Selective heterogeneous nucleation of gold nanoparticles on one-dimensional cadmium silicate for enhanced nonlinear optical responses
Haiyan Wanga,
Chan Zheng*ab and
Pinqiang Daiab
aSchool of Materials Science and Engineering, Fujian University of Technology, 3 Xueyuan Road, Fuzhou 350108, PR China. E-mail: czheng.fjut@gmail.com; Fax: +86-0591-22863283; Tel: +86-0591-22863283
bFujian Provincial Key Laboratory of Advanced Materials Processing and Application, 3 Xueyuan Road, Fuzhou 350108, PR China
First published on 2nd December 2016
Abstract
A facile route to uniformly assemble Au nanoparticles (NPs) on the surface of cadmium metasilicate (CdSiO3) nanowires (NWs) via a heterogeneous nucleation process was demonstrated. Transmission electron microscopy, X-ray diffraction, Fourier transform infrared spectroscopy, and UV-visible spectroscopy were performed to investigate the morphology, structure, and linear optical properties of the Au NPs/CdSiO3 NWs hybrid nanostructure. The results revealed that uniform decoration of the Au NPs on the surface of CdSiO3 NWs could be achieved by heterogeneous nucleation. Compared with the infrared bands of the CdSiO3 NWs, weaker characteristic infrared bands were observed upon assembly of the Au NPs on the CdSiO3 NWs due to an electron intraband transfer process in the 6sp band of the Au species. Additionally, the hybrid nanostructure displayed a red-shifted, relatively broader, and weaker surface plasmon resonance band compared with the Au NPs. These changes in the spectral bands could be attributed to the interparticle inter actions absorbed on the surface of the CdSiO3 NWs. The nonlinear optical (NLO) properties of Au NPs/CdSiO3 NWs were characterized using an open-aperture Z-scan technique with 4 ns laser pulses at 532 nm. The Au NPs/CdSiO3 NWs hybrid nanostructure displayed superior NLO responses than a suspension of carbon nanotubes, a benchmark optical limiter. Furthermore, the CdSiO3 NWs noncovalently functionalized with Au NPs offered superior performance over individual CdSiO3 NWs. The enhancement in NLO properties was mainly attributed to the combination of nonlinear scattering and nonlinear absorption induced by surface plasmon resonance effects by the Au NPs.
1. Introduction
Designing new nonlinear optical (NLO) materials is essential for the development of optoelectronic technologies, e.g., optical communication, optical limiting, optical data storage, information processing, and spatial light modulation and switching. This area has been at the forefront of research activities over the past several decades. Extensive research has been conducted and led to the discovery of three main classes of NLO materials: nonlinear absorption (NLA; i.e., reverse saturable absorbers, two-photon absorbers, and excited absorbers), nonlinear refraction, and nonlinear scattering (NLS) systems. However, none of these systems, when applied on its own, is able to fulfill the requirement of ideal nonlinearity such as large nonlinear nonlinearity, fast nonlinear responses, good optical quality and mechanical stability, easy preparation procedures, and low cost. Thus, there is a continuing pronounced interest in developing novel NLO materials with desirable physical properties and at a low cost. Diverse strategies have been developed to improve the NLO performance of well-known materials including element doping, NLO material incorporation into a solid-state matrix, and composite formation.1–3 Composites are a convenient approach to modify the NLO properties of existing NLO materials. A combination of multiple nonlinear mechanisms such as reverse saturable absorption (RSA), two-photon absorption (TPA), excited-state absorption, and NLS, achieved by covalent or noncovalent conjugation of two different NLO materials, often improve NLO properties through synergistic interactions.Among the potential NLO candidates, one-dimensional nanostructures are a subject of extensive interest because of their unique structural features and outstanding optical limiting (OL) properties.4 Like carbon nanotubes (CNTs) as the most intensively studied one-dimensional nanostructures, noble metal nanowires (NWs) and other one dimensional nanomaterials are also of great interest due to their potential applications in NLO fields.5,6 It is noted that, compared to one-dimensional nanomaterials, the NLO optical properties of two-dimensional nanomaterials, such as graphene, and other two-dimensional crystals also have attracted wide attention.7 However, most of the studies found that most of the two-dimensional nano-materials show a characteristic of saturated absorption. On the nanosecond time scale, some two-dimensional material dispersion liquid can exhibit the reverse saturable absorption characteristic due to the scattering effect caused by the thermal bubble. As a result, two-dimensional materials have been studied more and more as saturable absorption materials, and one-dimensional nanomaterials have been studied as optical limiting materials and have important applications in pulse shaping, mode-locking and laser protection.
In addition, the assembly of isotropic metal nanoparticles (NPs) and metal oxide NPs onto one-dimensional (1D) architectures has generated significant interest in various applications.8–10 Those unique applications take advantage of not only the specific physical and chemical properties of the anchoring NPs but also the high surface area, light weight, high mechanical strength, and excellent electrical and thermal transport properties of 1D supports. For example, phenyl-C61-butyric acid methylester (PCBM) doped into organic electro-optic (EO) materials have been used to enhance the dielectric constant and refractive index.11 The incorporation of various metal oxide nanostructures on CNTs surface results in composite materials that can be used as energy conversion or storage materials for applications in solar cells and super capacitors.12,13 Similarly, in the NLO field, the applications of such hybrids are mostly focused on metal NPs and metal oxide NPs depositing on the other nano-carrier systems. For example, Udayabhaskar et al.14 reported that the optical limiting behavior of the nanocomposites of ZnO:Au hetero structures was stronger than the pure ZnO owing to free carrier absorption taking place in Au NPs. A series of graphene oxide (GO)/noble metal (Au, Pt, and Pd) nanoparticle (NP) composites exhibited enhanced NLO and OL properties because of nonlinear scattering (NLS) effects.15 And compare with the individual counter parts and well known graphene composites was reported, ZnFe2O4-(15 wt%) decorated rGO could efficiently enhance the third-order NLO.16 However, reports on the NLO properties of 1D metal silicate nanowires (NWs) decorated with metal or metal oxide NPs are few as yet. 1D metal silicates have attracted great attention owing to their good thermal and chemical stability, environmental friendliness, tunable composition, low cost, and abundant supply, and they have found wide applications in the fields of heat-insulating materials, biomaterials, luminescence, and polymer fillers for composites.17–20 Very recently, we demonstrated the superior broadband OL performance of cadmium metasilicate (CdSiO3) NWs over that of benchmark CNTs and suggested that the composite material could be a good replacement for CNTs in OL applications.21 In addition, as recently demonstrated, metal NPs, such as gold and silver NPs, exhibit strong and fast response NLO properties.22–26 For example, the ultrafast transient response of gold nanoparticles significantly depended on particle shape, which supported the strong saturable absorption observed in nanorods and weak nonlinear response in nanoshells.27 The OL properties of metal NPs depend on the size of the NPs and their surrounding matrix or the solvent in which the NPs are suspended.22–26 Therefore, it is expected that combining 1D metal silicate NWs with optoelectronically active gold NPs would lead to some interesting and important effects including NLO enhancement.
In the present study, we present a facile method to assemble Au NPs on 1D CdSiO3 NWs towards developing new OL materials. It is widely believed that selective heterogeneous metal growth on 1D NWs can only be achieved by the creation of preferential nucleation sites on NWs through chemical modification.28 However, chemical modification of NWs under harsh acidic conditions can damage the structure of the NWs, thereby deteriorating the properties of the resulting nanodevice.29 Impregnation is the most widely used wet chemical method30 and is typically very simple to implement. It is thus an attractive option for synthesis. The selective deposition of Au NPs on CdSiO3 NWs by heterogeneous nucleation without pretreatment is illustrated in Scheme 1. This procedure can be extended to the preparation of novel 1D nanostructures decorated with metal NPs, for which a number of promising applications in a variety of fields can be envisioned. The NLO property of the as-prepared Au NPs/CdSiO3 NWs hybrid was subsequently examined and compared with that of Au NPs and CdSiO3 NWs.
2. Experimental
2.1 Synthesis of the Au NPs/CdSiO3 NWs hybrid nanostructure
2.2 Characterization
The morphologies of the samples were observed by transmission electron microscopy (TEM; JEM-2010, JEOL Ltd.) at an accelerating voltage of 200 kV. High-resolution TEM images were also acquired at an accelerating voltage of 200 kV. Images were acquired digitally on a Gatan multiple charge-coupled device camera. Crystallographic information of the samples was obtained via X-ray diffraction (XRD) conducted on a Bruker D8-Advance X-ray diffractometer using CuKα as a radiation source (λ = 1.5418 Å) operating at 40 kV and 30 mA. The 2θ range used was from 10° to 90°. The chemical groups present on the prepared samples were examined by Fourier transform infrared (FT-IR) spectroscopy conducted on a Shimadzu Nicolet 6700 FT-IR infrared spectrophotometer in the scanning range of 4000–400 cm−1, and the KBr pellet technique was used. Ultraviolet-visible (UV-vis) absorption spectroscopy was conducted to examine the optical properties of the samples. The analysis was conducted on a Shimadzu UV-2600 spectrophotometer, using a quartz cell with a 1 cm optical path length. All experiments were performed at room temperature.2.3 Z-scan measurement
The NLO and OL properties of the samples were determined by an open-aperture Z-scan technique31 using 4 ns pulses generated by a Q-switched Nd:YAG laser operating with a repetition rate of 1 Hz and at a wavelength of 532 nm. All measurements were conducted at room temperature. Each sample was dispersed in water and contained in quartz cells with a path length of 1 mm. The cuvettes were mounted on a translation stage that shifted each sample along the z-axis. The Z-scan system was calibrated by CS2, a standard NLO material, which presents pure nonlinear refraction, to ensure accuracy of all the experimental Z-scan results. The estimated uncertainly of the extracted coefficient was ±5%, mainly arising from energy fluctuation. The energy of a single pulse was approximately 200 μJ.3. Results and discussion
3.1 Morphology of the Au NPs/CdSiO3 NWs hybrid nanostructure
Fig. 1 presents representative TEM images of the CdSiO3 NWs (Fig. 1a–c) and Au NPs/CdSiO3 NWs hybrid nanostructure (Fig. 1d–f). Specifically, Fig. 1a revealed that the CdSiO3 NWs featured a coarse surface with an average width of ∼50 nm and length of up to several micrometers. TEM imaging of an individual CdSiO3 NW (Fig. 1b) revealed a homogeneous NPs coating on the surface of the CdSiO3 NW. To examine the crystalline nature of these NPs, high-resolution TEM analysis was performed. The distinct lattice fringes observed in Fig. 1c confirmed the crystalline nature of the NPs loaded on the CdSiO3 NWs. The lattice fringes had spacings of 2.189 and 2.241 Å, which respectively corresponded to those of the (312) and (−511) crystal planes of CdSiO3, which would provide heterogeneous nucleation sites for the deposition of Au NPs on CdSiO3 NWs. As confirmed by Fig. 1d–f, successful decorating of Au NPs on the surface of CdSiO3 NWs was realized. Au NPs with uniform size and shape dispersed homogeneously and adhered strongly to the CdSiO3 NWs surface. The high-resolution TEM image (Fig. 1f) of the hybrid structure displayed distinct lattice fringes. The latter had spacings of 2.66 Å, which matched the (311) crystal plane of monoclinic CdSiO3.32 Additionally, the presence of single crystalline cubic Au NPs with a clearly resolved (200) lattice fringe (spacing = 2.036 Å) was confirmed.33 These results indicated the occurrence of heterogeneous nucleation of the Au NPs on the CdSiO3 NWs surface, followed by growth of these nuclei, consequently forming a NP coating on the CdSiO3 NWs. The synthetic procedure provides a facile way to fabricate a series of novel NWs coated with metal or metal oxide NPs with potentially tailored electronic or optical properties. | ||
| Fig. 1 TEM and high-resolution TEM images of the (a)–(c) CdSiO3 NWs and (d)–(f) Au NPs/CdSiO3 NWs hybrid nanostructure. | ||
3.2 Structure of the Au NPs/CdSiO3 NWs hybrid nanostructure
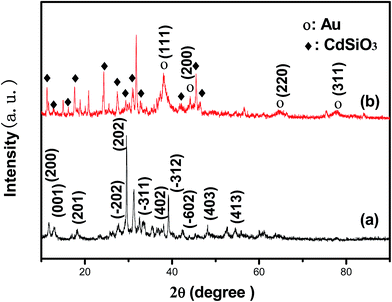
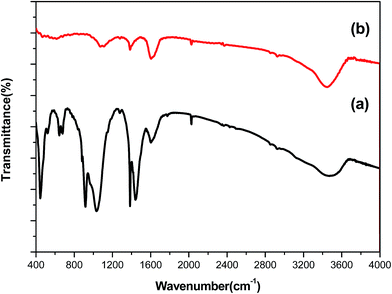
Fig. 2 shows the XRD patterns of the CdSiO3 NWs and Au NPs/CdSiO3 NWs hybrid nanostructure. The reflection pattern of the CdSiO3 NWs could readily be indexed to that of the monoclinic phase CdSiO3 (Fig. 2a), with lattice constants a = 1.510 nm, b = 0.363 nm, and c = 0.695 nm and space group of P21/a(14) (JCPDS 35-0810). The Au NPs/CdSiO3 NWs hybrid nanostructure displayed additional peaks at 2θ = 38.2°, 44.4°, 64.6°, and 77.5°, which could respectively be attributed to the (111), (200), (220), and (311) reflections of Au NPs (Fig. 2b), further confirming the deposition of Au NPs on the surface of the CdSiO3 NWs through heterogeneous nucleation process.FT-IR spectroscopy was used to investigate the microstructures of the as-prepared samples, and the results are presented in Fig. 3. The spectrum of the CdSiO3 NWs in Fig. 3a displayed a broad band from 840 to 1170 cm−1, corresponding to asymmetric stretching vibration of the Si–O–Si bond and stretching vibrations of terminal Si–O bonds. The peaks at 450–550 and 640 cm−1 were attributed to the symmetric stretching vibrations of Si–O–Si bridges.34 In addition, the band centered at 3440 cm−1 and the sharp band at 1670 cm−1 were assigned to O–H stretching and bending absorption, respectively. The small band located at 960 cm−1 was attributed to Si–OH vibration.35 Thus, the chemical composition analysis indicated the existence of [SiO4] tetrahedra in CdSiO3 NWs. Compared with the spectral bands of CdSiO3 NWs, the intensity of all the characteristic bands in the FT-IR spectrum of Au NPs/CdSiO3 NWs hybrid nanostructure was lower. This phenomenon was attributed to the occurrence of electron intraband transfer process in the 6sp band of Au species due to the SPR effect during assembly of the Au NPs on the CdSiO3 NWs.36 The SPR mainly results in the collective oscillation of electrons within the conduction band of the metal NPs. As the Au NPs are dispersed on the surface of the CdSiO3 NWs, the oscillation of Au NPs would causes the electron transfer between the CdSiO3 NWs and the Au NPs until the Fermi energy levels of are equal, because of the different levels of the Fermi energy levels of the two completely species. Therefore, the higher electron density of Au surface would lead to stronger SPR and electron transfer effect, consequently, the weaker Si–O bond strength and lower infrared vibrational spectrum of Au NPs/CdSiO3 NWs. Meanwhile, such a process would result in an increase in the localized surface electron density of the 6sp band of the Au species, thereby promoting the adsorption of Au on the CdSiO3 surface and weakening the Si–O bond strength (i.e., lower the infrared vibration of Si–O) through sp–π* back-donation.
3.3 Linear optical property of the Au NPs/CdSiO3 NWs hybrid nanostructure
The linear optical properties of the Au NPs, CdSiO3 NWs, and Au NPs/CdSiO3 NWs hybrid nanostructure were studied by UV-vis spectroscopy. As shown in Fig. 4a, the Au NPs displayed an absorption peak in the visible light region (526 nm), which could be attributed to the SPR of Au NPs.37,38 The SPR effect originates from the collective oscillation of electrons within the conduction band of the Au NPs (i.e., the intraband electron transfer) that resonate with the electromagnetic field of the incident light. In contrast, CdSiO3 NWs featured broad absorption with a maximum centered at ∼206 nm (Fig. 4b). After the assembly of Au NPs on the CdSiO3 NWs, a distinct red shift of the peak maxima, from 526 to 539 nm, was observed (Fig. 4c). Additionally, the peak was broader and less intense. The red shift, relative broadness, and low intensity of this band when compared with that displayed by Au NPs could be attributed to the interparticle interactions absorbed on the surface of the CdSiO3 NWs, as previously demonstrated.39 The absorption band appearing in the spectrum of the Au NPs/CdSiO3 NWs hybrid nanostructure confirmed the successful assembly of Au NPs on the CdSiO3 NWs. Furthermore, the absorption spectrum of the Au NPs/CdSiO3 NWs hybrid nanostructure did not feature any new absorption features, indicating that charge diffusion or electronic interaction did not occur between the CdSiO3 NWs and Au NPs in their ground state. | ||
| Fig. 4 UV-vis spectra of (a) Au NPs, (b) CdSiO3 NWs, and (c) Au NPs/CdSiO3 NWs suspended in aqueous solution at room temperature. | ||
3.4 NLO property of the Au NPs/CdSiO3 NWs hybrid nanostructure
In the present study, open-aperture Z-scan experiments were performed in the nanosecond regime at 532 nm to investigate the NLO properties of the Au NPs/CdSiO3 NWs hybrid nanostructure, and the linear transmittance of the Au NPs/CdSiO3 NWs hybrid nanostructure at 532 nm in 1 mm-thick cells was adjusted to 70%. CNTs are known as benchmark OL materials.40 Thus, their OL properties were also characterized under the same experimental conditions with the same linear transmittance as that used for examining the NLO properties of the Au NPs/CdSiO3 NWs hybrid nanostructure for direct comparison. The results are shown in Fig. 5a. In the open-aperture Z-scan, the transmittance of the sample was determined during its translation through the focal plane of at lightly focused beam. As the sample moves closer to the focus, the beam intensity increases and the nonlinear effect occurs, which then results in a decrease in transmittance because of RSA, TPA, and NLS. The depth of the valley in the Z-scan curve directly determines the NLO performance. Thus, as deduced from the scans, the NLO properties of the Au NPs/CdSiO3 NWs hybrid nanostructure were better than those of CNTs, indicating the potential application of the Au NPs/CdSiO3 NWs hybrid nanostructure in nonlinear optics.To investigate the differences in the NLO performance between CdSiO3 NWs and Au NPs/CdSiO3 NWs hybrid, their open-aperture Z-scans were measured, and the results are shown in Fig. 5b. At the focal point where the input fluence was maximum, the transmittances of CdSiO3 NWs and Au NPs/CdSiO3 NWs decreased to 57.8% and 47.5%, respectively. Thus, Au NPs/CdSiO3 NWs displayed a larger reduction in transmittance than CdSiO3 NWs. Therefore, the CdSiO3 NWs hybrid materials, noncovalently functionalized with Au NPs, are better candidates for applications in OL than individual CdSiO3 NWs. Using the Crank–Nicolson finite-difference scheme, the nonlinear extinction coefficient β can be fitted numerically to the following transmission equation for a third-order nonlinear process:31
 | (1) |
Here, q0(z,0) = βI0Leff, where I0 is the on-axis peak intensity at the focus z = 0, Leff = [1 − exp(−αl)]/α is the effective thickness of the sample, α is the linear absorption coefficient, and l is the sample thickness. The calculated β values at 532 nm for CdSiO3 NWs and Au NPs/CdSiO3 NWs were 2.11 and 2.84 cm GW−1, respectively. The larger β value of Au NPs/CdSiO3 NWs further highlights the advantage of the composite system over the CdSiO3 NWs.
Several mechanisms have been proposed to rationalize the NLO effect including TPA, free-carrier absorption, RSA, self-focusing/defocusing, and NLS.31 The mechanisms underlying the OL behavior in CdSiO3 NWs have been well studied and mainly originate from NLS.21 To confirm the occurrence of NLS effects in Au NPs/CdSiO3 NWs, NLS experiments were conducted on a CdSiO3 NWs suspension and a Au NPs/CdSiO3 NWs suspension using 532 nm laser pulses, and the results are presented in Fig. 6. Both samples displayed a strong scattering peak that was symmetric with respect to the focus, indicating the presence of strong NLS effects in both samples. However, the scattering was more pronounced in Au NPs/CdSiO3 NWs, as consistent with the superior NLO response of Au NPs/CdSiO3 NWs over that of CdSiO3 NWs. The enhanced NLS was attributed to the presence of Au NPs; NLS is known to be the dominant NLO mechanism in gold nanostructures.22–26 As the sample is excited by the laser, the absorbed photon energy expands the Au NPs into a microplasma state in the subnanosecond range and subsequently induces a scattering center. The absorbed heat is assumed to be transferred to the solvent to form microbubbles near the boiling temperature. These microplasma and microbubble scattering centers around the metal particles result in a stronger NLS and hence in enhanced NLO effects in Au NPs/CdSiO3 NWs compared with those in CdSiO3 NWs.
In addition to the NLS, we propose that NLA plays a major role in the optical nonlinearity of Au NPs/CdSiO3 NWs because of the SPR effects displayed by the Au NPs. In the hybrid structure, the SPR band was located at ∼526 nm, which was comparable with the excitation laser wavelength employed in the present study. The SPR of metal NPs often arises from the transition of the free electrons in the conduction band of the metal NPs upon laser pulse excitation. During the intraband transition, the ground-state electrons are promoted to the excited state. The excited electrons are free carriers, which possess a spectrum of energies after absorption, both kinetic and potential. Upon excitation of these electrons by a pulse close to the absorption peak, they do not oscillate at the same frequency as that of the unexcited electrons, thus causing the ground-state plasmon band to disappear or weaken41 and consequently enhancing the NLO effects.
Furthermore, it is noted that the physical dimension of the gold NPs will affect the optical properties of the hybrid structure. The research revealed that the intrinsic properties of metal nanostructures, such as SPR, can be tailored by controlling their size, shape, composition, crystallinity, and structure.42,43 Compared to the SPR of traditional Au nanoparticles centered at 520 nm, the SPR of Au nanostructures with other shapes (e.g., rods, plates, cubes) manifest special performance.44 These differences in linear optical properties between Au NPs and other Au nanostructures might importantly influence their nonlinear optical properties. Therefore, the nonlinear effect of Au NPs/CdSiO3 NWs hybrid would be affected by loading amount, size and shape of the gold nanoparticles. And the corresponding research work has being undergoing in our lab.
4. Conclusions
Facile assembly of Au NPs on CdSiO3 NWs was achieved by a heterogeneous nucleation method, which can readily be extended to fabricate novel metal or metal oxide NPs-decorated 1D nanostructure composites with potential applications in many fields. The TEM, XRD, and UV-vis absorption spectroscopy results confirmed the successful, uniform decoration of Au NPs on the surface of CdSiO3 NWs. The microstructure of the Au NPs/CdSiO3 NWs hybrid was investigated using FT-IR spectroscopy, and the results revealed that the intensities of the characteristic bands weakened owing to an electron intraband transfer process in the 6sp band of the Au species during the assembly process. The linear optical property of the Au NPs/CdSiO3 NWs hybrid was studied by UV-vis absorption spectroscopy. Compared with the spectrum of the Au NPs, that of the hybrid nanostructure featured a red-shifted, relatively broader, and weaker SPR band, which could be attributed to the interparticle interactions absorbed on the surface of the CdSiO3 NWs. The NLO properties were characterized using the open-aperture Z-scan technique with 4 ns laser pulses at 532 nm. The Au NPs/CdSiO3 NWs hybrid nanostructure displayed superior NLO responses than the CNTs suspension, a benchmark optical limiter. The CdSiO3 NWs hybrid material, noncovalently functionalized with Au NPs, offered superior performance over the individual CdSiO3 NWs. The enhancement in the NLO properties was attributed mainly to the combination of NLS and NLA induced by SPR effects of the Au NPs coating. The environmental friendliness, tunable composition, low cost, abundant supply, unique structure, and the excellent NLO properties of the prepared hybrid structure make the latter a potential candidate in the realm of OL and optical switch materials for photonic and optoelectronic devices.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 61108056), Major Projects of the University of Fujian Province (Grant No. 2015N5007), New Century Talent Support Program for Fujian Universities (Grant No. JA12226), and Talents Cultivation Program for Outstanding Young Scientists in Fujian Universities (Grant No. JA13208).References
- K. C. Chin, A. Gohel, W. Z. Chen, H. I. Elim, W. Ji, G. L. Chong, C. H. Sow and A. T. S. Wee, Gold and silver coated carbon nanotubes: an improved broad-band optical limiter, Chem. Phys. Lett., 2005, 409, 85–88 CrossRef CAS.
- J. F. Xu, M. Xiao, R. Czerw and D. L. Carroll, Optical limiting and enhanced optical nonlinearity in boron-doped carbon nanotubes, Chem. Phys. Lett., 2004, 389, 247–250 CrossRef CAS.
- Z. B. Liu, J. G. Tian, Z. Guo, D. M. Ren, F. Du, J. Y. Zheng and Y. S. Chen, Enhanced optical limiting effects in porphyrin-covalently functionalized single-walled carbon nanotubes, Adv. Mater., 2008, 20, 511–515 CrossRef CAS.
- S. Muhammad, H. L. Xu, R. L. Zhong, Z. M. Su, A. Al-Sehemi and A. Irfan, Quantum chemical design of nonlinear optical materials by sp2-hybridized carbon nanomaterials: issues and opportunities, J. Mater. Chem., 2013, 1, 5439–5449 RSC.
- H. L. Wang, L. F. Cui, Y. Yang, H. S. Casalongue, J. T. Robinson, T. Y. Liang, Y. Cui and H. J. Dai, Mn3O4-Graphene Hybrid as a High-Capacity Anode Material for Lithium Ion Batteries, J. Am. Chem. Soc., 2010, 132, 13978–13980 CrossRef CAS PubMed.
- M. Zhang, D. N. Lei, X. M. Yin, L. B. Chen, Q. H. Li, Y. G. Wang and T. H. Wang, Magnetite/graphene composites: microwave irradiation synthesis and enhanced cycling and rate performances for lithium ion batteries, J. Mater. Chem., 2010, 20, 5538–5543 RSC.
- F. Bonaccorso and Z. Sun, Solution processing of graphene, topological insulators and other 2d crystals for ultrafast photonics, Opt. Mater. Express, 2014, 4, 63–78 CrossRef.
- S. Iijima, Helical microtubules of graphitic carbon, Nature, 1991, 354, 56–58 CrossRef CAS.
- J. Wu, Y. J. Zhu, G. F. Cheng and Y. H. Huang, Microwave-assisted preparation of Ca6Si6O17(OH)2 and β-CaSiO3 nanobelts, Mater. Res. Bull., 2010, 45, 509–512 CrossRef CAS.
- D. M. Zhang, J. Chang and Y. Zeng, Fabrication of fibrous poly(butylene succinate)/wollastonite/apatite composite scaffolds by electrospinning and biomimetic process, J. Mater. Sci.: Mater. Med., 2008, 19, 443–449 CrossRef CAS PubMed.
- J. Wu, J. D. Luo, N. Cernetic, K. X. Chen, K. S. Chiang and A. K. Y. Jen, PCBM-doped electro-optic materials: investigation of dielectric, optical and electro-optic properties for highly efficient poling, J. Mater. Chem. C, 2016, 4, 10286–10292 RSC.
- H. L. Wang, L. F. Cui, Y. Yang, H. S. Casalongue, J. T. Robinson, T. Y. Liang, Y. Cui and H. J. Dai, Mn3O4-Graphene Hybrid as a High-Capacity Anode Material for Lithium Ion Batteries, J. Am. Chem. Soc., 2010, 132, 13978–13980 CrossRef CAS PubMed.
- M. Zhang, D. N. Lei, X. M. Yin, L. B. Chen, Q. H. Li, Y. G. Wang and T. H. Wang, Magnetite/graphene composites: microwave irradiation synthesis and enhanced cycling and rate performances for lithium ion batteries, J. Mater. Chem., 2010, 20, 5538–5543 RSC.
- R. Udayabhaskar, B. Karthikeyan, P. Sreekanthb and R. Philip, Enhanced multi-phonon Raman scattering and nonlinear optical power limiting in ZnO: Au nanostructures, RSC Adv., 2015, 5, 13590–13597 RSC.
- C. Zheng, W. Chen, Y. Y. Huang, X. Q. Xiao and X. Y. Ye, Graphene oxide-noble metal (Au, Pt, and Pd) nanoparticle composites as optical limiters, RSC Adv., 2014, 4, 39697–39703 RSC.
- M. Saravanan and G. T. C. Sabari, Enhanced nonlinear optical absorption and optical limiting properties of superparamagnetic spinel zinc ferrite decorated reduced graphene oxide nanostructures, Appl. Surf. Sci., 2016, 392, 904–911 Search PubMed.
- Y. Chen, M. Hanack, Y. Araki and O. Ito, Axially Modified Gallium Phthalocyanines and Naphthalocyanines for Optical Limiting, Chem. Soc. Rev., 2005, 34, 517–529 RSC.
- Y. Chen, M. Fujitsuka, S. M. O'Flaherty, M. Hanack, O. Ito and W. J. Blau, Strong Optical Limiting of Soluble Axially Substituted Gallium and Indium Phthalocyanines, Adv. Mater., 2003, 15, 899–902 CrossRef CAS.
- Y. Chen, Y. Lin, Y. Liu, J. Doyle, N. He, X. Zhuang, J. Bai and W. J. Blau, Carbon nanotube-based functional materials for optical limiting, J. Nanosci. Nanotechnol., 2007, 7, 1268–1283 CrossRef CAS PubMed.
- Y. Chen, N. He, J. J. Doyle, Y. Liu, X. Zhuang and W. J. Blaub, Enhancement of optical limiting response by embedding gallium phthalocyanine into polymer host, J. Photochem. Photobiol., A, 2007, 189, 414–417 CrossRef CAS.
- C. Zheng, C. C. Dai, L. Huang, W. Li and W. Z. Chen, Self-assembly of cadmium metasilicate nanowires as a broadband optical limiter, Opt. Mater., 2016, 54, 50–56 CrossRef CAS.
- J. Wang and W. J. Blau, Solvent Effect on Optical Limiting Properties of Single-Walled Carbon Nanotube Dispersions, J. Phys. Chem. C, 2008, 112, 2298–2303 CAS.
- R. Philip, G. R. Kumar, N. Sandhyarani and T. Pradeep, Picosecond optical nonlinearity in monolayer-protected gold, silver, and gold–silver alloy nanoclusters, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 13160–13166 CrossRef CAS.
- S. Porel, N. Venkatram, D. N. Rao and T. P. Radhakrishnan, In situ synthesis of metal nanoparticles in polymer matrix and their optical limiting applications, J. Nanosci. Nanotechnol., 2007, 7, 1887–1892 CrossRef CAS PubMed.
- G. Wang and W. Sun, Optical limiting of gold nanoparticle aggregates induced by electrolytes, J. Phys. Chem. B, 2006, 110, 20901–20905 CrossRef CAS PubMed.
- L. François, M. Mehran Mostafavi, J. Belloni, J. F. Delouis, J. Delaire and P. Feneyrou, Optical Limitation induced by Gold Clusters. 1. Size Effect, J. Phys. Chem. B, 2000, 104, 6133–6137 CrossRef.
- Y. Hua, K. Chandra, D. H. Dam, G. P. Wiederrecht and T. W. Odom, Shape-dependent Nonlinear Optical Properties of Anisotropic Gold Nanoparticles, J. Phys. Chem. Lett., 2015, 6, 4904–4908 CrossRef CAS PubMed.
- N. M. B. Neto, C. R. Mendonca, L. Misoguti and S. C. Zilio, Optical limiting of ultrashort pulses by carbon black suspension, Appl. Phys. B: Lasers Opt., 2004, 78, 1–3 CrossRef.
- K. C. Chin, A. Gohel, H. I. Elim, W. Chen, W. Ji, G. L. Chong, C. H. Sow and A. T. S. Wee, Modified carbon nanotubes as broadband optical limiting nanomaterials, J. Mater. Res., 2006, 21, 2758–2766 CrossRef CAS.
- J. Wang and W. J. Blau, Nonlinear optical and optical limiting properties of individual single-walled carbon nanotubes, Appl. Phys. B: Lasers Opt., 2008, 91, 521–524 CrossRef CAS.
- M. Sheik-Bahae, A. A. Said, T. H. Wei, D. J. Hagan and E. W. V. Stryland, Sensitive measurement of optical nonlinearities using single beam, IEEE J. Quantum Electron., 1990, 26, 760–769 CrossRef CAS.
- X. A. Zhang, B. Cao, Y. He and H. Jun, Silicon Nanowires Decorated with Au Nanoparticles and Their Spectroscopic Properties, Acta Chim. Sin., 2009, 67, 1277–1284 CAS.
- C. H. Li, Z. X. Yu, K. F. Yao, S. F. Ji and J. Liang, Nitrobenzene hydrogenation with carbon nanotube-supported platinum catalyst under mild conditions, J. Mol. Catal. A: Chem., 2005, 226, 101–105 CrossRef CAS.
- F. Wang, S. Arai and M. Endo, Metallization of multi-walled carbon nanotubes with copper by an electroless deposition process, Electrochem. Commun., 2004, 6, 1042–1044 CrossRef CAS.
- V. Georgakilas, V. Tzitzios, D. Gournis and D. Petridis, Attachment of Magnetic Nanoparticles on Carbon Nanotubes and Their Soluble Derivatives, Chem. Mater., 2005, 17, 1613–1617 CrossRef CAS.
- T. M. Day, P. R. Unwin, N. R. Wilson and J. V. Macpherson, Electrochemical Templating of Metal Nanoparticles and Nanowires on Single-Walled Carbon Nanotube Networks, J. Am. Chem. Soc., 2005, 127, 10639–10647 CrossRef CAS PubMed.
- Y. Zhang and H. Dai, Formation of Metal Nanowires on Suspended Single-Walled Carbon Nanotubes, Appl. Phys. Lett., 2000, 77, 3015–3017 CrossRef CAS.
- S. Banerjee and S. S. Wong, In situ quantum dot growth on multiwalled carbon nanotubes, J. Am. Chem. Soc., 2003, 125, 10342–10350 CrossRef CAS PubMed.
- M. A. Correa-Duarte, N. Sobal, L. M. Liz-Marzán and M. Giersig, Linear Assemblies of Silica-Coated Gold Nanoparticles Using Carbon Nanotubes as Templates, Adv. Mater., 2004, 16, 2179–2184 CrossRef CAS.
- Y. Chen, Y. Lin, Y. Liu, J. Doyle, N. He, X. Zhuang, J. Bai and W. J. Blau, Carbon nanotube-based functional materials for optical limiting, J. Nanosci. Nanotechnol., 2007, 7, 1268–1283 CrossRef CAS PubMed.
- S. L. Qu, Y. C. Gao, X. W. Jiang, H. D. Zeng, Y. L. Song, J. R. Qiu, C. S. Zhu and K. Hirao, Nonlinear absorption and optical limiting in gold-precipitated glasses induced by femtosecond laser, Opt. Commun., 2003, 224, 321–327 CrossRef CAS.
- F. Bonaccorso, M. Zerbetto, A. C. Ferrari and V. Amendola, Sorting Nanoparticles by Centrifugal Fields in Clean Media, J. Phys. Chem. C, 2013, 117, 13217–13229 CAS.
- T. P. Tyler, A. I. Henry, R. P. V. Duyne and M. C. Hersam, Improved Monodispersity of Plasmonic Nanoantennas via Centrifugal Processing, J. Phys. Chem. Lett., 2011, 2, 218–222 CrossRef CAS.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics?, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |