Enhanced biohydrogen production from beverage wastewater: process performance during various hydraulic retention times and their microbial insights
Periyasamy Sivagurunathan*a and
Chiu-Yue Lin*b
aDepartment of Environmental Engineering, Daegu University, Jillyang, Gyeongsan, Gyeongbuk 712-714, Republic of Korea. E-mail: contact2sivas12@gmail.com; cylin@fcu.edu.tw; Fax: +82-886-4-35072114; Tel: +82-886-4-24517250 ext. 6200
bDepartment of Environmental Engineering and Science, Feng Chia University, Taichung 40724, Taiwan
First published on 16th December 2015
Abstract
This study demonstrates the feasibility of continuous hydrogen production from beverage industrial wastewater (BW) in a continuously-stirred tank reactor (CSTR) using enriched mixed microflora (EMC) under mesophilic conditions. Various hydraulic retention times (HRT) (ranging from 6 to 1.5 h with an influent substrate concentration of 20 g Lhexose equivalent−1) have been evaluated to elucidate the peak hydrogen production rate (HPR) and operational stability of the bioreactor. The results show that a peak HPR of 37.5 L H2 per L per day was observed at HRT 1.5 h. In contrast, a maximum hydrogen yield (HY) of 1.62 mol H2 per mol hexose was attained at HRT 6 h. This HPR value was higher than those found using other organic wastewater sources reported in the literature. The major soluble metabolic products formed were butyric, lactic and acetic acid. The microbial community composition characterized using PCR-DGGE analysis revealed that Clostridium sp. was the dominant species. HRT-dependent trends influenced the HPR and HY. A peak energy production rate of 441 kJ L−1 d−1 was achieved at the lowest HRT (1.5 h) evaluated.
1. Introduction
Ever increasing oil prices, the scarcity of fossil fuel reserves, global warming and climate change are major driving forces for the search of environmentally friendly and renewable energy carriers. Over recent years, various biofuels (bioethanol, biobutanol, and biodiesel) have been proposed to reduce fossil-fuel consumption and carbon footprints.1 Among them, hydrogen seems to be more promising than other fuels because it produces only water after combustion, is carbon neutral and satisfies the environmental benefits of practicing as a fuel for a day-to-day life. Moreover, hydrogen can be produced biologically using anaerobic microorganisms from renewable organic waste materials such as industrial wastewater, lignocellulosic biomass and food waste.2–4In earlier studies, regarding the biohydrogen production, only glucose and sucrose were the widely studied substrates for continuous systems, which has now been realized as a non-economic process towards industrial scale applications. Therefore, exploitation of wastewater is recommended for cost-effective and sustainable bioprocesses. The utilization of wastewater for biohydrogen production has increased in recent years and is usually conducted using batch and continuous modes of operation.5 Most investigations on continuous hydrogen production from industrial wastewaters of cheese whey, coffee drink-manufacturing, condensed soluble molasses, tofu processing, sugary wastewater and molasses6–11 have been conducted in continuous stirred-tank reactors (CSTR) due to their improved mass-transfer and mixing. The selection of a cost-effective feed stock is an important criteria to achieve success during large scale H2 generation.
From an economical and industrial perspective, continuous operation is preferred, because it can save time and other capital costs, especially, while using organic wastewater as a feedstock. Previously, mixed cultures have been employed for hydrogen fermentation; however, their stability was not feasible, because major changes occur in the continuous operation due to shifts in the microbial community. This could be solved by preparing enriched mixed cultures (EMC), which has been proposed in some investigations,12,13 because EMC provide stability in the operation and are also mostly comprised efficient hydrogen producers.
There are several strategies used to improve bio-hydrogen productivity. Among them, evaluating the importance of HRT during its reduction in the continuous system is an important factor, which can regulate the metabolic flow of microorganisms and eliminate the non-hydrogen producing bacteria at lower flow rates. Our recent finding showed that BW could produce a stable HPR of 13 L L−1 d−1 at 8 h of HRT.14 Moreover, many other reports have shown that short HRTs (0.5–4 h) also have a significant effect on hydrogen production performance.7,9,15,16
Thus, to study the influence of HRT to further improve the HPR, this study aimed to find out the effect of HRTs (6 to 1.5 h) on continuous hydrogen production from BW using a selectively enriched mixed culture (EMC) as the seeding source. In addition, the corresponding changes in the microbial population were accessed via PCR-DGGE sequencing to elucidate the microbial niche. The information obtained herein is expected to be useful in developing future sustainable technologies for hydrogen production from cost-effective substrates (such as BW mentioned here).2
2. Materials and methods
2.1 Microbial source and wastewater composition
The acclimatized enriched mixed culture from a CSTR hydrogen production bioreactor (pH 5.5, volatile suspended solids = 3.23 g L−1) fed with BW at 8 h HRT14 was used as an inoculum source in this study. The characteristics of the beverage wastewater (BW) were pH 2.6–3.4, chemical oxygen demand (COD) 760–900 g L−1 and total reducing-sugar 660–750 g(hexose equivalent) L−1. From this raw wastewater feedstock, a 20 g total reducing-sugar(hexose equivalent) per L substrate solution was made for the experiments.2.2 Experimental setup for continuous operation
A schematic of the CSTR is shown in Fig. 1. A total volume of 2.5 L CSTR bioreactor with an effective working volume of 2.0 L was used. The start-up of the reactor was performed as mentioned in our previous study.14 The reactor was maintained at a constant temperature of 37 °C without pH control. A basal endo medium17 was used with the following ingredients (g L−1): 5.24, NH4HCO3; 6.72, NaHCO3; 0.125, K2HPO4; 0.1, MgCl2·6H2O; 0.015, MnSO4·6H2O; 0.025, FeSO4·7H2O; 0.005, CuSO4·5H2O; 0.00012, CoCl2·5H2O. The CSTR was operated for 157 days, with HRTs 6 h and 4 h maintained for 30 days after the post-start up, followed by 3 h HRT for 29 days, 2 h HRT for 45 days and 1.5 h for 23 days.2.3 Analytical methods
The biogas composition (H2 and CO2) was analyzed using gas chromatography equipped with a thermal conductivity detector (China Chromatograph 8700T). The biogas volume was measured using a wet-gas meter (Ritter, Bochum, Germany). The concentration of the soluble metabolic products (volatile fatty acids and ethanol) was detected using gas chromatography (Shimadzu GC-14A) equipped with a flame ionization detector (FID). The COD, pH, and concentration of volatile suspended solids (VSS) were measured according to the procedures described in standard methods.18 The dinitrosalicylic acid method was used to measure the total reducing-sugar concentration.19 The hydrogen yield was computed as mol H2 mol−1 hexose.2.4 Microbial community analysis
Microbial changes during different HRT operational conditions were assessed using the denaturing gradient gel electrophoresis (DGGE) technique. The total genomic DNA collected at HRT 6 h (day 24), 4 h (day 59), 3 h (day 85), 2 h (day 130), and 1.5 h (day 155) was extracted using a Blood & Tissue Genomic DNA Extraction Miniprep System (Viogene, Taiwan) following the manufacturer's instructions. The V6 regions of the bacterial 16S rRNA genes were subjected to polymerase chain reaction (PCR) amplification and DGGE analysis as per the method described elsewhere.202.5 Energy production analysis
The energy production rate (EPR) of biohydrogen and ethanol (EPR, kJ L−1 d−1) was calculated as follows:| H2 = HPR × HVH2 | (1) |
| EtOH = ethanol production rate × HVEtOH | (2) |
| The total energy production rate (TEPR, kJ L−1 d−1) = EPRH2 + EPREtOH | (3) |
3. Results and discussion
3.1 Process performances under various HRTs
Five HRTs (6, 4, 3, 2 and 1.5 h) were operated to investigate the fermentative hydrogen production performance of BW. Fig. 2 shows the profiles of the biogas production rate (BPR), HPR, hydrogen yield (HY), pH, H2 (%) and CO2 (%) under various operational HRT conditions. The performance of the reactor under steady-state conditions for each HRT is summarized in Table 1. The organic loading rate (OLR) was increased from 80 to 320 g L−1 dhexose equivalent−1 by shortening the HRT from 6 h to 1.5 h. The reactor was started-up with a batch operation mode for 24 h and then switched to a continuous mode at 6 h HRT. After the steady-state condition (the condition reaching stable hydrogen production with less than 10% deviation) was obtained, the HRT was gradually decreased to the other designed values of 4 h, 3 h, 2 h and 1.5 h with each HRT having their own steady-state conditions. As observed from (Fig. 2), the hydrogen content in the biogas mixture ranged from 39 to 45%, which is similar to the values found using condensed molasses solubles and molasses reported in the literature.10,15 The oxidation reduction potential (ORP) (mV) was observed in the range from −343 to −453 mV, which was close to the optimal value for hydrogen production as indicated.22 Furthermore, the pH range of 5.6–6.8 was favorable for metabolite and hydrogen production.23 In our study, during the HRT changes, the pH values lied in the range of 5.7–6.4, which favors stable and efficient hydrogen production from BW.| Parameters | Operational conditions | ||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| a TVFA, total volatile fatty acid = propionate + acetate + butyrate + lactate + formate; SMP, soluble metabolite product = ethanol + butanol + TVFA; VSS, volatile suspended solids. | |||||
| Steady-state days | 21–30 | 51–60 | 82–89 | 109–134 | 143–154 |
| Hydraulic retention time (h) | 6 | 4 | 3 | 2 | 1.5 |
| Organic loading rate (g substrate L−1 d−1) | 80 | 120 | 160 | 240 | 320 |
| Volumetric hydrogen production rate (L L−1 d−1) | 17.92 ± 0.19 | 21.65 ± 0.39 | 26.59 ± 0.34 | 32.85 ± 0.55 | 37.56 ± 0.75 |
| Hydrogen yield (mol mol−1 hexoseutilized) | 1.62 ± 0.03 | 1.30 ± 0.02 | 1.25 ± 0.02 | 1.06 ± 0.02 | 1.05 ± 0.03 |
| Substrate degradation rate (%) | 97.9 ± 0.99 | 97.41 ± 0.43 | 93.47 ± 0.73 | 92.0 ± 0.95 | 78.33 ± 1.26 |
| Ethanol (g COD per L) | 1.43 ± 0.09 | 1.52 ± 0.05 | 1.99 ± 0.15 | 1.65 ± 0.09 | 1.35 ± 0.20 |
| Butanol (g COD per L) | 0.25 ± 0.01 | 0.22 ± 0.01 | 0.79 ± 0.01 | 0.28 ± 0.01 | 0.51 ± 0.01 |
| Propionate (g COD per L) | 0.65 ± 0.01 | 0.89 ± 0.03 | 0.60 ± 0.04 | 0.54 ± 0.05 | 0.43 ± 0.01 |
| Acetate (g COD per L) | 4.89 ± 0.18 | 4.49 ± 0.17 | 3.76 ± 0.07 | 3.05 ± 0.07 | 2.26 ± 0.10 |
| Butyrate (g COD per L) | 9.89 ± 0.71 | 10.50 ± 0.25 | 9.47 ± 0.29 | 8.23 ± 0.59 | 6.48 ± 0.22 |
| Lactate (g COD per L) | 0.24 ± 0.10 | 1.44 ± 0.15 | 2.74 ± 0.15 | 3.49 ± 0.14 | 5.15 ± 0.24 |
| Formate (g COD per L) | 0.05 ± 0.01 | 0.04 ± 0.02 | 0.07 ± 0.01 | 0.03 ± 0.02 | 0.05 ± 0.01 |
| TVFA (g COD per L) | 15.74 ± 0.70 | 17.37 ± 0.74 | 16.53 ± 0.15 | 15.35 ± 0.10 | 14.35 ± 0.26 |
| SMP (g COD per L) | 17.43 ± 0.63 | 19.10 ± 0.62 | 19.33 ± 0.25 | 17.28 ± 0.19 | 16.22 ± 0.40 |
| VSS (g L−1) | 3.36 ± 0.12 | 4.55 ± 0.15 | 3.96 ± 0.20 | 3.47 ± 0.10 | 2.73 ± 0.18 |
| B/A ratio (%) | 0.80 ± 0.06 | 0.94 ± 0.05 | 1.00 ± 0.03 | 1.07 ± 0.01 | 1.14 ± 0.01 |
The HRT showed impacts on the hydrogen production rate (HPR) with shortening HRT (6 h to 1.5 h) gradually and increasing the HPR (17.9 to 37.5 L L−1 d−1). This result was similar to various reports on continuous hydrogen production and showed that a lower HRT favored higher hydrogen production rates due to the increased OLR.5 The maximum HPR (37.5 L L−1 d−1 at 320 g L−1 dhexose equivalent−1, HRT 1.5 h) obtained in this study was higher than the reported value of 9.8 L L−1 d−1 at HRT 3 h with a similar OLR of 320 g COD per L per day using a condensed molasses soluble substrate.24 In fact, in the continuous mode, the HPR was OLR dependent; increasing the OLR from 80 g L−1 dhexose equivalent−1 to 320 g L−1 dhexose equivalent−1 favored the hydrogen production rate but the HY was significantly affected. A peak HY of 1.62 mol mol−1 hexose was achieved at a low OLR (80 g L L −1 dhexose equivalent−1, 6 h HRT), whereas a lower HY of 1.05 mol mol−1 hexose was observed at a higher OLR (320 g L L−1 dhexose equivalent−1, 1.5 h HRT). The result of high HPR but low HY may be related to the changes in the hydrogen-producing microbial community structure.24 Table 2 shows the optimal operation conditions, maximal HPR and HY for various wastewater feedstocks used in continuous hydrogen production. As observed from Table 2, the HPR (37.5 L L−1 d−1) obtained in this study was higher than those reported using other wastewater feedstocks in continuous operation. The observed difference was mainly influenced by variations in the types of inoculum, substrate and other operational conditions (pH, OLR, HRT and temperature).
| Inoculum | Substrate | Reactor type | pH | OLR (g COD per L) | HRT (h) | Temp (°C) | HY (mol mol−1 substrate) | HPR (L L−1 d−1) | References |
|---|---|---|---|---|---|---|---|---|---|
| a AS – anaerobic sludge; AGS – anaerobic granular sludge; ADS – anaerobic digester sludge; EMC – enriched mixed culture; CWWW – cheese whey wastewater; CMS – condensed molasses soluble; TPWW – tofu processing wastewater; CSWW – corn syrup wastewater; BW – beverage wastewater; ** – g L−1; CSTR – continuously stirred tank reactor; MBR – membrane bioreactor; EGSB – expanded granular sludge bed reactor. | |||||||||
| AS | CMS | CSTR | 5.5 | 40 | 3 | 35 | 0.9 | 9.86 | 7 |
| ADS | TPWW | CSTR | 5.5 | 20 | 8 | 60 | 1.20 | 8.17 | 9 |
| ADS | TPWW | MBR | 5.5 | 43.4 | 4 | 60 | 1.45 | 19.86 | 9 |
| AS | Molasses | CSTR | 4.4 | 8 | 5 | 35 | N.A | 7.47 | 10 |
| AGS | CWWW | CSTR | 5.9 | 138.6** | 6 | 37 | 2.8 | 28.47 | 11 |
| AS | CMS | CSTR | 5.5 | 40 | 0.5 | 37 | 2.02 mmol H2 per g COD | 14.04 | 15 |
| ADS | Molasses | EGSB | 4.4 | 120 | 2 | 35 | 3.47 | 17.04 | 16 |
| ADS | CSWW | Novel reactor | 5.5 | 27 | 8 | 37 | 3.2 | 34 | 47 |
| AS | Beet sugar wastewater | CSTR | 4.5 | 18 | 8 | 35 | N.A | 10.8 | 48 |
| AS | TPWW | CSTR | 5.5 | 20 | 8 | 35 | N.A | 1.73 | 49 |
| EMC | BW | CSTR | 6.3 | 20** | 1.5 | 37 | 1.05 | 37.5 | This study |
3.2 Metabolic end products distribution
In acidogenic hydrogen fermentation, the HRT affects the production and distribution of soluble metabolic products (SMP). As shown in Table 1, butyrate and acetate were the major metabolic products, followed by lactate, ethanol, propionate and butanol. The SMP production varied significantly (in the range of 16.2–19.3 g COD per L) as the HRT was decreased from 6 h to 1.5 h with lower SMPs production (16.2 g COD per L) and biomass concentration (2.73 g VSS per L) observed at the shorter HRT of 1.5 h. Chen et al.25 also observed the similar phenomenon of decreased SMP production and biomass concentration at lower HRTs using a sucrose feedstock in a CSTR with the HPR being affected at low HRT.The volatile fatty acids distribution and their concentrations were HRT-dependent. Butyrate (HBu) accounted for 39.5% to 56.7% of the total SMPs indicating a butyrate-type fermentative pathway (Fig. 3). Butyrate concentrations were 9.89 to 10.50 g COD per L at HRT 6–4 h and then markedly decreased to 6.48 g COD per L upon reducing the HRT from 4 h to 1.5 h. The propionate concentration was observed to increase from 0.65 g COD per L to 0.89 g COD per L upon decreasing the HRT from 6 h to 4 h and decreased to 0.43 g COD per L at HRT 1.5 h. However, the acetate (HAc) concentration decreased gradually from 4.89 g COD per L to 2.26 g COD per L when the HRT was reduced from 6 h to 1.5 h. It was notable that the lactate concentration rose dramatically from 0.24 g COD per L to 5.15 g COD per L and accounted for 1.3% to 31.8% of the total SMP. The solvents ethanol and butanol accounted for 8% to 10.3% and 1.4% to 4.2%, respectively, with a negligible amount of formate (<0.4%).
| C6H12O6 + 4H2O → 2CH3COO− + 2HCO−3 + 4H+ + 4H2 | (4) |
| C6H12O6 + 2H2O → 2CH3CH2CH2COO− + 2HCO−3 + 3H+ + 2H2 | (5) |
| C6H12O6 + 2H2O → 2CH3CH2OH + 2HCO−3 + 2H+ | (6) |
| C6H12O6 → 2CH3CHOHCOO− + 2H+ | (7) |
| CH3CHOHCOO− + 0.4CH3COO− + 0.7H+ → 0.7CH3CH2CH2COO− + 0.6H2 + CO2 + 0.4H2O | (8) |
The distribution pattern of SMP as a function of HRT was dependent on the OLR and microbial community activity.26,27 The high HBu/SMP and HAc/SMP ratios and lower reduced end products/SMP ratio, observed in this study indicates an efficient hydrogen generation system. Moreover, butyrate and acetate production (eqn (4) and (5)), positively correlated to higher hydrogen production and their conversion ratio used to assess the hydrogen production performance.28,29 As mentioned in Table 1, the HY decreased (1.62 to 1.05 mol mol−1 hexose) as the HBu/HAc ratio increased (0.80 to 1.14) in the HRT range of 6–1.5 h, indicating a butyrate-mediated fermentative pathway was observed under the low HRTs, which significantly affected the HY. This HBu/HAc ratio value was consistent with the values previously reported and demonstrated a lower concentration ratio of butyrate to acetate was associated with higher HY.30,31
Previous studies also indicated that an increased OLR significantly affects hydrogen production and the distribution pattern of lactate and ethanol (eqn (6) and (7)).32,33 Moreover, coupled acetate and lactate pathways also exist (eqn (8)) with the formation of hydrogen, CO2 and butyrate.32 The increased lactate concentration of 5.15 g COD per L at 1.5 h HRT significantly affected the HY with a low value of 1.05 mol mol−1 hexose, whereas a higher yield of 1.62 mol mol−1 hexose was achieved at 6 h HRT with a low lactate concentration of 0.24 g COD per L. The observed difference was attributed to the short HRT, which did not allow enough time for the conversion of lactate to butyrate and hydrogen.32 The ethanol concentration did not vary significantly (in the range of 1.35–1.99 g COD per L) at the tested HRTs (6 to 1.5 h). These concentration values are at the same level to those previously reported7,15 using condensed molasses soluble in continuous hydrogen fermentation. Moreover, the production of lactate and ethanol under higher OLR (low HRT) was consistent with the findings of the previous studies.34,35
The COD mass balance at various HRTs under steady-state conditions were computed based on the distribution of the soluble metabolites, microbial biomass, and hydrogen (Table 3). The closure of COD balances of 87% to 99% indicates the accuracy of the experimental data. This proved that the reactor performance was reliable and the results were significant. In addition, this confirms that the measurements and analysis of the gaseous and liquid products were accurate. The observed limited variation (less than 13%) in the COD recovery could be attributed to the marginal error of the determination methods used.36
| HRT (h) | CODsub,ina (g COD per h) | CODsub,resb (g COD per h) | CODSMPc (g COD per h) | CODH2d (g COD per h) | CODbioe (g COD per h) | CODsumf (g COD per h) | COD balanceg (%) |
|---|---|---|---|---|---|---|---|
| a CODsub,in: g COD per h of influent substrate, calculated by (substrate concentration (mg COD per L) × feeding rate (L h−1)).b CODsub,res: g COD per h of residual substrate in the effluent, calculated by [CODsub,in × (1 − substrate utilization)].c CODSMP: g COD per h of soluble microbial products (SMP), calculated by (SMP concentration (mg COD per L) × feeding rate (L h−1)).d CODbio: g COD per h of biomass in the effluent, calculated by (mg cell per L × feeding rate (L h−1) × 1.42 mg COD per mg VSS per L), assuming that cell formula is C5H7O2N.50e CODH2: g COD per h of H2 evolved, calculated by (mol H2 per h × 16 g COD per g H2).f CODsum: g COD per h, sum of residual substrate + SMP + biomass + H2.g COD balance (%): [CODsum]/[CODsub,in] × 100. | |||||||
| 6 | 4.03 | 0.07 | 2.90 | 0.47 | 0.58 | 4.03 | 99.9 |
| 4 | 6.05 | 0.13 | 4.77 | 0.56 | 0.50 | 5.98 | 98.9 |
| 3 | 8.06 | 0.39 | 6.44 | 0.70 | 0.34 | 7.88 | 97.7 |
| 2 | 12.10 | 0.85 | 8.64 | 0.86 | 0.19 | 10.55 | 87.2 |
| 1.5 | 16.13 | 3.06 | 10.81 | 0.98 | 0.11 | 14.97 | 92.8 |
3.3 Microbial community variation at various HRTs
Fig. 4 shows the profile of the bacterial communities response under steady-state conditions of the different HRTs (6 to 1.5 h) determined using universal and Clostridium-specific bacterial primer sets. As shown in Fig. 4, the DGGE profiles were significantly different. It can be pointed out that changes in microbial community structure were attributed to the changes in HRT operation or in other words, caused the population shift in the mixed culture due to the wash-out of bacterial cells under the higher dilution rate (i.e. shorter HRT). In the DGGE band pattern analysis, each distinct band represented a specific species in the microbial population.37 The excised selected bands were subjected to DNA sequencing analysis and their results listed in Table 4. The homology strains were depicted in Fig. 5 and 6. Fig. 5 shows the genetic distance of the bacterial species obtained from BW at the different applied HRTs. There are six distinct divisions among the Clostridium species, whereas Klebsiella sp. and Ruminococcus sp. possess a single group within in the total microbial populations. Fig. 6 depicts the genetic distance of the Clostridial species based on the Clostridium specific primer sets. Among the Clostridium species, Clostridium butyricum possessed the superior dominance over the other species, which coincides with the butyrate-mediated fermentative metabolism of BW.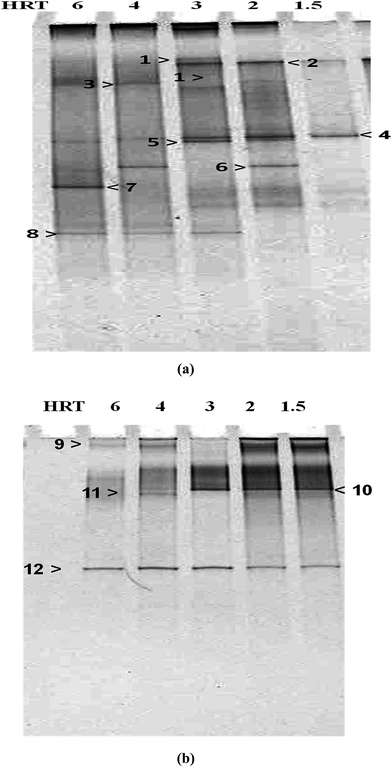 | ||
| Fig. 4 DGGE analyses of the reactor samples at various steady-state operational HRTs using primer sets: (a) universal eubacterial primer set and (b) Clostridium-specific primer set. | ||
| Primer sets | Band | Species | HRT | ||||
|---|---|---|---|---|---|---|---|
| 6 | 4 | 3 | 2 | 1.5 | |||
| a ‘+’ = appearance; ‘−’ = non appearance. | |||||||
| EUB 968f-GC & 1392r | 1 | Ruminococcus albus (accession no. NR_113032.1) | − | − | + | − | − |
| 2 | Clostridium butyricum (accession no. NR_042144.1) | − | − | + | + | + | |
| 3 | Clostridium tyrobutyricum (accession no. NR_044718.2) | + | + | + | + | − | |
| 4 | Clostridium butyricum (accession no. NR_042144.1) | + | + | + | + | + | |
| 5 | Clostridium pasteurianum (accession no. NR_104822.1) | − | − | + | + | − | |
| 6 | Clostridium acetobutylicum (accession no. NR_074511.1) | + | + | − | + | − | |
| 7 | Klebsiella oxytoca (accession no. NR_102982.1) | + | − | + | + | + | |
| 8 | Clostridium perfringens strain 13 (accession no. NR_074482.1) | + | + | + | − | − | |
| Chis 150f GC & ClostIr | 9 | Clostridium perfringens (accession no. JF499889) | + | + | − | + | + |
| 10 | Clostridium tyrobutyricum (accession no. NR_044718.2) | − | + | + | + | + | |
| 11 | Clostridium butyricum (accession no. NR_042144.1) | − | + | − | − | − | |
| 12 | Clostridium butyricum (accession no. NR_042144.1) | + | + | + | + | + | |
The bacterial communities prevailing at different HRTs were mainly composed of seven groups of bacteria, namely, Ruminococcus albus, Clostridium butyricum, C. tyrobutyricum, C. pasteurianum, C. acetobutylicum, C. perfringenes and Klebsiella oxytoca. At HRT 1.5 h with a peak HPR of 37.5 L L−1 d−1, four species (C. butyricum, C. tyrobutyricum, C. perfringenes and K. oxytoca) were observed. R. albus, C. pasteurianum and C. acetobutylicum did not appear at HRT 1.5 h. Basically, Clostridium sp. were useful microorganisms in dark fermentative hydrogen production. Nevertheless, the Clostridium and Klebsiella strains were the dominant hydrogen producers observed under low HRT conditions, which serve as the efficient hydrogen producers due to the wash-out of the other non-competitive bacteria under higher dilution rates.38 Klebsiella spp. has been reported as a potential facultative anaerobic hydrogen producer and were detected in continuous hydrogen production bioreactors fed with glucose, soft-drink wastewater or sucrose.37,39,40 Moreover, Klebsiella spp. in a reactor consumes O2 and assists to maintain a suitable anaerobic environment, which might favor the growth of O2-sensitive Clostridium sp. and then result in efficient hydrogen production.
The shift in microbial population (Fig. 4) at various HRTs showed a significant influence on the soluble metabolite distribution as well as hydrogen production performance. Ruminococcus albus was observed at HRT 3 h but further decreasing the HRT resulted in its disappearance. C. pasteurianum and C. acetobutylicum also disappeared at the low HRT of 1.5 h. The lower HY 1.05 mol mol−1 hexose at HRT 1.5 h was attributed to the wash-out of these populations under low HRT conditions. Moreover, C. butyricum, C. tyrobutryicum and C. perfringenes were the predominant populations observed at all the HRTs studied. The Clostridial species (C. butyricum, C. tyrobutyricum, C. perfringenes) detected at HRT 1.5 h have been reported as potential hydrogen-producing bacteria in continuous operations.15,32,38,41 In general, Clostridial spp. exhibits a butyrate-type hydrogen fermentation with the formation of acetate, lactate and ethanol.42 Klebsiella spp. exhibits a mixed acid type hydrogen fermentation with the cogeneration of ethanol and acetate. The results agree with the SMP analysis and imply that the dominant metabolites formed during BW fermentation were butyrate, lactate, acetate and ethanol. The optimal operational conditions (low HRT of 1.5 h and high OLR of 320 g L−1 dhexose equivalent−1) observed in this study favored the growth of efficient hydrogen producing bacteria and resulted in enhanced hydrogen production. The DGGE analysis clearly showed that HRT significantly affected the composition of microbial community structure during continuous hydrogen production.
3.4 Energy production rates
The energy production rates (EPR) at various HRTs were calculated based on the higher heating combustion value of hydrogen and ethanol. As shown in Table 5, the EPR values increased as the HRT decreased from 6 to 1.5 h. In other words, it was also due to the increased OLR (Table 5). A peak EPR (441 kJ L−1 d−1) was obtained at 1.5 h HRT, whereas a minimum EPR (222 kJ L−1 d−1) was observed at 6 h HRT. This was similar to that reported by Han et al.31 indicating that an increased OLR improved the energy production rate. The ethanol production rate dropped at 1.5 h HRT, whereas hydrogen production was not significantly affected at the low HRT of 1.5 h; this could be due to the changes in microbial community structure. The EPR obtained in this study was lower than the value of 457 kJ L−1 d−1 (ref. 43) found during CSTR operation using sugar beet molasses. However, it was higher than the value of 113 kJ L−1 d−1 (ref. 44) found using diluted sugar cane stillage wastewater. The EPR analysis showed that BW can be used as an efficient low-cost feedstock for sustainable bio-hydrogen and ethanol production.| HRT (h) | Production rates (mmol L−1 d−1) | Energy production rate (kJ L−1 d−1) | Total energy production rate (kJ L−1 d−1) | ||
|---|---|---|---|---|---|
| Hydrogen | Ethanol | Hydrogen | Ethanol | ||
| 6 | 533.93 | 14.5 | 201.84 | 20.35 | 222.20 |
| 4 | 705.75 | 14.9 | 243.79 | 21.65 | 265.44 |
| 3 | 852.40 | 15.85 | 300.80 | 28.39 | 329.18 |
| 2 | 1051.73 | 20.78 | 369.39 | 23.51 | 392.90 |
| 1.5 | 1291.57 | 17.21 | 422.35 | 19.17 | 441.52 |
3.5 Significance of the study
According to the experimental results, a low HRT with high OLR results in efficient hydrogen production. A maximum HPR (37.5 L L−1 d−1) was observed at lower HRT (1.5 h and OLR 320 g L−1 dhexose equivalent−1). Moreover, the HRT significantly influenced the microbial community structure, biomass concentration, soluble metabolic products distribution and hydrogen production performance in the CSTR bio-hydrogen fermentor. Therefore, using a short HRT to obtain a high OLR is a functional strategy for efficient hydrogen production from beverage wastewater.An increased lactate production (0.24–5.15 g COD per L) significantly affected the hydrogen yield but not the HPR; the reduction phenomenon of HY was attributed to the increased hydrogen partial pressure at high OLR. This high OLR diverted the metabolic flux towards lactate and ethanol, and decreased the activity of hydrogenase. As indicated by Kim et al.45 CO2 sparging can reduce the accumulation of lactate in a bioreactor, thus CO2 gas sparging could be a useful strategy to improve the hydrogen production performance during continuous operation.
Microbial community analysis demonstrated the dominance of efficient hydrogen-producing bacteria (C. butyricum, C. tyrobutyricum, C. perfringenes and K. oxytoca) at low HRT (1.5 h), which resulted in efficient hydrogen production from BW. However, a low biomass concentration of 2.7 g VSS per L was observed at 1.5 h HRT. The cell biomass wash-out under low HRT conditions is a common behavior in CSTR bioreactors due to the lack of a granule forming ability/immobilized structure to retain the biomass. Thus, immobilized cells operation is further recommended to study and compare the performance of hydrogen fermentation from BW.46
4. Conclusions
HRT affects the hydrogen production rate, hydrogen yield and liquid metabolite products of biohydrogen production from beverage wastewater in different HRT-dependent trends. A short HRT of 1.5 h can result in a peak hydrogen production rate and energy production rate. At HRT 1.5 h, the peak HPR and energy production rate were 37.5 L L−1 d−1 and 441 kJ L−1 d−1, respectively, with C. butyricum, C. tyrobutyricum, C. perfringenes and K. oxytoca being the dominant microflora. HY peaked at 6 h HRT with a value of 1.62 mol mol−1 hexose. This study shows that BW can be used as an efficient low-cost feedstock for sustainable bio-hydrogen production.Acknowledgements
The authors gratefully acknowledge the financial support by Taiwan's Bureau of Energy (Grant No. 102-D0616), Taiwan's National Science Council (NSC-102-2221-E-035-002-MY3 and NSC-102-2622E-035-016-CC1).References
- G. Kumar, P. Sivagurunathan, S. H. Kim, P. Bakonyi and C. Y. Lin, Arabian J. Sci. Eng., 2015, 40, 15–22 CrossRef CAS.
- W. Han, B. Wang, Y. Zhou, D. X. Wang, Y. Wang, L. R. Yue, Y. F. Li and N. Q. Ren, Bioresour. Technol., 2012, 110, 219–223 CrossRef CAS PubMed.
- G. Kumar, P. Bakonyi, S. Periyasamy, S. H. Kim, N. Nemestóthy and K. Bélafi-Bakó, Renewable Sustainable Energy Rev., 2015, 44, 728–737 CrossRef CAS.
- W. Han, D. N. Liu, Y. W. Shi, J. H. Tang, Y. F. Li and N. Q. Ren, Bioresour. Technol., 2015, 180, 54–58 CrossRef CAS PubMed.
- C. Y. Lin, C. H. Lay, B. Sen, C. Y. Chu, G. Kumar, C. C. Chen and J. S. Chang, Int. J. Hydrogen Energy, 2012, 37, 15632–15642 CrossRef CAS.
- Y. Ueno, S. Otsuka and M. Morimoto, J. Ferment. Bioeng., 1996, 82, 194–197 CrossRef CAS.
- C. H. Lay, J. H. Wu, C. L. Hsiao, J. J. Chang, C. C. Chen and C. Y. Lin, Int. J. Hydrogen Energy, 2010, 35, 13445–13451 CrossRef CAS.
- K. W. Jung, D. H. Kim and H. S. Shin, Int. J. Hydrogen Energy, 2010, 35, 13370–13378 CrossRef CAS.
- M. S. Kim, D. Y. Lee and D. H. Kim, Int. J. Hydrogen Energy, 2011, 36, 8712–8718 CrossRef CAS.
- B. Wang, Y. Li and N. Ren, Int. J. Hydrogen Energy, 2013, 38, 4361–4367 CrossRef CAS.
- G. Davila-Vazquez, C. B. Cota-Navarro, L. M. Rosales-Colunga, A. de León-Rodríguez and E. Razo-Flores, Int. J. Hydrogen Energy, 2009, 34, 4296–4304 CrossRef CAS.
- P. Sivagurunathan, K. Gopalakrishnan and C. Y. Lin, Curr. Biochem. Eng., 2014, 1, 92–98 CrossRef CAS.
- B. Sen and R. R. Suttar, Int. J. Hydrogen Energy, 2012, 37, 15588–15597 CrossRef CAS.
- P. Sivagurunathan, B. Sen and C. Y. Lin, Int. J. Hydrogen Energy, 2014, 39, 19232–19241 CrossRef CAS.
- C. Y. Chu, S. Y. Wu, P. C. Hsieh and C. Y. Lin, Int. J. Hydrogen Energy, 2011, 36, 14078–14085 CrossRef CAS.
- W. Q. Guo, N. Q. Ren, X. J. Wang, W. S. Xiang, Z. H. Meng, J. Ding, Y. Y. Qu and L. S. Zhang, Int. J. Hydrogen Energy, 2008, 33, 4981–4988 CrossRef CAS.
- G. Endo, T. Noike and T. Matsumoto, Proc. Am. Soc. Civ. Eng., 1982, 325, 61–68 CrossRef.
- A. W. W. A, American Public Health Association, and Water Environment Federation: Standard methods for the examination of water and wastewater, APHA, AWWA, and WEF, Washington, D.C., USA, 20th edn, 1998 Search PubMed.
- G. L. Miller, Anal. Chem., 1959, 31, 426–428 CrossRef CAS.
- P. Sivagurunathan, B. Sen and C. Y. Lin, J. Biosci. Bioeng., 2014, 117, 222–228 CrossRef CAS PubMed.
- A. A. Robert, Physical chemistry, John Wiley & Sons, New York, 6th edn, 1991 Search PubMed.
- C. Li and H. H. P. Fang, Crit. Rev. Environ. Sci. Technol., 2007, 37, 1–39 CrossRef CAS.
- S. Y. Wu, C. N. Lin and J. S. Chang, Biotechnol. Prog., 2003, 19, 828–832 CrossRef CAS PubMed.
- J. J. Chang, J. H. Wu, F. S. Wen, K. Y. Hung, Y. T. Chen, C. L. Hsiao, C. Y. Lin and C. C. Huang, Int. J. Hydrogen Energy, 2008, 33, 1579–1585 CrossRef CAS.
- C. C. Chen, B. Sen, Y. S. Chuang, C. J. Tsai and C. H. Lay, J. Cleaner Prod., 2012, 32, 236–243 CrossRef CAS.
- R. J. Zoetemeyer, A. J. C. M. Matthijsen, A. Cohen and C. Boelhouwer, Water Res., 1982, 16, 633–639 CrossRef CAS.
- S. van Ginkel and B. E. Logan, Environ. Sci. Technol., 2005, 39, 9351–9356 CrossRef CAS PubMed.
- S. Zhang, Y. Lee, T. H. Kim and S. J. Hwang, Bioresour. Technol., 2013, 141, 227–232 CrossRef CAS PubMed.
- M. F. Arooj, S. K. Han, S. H. Kim, D. H. Kim and H. S. Shin, Int. J. Hydrogen Energy, 2008, 33, 3289–3294 CrossRef CAS.
- Y. Mu, H. Q. Yu and Y. Wang, Chemosphere, 2006, 64, 350–358 CrossRef CAS PubMed.
- C. Y. Lin, C. H. Hung, C. H. Chen, W. T. Chung and L. H. Cheng, Process Biochem., 2006, 41, 1383–1390 CrossRef CAS.
- J. H. Jo, D. S. Lee, D. Park and J. M. Park, Bioresour. Technol., 2008, 99, 6666–6672 CrossRef CAS PubMed.
- N. Q. Ren, H. Chua, S. Y. Chan, Y. F. Tsang, Y. J. Wang and N. Sin, Bioresour. Technol., 2007, 98, 1774–1780 CrossRef CAS PubMed.
- K. W. Jung, D. H. Kim, M. Y. Lee and H. S. Shin, Int. J. Hydrogen Energy, 2012, 37, 7473–7481 CrossRef CAS.
- Y. Akutsu, Y. Y. Li, H. Harada and H. Q. Yu, Int. J. Hydrogen Energy, 2009, 34, 2558–2566 CrossRef CAS.
- B. E. Rittmann and P. L. McCarty, Environmental Biotechnology: Principles and Applications, Tata McGraw Hill Education Private Limited, 2012 Search PubMed.
- C. H. Hung, K. S. Lee, L. H. Cheng, Y. H. Huang, P. J. Lin and J. S. Chang, Appl. Microbiol. Biotechnol., 2007, 75, 693–701 CrossRef CAS PubMed.
- S. Y. Wu, C. H. Hung, C. N. Lin, H. W. Chen, A. S. Lee and J. S. Chang, Biotechnol. Bioeng., 2006, 93, 934–946 CrossRef CAS PubMed.
- G. Peixoto, N. K. Saavedra, M. B. A. Varesche and M. Zaiat, Int. J. Hydrogen Energy, 2011, 36, 8953–8966 CrossRef CAS.
- S. Y. Wu, C. H. Hung, C. Y. Lin, P. J. Lin, K. S. Lee, C. N. Lin, F. Y. Chang and J. S. Chang, Int. J. Hydrogen Energy, 2008, 33, 1542–1549 CrossRef CAS.
- Y. Huang, W. Zong, X. Yan, R. Wang, C. L. Hemme, J. Zhou and Z. Zhou, Appl. Environ. Microbiol., 2010, 76, 3387–3390 CrossRef CAS PubMed.
- X. Zhao, D. Xing, N. Fu, B. Liu and N. Ren, Bioresour. Technol., 2011, 102, 8432–8436 CrossRef CAS PubMed.
- W. Z. Han, H. Chen, X. Yao and Y. Li, J. Biomed. Biotechnol., 2011, 2011 Search PubMed.
- S. C. Santos, P. R. Ferreira Rosa, I. K. Sakamoto, M. B. Amâncio Varesche and E. L. Silva, Int. J. Hydrogen Energy, 2014, 39, 9000–9011 CrossRef CAS.
- D. H. Kim, S. K. Han, S. H. Kim and H. S. Shin, Int. J. Hydrogen Energy, 2006, 31, 2158–2169 CrossRef CAS.
- P. Sivagurunathan, B. Sen and C.-Y. Lin, Appl. Energy, 2015, 147, 1–9 CrossRef CAS.
- H. Hafez, G. Nakhla and H. E. Naggar, Energies, 2009, 2, 445–455 CrossRef CAS.
- G. Zhu, C. Liu, J. Li, N. Ren, L. Liu and X. Huang, Front. Environ. Sci. Eng. China, 2013, 7, 143–150 CrossRef CAS.
- C. H. Lay, B. Sen, S. C. Huang, C. C. Chen and C. Y. Lin, Renewable Energy, 2013, 58, 60–67 CrossRef CAS.
- G. Tchobanoglous, F. L. Burton and H. D. Stensel, Wastewater engineering: Treatment and reuse, McGraw-Hill, Boston, 4th edn, 2003 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |





