ACS-22, a protein homologous to mammalian fatty acid transport protein 4, is essential for the control of the toxicity and translocation of multi-walled carbon nanotubes in Caenorhabditis elegans†
Lingtong Zhi,
Wei Fu,
Xiong Wang and
Dayong Wang*
Key Laboratory of Environmental Medicine Engineering in Ministry of Education, Medical School, Southeast University, Nanjing 210009, China. E-mail: dayongw@seu.edu.cn
First published on 18th December 2015
Abstract
Biodistribution and translocation are important for the generation of the toxicity of multi-walled carbon nanotubes (MWCNTs) in organisms. However, we know little about the molecular mechanisms of MWCNT translocation. In Caenorhabditis elegans, the acs-22 gene encodes a protein homologous to mammalian FATP4 (fatty acid transport protein 4). In this study, we employed a C. elegans in vivo assay system to investigate the possible function of ACS-22 in regulating the toxicity and translocation of MWCNTs. Prolonged exposure to MWCNTs significantly decreased transcriptional expression of the acs-22 gene. Loss-of-function mutation of the acs-22 gene strengthened MWCNT toxicity to the functions of both primary targeted organs such as intestine and secondary targeted organs such as neurons and reproductive organs; however, overexpression of the acs-22 gene reduced the toxicity of MWCNTs to the functions of both primary and secondary targeted organs. Loss-of-function mutation of the acs-22 gene enhanced the distribution and translocation of MWCNTs in both primary and secondary targeted organs, whereas overexpression of the acs-22 gene inhibited the distribution of MWCNTs in primary targeted organs and prevented translocation of MWCNTs into secondary targeted organs. Moreover, our results demonstrated the important function of ACS-22 in intestine to be in limiting the toxicity and translocation of MWCNTs. Therefore, our data suggest an essential role of ACS-22 in regulating the toxicity and translocation of MWCNTs in nematodes. Our results will provide important clues for further examination of the molecular mechanisms of translocation of MWCNTs in organisms.
Introduction
Carbon nanotubes (CNTs), which are amongst the most important engineered nanomaterials (ENMs), have been produced in bulk for many diverse purposes. Along with the production and application of CNTs, both in vitro and in vivo studies have suggested the potential adverse effects of CNTs on human health and on environmental organisms.1–4 For the cellular mechanisms of CNT toxicity, induction of oxidation and bioavailability are considered to play important roles.4–6 Evidence has suggested that CNTs can be translocated into targeted organs or cells.5,7–9 The cellular mechanisms for the distribution and translocation of CNTs have been examined.10 However, the molecular basis of the distribution and translocation of CNTs is still largely unclear.Caenorhabditis elegans is a typical model animal, and can offer a system suited for asking in vivo toxicological questions with relevance at the organism level.11,12 Previous studies have investigated the in vivo toxicity of CNTs, including both single-walled carbon nanotubes and multi-walled carbon nanotubes (MWCNTs), and the underlying cellular and molecular mechanisms in nematodes.13–17 MWCNTs, one class of CNTs, consist of many single-walled nanotubes stacked one inside the other. In nematodes, MWCNT exposure can cause toxicity to the functions of both primary targeted organs, such as intestine, and secondary targeted organs, such as neurons and reproductive organs.14,15,17 Some microRNAs (miRNAs) were further identified to be involved in the control of MWCNT toxicity in nematodes.16 Meanwhile, it has been shown that MWCNTs can be translocated into both the primary targeted organs such as intestine and the secondary targeted organs such as reproductive organs in nematodes.14,15 However, the underlying molecular mechanisms of MWCNT translocation in nematodes are still unclear.
In C. elegans, the acs-20 and acs-22 genes encode proteins homologous to mammalian FATP4 (fatty acid transport protein 4), a key factor involved in forming the stratum corneum barrier.18 Previous study has implied that ACS-20 and ACS-22 may be involved in the control of function of biological barriers in nematodes.18 We then asked whether ACS-20 and/or ACS-22 are involved in the control of toxicity and translocation of MWCNTs. In the present study, we employed the C. elegans in vivo assay system to investigate the possible function of ACS-20 and/or ACS-22 in regulating toxicity and translocation of MWCNTs. Our results demonstrated the essential role of ACS-22 in regulating the function of the intestinal barrier against MWCNT toxicity. Our data will provide important clues for the in-depth elucidation of the underlying molecular mechanisms of translocation of ENMs in organisms.
Materials and methods
Chemicals
The MWCNTs used in this study were from Shenzhen Nanotech Port Co. Ltd (Shenzhen, China). The morphology of MWCNTs in K-medium was examined by transmission electron microscopy (TEM, JEM-200CX, JEOL, Japan). The zeta potential of MWCNTs was analyzed using a Nano Zetasizer (Nano ZS90, Malvern Instruments, UK). Impurities were determined by elemental inductively coupled plasma mass spectrometry.MWCNTs were dispersed in K-medium to prepare a stock solution (1 mg mL−1). They were further sonicated for 30 min (40 kHz, 100 W) and diluted with K-medium to the concentration used in the study (1 mg L−1) just prior to exposure.14,15
C. elegans maintenance
The nematodes used were wild-type N2, mutants of acs-20(tm3232) (a loss-of-function mutation of the acs-20 gene) and acs-22(tm3236) (a loss-of-function mutation of the acs-22 gene), and transgenic strains of acs-22(tm3236)Ex(Pges-1-acs-22), Ex(Pdpy-30-acs-22) and Ex(Pges-1-acs-22), which were maintained at 20 °C on nematode growth medium plates seeded with Escherichia coli OP50 as described previously.11 Some nematode strains used in this study were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). Gravid nematodes were washed off the plates into centrifuge tubes, and lysed with a bleaching mixture (0.45 mol L−1 NaOH, 2% HOCl). Age-synchronous populations of L1-larvae were obtained as described previously.19MWCNT exposure
To evaluate MWCNT toxicity, MWCNT exposure (prolonged exposure) was performed during development from L1-larvae to young adults (approximately 3.5 days) in 12-well sterile tissue culture plates at 20 °C in the presence of food (OP50). After exposure, nematodes were used for toxicity assessment, with reproduction, locomotion behavior, and intestinal reactive oxygen species (ROS) production as the endpoints.Toxicity assessment
Reproduction was assessed by the endpoint of brood size. The method was performed as described previously.20 The number of offspring at all stages beyond the egg was counted. Twenty nematodes were examined per treatment, and three replicates were performed.Locomotion behavior was evaluated by the endpoints of head thrash and body bend. The methods were performed as described previously.21,22 A head thrash was defined as a change in the direction of bending at the mid body. A body bend was counted as a change in the direction of the part of the nematodes corresponding to the posterior bulb of the pharynx along the y axis, assuming that the nematode was traveling along the x axis. Twenty nematodes were examined per treatment, and three replicates were performed.
The method for ROS induction was performed as described previously.23,24 To examine intestinal ROS production, nematodes were transferred to 1 μmol L−1 of 5′,6′-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) in 12-well sterile tissue culture plates and incubated for 3 h at 20 °C. Nematodes were mounted on 2% agar pads for examination with a laser scanning confocal microscope (Leica, TCS SP2, Bensheim, Germany) at an excitation wavelength of 488 nm and with a 510 nm emission filter. Relative fluorescence intensities of the intestines were semi-quantified. The relative quantification of fluorescence signals labeling ROS production in comparison to the autofluorescence in nematodes without labeling was determined. The fluorescence intensity of anterior intestine was collected in an Image J system. Thirty nematodes were examined per treatment, and three replicates were performed.
Distribution and translocation of MWCNTs
To investigate the distribution and translocation of MWCNTs in nematodes, Rhodamine B (Rho B) was loaded on MWCNTs by mixing Rho B solution (1 mg mL−1, 0.3 mL) with an aqueous suspension of MWCNTs (0.1 mg mL−1, 5 mL) as previously described.14,15 Unbound Rho B was removed by dialysis against distilled water over 72 h. The resulting MWCNTs/Rho B was stored at 4 °C. The examined nematodes were incubated with MWCNTs/Rho B at a concentration of 1 mg L−1 for 3 h, and washed with M9 buffer. Nematodes were observed under a fluorescence microscope. Rho B staining alone served as the control.DNA constructs and germline transformation
To generate the entry vector carrying a promoter sequence, the promoter region for the dpy-30 gene expressed in all cells (Pdpy-30) or the ges-1 gene specifically expressed in intestine (Pges-1) was amplified by PCR from wild-type C. elegans genomic DNA, and then inserted into the pPD95_77 vector in the sense orientation. acs-22 (the isoform of D1009.1a) cDNA was amplified by PCR. The sequence of the amplified acs-22 cDNA was verified by sequencing, and then inserted into the corresponding entry vector carrying the dpy-30 or ges-1 promoter sequence. PCR was performed using primers for Pdpy-30 (forward primer, 5′-ATACTGCAGCTTCTAAACGAAACTGCC-3′; reverse primer, 5′-ATAGGATCCATCAGCCATCTTGGTTTT-3′), Pges-1 (forward primer, 5′-CGGTCTAGAGTTTGTTATCATTGTCCA-3′; reverse primer, 5′-CATGGATCCCATCTGAATTCAAAGATA-3′), and acs-22 (forward primer, 5′-CTAGGTACCATGAGGGAAATGCCGGAC-3′; reverse primer, 5′-CGCCTCGAGTTAAATGCGATCATAAAC-3′). Germline transformation was performed as described previously by co-injecting the transforming DNA at a concentration of 10–40 μg mL−1 and the marker DNA of unc-119(+) at a concentration of 60 μg mL−1 into the gonad of unc-119(ed3) mutant nematodes.25Nile red staining
The methods were performed as described previously.15 Nile red (Molecular Probes, Eugene, OR) was dissolved in acetone to produce a 0.5 mg mL−1 stock solution and stored at 4 °C. Stock solution was freshly diluted in 1 × PBS buffer to 1 μg mL−1, and 150 μL of the diluted solution was used for Nile red staining. Twenty nematodes were examined per treatment, and three replicates were performed.Analysis of triglyceride content
Lipid of nematodes was extracted by the method described previously.26 The triglyceride content was measured using an enzymatic kit (Wako Triglyceride E-test, Wako Pure Chemical Ltd, Osaka, Japan). Ten replicates were performed.Reverse-transcription and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from nematodes using Trizol (Invitrogen, UK) according to the manufacturer's protocols. After assessment of purity and concentration of RNAs by OD260/280 in a spectrophotometer, cDNA synthesis was performed in a 12.5 μL reaction volume containing 625 ng of total RNA, 0.5 mmol L−1 reverse-transcript primers, 50 mmol L−1 Tris–HCl, 75 mmol L−1 KCl, 3 mmol L−1 MgCl2, 10 mmol L−1 dithiothreitol, 20 units of ribonuclease inhibitor and 100 U of reverse transcriptase (Takara, China). Relative expression levels of genes were determined by real-time PCR in an ABI 7500 real-time PCR system with Evagreen (Biotium, USA). Reactions were performed in triplicate. Relative quantification of targeted genes in comparison to the reference tba-1 gene encoding a tubulin was determined. The final results were expressed as the relative expression ratio between the targeted gene and the reference gene. The primer information is shown in Table S1.†Statistical analysis
All data in this article are expressed as means ± standard error of the mean (SEM). Graphs were generated using Microsoft Excel software (Microsoft Corp., Redmond, WA). Statistical analysis was performed using SPSS 12.0 software (SPSS Inc., Chicago, USA). Differences between groups were determined using analysis of variance (ANOVA). The probability levels of 0.05 and 0.01 were considered to be statistically significant.Results
Properties of MWCNTs
After sonication, the length of MWCMTs was 572 ± 245 nm, and the diameter of MWCNTs was 10–20 nm (Fig. 1A). The zeta potential of MWCNTs in K-medium was −33.2 ± 2.2 mV (Fig. 1B). The purity of MWCNTs was 98.94%, and Ni and Fe impurities at concentrations of not more than 0.2% were present in MWCNTs (Fig. 1B). | ||
| Fig. 1 Properties of MWCNTs. (A) TEM picture of MWCNTs in K-medium after sonication. (B) Some properties of MWCNTs. | ||
Mutation of acs-22 gene resulted in more severe MWCNT toxicity to the functions of both primary and secondary targeted organs in nematodes
To further examine the possible function of the acs-20 or acs-22 gene in regulating MWCNT toxicity, we employed acs-20(tm3232) and acs-22(tm3236) mutants to compare MWCNT toxicity between exposed wild-type and exposed acs-20 or acs-22 mutant nematodes. Interestingly, after prolonged exposure, a more severe decrease in locomotion behavior as reflected by endpoints of head thrash and body bend was observed in MWCNT (1 mg L−1)-exposed acs-22(tm3236) mutant compared with MWCNT (1 mg L−1)-exposed wild-type nematodes (Fig. 2A). Similarly, a more severe reduction in brood size was found in MWCNT (1 mg L−1)-exposed acs-22(tm3236) mutants compared with MWCNT (1 mg L−1)-exposed wild-type nematodes (Fig. 2B). In contrast, loss-of-function mutation of the acs-20 gene did not significantly affect the toxicity of MWCNTs (1 mg L−1) in terms of locomotion behavior and brood size of nematodes (Fig. 2C and D). These results suggest that mutation of acs-22 gene induced more severe toxicity to the functions of secondary targeted organs in exposed nematodes.Moreover, we observed that a more severe induction of intestinal ROS production was formed in MWCNT (1 mg L−1)-exposed acs-22(tm3236) mutants compared with MWCNT (1 mg L−1)-exposed wild-type nematodes (Fig. 3A). In contrast, loss-of-function mutation of acs-20 gene did not significantly influence the toxicity of MWCNTs (1 mg L−1) in inducing intestinal ROS production in nematodes (Fig. 3B). Therefore, our results imply that mutation of acs-22 gene led to a more severe toxicity to the functions of both primary and secondary targeted organs in exposed nematodes.
Mutation of the acs-22 gene strengthened the distribution of MWCNTs in the body of exposed nematodes
Considering the fact that biodistribution and translocation are crucial factors for the toxicity of MWCNTs, we further loaded Rho B on MWCNTs to prepare MWCNTs/Rho B. After exposure, we observed that there was a more pronounced MWCNTs/Rho B distribution in both primary targeted organs such as pharynx and intestine and secondary targeted organs such as spermatheca in exposed acs-22(tm3236) mutants compared with exposed wild-type nematodes (Fig. 4 and S1†). Mutation of the acs-22 gene did not alter the distribution region of MWCNTs in nematodes (Fig. 4), suggesting that ACS-22 may function to regulate the translocation capacity of MWCNTs through the intestinal barrier. In contrast, mutation of the acs-20 gene did not obviously affect the distribution and translocation of MWCNTs/Rho B in nematodes (Fig. S2†). Exposure to Rho B resulted in the relatively equal distribution of fluorescence in tissues of wild-type, acs-20(tm3232), or acs-22(tm3236) mutant nematodes (Fig. S3†). In addition, stronger fluorescence signals of Rho B were observed in acs-20(tm3232) or acs-22(tm3236) mutant nematodes compared with those in wild-type nematodes (Fig. S3†). Therefore, mutation of the acs-22 gene enhanced the biodistribution and translocation of MWCNTs in the body of nematodes.Overexpression of acs-22 gene in all cells or in intestine reduced MWCNT toxicity to the functions of both primary and secondary targeted organs in nematodes
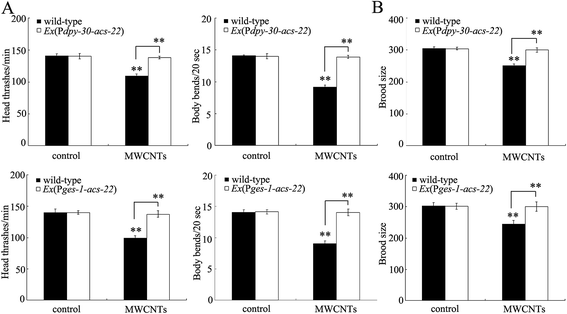
In order to further confirm the function of ACS-22 in regulating MWCNT toxicity, we first overexpressed acs-22 in all cells in nematodes with the aid of the dpy-30 promoter. After prolonged exposure, we found that the toxic effects of MWCNT (1 mg L−1) exposure on the locomotion behavior of nematodes were obviously inhibited by overexpression of the acs-22 gene in all cells (Fig. 5A). Similarly, the toxic effect of MWCNT (1 mg L−1) exposure on the brood size of nematodes was also noticeably suppressed by overexpression of acs-22 gene in all cells (Fig. 5B). Moreover, after prolonged exposure, we observed that the toxic effect of MWCNT (1 mg L−1) exposure in inducing intestinal ROS production was obviously inhibited by the overexpression of acs-22 gene in all cells in nematodes (Fig. 6A). These data suggest that overexpression of acs-22 gene in all cells can reduce MWCNT toxicity to the functions of both primary and secondary targeted organs in nematodes.Considering the crucial role of intestinal barrier against the toxicity of ENMs in nematodes,14,27,28 we further constructed transgenic nematodes overexpressing the acs-22 gene in intestine. Interestingly, we found that the toxic effects of MWCNTs (1 mg L−1) on both locomotion behavior and brood size could be significantly inhibited by the overexpression of acs-22 gene in intestine in nematodes (Fig. 5). Similarly, the toxic effect of MWCNTs (1 mg L−1) in inducing intestinal ROS production was also noticeably suppressed by the overexpression of acs-22 gene in intestine in nematodes (Fig. 6B). Therefore, overexpression of the acs-22 gene in intestine may potentially be able to prevent MWCNT toxicity to the functions of both primary and secondary targeted organs in nematodes.
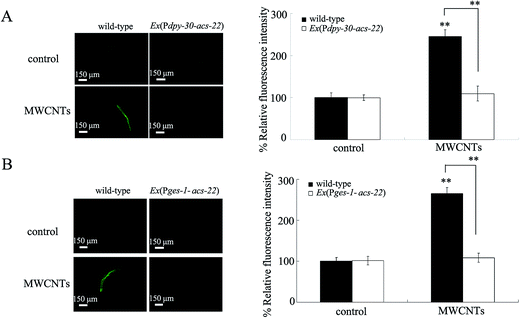
Overexpression of acs-22 gene reduced the distribution of MWCNTs in the body of exposed nematodes
We next investigated the effect of overexpressing the acs-22 gene on the distribution and translocation of MWCNTs in nematodes. Compared with the distribution pattern of MWCNTs/Rho B in wild-type nematodes, we observed that MWCNTs/Rho B was mainly distributed in the pharynx and intestine in nematodes overexpressing the acs-22 gene in all cells or in intestine (Fig. 4 and S1†). In addition, there was only a very moderate amount of MWCNTs/Rho B distributed in the pharynx and intestine in nematodes overexpressing the acs-22 gene in all cells or in intestine (Fig. 4 and S1†). Exposure to Rho B resulted in a relatively equal distribution of fluorescence in tissues of wild-type nematodes or nematodes overexpressing the acs-22 gene in all cells or in intestine (Fig. S3†). Therefore, overexpression of the acs-22 gene in all cells or in intestine may further reduce the biodistribution and translocation of MWCNTs in the body of nematodes.Expression of acs-22 in intestine rescued the susceptible phenotype of acs-22 mutant nematodes exposed to MWCNTs
To confirm the crucial function of ACS-22 in intestine in regulating MWCNT toxicity, the acs-22 gene was expressed in the intestine of acs-22(tm3236) mutant nematodes. We found that expression of the acs-22 gene in the intestine could rescue the susceptibility of acs-22(tm3236) mutant nematodes to the toxic effects of MWCNT on locomotion behavior or brood size (Fig. S4A and B†). Moreover, expression of the acs-22 gene in the intestine could further rescue the susceptibility of acs-22(tm3236) mutant nematodes to MWCNT toxicity in inducing ROS production (Fig. S4C†). These results confirmed the crucial role of intestinal ACS-22 in regulating MWCNT toxicity in nematodes.Mutation of the acs-22 gene enhanced the intestinal permeability in MWCNT-exposed nematodes
To further determine the underlying mechanism for acs-22 gene in regulating the toxicity and translocation of MWCNTs, we investigated the permeability of primary targeted organs in wild-type and acs-22 mutant nematodes. It has been shown that MWCNTs can increase the intestinal permeability in nematodes.14,15 We used the lipophilic fluorescent dye Nile red to stain the examined nematodes. First of all, we found that mutation of the acs-22 gene induced an increased relative fluorescence intensity of Nile red signals in intestine compared with wild-type N2 (Fig. 7A), implying that mutation of the acs-22 gene may cause intestinal hyperpermeability in nematodes. Moreover, we observed that the MWCNT-exposed acs-22(tm3236) mutant exhibited an increased relative fluorescence intensity of Nile red signals in intestine compared with MWCNT-exposed wild-type N2 (Fig. 7A). Considering the fact that Nile red can also be used to label fat storage,29,30 we further analyzed the triglyceride content of nematodes. Mutation of the acs-22 gene did not significantly affect the triglyceride content of nematodes (Fig. 7B). In addition, MWCMTs exposure did not significantly influence the triglyceride content of wild-type or acs-22 mutant nematodes (Fig. 7B). These results suggest that mutation of acs-22 gene may enhance the intestinal permeability in MWCNT-exposed nematodes.Overexpression of the acs-22 gene in intestine maintained the normal intestinal permeability in MWCNT-exposed nematodes
Again, we compared the intestinal permeability between MWCNT-exposed wild-type and MWCNT-exposed nematodes overexpressing the acs-22 gene in intestine. Overexpression of the acs-22 gene in intestine did not significantly affect relative fluorescence intensity of Nile red signals in intestine compared with wild-type N2 (Fig. 7A), implying that overexpression of the acs-22 gene in intestine may not alter the normal intestinal permeability in nematodes. Moreover, we found that overexpression of the acs-22 gene in intestine significantly inhibited the relative fluorescence intensity of Nile red signals in intestine in MWCNT-exposed nematodes (Fig. 7A). Meanwhile, overexpression of acs-22 gene did not significantly influence the triglyceride content of nematodes (Fig. 7B). In addition, MWCMT exposure also did not significantly affect the triglyceride content of wild-type or nematodes overexpressing the acs-22 gene in intestine (Fig. 7B). These results suggest that overexpression of the acs-22 gene may be able to maintain the normal intestinal permeability in MWCNT-exposed nematodes.MWCNT exposure decreased the expression of the acs-22 gene in nematodes
We finally investigated the effect of MWCNT exposure on expression of the acs-20 and acs-22 genes in nematodes. After prolonged exposure, we found that MWCNTs (1 mg L−1) significantly decreased the transcriptional expression of the acs-22 gene in nematodes (Fig. S5†). In contrast, prolonged exposure to MWCNTs (1 mg L−1) did not significantly alter the transcriptional expression of the acs-20 gene in nematodes (Fig. S5†).Discussion
Biodistribution and translocation are very important for the effects of toxicity of MWCNTs to organisms.10,12 Previous studies have observed the distribution and translocation of CNTs in targeted organs or cells.5,7–9 However, in contrast to the information on cellular mechanisms of MWCNT translocation, we know little about the molecular mechanisms of MWCNT translocation in organisms. The protein products encoded by the acs-20 and acs-22 genes are homologous to mammalian FATP4, which was deduced as a key factor involved in forming the stratum corneum barrier.18 However, the exact functions of ACS-22 are still largely unclear. Regarding these two genes, we found that prolonged exposure to MWCNTs (1 mg L−1) could significantly decrease the expression level of the acs-22 gene (Fig. S5†), implying that the acs-22 gene may be involved in the control of toxicity and translocation of MWCNTs in nematodes. Similarly, a recent report indicated that exposure to graphene oxide also decreased the expression level of acs-22 in nematodes.27In this study, we employed both the loss-of-function mutant acs-22(tm3236) and nematodes overexpressing the acs-22 gene to examine the possible function of the acs-22 gene in the control of MWCNT toxicity. Our results demonstrated that mutation of the acs-22 gene resulted in a susceptible property of nematodes to MWCNT toxicity on the functions of both primary targeted organs such as intestine and secondary targeted organs such as neurons and reproductive organs, compared with wild-type nematodes (Fig. 2 and 3). In contrast, overexpression of the acs-22 gene in all cells caused resistance of nematodes to the toxic effects of MWCNT on the functions of both primary targeted organs such as intestine and secondary targeted organs such as neurons and reproductive organs, compared with wild-type nematodes (Fig. 5 and 6). Therefore, our results suggest that ACS-22 may negatively regulate MWCNT toxicity in nematodes.
Moreover, we used both the loss-of-function mutant acs-22(tm3236) and nematodes overexpressing acs-22 gene to examine the possible function of the acs-22 gene in the control of distribution and translocation of MWCNTs. Interestingly, we observed that mutation of the acs-22 gene enhanced the distribution and translocation of MWCNTs in the targeted organs (both the primary and the secondary targeted organs) (Fig. 4 and S1†). In contrast, overexpression of the acs-22 gene in all cells suppressed the distribution of MWCNTs in primary targeted organs and prevented the translocation of MWCNTs into the secondary targeted organs (Fig. 4 and S1†). Therefore, we here further provide direct evidence to prove the crucial role of ACS-22 in the control of distribution and translocation of specific ENMs in organisms.
Regarding the possible cellular mechanisms mediated by the acs-22 gene in regulating MWCNT toxicity and translocation, our results suggested that ACS-22 is involved in the control of the functional state of the intestinal barrier. Mutation of the acs-22 gene induces a hyperpermeable property of intestine in nematodes (Fig. 7).27 This intestinal hyperpermeability in acs-22 mutant nematodes strengthened the translocation of MWCNTs into the body through the intestinal barrier (Fig. 4), and enhanced the toxicity of MWCNTs to the functions of both primary and secondary targeted organs (Fig. 2 and 3). The induced oxidative stress may function together with the increased MWCNT translocation in the generation of enhanced toxicity of MWCNTs to nematodes (Fig. 3). In contrast, the nematodes overexpressing the acs-22 gene in all cells exhibited a resistance to the toxic effects of MWCNT on the functions of both primary and secondary targeted organs (Fig. 5 and 6), which may be largely due to the inhibition of MWCNT translocation into the secondary targeted organs and the maintenance of a normal intestinal barrier in nematodes overexpressing the acs-22 gene in all cells (Fig. 4 and 7).
In C. elegans, the acs-22 gene is predominantly expressed in the intestine.18 In this study, we found that the intestine-specific activity of ACS-22 is required for the control of the functional state of the intestinal barrier. Overexpression of ACS-22 in intestine could sustain the normal functional state of the intestinal barrier (Fig. 7). Moreover, the intestine-specific activity of ACS-22 is further required for the control of MWCNT toxicity and translocation. Overexpression of ACS-22 in intestine could result in a resistant property of nematodes to MWCNT toxicity and suppression of the translocation of MWCNTs into the secondary targeted organs in nematodes (Fig. 4–6). Therefore, ACS-22 can act at least in intestine to regulate the toxicity and translocation of MWCNTs by influencing the functional state of the intestinal barrier.
ACS-20 and ACS-22 are two members of the FATP4 family. Previous study has shown that mutation of acs-20 gene can cause a hyperpermeable property of nematodes to Nile red.18 However, in this study, we found that mutation of acs-20 gene did not obviously affect the toxicity and translocation of MWCNTs in nematodes (Fig. 2–4). One of the important possibilities is that acs-20 gene is predominantly expressed in the epidermis of nematodes, and may affect the functional state of the epidermal barrier.18 Another possibility is that the size of MWCNTs may not allow the translocation of MWCNTs into the body through an epidermal barrier with altered permeability. More importantly, we did not observe the dysregulation of the acs-20 gene in MWCNT-exposed nematodes (Fig. S5†).
Conclusions
In conclusion, we examined the possible function of ACS-22, a protein homologous to mammalian FATP4, in the control of toxicity and translocation of MWCNTs in the C. elegans in vivo assay system. Our results suggest that the acs-22 gene may negatively regulate the toxicity and translocation of MWCNTs in nematodes. Mutation of the acs-22 gene induced susceptibility of nematodes to MWCNT toxicity and strengthened the distribution and translocation of MWCNTs into targeted organs. However, overexpression of the acs-22 gene in all cells or in intestine resulted in resistance of nematodes to MWCNT toxicity, suppressed the distribution of MWCNTs in primary targeted organs, and inhibited the translocation of MWCNTs into secondary targeted organs through the intestinal barrier. Our results suggest the crucial role of ACS-22 in intestine in the control of toxicity and translocation of MWCNTs, which provides important clues for the further examination of the molecular mechanisms of translocation of specific ENMs in organisms.Conflict of interest
None of the authors have any conflicting interests.Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (no. 81172698).References
- K. Donaldson, R. Aitken, L. Tran, V. Stone, R. Duffin, G. Forrest and A. Alexanders, Toxicol. Sci., 2006, 92, 5–22 CrossRef CAS PubMed.
- A. Helland, P. Wick, A. Koehler, K. Schmid and C. Som, Environ. Health Perspect., 2007, 115, 1125–1131 CrossRef CAS PubMed.
- L. A. Mitchell, F. T. Lauer, S. W. Burchiel and J. D. McDonald, Nat. Nanotechnol., 2009, 4, 451–456 CrossRef CAS PubMed.
- Y. Liu, Y. Zhao, B. Sun and C. Chen, Acc. Chem. Res., 2013, 46, 702–713 CrossRef CAS PubMed.
- H. C. Nerl, C. Cheng, A. E. Goode, S. D. Bergin, B. Lich, M. Gass and A. E. Porter, Nanomedicine, 2011, 6, 849–865 CrossRef CAS PubMed.
- J. Kayat, V. Gajbhiye, R. K. Tekade and N. K. Jain, Nanomedicine: Nanotechnology, Biology and Medicine, 2011, 7, 40–49 CrossRef CAS PubMed.
- C. Lam, J. T. James, R. McCluskey and R. L. Hunter, Toxicol. Sci., 2004, 77, 126–134 CrossRef CAS PubMed.
- J. Muller, F. Huaux, N. Moreau, P. Misson, J. Heilier, M. Delos, M. Arras, A. Fonseca, J. B. Nagy and D. Lison, Toxicol. Appl. Pharmacol., 2005, 207, 221–231 CrossRef CAS PubMed.
- Z. Liu, C. Davis, W. Cai, L. He, X. Chen and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 1410–1415 CrossRef CAS PubMed.
- Q. Mu, D. L. Broughton and B. Yan, Nano Lett., 2009, 9, 4370–4375 CrossRef CAS PubMed.
- S. Brenner, Genetics, 1974, 77, 71–94 CAS.
- Y.-L. Zhao, Q.-L. Wu, Y.-P. Li and D.-Y. Wang, RSC Adv., 2013, 3, 5741–5757 RSC.
- P. Chen, K. Hsiao and C. Chou, Biomaterials, 2013, 34, 5661–5669 CrossRef CAS PubMed.
- Q.-L. Wu, Y.-X. Li, Y.-P. Li, Y.-L. Zhao, L. Ge, H.-F. Wang and D.-Y. Wang, Nanoscale, 2013, 5, 11166–11178 RSC.
- A. Nouara, Q.-L. Wu, Y.-X. Li, M. Tang, H.-F. Wang, Y.-L. Zhao and D.-Y. Wang, Nanoscale, 2013, 5, 6088–6096 RSC.
- Y.-L. Zhao, Q.-L. Wu, Y.-P. Li, A. Nouara, R.-H. Jia and D.-Y. Wang, Nanoscale, 2014, 6, 4275–4284 RSC.
- C.-J. Shu, X.-M. Yu, Q.-L. Wu, Z.-H. Zhuang, W.-M. Zhang and D.-Y. Wang, RSC Adv., 2015, 5, 8942–8951 RSC.
- E. Kage-Nakadai, H. Kobuna, M. Kimura, K. Gengyo-Ando, T. Inoue, H. Arai and S. Mitani, PLoS One, 2010, 5, e8857 Search PubMed.
- S. Donkin and P. L. Williams, Environ. Toxicol. Chem., 1995, 14, 2139–2147 CrossRef CAS.
- Q. Rui, Y.-L. Zhao, Q.-L. Wu, M. Tang and D.-Y. Wang, Chemosphere, 2013, 93, 2289–2296 CrossRef CAS PubMed.
- Q.-L. Wu, Y.-L. Zhao, J.-P. Fang and D.-Y. Wang, Nanoscale, 2014, 6, 5894–5906 RSC.
- Y.-L. Zhao, Q. Liu, S. Shakoor, J. R. Gong and D.-Y. Wang, Toxicol. Res., 2015, 4, 270–280 RSC.
- Q.-L. Wu, Y.-L. Zhao, G. Zhao and D.-Y. Wang, Nanomedicine: Nanotechnology, Biology and Medicine, 2014, 10, 1401–1410 CrossRef CAS PubMed.
- L.-M. Sun, Z.-Q. Lin, K. Liao, Z.-G. Xi and D.-Y. Wang, Sci. Total Environ., 2015, 512–513, 251–260 CrossRef CAS PubMed.
- C. Mello and A. Fire, Methods Cell Biol., 1995, 48, 451–482 CrossRef CAS PubMed.
- E. G. Bligh and W. J. Dyer, Can. J. Biochem. Physiol., 1959, 37, 911–917 CrossRef CAS PubMed.
- Z.-F. Liu, X.-F. Zhou, Q.-L. Wu, Y.-L. Zhao and D.-Y. Wang, RSC Adv., 2015, 5, 94257–94266 RSC.
- Y.-L. Zhao, X.-M. Yu, R.-H. Jia, R.-L. Yang, Q. Rui and D.-Y. Wang, Sci. Rep., 2015, 5, 17233 CrossRef CAS PubMed.
- Q.-L. Wu, Q. Rui, K.-W. He, L.-L. Shen and D.-Y. Wang, Neurosci. Bull., 2010, 26, 104–116 CrossRef CAS PubMed.
- J.-N. Yang, Y.-L. Zhao, Y.-W. Wang, H.-F. Wang and D.-Y. Wang, Toxicol. Res., 2015, 4, 1498–1510 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23543j |
| This journal is © The Royal Society of Chemistry 2016 |