in 1 min, followed by equilibration for 5 min. Free punicic acid and monoglycerides of punicic acid eluted at 14.39 and 15.24 min, respectively.
2.9 Cytotoxicity of nanodispersions
| Inhibition rate = (ODc − ODt)/ODc × 100% |
In this equation, the ODc and ODt represent the OD 490 nm values of control group and the treatment group, respectively.
2.10 Statistical analysis
The number of independent replica was listed individually for each experiment. Note that where applicable, all data are mean ± SD. The analysis of data for cell proliferation was performed by one-way factorial analysis of variances (ANOVA) and multiple comparisons (Fisher's method as Post-Hoc test, p < 0.05).213. Results and discussion
3.1 Synthesis and characteristics of monoglycerides of punicic acid
The structure of the as-prepared sample was characterized by FTIR, NMR and UV spectra. Fig. 1 shows the FTIR spectra of punicic acid, glycerol and monoglycerides of punicic acid.The corresponding infrared adsorptions arising from the functional groups in samples are shown in Table 1. The characteristic peak of hydroxide group in glycerol appears at 1038 cm−1. Punicic acid shows the characteristic peaks of carboxyl at about 1688 cm−1. Moreover, the typical adsorption peaks of conjugated double bond appear at 991 and 725 cm−1, respectively. By comparison with the spectrum of punicic acid, the disappearance of carboxyl at 1688 cm−1 and the appearance of ester bond stretching peak at 1732 cm−1 could demonstrate the occurrence of condensation reaction between glycerol and punicic acid.
| Frequency (cm−1) | Functional group and mode of vibration | Glycerol | Punicic acid | Monoglycerides of punicic acid |
|---|---|---|---|---|
| 725, 991 | Conjugated ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH bending CH bending |
− | + | + |
| 1038 | –O–H stretching | + | − | + |
| 1463 | –CH (–CH2–, CH3) bending | + | + | + |
| 1688 | –COOH stretching | − | + | − |
| 1732 | –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester Fermi resonance O ester Fermi resonance |
− | − | + |
| 2857–2934 | CH (–CH2–) stretching | + | + | + |
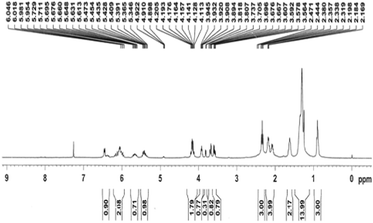
Fig. 2 indicates the 1H-NMR spectrum of monoglycerides of punicic acid. It shows signals at δH 5.0–7.0 ppm, which correspond to the shifts of olefinic protons. The signals at δH 3.894–3.945 ppm can be assigned to chemical shifts of protons in the ester bonds, suggesting the successful condensation between glycerol and punicic acid. In addition, it is clear that both hydroxyl protons appear at δH 2.319 ppm (–CH2OH–) and δH 4.113–4.205 ppm (–CHOH–), respectively. This proved the formation of monoglycerides of punicic acid.
Moreover, the UV spectra of punicic acid and monoglycerides of punicic acid nanodispersions in water were compared as shown in Fig. 3A. Punicic acid exhibits UV adsorption peaks at 263 nm, 273 nm and 284 nm, respectively. It is notable that the UV adsorption properties of conjugated ester bonds in monoglycerides of punicic acid are not affected after being dispersed to nanoscale.
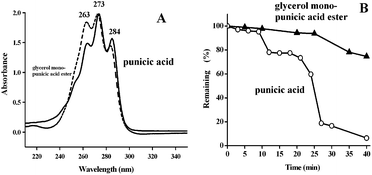 | ||
| Fig. 3 (A) UV spectra of punicic acid and monoglycerides of punicic acid; (B) oxidative stability comparison of free punicic acid (closed circles) and monoglycerides of punicic acid (open triangles). | ||
3.2 Morphology of nanodispersions
The morphology and mean particle sizes of dispersions from glycerol mono-punicic acid in water were observed by HRTEM and laser light-scattering method (Fig. 4). It can be seen that monoglycerides of punicic acid nanodispersions are granular and have about 23 nm size in diameter.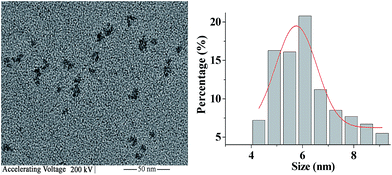 | ||
| Fig. 4 HRTEM (A) of the corresponding particle size distribution (B) of monoglycerides of punicic acid. | ||
Inview of the amphipathicity of glycerol mono-punicic acid, the possible structure of such global nanoparticle was speculated in Fig. 5. We speculate that the long hydrophobic alkyl tail with conjugated triene system faces inward and the glycerol polar hydroxyl group head stretches outside in water (Fig. 5). The whole system then can form a spherical particle in nano scales. It seems that the long hydrophobic alkyl tail aggregates together and is protected by the glycerol polar hydroxyl group.
3.3 Stability of monoglycerides of punicic acid in air at 50 °C
In order to assess the effects of glycerol modification on the oxidative stability of punicic acid, monoglycerides of punicic acid were oxidized in air at 50 °C and the free punicic acid was used as the control. It was found that under the present oxidative condition (in air at 50 °C), free punicic acid is unstable. Note that about 40.3% of free punicic acid was lost for 25 min (Fig. 3B). The percentage of the oxidized punicic acid got to 93.3% only when oxidizing for 36 min. After the modification of glycerol, the oxidative stability of conjugated triene structure is significantly improved comparatively. It can be seen that only 6.3% and 25.3% of monoglycerides of punicic acid were oxidized in 25 min and 40 min, respectively (Fig. 3B); i.e., the formation of the ester group based on the modification of glycerol increased the oxidative stability of conjugated triene system of punicic acid greatly.3.4 Responses of NIH3T3 cells to nanodispersions
In this study, we exposed mouse embryonic fibroblast cell line NIH3T3 to nanodispersions of monoglycerides of punicic acid. Cells responses were then studied by metabolic and morphological methods. Fig. 6 shows the inhibition rate of glycerol, punicic acid and monoglycerides of punicic acid nanodispersions on the metabolism of NIH3T3 cells measured by the MTT method, which is associated with the function of mitochondria. It was found glycerol has a slightly inhibitory effect on the metabolism of NIH3T3 cells. The inhibition rate of glycerol increases with the increase of sample content, but the maximum value is only about 6.6% when the sample content gets to 1 mg ml−1. The IC50 is calculated as the killing concentration inducing 50% inhibition. As for glycerol, the IC50 for NIH3T3 cells is 8896.0 mg ml−1, suggesting almost no toxicity. After exposure to punicic acid or monoglycerides of punicic acid nanodispersions, the metabolism of NIH3T3 cells is significantly inhibited. The IC50 value reaches 134.7 μg ml−1 and 167.1 μg ml−1, respectively; moreover, almost a complete inhibition on the metabolism of NIH3T3 cells can be observed when the content of punicic acid or monoglycerides of punicic acid nanodispersions gets to 0.2 mg ml−1.The distribution and morphology of cells were observed by an optical microscope. Fig. 7 shows the number and distribution of NIH3T3 cells after exposure to glycerol, punicic acid and monoglycerides of punicic acid nanodispersions for 48 h, respectively. The cells grow well in the absence of any additive and the cell number is the most compared with that in other groups (Fig. 7A). Moreover, cells develop a spindle-like morphology and produce long neuritis. In the presence of glycerol, the cells morphology has no significant change (Fig. 7B). However, when the glycerol content reaches 0.2 mg ml−1, the cells number slightly decreases (Fig. 7B3). As for punicic acid and monoglycerides of punicic acid nanodispersions, both the number and morphology of cells obviously change (Fig. 7C and D). Generally, the cells number gradually decreases with the increase of the additive content and only several cells could be observed when the content gets to 0.2 mg ml−1. Moreover, cells contract and form a round shape after exposure to punicic acid or monoglycerides of punicic acid nanodispersions for 48 h, respectively. Contractive cells also gradually increase with the increase of the sample concentration. However, there are no significant differences between punicic acid group and monoglycerides of punicic acid nanodispersions group.
3.5 Stability of nanodispersions in cell nutrient solution and simulated intestinal fluid at 37 °C
Since both nanodispersions and free punicic acid had special effects on cytotoxicity in solutions but not in air, it was necessary then to study their oxidative stability in cell nutrient solution at 37 °C. It was found that under the present condition, oxidative speed of free punicic acid was not very fast, about 54.24% of free punicic acid was lost for 38 h (Fig. 8). It seems that the temperature had great effects on its oxidation speed. In addition, after modification and dispersion into nanoscales, the nanodispersions showed higher stability than that of free punicic acid. It was found that only 25% monoglycerides of punicic acid was lost under the same condition; i.e., the nanodispersions can also protect conjugated triene system from oxidation. According to its microscopic structure described above, we deduced the possible reason was as below: the glycerol polar hydroxyl group of monoglycerides of punicic acid can stand outside in water, and then protect partly long hydrophobic alkyl tail containing conjugated triene system inside the nano particles. | ||
| Fig. 8 Oxidative stability comparison of free punicic acid (closed square) and nanodispersions (closed circles). | ||
As a kind of polyunsaturated fatty acid, it was important to realize the characteristics of digestion and absorption of punicic acid and its modification products. In this study, their digestion in simulated intestinal fluid was also studied. It was found the nanodispersions of monoglycerides of punicic acid can be hydrolyzed by pancreatin to free punicic acid. It took about 2 hours to hydrolyze 0.2 mg monoglycerides of punicic acid completely (Fig. 9).
According to the present results, both monoglycerides of punicic acid and its nanodispersions were more stable than free punicic acid in air and solution. Such a modification method then can provide a new way of protection and will be very helpful for activities such as production, storage, and transportation. Since the monoglycerides of punicic acid can be hydrolyzed to free punicic acid by intestinal juice, it then can be used as a precursor of free punicic acid.
Since hydrophilic glycerol exhibited a slight inhibition on the metabolism of NIH3T3 cells in a dose-dependent mode and had no effect on the cells' spread under the tested concentrations (Fig. 5 and 6), it was chosen to modify the punicic acid. With the increase in oxidative stability, the cytotoxicity of nanodispersions to NIH3T3 cell was not weakened. The possible reasons perhaps were as below: (i) the interaction of nanodispersions with cell membrane is different from free punicic acid, due to its nano particle structure and two hydroxyl groups; and (ii) the bioavailability of nanodispersions increased with increased solubility in body fluid.
4. Conclusions
In conclusion, the oxidative stability of free punicic acid increased by modifying the carboxyl using glycerol. The obtained amphipathic monoglycerides of punicic acid can be dispersed and form a kind of nanodispersions. Such nanodispersions showed higher oxidative stability, higher solubility in water and also can be hydrolyzed by pancreatin. Therefore, this nanodispersions and monoglycerides of punicic acid can be used as precursor of free punicic acid.The most important result was that the cytotoxicity of such precursor to NIH3T3 cells was also as strong as that of free punicic acid. The present study has identified a way that can increase the oxidative stability and also maintain the cytotoxicity simultaneously. Consequently, the biological activity of monoglycerides of punicic acid nanodispersion in vivo is worth waiting for.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31000774 and 20971039) and the Program for Innovative Research Team in University of Henan Province (2012IRTSTHN006) and the Program for Chang-jiang Scholars and Innovative Research Team in University (IRT1061). Moreover, we also thank Xinxiang R&J Chemistry and Biotechnology Com. Ltd. for their help in the biological activity analysis.Notes and references
- H. Abbasi, K. Rezaei and L. Rashidi, J. Am. Oil Chem. Soc., 2008, 85, 83–89 CrossRef CAS PubMed.
- Y. Cao, J. Chen, L. Yang and Z. Y. Chen, J. Nutr. Biochem., 2009, 20, 685–693 CrossRef CAS PubMed.
- J. Chen, Y. Cao, H. Gao, L. Yang and Z. Y. Chen, Chem. Phys. Lipids, 2007, 150, 136–142 CrossRef CAS PubMed.
- Y. Yasui, M. Hosokawa, H. Kohno, T. Tanaka and K. Miyashita, Anticancer Res., 2006, 26, 1855–1860 CAS.
- K. Koba, J. Imamura, A. Akashoshi, J. Kohno-Murase, S. Nishizono, M. Iwabuchi, K. Tanaka and M. Sugano, J. Agric. Food Chem., 2007, 55, 3741–3748 CrossRef CAS PubMed.
- R. Suzuki, R. Noguchi, T. Ota, M. Abe, K. Miyashita and T. Kawada, Lipids, 2001, 36, 477–482 CrossRef CAS.
- K. Arao, H. Yotsumoto, S. Y. Han, K. Nagao and T. Yanagita, Biosci., Biotechnol., Biochem., 2004, 68, 395–397 CrossRef PubMed.
- I. O. C. M. Vroegrijk, J. A. Diepen, S. Berg, I. Westbroek and H. Keizer, Food Chem. Toxicol., 2011, 49, 1426–1430 CrossRef CAS PubMed.
- K. Arao, Y. M. Wang, N. Inoue, J. Hirata, J. Y. Cha, K. Nagao and T. Yanagita, Lipids Health Dis., 2004, 3, 24 CrossRef PubMed.
- B. K. McFarlin, K. A. Strohacker and M. L. Kueht, Br. J. Nutr., 2009, 102, 54–59 CrossRef CAS PubMed.
- L. Yang, Y. Cao, J. N. Chen and Z. Y. Chen, J. Agric. Food Chem., 2009, 57, 4212–4217 CrossRef PubMed.
- I. Lalush, H. Bar, I. Zakaria, S. Eichler and E. Shimoni, Biomacromolecules, 2005, 6, 121–130 CrossRef CAS PubMed.
- C. W. Park, S. J. Kim, S. J. Park, J. H. Kim, J. K. Kim, G. B. Park, J. O. Kim and Y. L. HA, J. Agric. Food Chem., 2002, 50, 2977–2983 CrossRef CAS PubMed.
- P. Bougnoux, N. Hajjaji, K. Maheo, C. Couet and S. Chevalier, Prog. Lipid Res., 2010, 49, 76–86 CrossRef CAS PubMed.
- N. Anarjan, I. A. Nehdi and C. P. Tan, Chem. Cent. J., 2013, 7, 127 CrossRef PubMed.
- Y. Cao, L. Yang, H. L. Gao, J. N. Chen, Z. Y. Chen and Q. S. Ren, Chem. Phys. Lipids, 2007, 145, 128–133 CrossRef CAS PubMed.
- H. J. Wang, Y. Cao, C. Cao, Y. Y. Sun, X. H. Yu, L. F. Zhu and L. Yang, ACS Appl. Mater. Interfaces, 2011, 3, 2755–2763 CAS.
- Y. Cao, M. L. He, Y. H. Zhang and H. J. Wang, Micro Nano Lett., 2011, 6, 874–877 CAS.
- H. J. Wang, X. H. Yu, Y. Cao, B. Zhou and C. F. Wang, J. Inorg. Biochem., 2012, 113, 40–46 CrossRef CAS PubMed.
- M. C. Alley, D. A. Scudiero, A. Monks, M. L. Hursey, M. J. Czerwinski, D. L. Fine, B. J. Abbott, J. G. Mayo, R. H. Shoemaker and M. R. Royd, J. Cancer Res., 1988, 48, 589–601 CAS.
- Y. Cao, H. J. Wang, C. Cao, Y. Y. Sun, L. Yang, B. Q. Wang and J. G. Zhou, J. Nanopart. Res., 2011, 13, 2759–2767 CrossRef.
| This journal is © The Royal Society of Chemistry 2014 |