Crystallizable and tough aliphatic thermoplastic poly(ether urethane)s synthesized through a non-isocyanate route
Abstract
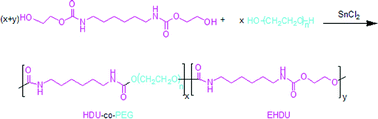
A simple non-isocyanate route synthesizing aliphatic thermoplastic poly(ether urethane)s (PEUs) with good thermal and mechanical properties is described. Melt transurethane polycondensation of 1,6-bis(hydroxyethyloxy carbonyl amino)hexane (BHCH) with two ethylene glycol oligomers, i.e. triethylene glycol (3EG) and tetraethylene glycol(4EG), was conducted, and high molecular weight PEUs were prepared. The PEUs were characterized by gel permeation chromatography, FT-IR, 1H-NMR, differential scanning calorimetry, thermogravimetric analysis, wide angle X-ray scattering, and tensile tests. The PEUs exhibited Mn above 32 000, Mw above 54 300, Tg ranging from 11.2 °C to 28.2 °C, Tm from 103.7 °C to 150.6 °C, and initial decomposition temperature over 237.4 °C. A PEU prepared at a BHCH/4EG molar ratio of 5 : 1 exhibited the best mechanical properties with tensile strength of 32.82 MPa and strain at break of 151.82%. Some urea linkages were formed due to a back-biting side reaction in the transurethane polycondensation.

 Please wait while we load your content...
Please wait while we load your content...