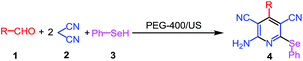Ultrasound assisted multicomponent reactions: a green method for the synthesis of highly functionalized selenopyridines using reusable polyethylene glycol as reaction medium†
Md. Nasim Khana,
Shaik Karamthullaa,
Lokman H. Choudhury*a and
Md. Serajul Haque Faizi b
b
aDepartment of Chemistry, Indian Institute of Technology Patna, Patna-800 013, Bihar, India. E-mail: lokman@iitp.ac.in; Fax: +91 612 2277383; Tel: +91 916 122552038
bDepartment of Chemistry, Indian Institute of Technology Kanpur, Kanpur-208 016, Uttar Pradesh, India
First published on 19th February 2015
Abstract
A simple and benign one-pot protocol for the synthesis of 2-amino selenopyridine derivatives 4 has been developed using ultrasound assisted multicomponent reactions of aldehydes, malononitrile and benzeneselenol in polyethylene glycol (PEG-400). Under similar reaction conditions, sterically hindered o,o′-disubstituted aromatic aldehydes provide the corresponding functionalized seleno dihydropyridines 5. In this process in total four new bonds (two C–C, one C–N and one C–Se) forms in one pot.
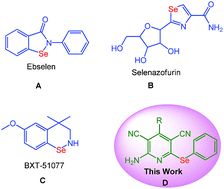
Selenium is one of the most important nutritional elements both for animals and human beings.1 Organoselenium compounds exhibit wide range of pharmacological and biological activities such as antioxidants, enzyme modulators, antimicrobials, antitumor, antiviral, antihypertensive agents, cytokine inducers, anticancer agent etc.2 Although selenium containing heterocycles posses considerable amount of cyctotoxic properties still they are less explored which may be due to their low stability compared to their sulphur analogues. Very recently, development of organoselenium compounds have gained considerable interest in synthetic organic chemistry and several novel organoselenides have been reported in the literature.3 A few representative Se containing heterocyles having promising medicinal properties are depicted in Fig. 1. Ebselen (A) is a mimic of glutathione peroxidase and which has been investigated for possible treatment of reperfusion injury, stroke, hearing loss, tinnitus and bipolar disorder.4 Similarly, Selenazofurin (B) containing the five membered selenazole ring is highly effective against both viral infections and animal tumors.5 Among several BTX series, BTX-51077 (C) is an analogue of ebselen that has been found to be efficient inhibitors of the TNFα-induced proinflammatory responses of endothelial cells.6 Functionalized pyridines are abundant in various bioactive natural as well as synthetic compounds.7 Considering the importance of functionalized pyridines and Se-containing heterocycles we were motivated to design a simple and efficient method for the preparation of selenopyridine derivatives (D) as shown in Fig. 1.
Literature studies have revealed that methods for the preparation of selanyl intact pyridines are still limited.8 Most of the literature reported methods use nucleophilic substitution of halopyridines using either diselenides or salt of selenols. To the best of our knowledge selenols have not been explored fully in multicomponent reactions. Thus there are lot of scope to design new multicomponent reactions using directly selenols for the facile access of pyridine tethered selenides. From green chemistry point of view designing an atom and step economic method under eco-friendly conditions is one of the very important requirements in organic synthesis. Multicomponent reactions (MCRs) in which more than two reactants react in a single step to form multiple bonds has become very popular strategy for the synthesis of library of molecules within a short time. Unconventional media with high boiling points are considered as ecofriendly solvents in organic synthesis and recently it has gained considerable attention in MCRs.9 PEG is one of the most explored unconventional media in organic synthesis.10 Recently we have used PEG as a reaction medium cum promoter in MCRs for the synthesis of functionalized dihydropyridines.11
Similarly, we have also recently developed catalyst free MCRs for the synthesis of functionalized pyrroles12 and thiazoles.13 In continuation of our work on design of MCRs14 in this paper we have reported an ultrasound assisted multicomponent reactions of aldehydes, malononitrile and phenylselenol in PEG-400 as shown in Scheme 1.
Polyethylene glycols (PEG) posses a wide range of virtues such as low volatility, good solubilising power, high boiling point reactions, nonhazardous, water solubility and ease of workup.15 In addition, it is readily available with very low cost, and suitable for energy dissipation with ultrasonication and microwaves. Among the several class of polyethylene glycols, PEG-400 is a hydrophilic polymer and acts as an efficient reaction medium for the synthesis of a large number of organic transformations.16 In continuation with our endeavour to further explore PEGs in MCRs for green synthesis we turned our attention to design a MCR for the synthesis of functionalized amino pyridines tethered with phenyselenium using PEG as reaction medium. In ultrasound assisted reactions, the cavitations process generates energetic bubbles that absorbs energy from the wave of ultrasound which then collapse with pressure changes and high temperature that results in concentration of high-energy particles and leads to intermolecular reactions.17
Taking cue from our previous work for the synthesis of 2-amino-3,5-dicyano-6-thiopyridines18 we wanted to use a similar approach for selenopyridines using phenyl selenium in place of thiol. Considering a wide range of applications of thiopyridines,19 several catalytic methods are available for the multicomponent synthesis of 2-amino-3,5-dicyano-6-thiopyridines however, synthesis of corresponding selenopyridines have not been yet explored. It is believed that these Se-containing pyridines will also attract the attention of medicinal chemists and may exhibit very useful medicinal properties.
In the initial investigation, the reaction of 4-methoxybenzaldehyde (1.0 mmol), malononitrile (2.0 mmol) and benzeneselenol (1.0 mmol) was chosen as a model reaction. Under catalyst-free conditions and at room temperature, the above reagents in ethanol provided the desired product 4b only in trace amount (Table 1, entry1). Attempted refluxing conditions did not provide any advantage (Table 1, entry 2). Next we tried this model reaction in ethanol in the presence of a catalytic amount (10 mol%) of various organocatalysts such as triethylamine, L-proline and DABCO under reflux conditions. In all these cases the yields obtained were higher than the catalyst-free conditions; however the observed yields were below 30% (Table 1, entries 3–5). Even in the presence of (10 mol%) NaOH the reaction did not provide good yield. Interestingly, by using imidazole (10 mol%) as catalyst, significant improvement in yield was observed (Table 1, entry 7). Next we wanted to explore glycols such as glycerol or PEGs to optimize this reaction. Under catalyst free conditions when the model reaction was performed at room temperature using glycerol and PEG-200 as reaction medium the observed yields were only 15 and 20% respectively. Interestingly, when PEG-400 was used as reaction medium it provided 40% yield of desired selenopyridine at room temperature within 8 h. After having this encouraging result using PEG-400, we wanted to optimize the reaction condition by changing reaction temperature. Catalyst free reaction in PEG-400 at 100 °C shows decrease in the reaction time as well as increase in yield compared to the room temperature reaction. When the model reaction in PEG-400 medium was subjected to microwave heating at 100 °C for 15 minutes using a microwave reactor, very poor yield was observed (Table 1, entry 12). Interestingly a trial reaction of this model substrates in PEG-400 medium under catalyst free conditions and in the presence of ultrasound irradiation showed very significant increase in yield (82%) within 5 h (Table 1, entry13). The effect of temperature was also studied and we observed that increase in temperature from 25 °C to 50 °C reduces the reaction time but also reduces the yield of the desired product. Under solvent free and US conditions the desired product was observed only in trace amount even after 7 h reaction time (Table 1, entry 15). Finally, from Table 1, we realized that the optimum conditions to obtain good yield for the desired product is PEG-400 as a solvent in the presence of ultrasound irradiation at room temperature without any inert atmosphere.
| Entry | Catalyst | Solvent | Time/(h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 4-methoxybenzaldehyde (1.0 mmol), malononitrile (2.0 mmol), benzeneselenol (1.0 mmol) in PEG-400 (2.0 ml) sonicated at rt.b Isolated yields. | ||||
| 1 | No catalyst | EtOH/rt | 10 | Trace |
| 2 | No catalyst | EtOH/reflux | 10 | Trace |
| 3 | TEA | EtOH/reflux | 6 | 20 |
| 4 | L-proline | EtOH/reflux | 7 | 15 |
| 5 | DABCO | EtOH/reflux | 5 | 25 |
| 6 | NaOH | EtOH/reflux | 2 | 20 |
| 7 | Imidazole | EtOH/reflux | 2 | 76 |
| 8 | No catalyst | Glycerol/rt | 9 | 10 |
| 9 | No catalyst | PEG-200/rt | 9 | 15 |
| 10 | No catalyst | PEG-400/rt | 8 | 40 |
| 11 | No catalyst | PEG-400/100 °C | 3 | 65 |
| 12 | No catalyst | PEG-400/MW | 0.25 | 20 |
| 13 | No catalyst | PEG-400/US/rt | 5 | 82 |
| 14 | No catalyst | PEG-400/US/50 °C | 4 | 75 |
| 15 | No catalyst | Solvent free/US/rt | 7 | Trace |
With the optimized conditions in hand, we turned our attention to investigate the scope and general applicability of this methodology by carrying out the synthesis of 2-amino-4-aryl/alkyl-6-(phenylselanyl)pyridine-3,5-dicarbonitriles using different aldehydes (Table 2).
| Entry | R | Ph | Producta | Time (hour) | Yieldb (%) |
|---|---|---|---|---|---|
| a All products were fully characterized by IR, 1H NMR, 13C NMR and elemental analysis.b Isolated yield. | |||||
| 1 | C6H5 | C6H5 | 4a | 5 | 76 |
| 2 | 4-OMeC6H4 | C6H5 | 4b | 4 | 82 |
| 3 | 3-OPh-C6H4 | C6H5 | 4c | 4 | 84 |
| 4 | 4-Br-C6H4 | C6H5 | 4d | 5 | 71 |
| 5 | 4-CN-C6H4 | C6H5 | 4e | 3 | 85 |
| 6 | Thiophene-carboxaldehyde | C6H5 | 4f | 4.5 | 87 |
| 7 | C6H5CH2 | C6H5 | 4g | 9 | 67 |
| 8 | 4-Me-C6H4 | C6H5 | 4h | 8 | 68 |
| 9 | 3-Cl-C6H4 | C6H5 | 4i | 5 | 81 |
| 10 | 3,4-OMe-C6H3 | C6H5 | 4j | 7 | 86 |
| 11 | 3,4-Cl-C6H3 | C6H5 | 4k | 7 | 78 |
A wide range of aromatic aldehydes tethered with either electron-withdrawing or electron-donating groups underwent this multicomponent reaction and the corresponding selenopyridines were obtained in moderate to good yields (Table 2, entries 1–5 and 8–11). Heteroaromatic aldehyde such as thiophene-2-carbaldehyde (Table 2, entry 6) also provided the corresponding selenopyridine in 87% isolated yield using the same reaction conditions. Aliphatic aldehyde such as phenylacetaldehyde was also tested and was found to be suitable in this multicomponent reaction to obtain the desired selenopyridines (Table 2, entry 7). It is noteworthy to mention that when sterically hindered o,o′-disubstituted aldehydes were tested, it gave the final product as unaromatized 1,4-dihydroselenopyridines (Table 3, entries 1 and 2).
All the products were fully characterized by IR, 1H NMR, 13C NMR spectra and by elemental analysis. The molecular structure of a representative compound 4b was established unambiguously by single crystal X-ray diffraction (Fig. 2). The compound 4b was recrystallized in acetonitrile and the crystal belongs to P21/n space group with Z: 4 Z′: 0. In this molecule intramolecular hydrogen bonding has been observed between the ‘N3’of nitrile with the ‘H1’ of the –NH2 due to their close proximity (Fig. 3).
It is noteworthy to mention that in all the cases the reactions were found remarkably clean and isolation of the products was easy.
Next we attempted to check the recyclability of the solvent (PEG) in this methodology. The reaction of 4-methoxybenzaldehyde (1.0 mmol), malononitrile (2.0 mmol) and benzeneselenol (1.0 mmol) in 2.0 ml PEG-400 for the synthesis of 4b was chosen as the model reaction for this purpose. After completion of the reaction, 2.0 ml ethanol was added for the precipitation of the product. The product was separated by filtration and ethanol was removed from the filtrate using rotary evaporator. Finally recovered PEG was washed with diethyl ether to obtain clean PEG-400. This recovered PEG was further used for the next cycle and the same procedure was repeated two more times without significant loss of activity. The % of yield obtained in different runs was 82 (1st), 82 (2nd) and 81% (3rd) respectively.
On the basis of the above results a plausible reaction mechanism has been shown in Scheme 2. We believe that in the initial step Knoevenagel condensation occurs in the presence of PEG-400. It is assumed that PEG activates both aldehyde and malononitrile to form yilidine intermediate. Next step is the simultaneous nucleophilic attack of the benzeneselenol and another equivalent of malononitrile for simultaneous intramolecular cyclization and tautomerization leading to the formation of intermediate 1,4-dihydroselenopyridine. The intermediate finally undergoes aerial oxidation and providing the final product.
 | ||
| Scheme 2 Proposed mechanism for the synthesis of 2-amino-4-aryl/alkyl-6-(phenylselanyl)pyridine-3,5-dicarbonitrile derivatives. | ||
In case of sterically hindered o,o′-disubstituted aldehydes the reaction stops at the 1,4-dihydroselenopyridine stage, which may be due to the steric crowding in the dihydropyridine intermediate.
In conclusion, we have developed a simple and efficient multicomponent reaction using PEG-400 as a reusable green solvent assisted by ultrasonication for the easy access to a series of selenopyridine derivatives. The virtues of this synthetic methodology are: a mild single step reaction conditions without metal catalyst or volatile organic solvent. The isolated products are pure enough for the characterization without any column chromatography. Considering the presence of selenium element with pyridine moiety in these products, this type of molecules may be useful in medicinal chemistry.
L.H.C. is grateful to DST, New Delhi for the partial financial assistance under the fast track scheme with sanction no. SR/FT/CS-042/2009. Authors are also thankful to IIT Patna for providing the general research facility to carry out this work along with partial financial assistance. M.N.K. and S.K are thankful to CSIR New Delhi, for their Senior Research Fellowships. We are also grateful to SAIF-Panjab University, Chandigarh for providing analytical facilities.
References and notes
- R. K. Bruce, Encyclopaedia of Inorganic Chemistry, John Wiley and Sons, 2nd edn, 2005, vol. 10, p. 1 Search PubMed.
- (a) C. M. Weekley and H. H. Harris, Chem. Soc. Rev., 2013, 42, 8870 RSC; (b) B. K. Sarma and G. Mugesh, J. Am. Chem. Soc., 2005, 127, 11477 CrossRef CAS PubMed; (c) D. Plano, Y. Baquedano, E. Ibanez, I. Jimenez, J. A. Palop, J. E. Spallholz and C. Sanmartin, Molecules, 2010, 15, 7292 CrossRef CAS PubMed; (d) M. Soriano-Garcia, Curr. Med. Chem., 2004, 12, 1657 CrossRef; (e) S. R. V. Madhunapantula, D. Desai, A. Sharma, S. J. Huh, S. Amin and G. P. Robertson, Mol. Cancer Ther., 2008, 7, 1297 CrossRef CAS PubMed; (f) J. Mlochowski, K. Kloc, R. Lisiak, P. Potaczek and H. Wojtowicz, ARKIVOC, 2007, vi, 14 Search PubMed; (g) S. T. Manjare, S. Kim, W. D. Heo and D. G. Churchill, Org. Lett., 2014, 16, 410 CrossRef CAS PubMed; (h) C. Narajji, M. D. Karvekar and A. K. Das, Indian J. Pharm. Sci., 2007, 69, 344 CrossRef CAS PubMed; (i) P. L. Tran, N. Lowry, T. Campbell, T. W. Reid, D. R. Webster, E. Tobin, A. Aslani, T. Mosley, J. Dertien, J. A. Colmer-Hamood and A. N. Hamood, Antimicrob. Agents Chemother., 2012, 56, 972 CrossRef CAS PubMed; (j) N. Rajesh, Mini-Rev. Med. Chem., 2008, 8, 657 CrossRef , references cited therein; (k) J.-I. Lee, H. Nian, A. J. L. Cooper, R. Sinha, J. Dai, W. H. Bisson, R. H. Dashwood and J. T. Pinto, Cancer Prev. Res., 2009, 2, 683 CrossRef CAS PubMed.
- (a) R. F. S. Canto, F. A. R. Barbosa and V. Nascimento, Org. Biomol. Chem., 2014, 12, 3470 RSC; (b) C. Pizzo and S. G. Mahler, J. Org. Chem., 2014, 79, 1856 CrossRef CAS PubMed; (c) A. Sperança, B. Godoi and G. Zeni, J. Org. Chem., 2013, 78, 1630 CrossRef PubMed; (d) M. Elsherbini, W. S. Hamama, H. H. Zoorob, D. Bhowmick, G. Mugesh and T. Wirth, Heteroat. Chem., 2014, 25, 320 CrossRef CAS; (e) S. Braverman, M. Cherkinsky, Y. Kalendar, H. E. Gottlieb, E. M. Mats, A. Gruzman, I. Goldberg and M. Sprecher, J. Phys. Org. Chem., 2013, 26, 102 CrossRef CAS; (f) X. Pan, J. Zhu, J. Zou, Z. Zhang, Z. Cheng, N. Zhou, W. Zhang and X. Zhu, Org. Lett., 2012, 14, 6170 CrossRef CAS PubMed; (g) B. Alcaide, P. Almendros, A. Luna, G. Gomez-Campillos and M. R. Torres, J. Org. Chem., 2012, 77, 3549 CrossRef CAS PubMed; (h) V. P. Singh, H. B. Singh and R. J. Butcher, Chem. Commun., 2011, 47, 7221 RSC.
- (a) M. Parnham and H. Sies, Expert Opin. Invest. Drugs, 2000, 9, 607 CrossRef CAS PubMed; (b) T. Yamaguchi, K. Sano, K. Takakura, I. Saito, Y. Shinohara, T. Asano and H. Yasuhara, Stroke, 1998, 29, 12 CrossRef CAS; (c) J. Kil, C. Pierce, H. Tran, R. Gu and E. D. Lynch, Hear. Res., 2007, 226, 44 CrossRef CAS PubMed; (d) N. Singh, A. C. Halliday, J. M. Thomas, O. V. Kuznetsova, R. Baldwin, E. C. Y. Woon, P. K. Aley, I. Antoniadou, T. Sharp, S. R. Vasudevan and G. C. Churchill, Nat. Commun., 2013, 4, 1332 CrossRef PubMed.
- (a) G. Gebeyehu, V. E. Marquez, A. V. Cott, D. A. Cooney, J. A. Kelley, H. N. Jayaram, G. S. Ahluwalia, R. L. Dion, Y. A. Wilson and D. G. Johns, J. Med. Chem., 1985, 28, 99 CrossRef CAS; (b) S. K. Wray, R. H. Smith, B. E. Gilbert and V. Knight, Antimicrob. Agents Chemother., 1986, 29, 67 CrossRef CAS; (c) J. J. Kirsi, J. A. North, P. A. McKernan, B. K. Murray, P. G. Canonico, J. W. Huggins, P. C. Srivastava and R. K. Robins, Antimicrob. Agents Chemother., 1983, 24, 353 CrossRef CAS; (d) H.-J. Lee, K. Pawlak, B. T. Nguyen, R. K. Robins and W. Sadee, Cancer Res., 1985, 45, 5512 CAS.
- (a) M. Moutet, P. D'Alessio, P. Malette, V. Devaux and J. Chaudiere, Free Radical Biol. Med., 1998, 25, 270 CrossRef CAS; (b) P. D'Alessio, M. Moutet, E. Coudrier, S. Darquenne and J. Chaudiere, Free Radical Biol. Med., 1998, 24, 979 CrossRef.
- M. Schwoerer and H. C. Wolf, Organic Molecular Solids, Wiely VCH, Weinheim, 2005 Search PubMed.
- (a) S. Thurow, R. Webber, G. Perin, E. J. Lenardao and D. Alves, Tetrahedron Lett., 2013, 54, 3215 CrossRef CAS PubMed; (b) S. H. Abdel-Hafez, S. A. Abdel-Mohsen and Y. A. El-Ossaily, Phosphorus, Sulfur Silicon Relat. Elem., 2006, 181, 2297 CrossRef CAS; (c) F. Luis, V. J. Jose, A. M. Isabel, L. Antonio and S. J. Luis, Heterocycles, 1988, 27, 2125 CrossRef PubMed; (d) A. Dandapat, C. Korupalli, D. J. C. Prasad, R. Singh and G. Sekar, Synthesis, 2011, 2297 CAS; (e) Y. Li, H. Wang, X. Li, T. Chen and D. Zhao, Tetrahedron, 2010, 66, 8583 CrossRef CAS PubMed; (f) N. Taniguchi and T. Onami, J. Org. Chem., 2004, 69, 915 CrossRef CAS PubMed; (g) C. S. Freitas, A. M. Barcellos, V. G. Ricordi, J. M. Pena, G. Perin, R. G. Jacob, E. J. Lenardão and D. Alves, Green Chem., 2011, 13, 2931 RSC; (h) K. K. Bhasin, S. Doomra, G. Kaur, E. Arora, N. Singh, Y. Nagpal, R. Kumar, T. M. Klapoetke and S. K. Mehta, Phosphorus, Sulfur Silicon Relat. Elem., 2008, 183, 992 CrossRef CAS.
- (a) R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958 RSC; (b) M. Li, A. Taheri, M. Liu, S. Sun and Y. Gu, Adv. Synth. Catal., 2014, 356, 537 CrossRef CAS; (c) Y. Gu and F. Jerome, Chem. Soc. Rev., 2013, 42, 9550 RSC; (d) Y. Gu, Green Chem., 2012, 14, 2091 RSC; (e) P. G. Jessop, Green Chem., 2011, 13, 1391 RSC; (f) P. Pollet, E. A. Davey, E. E. U. Benavides, C. A. Eckert and C. L. Liotta, Green Chem., 2014, 16, 1034 RSC; (g) M. B. Gawande, V. D. B. Bonifacio, R. Luque, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522 RSC.
- (a) A. Nagaraju, B. J. Ramulu, G. Shukla, A. Srivastava, G. K. Verma, K. Raghuvanshi and M. S. Singh, Green Chem., 2015, 17, 950 RSC; (b) V. V. Kouznetsov, D. R. M. Arenas and A. R. R. Bohorquez, Tetrahedron Lett., 2008, 49, 3097 CrossRef CAS PubMed; (c) G.-p. Lu, L.-Y. Zeng and C. Cai, Green Chem., 2011, 13, 998 RSC; (d) M. M. Bassaco, M. P. Fortes, D. F. Back, T. S. Kaufman and C. C. Silveira, RSC Adv., 2014, 4, 60785 RSC; (e) S. Fatma, D. Singh, P. Mishra, P. K. Singh, P. Ankit, M. Singh and J. Singh, RSC Adv., 2013, 3, 22527 RSC.
- S. Pal, V. Singh, P. Das and L. H. Choudhury, Bioorg. Chem., 2013, 48, 8 CrossRef CAS PubMed.
- S. Karamthulla, S. Pal, M. N. Khan and L. H. Choudhury, Synlett, 2014, 25, 1926 CrossRef CAS PubMed.
- S. Karamthulla, S. Pal, M. N. Khan and L. H. Choudhury, RSC Adv., 2014, 4, 37889 RSC.
- (a) S. Pal, L. H. Choudhury and T. Parvin, Mol. Diversity, 2012, 16, 129 CrossRef CAS PubMed; (b) S. Karamthulla, S. Pal, M. N. Khan and L. H. Choudhury, RSC Adv., 2013, 3, 15576 RSC; (c) S. Pal, M. N. Khan, S. Karamthulla, S. J. Abbas and L. H. Choudhury, Tetrahedron Lett., 2013, 54, 5434 CrossRef CAS PubMed; (d) M. N. Khan, S. Pal, S. Karamthulla and L. H. Choudhury, RSC Adv., 2014, 4, 3732 RSC; (e) S. Pal, M. N. Khan, S. Karamthulla and L. H. Choudhury, RSC Adv., 2013, 3, 15705 RSC.
- (a) J. Chen, S. K. Spear, J. G. Huddleston and R. D. Rogers, Green Chem., 2005, 7, 64 RSC; (b) D. J. Gravert and K. D. Janda, Chem. Rev., 1997, 97, 489 CrossRef CAS PubMed.
- (a) J. Chen, Y. Zhang, W. Hao, R. Zhang and F. Yi, Tetrahedron, 2013, 69, 613 CrossRef CAS PubMed; (b) R. G. Lara, P. C. Rosa, L. K. Soares, M. S. Silva, R. G. Jacob and G. Perin, Tetrahedron, 2012, 68, 10414 CrossRef CAS PubMed; (c) K. S. Feu, A. F. de la Torre, S. Silva, M. A. F. de Moraes Junior, A. G. Correa and M. W. Paixao, Green Chem., 2014, 16, 3169 RSC.
- (a) T. J. Mason, Chem. Soc. Rev., 1997, 26, 443 RSC; (b) R. B. N. Baig and R. S. Verma, Chem. Soc. Rev., 2012, 41, 1559 RSC; (c) G. Cravotto and P. Cintas, Chem.–Eur. J., 2007, 13, 1902 CrossRef CAS PubMed.
- M. N. Khan, S. Pal, L. H. Choudhury and T. Parvin, RSC Adv., 2012, 2, 12305 RSC.
- (a) S. K. Srivastava, R. P. Tripathi and R. Ramachandran, J. Biol. Chem., 2005, 280, 30273 CrossRef CAS PubMed; (b) H. Harada, S. Watanuki, T. Takuwa, K. Kawaguchi, T. Okazaki, Y. Hirano and C. Saitoh, PCT Int. Appl. WO 2002006237 A1 20020124, 2002; (c) H. Chen, W. Zhang, R. Tam and A. K. Raney, PCT Int. Appl. WO 2005058315 A1 20050630, 2005; (d) M. A. Azuine, H. Tokuda, J. Takayasu, F. Enjyo, T. Mukainaka, T. Konoshima, H. Nishino and G. J. Kapadia, Pharmacol. Res., 2004, 49, 161 CrossRef CAS PubMed; (e) J. M. Quintela, C. Peinador, M. C. Veiga, L. M. Botana, A. Alfonso and R. Riguera, Eur. J. Med. Chem., 1998, 33, 887 CrossRef CAS; (f) L. C. W. Chang, J. K. von Frijtag Drabbe Kunzel, T. Mulder-Krieger, R. F. Spanjersberg, S. F. Roerink, G. van den Hout, M. W. Beukers, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2005, 48, 2045 CrossRef CAS PubMed.
- ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: Reaction conditions and spectra. CCDC 1004592. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra02403j |
| This journal is © The Royal Society of Chemistry 2015 |