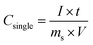Three-dimensional graphene nanosheets/carbon nanotube paper as flexible electrodes for electrochemical capacitors
Xiaoliang Yun,
Jie Wang,
Laifa Shen,
Hui Dou and
Xiaogang Zhang*
Jiangsu Key Laboratory of Materials and Technology for Energy Conversion, College of Material Science and Engineering, Nanjing University of Aeronautics and Astronautics, Nanjing, China. E-mail: azhangxg@163.com; Fax: +86 025 52112626; Tel: +86 025 52112918
First published on 11th February 2015
Abstract
In this paper, a flexible film electrode was prepared by depositing graphene nanosheets on carbon nanotube paper as a self-standing electrode for high-performance electrochemical capacitors. The composite film has a layered structure, where carbon nanotubes were efficiently intercalated in the layer of graphene nanosheets. The hierarchical structure of graphene nanosheet/carbon nanotube paper electrodes shows lower resistance to ions and electron transport. The unique electrodes exhibit high-rate capability and long-term cycling ability. A quasi-rectangular shape of CV curve can still be maintained even at a high scan rate of 10 V s−1. The capacitance decay was only 6.9% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a current density of 6.4 mA cm−2.
000 cycles at a current density of 6.4 mA cm−2.
Introduction
Recently, applications of portable and flexible devices such as flexible touch screens and solar cells have become daily essentials.1 These technologies have dramatically stimulated the development of related technologies, including the design and construction of energy-storage devices.2,3 Electrochemical capacitors are high-power energy-storage systems,4–8 where charges are stored in the interface of electrolyte and electrode material through rapid and reversible adsorption/desorption of ions.9–11 Much effort has also been made to develop thin-film supercapacitors with high capacity, light weight and flexibility.12,13 Theoretically, the two-dimensional (2D) extension of thin-film supercapacitors could substantially reduce deformation resistance from the vertical direction, ultimately making the entire device thin, flexible, and easy to fold and twist. The diffusion distance of electrolyte ions in thin-film supercapacitors would also be shorter than in their counterparts.Graphene, a two-dimensional monolayer of sp2-bonded carbon atoms, has attracted increasing attention in recent years, mainly due to its extraordinarily high electrical and thermal conductivities, great mechanical strength, large specific surface area, and potentially low manufacturing cost.14–17 The intrinsic capacitance of single-layer graphene reaches ∼21 μF cm−2 when the entire surface area is used.18 Graphene film materials derived from graphite oxide (GO) and other carbon-based materials have exhibited excellent properties in various aspects, including high specific capacitance, long cycle stability, energy density, and power density. Recent efforts have been mainly focused on developing novel electrode materials for thin-film supercapacitors, including reduced graphene oxide film (462 μF cm−2), polystyrene-based hierarchical porous carbon (28.7 μF cm−2), active carbon (10 μF cm−2) and carbon fabric (1 mF cm−2).19–24 However, studies aiming to further improve the area capacitance of thin-film supercapacitors are relatively sparse.25–28 It is highly desirable to develop a facile method to prepare new graphene-based, two-dimensional materials for capacitors. Graphene-based electrodes offer higher densities owing to tight stacking of graphene sheets, which can prevent ions from accessing surface area and limit performance at high power.17,29–31 Liu et al. successfully prepared a new graphene foam/carbon nanotube hybrid film for use as an efficient flexible electrode material in asymmetric supercapacitor devices.31 Therefore, a major challenge with graphene-based electrodes is developing novel structures that allow access to the surface area by incorporating sufficient porosity, utilizing spacers such as carbon spheres between graphene sheets to increase spacing distance, which increase the capacitance of graphene electrodes by 70% in aqueous electrolyte. Dryfe et al. and Zhu et al. successfully prepared graphene on carbon cloth via an electrophoretic deposition process for use as efficient flexible electrode materials in supercapacitor devices.16,27 However, highly irreversible agglomeration and precipitation of the graphene and poor capacitive behavior of these hybrid fibers rendered them far from ideal for commercial purposes.
Herein, we demonstrate a facile method to fabricate an ultralight and highly conductive graphene nanosheets/carbon nanotube paper (GN/CNTP) composites as high-rate free-standing flexible electrodes for electrochemical capacitors. Highly conductive carbon nanotube papers (CNTP) were chosen as a flexible three-dimensional conductive substrate, which can serve as an effective “spacer” to prevent the restacking of graphene nanosheets. Owing to interaction of π–π and functional groups between graphene oxide and carbon nanotubes, reduced-layer graphene nanosheets are deposited on both surfaces of carbon nanotube paper, inhibiting irreversible agglomeration and precipitation of graphene nanosheets. The final nanocomposites exhibited a capacity of 18.1 mF cm−2 according to the discharge curve at a current density of 3.2 mA cm−2. The result indicates that this kind of composite design provides guidelines for fabricating electrode architectures to enhance electrochemical performance for supercapacitors.
Results and discussion
Scheme 1 shows an illustration of fabricating the few-layer GN/CNTP for supercapacitor electrodes through a two-step process. A typical GN/CNTP composite electrode (Fig. 1a) can be prepared by immersing a pie of carbon nanotube paper (Fig. 1b) in a suspension of GO (6 mg mL−1). Owing to interaction of π–π and functional groups between graphene oxide and carbon nanotubes, graphene nanosheets are deposited on the surface of carbon nanotube paper.14,32 The resulting graphene nanosheet/carbon nanotube paper composite film is then immersed in ascorbic acid (VC) solution (10 mg mL−1) overnight and subsequently heated at 60 °C for 2 h. During this step, GO sheets are reduced to form graphene on the surface of carbon nanotube paper.33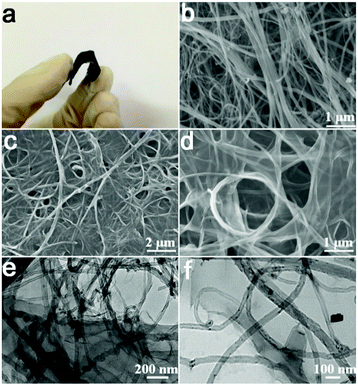 | ||
| Fig. 1 (a) Digital photograph of GN/CNTP, (b) SEM images of CNTP (c and d) SEM images, and (e and f) TEM images of stacked GN/CNTP at different magnifications. | ||
Fig. 1a shows the images of GN deposited on CNTP, in which GN and CNTP have formed integrated films. Fig. 1b shows that the CNTP overlap to appear as a fabric. The SEM images (Fig. 1c and d) of GN/CNTP clearly exhibit that GN has been successfully deposited on the surface of CNTP. The GN are well adhered to both sides of the CNTP. Each CNTP is sandwiched between graphene nanosheets (Fig. 1e and f). This layer-by-layer (LBL) structure alleviates agglomeration of 2D carbon nanomaterials and maximizes the utilization of the surface of the graphene nanosheets to guarantee permeation of electrolyte ions. The CNT layers can be served as well-defined porous spacer that not only prevents graphene film restacking but also provides sufficient separation between the wrinkled graphene film to facilitate electrolyte ion transfer.
The chemical structure of pure CNTP, GO/CNTP and GN/CNTP was further investigated via by Raman. The Raman spectra of pure CNTP, GN/CNTP, or GO/CNTP have two prominent bands around 1340 cm−1 and 1580 cm−1, assigned to the D and G bands of carbon, respectively. The G band is related to graphitic carbon and the D band is associated with the structural defects or partially disordered structures of graphitic domains.34–36 The ID/IG ratio provides a sensitive measure of the disorder and crystallite size of the graphitic layers.37 The ID/IG value of GO/CNTP was calculated to be 0.75, while that of GN/CNTP was 1.04. This result indicates that the oxidized areas of GO sheets were partly restored upon reduction with VC, forming small conjugated domains. The higher ID/IG ratio indicates a high proportion of disorder in GN (the defects associated with edge surface and vacancies of graphene nanosheets), which are beneficial for electrochemical capacitance.38 The Raman spectra of pure CNTP and GO/CNTP are different, implying that components have different structures, mainly owing to GO film deposits.
Fourier transform infrared (FT-IR) spectrometry was used to identify the functional groups of CNTP, GO/CNTP and GN/CNTP. As shown in Fig. 2b, the most characteristic features in the FTIR spectrum of GN are adsorption bands corresponding to CO stretching at 1721 cm−1, O–H vibration at 1412 cm−1, and a broad and intense O–H stretching at 3400 cm−1. After reduction, the –OH bands at 3420 and 1412 cm−1 were significantly reduced and that of the –CO groups at 1721 cm−1 disappeared due to reduction of the oxygen group in GN. The powder X-ray diffraction (XRD) pattern of as-prepared GO, CNTP and GN/CNTP is shown in Fig. 3. The diffraction peaks of CNTP around 26° are attributed to the (002) graphite diffraction peaks.39,40 The GO (002) diffraction peak is much less intense than that of CNTP because the graphite structure is exfoliated by the Hummer method. The (002) diffraction peak of GN/CNTP is much less intense than that of CNTP but stronger than that of GO because CNTP has intercalated between the graphene layers.41
To investigate the electrochemical performance of the obtained GN/CNTP as an electrode material for supercapacitors, they were evaluated by cyclic voltammetry (CV) and galvanostatic charge/discharge measurements in a three-electrode system. An identical GN/CNTP composite film was directly used as the working electrodes without binders or conducting additives. The representative CV curves of the electrode in 6 M KOH aqueous solution over the range of −1.0–0 V (Fig. 4a) at different scan rates, are close to the capacitive curve shape expected, especially at low scan rates. With the increase in scan rate, the rectangular curve shape of the sample remains, even up to 10 V s−1. The CV curves of GN/CNTP remained rectangular as the potential scan rate increased from 1 to 10 V s−1, indicating good charge propagation at electrode/electrolyte interfaces following the mechanism of electric double-layer capacitors.42 This high-power property is ascribed to their high conductivity and structural/textural merits. The abundant opened CNTs favor ion access to the active surface, facilitating fast charge transfer. Results demonstrate that the GN/CNTP film displayed improved rate capability.
The discharge curves of GN/CNTP measured at current densities in the range of 3.2–24.0 mA cm−2 show straight lines in the region of −1–0 V. The gravimetric specific capacitance for the three-electrode cell was obtained from the galvanostatic charge/discharge curves as:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a current density of 6.4 mA cm−2, the capacitive retention is still as high as 93.1%. In comparison, the cycling stability of pure CNTP is presented in Fig. 4f, which demonstrates an outstanding cycling stability with 90% capacity remaining after 10
000 cycles at a current density of 6.4 mA cm−2, the capacitive retention is still as high as 93.1%. In comparison, the cycling stability of pure CNTP is presented in Fig. 4f, which demonstrates an outstanding cycling stability with 90% capacity remaining after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 continuous cycles.
000 continuous cycles.
Conclusions
In summary, we have proposed a simple layer-by-layer approach for construction of 2D planar ultrathin GN and CNTP as flexible electrodes for supercapacitors. This 2D planar architecture allows the formation of an efficient electrical double layer and the utilization of the maximum active surface area of GN. The CNTP as a porous physical spacer could enhance the electrical conductivity and reduce the agglomeration of GN along the out-of-plane axis. Electrochemical tests show that a quasi-rectangular shape of CV curve can still maintained even at 10 V s−1. The capacitance loss was only 6.9% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a current density of 6.4 mA cm−2. These promising results demonstrate that such relative easy synthesis, low cost, and macroscopic-scale electrode materials have great potential in the fabrication of high-class supercapacitor devices for practical applications.
000 cycles at a current density of 6.4 mA cm−2. These promising results demonstrate that such relative easy synthesis, low cost, and macroscopic-scale electrode materials have great potential in the fabrication of high-class supercapacitor devices for practical applications.
Experimental section
Methods
Characterization
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were carried out with a JEOL JSM-6380LV FESEM and JEOL JEM-2010, respectively. Powder X-ray diffractions (XRDs) were studied via a Bruker D8 Advance X-ray diffractometer using Cu Kα radiation. Fourier transform infrared (FT-IR) spectrometry measurements were recorded on a Nicolet 750. The Raman spectra were measured by using a Jobin Yvon HR800 confocal Raman system with 632.8 nm diode-laser excitation on a 300 S mm−1 line grating at RT.Electrochemical measurements
Cyclic voltammetry (CV) and galvanostatic charge/discharge behaviors of GN/CNTP electrodes were investigated on a CHI 660A electrochemical workstation (Shanghai Chenhua, China) in a conventional three-electrode system with 6 M KOH aqueous solution as the electrolyte. Platinum foil and a saturated calomel electrode (SCE) were used as counter and reference electrodes. The working electrodes were prepared by mixing active material (1 cm2). After coating the film on foamed Ni grids (1 cm × 1 cm), the electrodes were dried at 60 °C for several hours before pressing under a pressure of 10 MPa.Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program) (no. 2014CB239701), National Natural Science Foundation of China (nos 21173120, 21103091, and 51372116), National Natural Science Foundation of Jiangsu Province (no. BK2011030), the Fundamental Research Funds for the Central Universities of NUAA (NP2014403), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- X. Lee, T. T. Yang, X. Li, R. J. Zhang, M. Zhu, H. Z. Zhang, D. Xie, J. Q. Wei, M. L. Zhong, K. L. Wang, D. H. Wu, Z. H. Li and H. W. Zhu, Appl. Phys. Lett., 2013, 102, 162113 Search PubMed.
- J. L. Liu, M. H. Chen, L. L. Zhang, J. Jiang, J. X. Yan, Y. Z. Huang, J. Y. Lin, H. J. Fan and Z. X. Shen, Nano Lett., 2014, 14, 7180–7187 CrossRef CAS PubMed.
- J. L. Liu, J. Sun and L. A. Gao, J. Phys. Chem. C, 2010, 114, 19614–19620 CAS.
- C. G. Liu, Z. N. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863–4868 CrossRef CAS PubMed.
- J. J. Yoo, K. Balakrishnan, J. S. Huang, V. Meunier, B. G. Sumpter, A. Srivastava, M. Conway, A. L. M. Reddy, J. Yu, R. Vajtai and P. M. Ajayan, Nano Lett., 2011, 11, 1423–1427 CrossRef CAS PubMed.
- C. Z. Meng, C. H. Liu, L. Z. Chen, C. H. Hu and S. S. Fan, Nano Lett., 2010, 10, 4025–4031 CrossRef CAS PubMed.
- L. B. Hu, M. Pasta, F. La Mantia, L. F. Cui, S. Jeong, H. D. Deshazer, J. W. Choi, S. M. Han and Y. Cui, Nano Lett., 2010, 10, 708–714 CrossRef CAS PubMed.
- G. Y. Xu, B. Ding, P. Nie, L. F. Shen, J. Wang and X. G. Zhang, Chem.–Eur. J., 2013, 19, 12306–12312 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed.
- A. K. Mondal, B. Wang, D. W. Su, Y. Wang, S. Q. Chen, X. G. Zhang and G. X. Wang, Mater. Chem. Phys., 2014, 143, 740–746 CrossRef CAS PubMed.
- C. Z. Wu, X. L. Lu, L. L. Peng, K. Xu, X. Peng, J. L. Huang, G. H. Yu and Y. Xie, Nat. Commun., 2013, 4, 2431 Search PubMed.
- M. Kaempgen, C. K. Chan, J. Ma, Y. Cui and G. Gruner, Nano Lett., 2009, 9, 1872–1876 CrossRef CAS PubMed.
- D. S. Yu and L. M. Dai, J. Phys. Chem. Lett., 2010, 1, 467–470 CrossRef CAS.
- Q. Wu, Y. X. Xu, Z. Y. Yao, A. R. Liu and G. Q. Shi, ACS Nano, 2010, 4, 1963–1970 CrossRef CAS PubMed.
- D. H. Wang, R. Kou, D. Choi, Z. G. Yang, Z. M. Nie, J. Li, L. V. Saraf, D. H. Hu, J. G. Zhang, G. L. Graff, J. Liu, M. A. Pope and I. A. Aksay, ACS Nano, 2010, 4, 1587–1595 CrossRef CAS PubMed.
- H. R. Byon, S. W. Lee, S. Chen, P. T. Hammond and Y. Shao-Horn, Carbon, 2011, 49, 457–467 CrossRef CAS PubMed.
- J. L. Xia, F. Chen, J. H. Li and N. J. Tao, Nat. Nanotechnol., 2009, 4, 505–509 CrossRef CAS PubMed.
- H. S. Teng, Y. J. Chang and C. T. Hsieh, Carbon, 2001, 39, 1981–1987 CrossRef CAS.
- Y. C. Cao, M. Zhu, P. X. Li, R. J. Zhang, X. M. Li, Q. M. Gong, K. L. Wang, M. L. Zhong, D. H. Wu, F. Lin and H. W. Zhu, Phys. Chem. Chem. Phys., 2013, 15, 19550–19556 RSC.
- F. C. Wu, R. L. Tseng, C. C. Hu and C. C. Wang, J. Power Sources, 2005, 144, 302–309 CrossRef CAS PubMed.
- W. Xing, S. Z. Qiao, R. G. Ding, F. Li, G. Q. Lu, Z. F. Yan and H. M. Cheng, Carbon, 2006, 44, 216–224 CrossRef CAS PubMed.
- Z. H. Li, D. C. Wu, Y. R. Liang, F. Xu and R. W. Fu, Nanoscale, 2013, 5, 10824–10828 RSC.
- C. W. Huang, C. H. Hsu, P. L. Kuo, C. T. Hsieh and H. S. Teng, Carbon, 2011, 49, 895–903 CrossRef CAS PubMed.
- J. Yan, T. Wei, B. Shao, F. Q. Ma, Z. J. Fan, M. L. Zhang, C. Zheng, Y. C. Shang, W. Z. Qian and F. Wei, Carbon, 2010, 48, 1731–1737 CrossRef CAS PubMed.
- J. Yan, Z. J. Fan, T. Wei, W. Z. Qian, M. L. Zhang and F. Wei, Carbon, 2010, 48, 3825–3833 CrossRef CAS PubMed.
- Z. Q. Niu, L. Zhang, L. Liu, B. W. Zhu, H. B. Dong and X. D. Chen, Adv. Mater., 2013, 25, 4035–4042 CrossRef CAS PubMed.
- X. H. Cao, Y. M. Shi, W. H. Shi, G. Lu, X. Huang, Q. Y. Yan, Q. C. Zhang and H. Zhang, Small, 2011, 7, 3163–3168 CrossRef CAS PubMed.
- Y. Wang, Z. Q. Shi, Y. Huang, Y. F. Ma, C. Y. Wang, M. M. Chen and Y. S. Chen, J. Phys. Chem. C, 2009, 113, 13103–13107 CAS.
- M. D. Stoller, S. J. Park, Y. W. Zhu, J. H. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed.
- J. L. Liu, L. L. Zhang, H. B. Wu, J. Y. Lin, Z. X. Shen and X. W. Lou, Energy Environ. Sci., 2014, 7, 3709–3719 CAS.
- Q. Cheng, J. Tang, J. Ma, H. Zhang, N. Shinya and L. C. Qin, Phys. Chem. Chem. Phys., 2011, 13, 17615–17624 RSC.
- J. Chen, K. X. Sheng, P. H. Luo, C. Li and G. Q. Shi, Adv. Mater., 2012, 24, 4569–4573 CrossRef CAS PubMed.
- D. Graf, F. Molitor, K. Ensslin, C. Stampfer, A. Jungen, C. Hierold and L. Wirtz, Nano Lett., 2007, 7, 238–242 CrossRef CAS PubMed.
- V. Lee, L. Whittaker, C. Jaye, K. M. Baroudi, D. A. Fischer and S. Banerjee, Chem. Mater., 2009, 21, 3905–3916 CrossRef CAS.
- X. B. Cao, D. P. Qi, S. Y. Yin, J. Bu, F. J. Li, C. F. Goh, S. Zhang and X. D. Chen, Adv. Mater., 2013, 25, 2957–2962 CrossRef CAS PubMed.
- S. Sampath, A. N. Basuray, K. J. Hartlieb, T. Aytun, S. I. Stupp and J. F. Stoddart, Adv. Mater., 2013, 25, 2740–2745 CrossRef CAS PubMed.
- K. Gao, Z. Shao, X. Wu, X. Wang, Y. Zhang, W. Wang and F. Wang, Nanoscale, 2013, 5, 5307–5311 RSC.
- D. S. Zhang, T. T. Yan, L. Y. Shi, Z. Peng, X. R. Wen and J. P. Zhang, J. Mater. Chem., 2012, 22, 14696–14704 RSC.
- Z. Y. Guo, J. Wang, F. Wang, D. D. Zhou, Y. Y. Xia and Y. G. Wang, Adv. Funct. Mater., 2013, 23, 4840–4846 CAS.
- D. Zhang, T. Yan, L. Shi, Z. Peng, X. Wen and J. Zhang, J. Mater. Chem., 2012, 22, 14696 RSC.
- Z. S. Wu, Y. Sun, Y. Z. Tan, S. B. Yang, X. L. Feng and K. Mullen, J. Am. Chem. Soc., 2012, 134, 19532–19535 CrossRef CAS PubMed.
- Z. Q. Niu, W. Y. Zhou, J. Chen, G. X. Feng, H. Li, W. J. Ma, J. Z. Li, H. B. Dong, Y. Ren, D. A. Zhao and S. S. Xie, Energy Environ. Sci., 2011, 4, 1440–1446 CAS.
- D. Pech, M. Brunet, H. Durou, P. H. Huang, V. Mochalin, Y. Gogotsi, P. L. Taberna and P. Simon, Nat. Nanotechnol., 2010, 5, 651–654 CrossRef CAS PubMed.
- M. F. El-Kady, V. Strong, S. Dubin and R. B. Kaner, Science, 2012, 335, 1326–1330 CrossRef CAS PubMed.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |