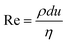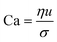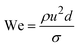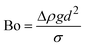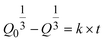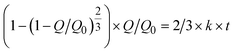Revolutionizing targeting precision: microfluidics-enabled smart microcapsules for tailored delivery and controlled release
Lingling
Ren
,
Shuang
Liu
,
Junjie
Zhong
 * and
Liyuan
Zhang
* and
Liyuan
Zhang
 *
*
School of Petroleum Engineering, China University of Petroleum (East China), Qingdao, Shandong, China. E-mail: zhongjunjie@upc.edu.cn; 20210085@upc.edu.cn
First published on 15th January 2024
Abstract
As promising delivery systems, smart microcapsules have garnered significant attention owing to their targeted delivery loaded with diverse active materials. By precisely manipulating fluids on the micrometer scale, microfluidic has emerged as a powerful tool for tailoring delivery systems based on potential applications. The desirable characteristics of smart microcapsules are associated with encapsulation capacity, targeted delivery capability, and controlled release of encapsulants. In this review, we briefly describe the principles of droplet-based microfluidics for smart microcapsules. Subsequently, we summarize smart microcapsules as delivery systems for efficient encapsulation and focus on target delivery patterns, including passive targets, active targets, and microfluidics-assisted targets. Additionally, based on release mechanisms, we review controlled release modes adjusted by smart membranes and on/off gates. Finally, we discuss existing challenges and potential implications associated with smart microcapsules.
1. Introduction
A microcapsule is an inner core droplet that is usually surrounded by an outer polymeric shell. The inner core provides desired space and entraps diverse active materials, while the shell acts as an effective barrier that separates the core from ambient environments.1 Droplet-based microfluidics is the most effective technology for fabricating microcapsules owing to its precise manipulation of fluids on a low-energy-demand micrometer scale.2–4 Under the balanced forces between immiscible fluid phases, emulsion droplets are produced and subsequently converted into microcapsules by solidifying the shell.5,6 However, practical conditions are complex and variable, wherein traditional microcapsules may not always be suitable for their intended applications, particularly when the co-encapsulation of multiple active materials and point-in-time release are required.7,8 To address these challenges, it is crucial to fabricate smart microcapsules that can achieve the controlled release of encapsulants at specific and pre-designed sites. The biggest advantage of microfluidics-assisted smart microcapsules is that their membranes can be customized to meet specific needs for diverse applications. Smart microcapsules have become promising candidates for controllably encapsulating, transporting, and releasing various active materials in versatile applications, including agricultural,9,10 food,11,12 energy,13,14 biomedical15,16 cosmetic,17,18 and chemical industries.19,20Desirable characteristics in smart microcapsules as promising delivery systems are associated with encapsulation capacity, retention, targeted delivery capability, and the controlled release of active encapsulants.21 Microfluidics-assembled smart microcapsules with engineering structures have been widely explored, such as core–shell microcapsules protecting encapsulants from degradation,22,23 multiple separate compartments enabling co-encapsulation and drug synergy,24,25 and responsive membranes achieving stimulus-triggered release to target sites.26–28 Although there are excellent reviews published on smart microcapsules, some highlight a particular class, such as stimulus-responsive property,29 smart membranes,30 or smart gating,31 and others focus on specific applications.9,15 In the preparation of smart microcapsules, an important step is not only the selection of an encapsulation carrier for an encapsulant based on final applications, but also the target delivery and controlled release performance, including sites and modes of release. However, reported papers mainly focus on microcapsules transported under specific conditions and then stimulated under external conditions, which is known as passive target. It is of great significance to summarize the most recent advances in microfluidics-assisted smart microcapsules using active target modes as delivery systems in a controlled release manner.
The main focus of this review is to summarize a droplet-based microfluidics emulsion templated smart microcapsule in terms of fabrication, target delivery and controlled release. The “smart” of “smart microcapsule” encompasses two essentials: specific target and controlled release. We begin by briefly introducing droplet formation, the selection of suitable microfluidics materials and flexible devices, and the advantages of droplet-based microfluidics. We introduce smart microcapsules as delivery systems, including single-, double-, and high-order emulsion templates and target delivery patterns, including passive and active targets. In addition, the release mechanisms are used as the starting point to present the controlled release procedures adjusted by the smart whole membrane and smart on/off gates. Finally, we discuss the existing challenges and outlooks associated with smart microcapsules.
2. Droplet-based microfluidics for emulsion-templated smart microcapsules
2.1 Mechanisms for emulsion-droplet formation
When two mutually immiscible fluids are introduced into the microfluidics channel, emulsion droplets are produced because of fluid instabilities.32–34 This phenomenon can be explained by Rayleigh–Plateau instability, where surface tension forces seek to minimize the interfacial area, causing the introduced fluid to become unstable and to form droplets when the surface instability is large enough.35 The process of droplet formation is a result of a well-controlled balance between various forces acting on the fluid flow in the microscale space, characterized by inertial force, viscous force, gravity, and interfacial tension according to the continuity equation.36–38 Dimensionless numbers are powerful tools for comparing the relative predominance of various forces and obtaining the necessary fundamental relations in different flow regimes. For example, the occurrence conditions for the dripping regime are 10−2 < Ca < 1 and We < 1, while that of the jetting regime is Ca + We ≥ 1.32Table 1 shows the commonly used dimensionless numbers in microfluidics droplet generation, and more detailed information about dimensionless numbers and droplet formation can be found in other reports.4,392.2 Microfluidics devices for emulsion-droplet formation
| Materials | Advantages | Disadvantages | Emulsion structures | Surface modification techniques | Technique characteristics | Ref. |
|---|---|---|---|---|---|---|
| Oil (O), water (W), oil-in-water (O/W), water-in-oil (W/O), oil-in-water-in-oil (O/W/O), water-in-oil-in-water (W/O/W). | ||||||
| PDMS | Gas permeability, transparency, biocompatibility, flexibility, easy molding, reproducibility | The poor mechanical property, low surface free energy; instability, deformability | W/O/W, O/W/O | Plasma treatment | A simple and versatile method but the short duration of hydrophilic | 51 and 52 |
| W/O/W | Plasma treatment + coating polyvinyl alcohol (PVA) | Improved plasma treatment and the contact angle was 21.0 ± 3.2° for 30 days | 53 | |||
| W/O, W/O/W | Adding the surfactant Silwet L-77, Pluronic F-127 | Easy to use and the contact angles of the water droplets can be controlled at 52–85° for 10–30 days | 34 and 54 | |||
| O/W, W/O/W | Sequential layer-by-layer deposition of polyelectrolytes | Allow generating emulsions even after several months | 39 | |||
| W/O | Coating with a glass-like layer using sol–gel chemistry | Better precision control and chemical robustness, easy to carry out but suffer from poor durability | 55 | |||
| W/O/W, O/W/O | Covalent with silane + grafting acrylic acid | Spatially modulates the wettability and high contrast spatial patterning | 56 | |||
| Glass | High rigidity, high chemical stability, high strength, impermeability, transparency, easy surface modification, ensure true 3D flow | Limited geometries, limited emulsion size | W/O | Coating PMMA film and silane + laser-induced backside wet etching | Hybrid structures; the ridge is hydrophilic, and the inner surface is hydrophobic | 57 |
| W/O/W, O/W/O | Coating octadecyltrichlorosilane + local UV irradiation for hydrophilization | Locally controlling; forming multiple emulsion droplets | 58 and 59 | |||
| W/O/W, O/W/O | Coating n-octadecyltrimethoxysilane and 2-[methoxy(polyethyleneoxy)6-9-propyl]tris(dimethylamino)silane | Hybrid property of the hydrophilic and hydrophobic surfaces | 60 | |||
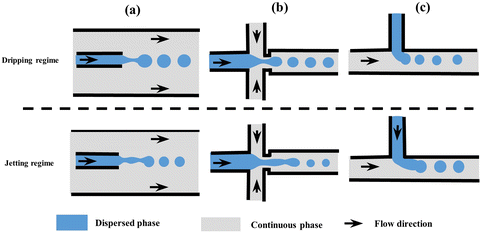 | ||
| Fig. 1 Schematic of microfluidic droplet generators with dripping and jetting regimes (not to scale). (a) Co-flow, (b) flow-focusing, and (c) T-junction. Modified with permission from ref. 63. Copyright 2013, IOP Publishing Ltd. Printed in the UK and USA. | ||
| Geometries | Emulsion structures | Mean size (μm) | Coefficient of variation | Ref. |
|---|---|---|---|---|
| Cross-flow | W/O | 90–120 | <5% | 36 |
| O/W, W/O | 131.5 | 1.35% | 74 | |
| O/W | 96.4 | 1.3% | 75 | |
| Co-flow | O/W/O | 156 | 3.88% | 76 |
| W/O | 210 | 1.20% | 77 | |
| O/W/O | 270 | 1.38% | 78 | |
| O/W | 2–200 | <3% | 79 | |
| Flow-focusing | O/W/O | 198.2 | 2.4% | 80 |
| O/W | 10–50 | 3.9% | 81 | |
| W/O | 153 | 5% | 82 | |
| One-step | O/W/O/W | 92 | 2% | 83 |
| O/W/O | 181 | 1.5% | 84 | |
| W/O/W | 160 | 1.6% | 85 | |
| O/W | 107 | 3.1% | 86 | |
| W/O/W/O | 181 | 2% | 87 |
Freely combined with the three shear-induced geometries, multi-step, one-step, and high-throughput microfluidics devices were designed for innovative droplet generation, as shown in Fig. 2. Generally, a multi-step microfluidics device is used to connect multiple basic geometries with opposite wettability in series, such as two consecutive cross-flow devices,64 two flow-focusing devices,65 and four co-flow devices.66 However, it is difficult for the multi-step device to precisely control spatial wettability; thus, full control over the relative sizes of the core and the shell of the emulsions is challenging. A one-step microfluidics device can be fabricated by simultaneously converging three phases into one point and providing high precision for aggregate formation. For example, a facial one-step microfluidics device was designed for the quick production of smart microcapsules with controllability and scalability, enhancing the ability to control the process of emulsion generation.67,68 However, a good alignment of the tubes is necessary on a micron scale, which is a labor-intensive process. To resolve this problem, a novel design without manual adjustment for coaxial alignment was fabricated by simply inserting an annular capillary array into a collection channel, which maintained a fixed coaxial alignment and allowed the innermost flow to be sheathed by the middle phase.69 Moreover, the high-throughput generation of microcapsules has been achieved using parallelized microfluidics flow-focusing devices.70 A reported parallelized device for mass production was multiple parallel droplet generators coupled to only two inlets and achieved a throughput of 8.2 L h−1.71 Using parallel droplet microfluidic, Headen et al. achieved 600% increased throughput for cell encapsulation compared to single-droplet device.72 Additionally, Shin et al. designed a multi-layer drop maker geometry to fulfill the mass production of droplets for the remediation of heavy crude oils.73
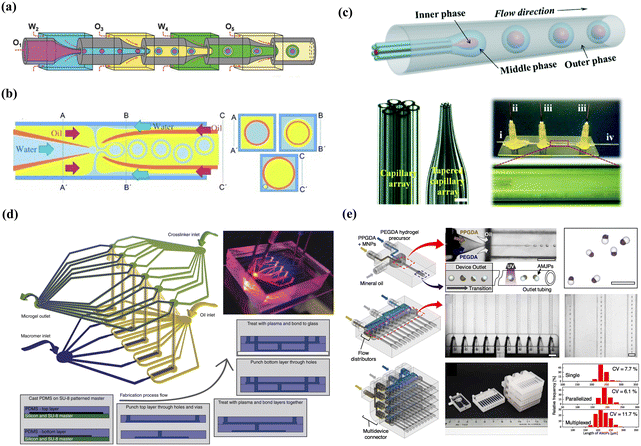 | ||
| Fig. 2 Combined microfluidics devices: (a) multi-step microfluidics device. Reprinted with permission from ref. 66. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) One-step microfluidics and the three cross-sectional schematics (A–A’, B–B’, and C–C’) are included for clarity. Reprinted with permission from ref. 88. Copyright 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) Advanced one-step microfluidics and the i–iv are the inner, middle, outer and collection channels, respectively. Reprinted with permission from ref. 69. Copyright 2014 Royal Society of Chemistry. (d) Parallel microfluidics device. Reprinted with permission from ref. 72. Copyright 2017, Springer Nature. (e) Multi-layer microfluidics devices. Reprinted with permission from ref. 73. Copyright 2023 Elsevier B.V. | ||
2.3 Advantages of droplet-based microfluidics for smart microcapsules
Compared with traditional emulsification technologies that rely on bulk properties of emulsions and output energy, microfluidics technology offers significant advantages for generating different emulsions with the desired dispersity and size on a low-energy-demand micrometer scale.4 Through laminar fluidic patterns and dominant convection effects, microfluidics techniques enable precise control of fluid dynamics, contributing to the highly monodisperse and controllable size emulsion droplets. For example, droplets obtained from traditional methods exhibit a CV of 7–15%, while microfluidics droplets achieve much lower values of 1–5%.89,90 Moreover, different droplet diameters ranging from 5 to 1000 μm can be controlled in microfluidics by precise control of the relative flow rates between the two phases.91 Reports showed that decreasing the flow rate of the outer phase from 8000 μL h−1 to 2000 μL h−1 reduces droplet size from 60 μm to 40 μm and maintained droplets with a CV as low as 1.3%.61,91,92 However, by adjusting the flow rate to control the size of droplets, a problem that needs to be considered is that the ultrahigh flow rate can block and stagnate the channels, causing excessive material consumption and droplet instability.36By precisely manipulating composition and tailorable interfaces between continuous and dispersed phases, microfluidics techniques offer flexibility in achieving shape-controlled microcapsules. Typically, channel geometries are commonly employed to manipulate the shape of emulsion droplets. An example is the helical-shaped microcapsules fabricated using two square capillaries to connect the injection, transition, and transformation tubes under the liquid rope coiling effect.92 A Y-shaped channel and planar sheath-flow geometry were utilized to produce biphasic Janus droplets.93 Alternatively, precisely tailoring interfacial tension of multiple interfaces by microfluidics offers a promising approach to fabricating microcapsules with flexible shapes, including Janus,94 snowman-like95 and dumbbell-shaped.96 However, non-spherical-shaped microcapsules are unstable owing to the interfacial minimum free energy effect. To enhance droplet formation stability, an obstacle-assisted microfluidics device was designed by incorporating an obstacle into the outlet channel.97 In addition, using a polyethylene glycol-modified protein-surfactant to stabilize microcapsules, non-spherical emulsion droplets could be stable against coalescence for months and maintained non-spherical shapes for hours, which offered new opportunities for shape-relevant studies.98
The free combination of basic geometries endows the fabrication of microcapsules with more sophisticated structures and morphologies that cannot be achieved by traditional fabrication methods.99 Owing to the excellent controllability and remarkable scalability of microfluidics, multiple compartments could be designed with specific core numbers, ratios, and sizes, benefiting from the synergistic encapsulation of multiple materials.100 The greater potential of microfluidics lies in synthetic cells with physiologically relevant environments by applying a high-throughput microfluidics method.101 Meanwhile, microfluidics systems provide more stable reaction conditions and reduce reagent consumption, thereby efficiently preventing cross-contamination and simplifying post-processing.102
3. Smart microcapsules for target delivery
Delivery systems are the prerequisite and key to the success of target delivery. In this chapter, we begin by summarizing emulsion-templated microcapsules as delivery systems, followed by introducing the target delivery patterns, including passive target, active target, and microfluidic-assisted target.3.1 Emulsion templated microcapsules as delivery systems
An ideal delivery system should encapsulate the encapsulants as efficiently as possible and overcome a series of barriers to easy transport to the target sites. Emulsion-templated microcapsules manufactured through emulsion templating and subsequent solidification are promising candidates for delivery systems owing to their flexible manipulation to functionalize the separated phases and interfaces by physical/chemical processes. The emulsion templates typically include single emulsion, double emulsion, and high-order emulsion. We summarized the emulsion-templated microcapsules as delivery systems to encapsulate different active materials for diverse applications in Table 4.| Structures | Key materials | Emulsification | Encapsulants | Characteristics | Applications | Ref. |
|---|---|---|---|---|---|---|
| O/W | Poly(lactic-co-glycolic acid) (PLGA) in dichloromethane/1% PVA | Evaporation of solvent | Bupivacaine | Biodegradable matrix; lower initial burst | Drug delivery | 103 |
| W/O | 4 wt% biocide, 40 wt% PEGDA, 1 wt% photoinitiator, fluorescein sodium salt/98 wt% dodecane, 2 wt% EM 90 | UV polymerization | Grotamar71 | Microcapsules maintain their shapes for more than 6 weeks in oil and exhibit antimicrobial activity | Agriculture industry | 104 |
| O/W | Soybean oil containing crosslinking agent terephthalaldehyde/chitosan aqueous solution | Interface crosslinking reaction | Tea tree oil | The storage time can be up to 150 days | Personal-care | 105 |
| O/W | Methyl methacrylate, isophorone diisocyanate, azobisisobutyronitrile, pentaerythritol tetraacrylate, Span 80/Tween 80, diethylenetriamine | Interfacial polymerization and suspension-like polymerization | N-Hexadecane | The encapsulation ratio of 94.5% | Energy storage | 106 |
| W/O | 20 wt% PEGDA, 10 wt% NaSS, 20 wt% glycerin, photoinitiator 2 wt% Darocur 1173/paraffin oil, 0.5 wt% Abil EM 90 | Ultraviolet (UV) polymerization | Bacillus subtilis | The encapsulation efficiency of bacterial was almost 100% | Enhanced oil recovery | 107 |
| W/O/W | 2 w/v%, PVA/dichloromethane, 7 w/v% PLGA, 0.7 w/v% rifampicin, 5 v/v% span 80/2 w/v% PVA | Solvent evaporation | Rifampicin | The drug encapsulation efficiency is 78.5 ± 1.1% | Drug delivery | 108 |
| O/W/O | Soybean oil/Pluronic F127, glycerin/soybean oil, poly (N-methylolacrylamide) | Free radical polymerization | Sodium dodecyl benzene sulfonate | The efficient encapsulation avoids the adsorption loss of surfactants | Enhanced oil recovery | 13 |
| O/W/O | Soybean oil, benzyl benzoate, Sudan III/sodium alginate, calcium-ethylenediaminetetraacetic acid, D-(+)-gluconic acid δ-lactone/soybean oil, 5 w/v% PGPR 90 | Solvent diffusion-ionic crosslinking | Citral | Under mild conditions, without high temperature, UV irritation, or acetic acid | Food antioxidant | 76 |
| W/W/W | 15 wt% dextran, 0.5 wt% poly(diallyldimethyl ammonium chloride)/17 wt% polyethylene glycol/polyethylene glycol, polystyrene sodium sulfate | Electrostatic attraction | Platelet-derived growth factor-BB | Without organic solvents; maintain the biological activities of proteins | Sensitive biomarkers | 68 |
| O–W–O | 8% w/v PEG (35 kDa), 17% OptiPrep densifier/4–8% w/v 4-arm maleimide,10 mM triethanolamine/mineral oil, 0.5 v/v% Span-80 | Michael addition-mediated gelation | Hepatocyte cells | Encapsulants maintained inside the microcapsules for over ten days | Cell culture | 109 |
| W/O/W | Sodium alginate/ethyl cellulose/PVA | Ionic cross-linking and solvent evaporation | Phycocyanin | Encapsulation efficiency of up to 98% | Colon-targeted delivery | 110 |
| W/O/W | Diethyl phthalate, diisodecyl phthalate, pentane, cyclopentane, cyclohexane, heptane/37 wt% glycidyl methacrylate, 24 wt% ethylene glycol methacrylate, 4.5 wt% 2-hydroxy-2-methylpropiophenone, 1 w/w% 2-hydroxy-2-methylpropiophenone/2 wt% PVA | UV polymerization | Nile red and fluorescein salt | Strong microcapsules; encapsulating materials in the core and shell | Applications requiring mechanical stability | 111 |
| W/O/W | 5% PVA and 4% Tween 80/photocurable oil/5% PVA | UV polymerization | α-Pinene | After approximately 2.8 days of dispersion in water, 85 ± 8% of α-pinene remained in microcapsules | Cosmetic industry | 112 |
| W/W/O | NIPAm-poor, Span 80/NIPAm-rich, oleophilic, Pluronic F-127, glucose oxidase-loaded silica nanocontainers/silicon oil, 5 v/v% DC749 | UV polymerization | Glucose oxidase-loaded silica nanocontainers | The outer shell serves as the gateway for the transport of smaller molecules; controllable enzymatic reactions | Glucose sensors | 113 |
| W/O/W | Glycerol/fatty glycerides/glycerol, PVA | Liquid-to-solid phase transition | FITC-dextran | These microcapsules remain stable at room temperature for at least six months, with uniform mechanical properties | Multiple compartments and robust microcapsules are desired | 114 |
| W/O/W | Poly(n-vinyl caprolactam), 20 w/w% PVA/ethoxylated trimethylolpropane tri acylate, 1 w/w% photoinitiator/10 w/w% PVA | UV polymerization | Kinetic hydrate inhibitors | Release encapsulant only at temperature responsible for hydrate formation under shear flow | Deepwater oil and gas production | 115 |
| W/O/W/O | The aqueous solution containing hydrophilic actives/hexadecane/10% PEGDA, 2% PVA/2% Span 80, mineral oil | UV polymerization | Erioglaucine or fluorescein sodium salt | The hydrophilic cargoes exhibited 3 months of retention | Biomedicine | 116 |
| O/W/O/W | α-Pinene/2% PVA/photocurable ethoxylated trimethylolpropane triacrylate/10% PVA | UV polymerization | α-Pinene | The encapsulation efficiency is above 95% | Fragrances application | 83 |
| (W/O)/W/O | Nanoparticles/1% w/v Pluronic F127, NIPAM, N,N′-methylenebisacrylamide, 2,2′-azobis(2-amidinopropane) dihydrochloride/soybean oil, 8 w/v% PGPR 90 | UV polymerization | FluoSphereÒ beads | Nanoparticles are encapsulated in microcapsules without any leakage before sites on demand | Pharmaceutical industry | 117 |
 | ||
| Fig. 3 Single emulsion-templated microparticles with homogeneous structures or heterogeneous structures. (a) Microfluidics device for single emulsion-templated microparticles with a homogeneous structure. Reprinted with permission from ref. 119. Copyright 2023, American Chemical Society. (b) Microfluidics device with a θ-shaped injection tube for single emulsion-templated microparticles with heterogeneous structures. Reprinted with permission from ref. 120. Copyright 2023, Elsevier B.V. | ||
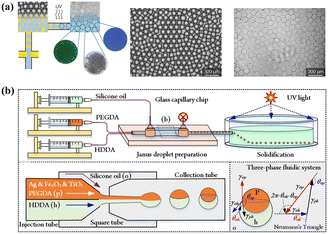
Generally, the commonly used single emulsions include W/O and O/W. The former is suitable for water-soluble cargo, such as Grotamar104 and the latter works well for hydrophobic cargo, such as curcumin.86 Using W/O emulsions as the protector to encapsulate cells, cell-laden delivery systems are significant for in situ delivery in tissue engineering.126
Moreover, W/W emulsions can be used to encapsulate active materials.127 However, the low interfacial tension in W/W emulsion makes droplet formation difficult and not conducive to encapsulate. To resolve this problem, mechanical shaking was introduced to the device to fabricate stable W/W emulsions, which provide small pulses and facilitate jet break-up.128 In addition, protein particles129 and polydopamine particles130 are used to stabilize W/W emulsions. Interfacial precipitation and interfacial gelation could also be introduced to enhance the encapsulation efficiency of W/W emulsions.128
Co-encapsulating hydrophilic and hydrophobic materials in single emulsion templated delivery systems poses a challenge owing to the nature of the emulsion core (hydrophilic or hydrophobic). Incorporating micro/nanoparticles in the matrix offers a promising solution to address this issue and enhances encapsulation efficiency without chemical conjugation. For instance, embedding porous silicon particles into the dispersed oil phase of O/W microcapsules to encapsulate hydrophilic atorvastatin and hydrophobic celecoxib is directly added to the oil phase.131 Another effective approach involves using halloysite nanotubes embedded in a polymer matrix to achieve the co-encapsulation of drugs with different physicochemical properties.117
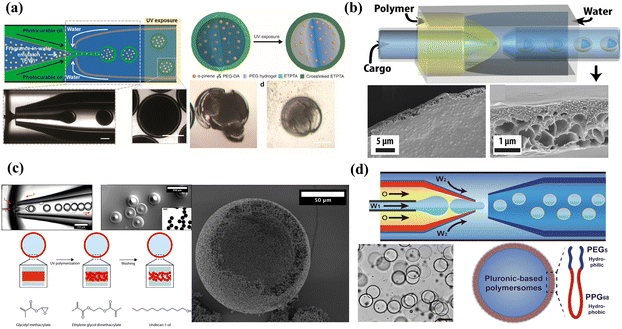 | ||
| Fig. 4 Microcapsules with different shells: (a) microcapsules with dense shells. Reprinted with permission from ref. 112. Copyright 2016, American Chemical Society. (b) Microcapsules with porous shells. Reprinted with permission from ref. 134. Copyright 2020, American Chemical Society. (c) Microcapsules with colloidal particle shells. Reprinted with permission from ref. 135. Copyright 2017, American Chemical Society. (d) Microcapsules with polymersome shells. Reprinted with permission from ref. 136. Copyright 2022, Springer Nature. | ||
Microcapsules with dense shells have the properties of low permeability and mechanical and chemical stability, which are suitable for delivery systems that require highly efficient encapsulation, long-term storage/isolation without leakage, and no need for molecular exchange in the delivery process. The polymerization and consolidation of polymers are appropriate methods for dense shell formation. Some active encapsulants with poor chemical stability are commonly encapsulated in delivery systems with dense shells. For example, an encapsulation ratio of 94.5% of the thermal energy storage system was developed to encapsulate N-hexadecane by hybrid and sense polymer shells of polyurea and poly(methyl methacrylate).106 Delivery systems with dense shells also play a key role in mitigating volatile materials.132 A typical example is the fragrance-in-water emulsion-assisted microcapsules, in which a dense shell was fabricated by copolymerizing trimethylolpropane ethoxylate triacrylate and PEGDA and achieving an encapsulation efficiency of α-pinene above 95%.83 To avoid the adsorption loss of surfactants for enhancing oil recovery, the microcapsule with a dense shell was a promising delivery system for targeting the delivery of surfactant to the residual oil.13 Delivery systems with dense and stable shells are also necessary to protect the structure and activity of proteins from the negative influence of the gastrointestinal environment. By introducing hydrogen bonds between molecules to construct the stable shell, a high encapsulation efficiency of up to 98% was achieved, and the stability and bioavailability of phycocyanin were improved.110 The dense shell thickness is an important parameter for mechanical properties and can be adjusted by microfluidics precursor concentration, flow rate, and viscosity. Homogeneous shell thickness is conducive to maintaining mechanical stability but requires higher osmotic pressure for encapsulant release, which may not be suitable for certain biomedical applications. An inhomogeneous dense shell was fabricated by adjusting the flow rate ratio of the middle and inner phases.133 Under low osmotic pressure conditions, the weakest spot of the inhomogeneous shell swells and eventually ruptures, allowing for encapsulant release.
Semipermeable microcapsules with porous shells are commonly used to selectively encapsulate active materials and ensure the interaction between the internal and the surrounding environment, especially for cell encapsulation. Porous membranes could be fabricated by inner droplets as templates, then creating pores in the shell or directly forming a porous shell.137,138 The permeability of the porous shell is determined by the porosity and the size of pores, which allow transporting the smaller materials compared to the pore size. For example, using butyl-acetate as a porogen, semi-permeable biocompatible microcapsules with pore diameters below 30 nm were fabricated through polymerization-induced phase separation, which could encapsulate proteins and enzymes larger than 32.7 kDa and allow for the permeation of smaller molecules.139 Using polyethylene oxide as the porogen, β-cell with high cell viability (>90%) was encapsulated in the delivery system with a porous alginate shell, which protected β cells from immune rejection and allowed the exchange of small molecular nutrients during transplantation.140 The commonly used porogens are hydrocarbon waxes, carbohydrates, gelatin, and sugars.141 However, caution must be exercised to remove the porogen to prevent negative effects on morphology.89 Therefore, permanent geometric templates and self-assembling dendrimer-dye complexes have been introduced to enable the production of monodisperse porous microspheres with well-defined pores.141 It is difficult for porous delivery systems to combine permeability, selectivity, and mechanical stability. To balance these three features, a phase-inversion technique was introduced to fabricate a strong and permeable porous shell. An example is the asymmetric graded macroporous shell designed by Wu et al., and in their report, the microcapsules unbuckle slowly and recover a spherical shape in high osmotic pressure (PEG-6000, 0.1 mol L−1).134
The colloidal particles adsorbed to emulsion templates are connected into a densely packed colloidal particle shell, which could be termed a colloidal microcapsule.142,143 Delivery systems with colloidal particle shells are preferred for encapsulating active materials that require ultrahigh porosity, a large contact surface area, and internal structural control.144 Inorganic and organic nanoparticles could be used to form colloidal shells, whose pore size could be studied by monitoring dye molecules with different hydrodynamic diameters.145,146 The permeability of the colloidal shell can be controlled through the radii of the coating particles and post-fabrication treatment.147 The pore size between particles is about 10% of the radius of particles.148 The pore size is usually more than 50 nm by colloidal self-assembly or phase separation of polymers, while the microphase separation of block polymers provides a pore size of 5–50 nm.149–151 Encapsulants, which are smaller in size, can diffuse through these shells. For instance, a porous shell made of 20 nm shellac nanoparticles crosslinked by telechelic polymers can allow for the transport of 1 nm rhodamine B while encapsulating 60 nm particles in the cores.152 In particular, when the molecular size is significantly smaller than the pore size, the transport rate of the encapsulants is not affected by the size of the particles that form the shell.153 To enhance the mechanical property of the colloidal shell, low diluent concentrations and densely interconnected particle networks were designed and exhibited a force at a break up to 200 mN.135 Instead of self-assembly to form densely packed colloidal particles, electrostatic interactions were introduced to complex the negatively charged shellac nanoparticles and the positively charged telechelic polymer, thus developing a porous ultrathin shell for selective permeability.152
Polymersomes (also referred to as polymeric vesicles) are vesicles with membranes composed of bilayers of macromolecular amphiphilic block-copolymers, diblock, triblock, graft, and dendritic copolymers.154 Polymersomes serve as delivery systems that exhibit high stability, versatility, and the capacity to simultaneously encapsulate hydrophilic and hydrophobic materials.155 The hydrophilic substances are usually encapsulated in their aqueous lumen while the hydrophobic molecules are encapsulated within their membranes owing to the adjusted chemical composition and thickness of membranes. The thickness of the membrane could be adjusted by tuning the length and composition of the amphiphilic block-co-polymers, ranging from 2 nm to 47 nm.156 The thicker membranes allow for more efficient entrapment of hydrophobic drugs as well as smaller nanoparticles. For example, hydrophobic gold nanoparticles (9 ± 2 nm) were incorporated within the hydrophobic portion of the shell to achieve laser light trigger release, while nanoparticles above 7 nm could not be encapsulated within the membranes of liposomes.157 Compared to liposomes, the dilemma of polymersomes is the modulation of stability and permeability.158 With the precise control of microfluidics, Pluronic L121 polymersomes with stable and semi-permeable properties were fabricated and achieved spatiotemporal control of enzymatic reactions in artificial cell-like, enabling the formation of artificial cell models.136
To simultaneously encapsulate different materials in a single vehicle without cross-contamination, multicompartment microcapsules are good candidates for delivery systems. Multicompartment microcapsules can be categorized as concentric multi-compartments (multi-shells)159 and parallelly multi-compartments (multi-cores).100 For double emulsions, concentric multiple compartments usually refer to the simultaneous encapsulation of the core and shell.160 Multi-core delivery systems could be fabricated by employing multiple-inner channels in a microfluidics device24 (Fig. 5(a) and (b)), or by designing hierarchical and scalable microfluidics devices (Fig. 5(c)). By flowing quantum dots in one inner channel and ferric oxide in the other inner channel, multi-core anisotropic magnetic microcapsules were fabricated, which encapsulated quantum dots and ferric-oxide in separate cores.161 Deng et al. utilized independent droplet streams and adjusted interfacial energies to produce multicompartment liposomes.162 By changing the relevant flow rates of drop maker fluids and thus changing the formation rates and numbers of droplets, microcapsules with controlled array quadruple cores were produced.100 In addition, five separate internal channels in a microfluidics device were used to produce smart microcapsules with five cores.24
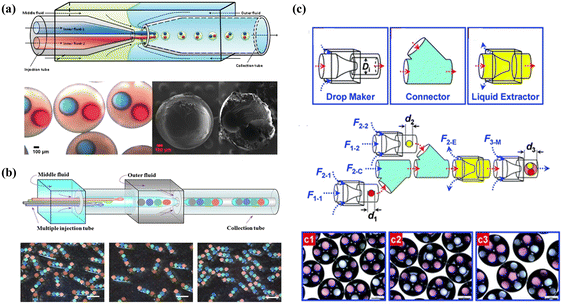 | ||
| Fig. 5 Double emulsion templated microcapsules with multi-cores fabricated using different microfluidics devices. (a) Microcapsules with two components fabricated using two inner channels in a microfluidics device. Reprinted with permission from ref. 114. Copyright 2010, American Chemical Society. (b) Rod-like microcapsules with position-indexed photonic crystal cores fabricated using multiple inner channels in a microfluidics device. Reprinted with permission from ref. 24. Copyright 2012, Springer Nature. (c) Microcapsules with quadruple-component (c1), quintuple-component (c2), and sextuple-compoment (c3) fabricated using different combinations of building blocks. Reprinted with permission from ref. 7. Copyright 2011, the Royal Society of Chemistry. | ||
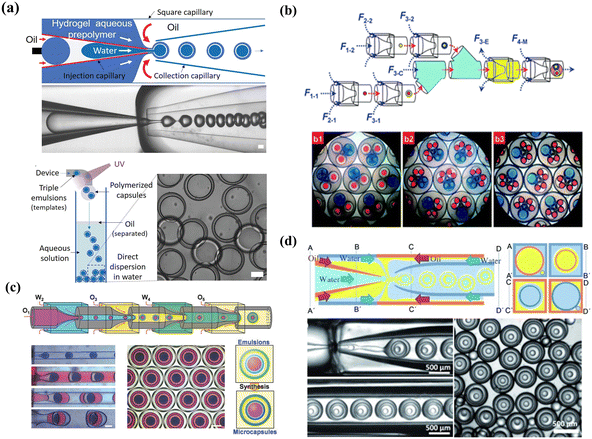 | ||
| Fig. 6 High-order emulsion-templated microcapsules fabricated using different microfluidics devices. (a) W/O/W/O triple emulsion templated microcapsules fabricated using a one-step microfluidics device. Reprinted with permission from ref. 116. Copyright 2020, Wiley-VCH GmbH. (b) O/W/O/W triple emulsion templated microcapsules fabricated using a multi-step microfluidics device with a combination of three building blocks. b1 and b2 are triple emulsions containing two different double emulsions respectively. b3 is triple emulsions containing one single emulsion and two double emulsions. Reprinted with permission from ref. 7. Copyright 2011, the Royal Society of Chemistry. (c) O/W/O/W/O quadruple emulsion templated Trojan-horse-like microcapsules fabricated using a multi-step microfluidics device. Reprinted with permission from ref. 66. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA. (d) W/O/W/O/W quadruple emulsion templated microcapsules fabricated using a one-step microfluidics device and the four cross-sectional schematics (A–A’, B–B’, and C–C’) are included for clarity. Reprinted with permission from ref. 88 Copyright 2011, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
In addition to triple emulsion templates, quadruple emulsion templated microcapsules, especially concentric multi-compartments, were fabricated for efficient encapsulation. Using W1/O2/W3/O4/W5 quadruple emulsions as templates, Lee et al. manufactured multi-shell microcapsules with concentric multi-compartments for encapsulating distinct reagents.164 To enhance encapsulation efficiency, the W1/O2/W3 double-emulsion drops flow through a core stream of the outer core–sheath flow W3/O4, and the O2 and O4 were fabricated by ultrathin shells. Another novel Trojan-horse-like delivery system with concentric multi-compartments was reported by applying one-step quadruple template synthesis, in which nested inner core and outer compartments could separately encapsulate different materials by incorporating two stimulus-responsive functional shells into their inner and outer aqueous layers.66
3.2 Smart microcapsules for target delivery
Physicochemical properties-mediated passive target. Physicochemical property-mediated passive target could be adjusted by size,165,166 charge,167 shape,168 and rigidity169 of carriers to achieve local accumulation at the target sites. Optimization of these parameters helps to enhance targeting accumulation and maximize delivery efficacy.167 The primary factor is the carrier size, especially for the enhanced permeability and retention (EPR) effect in drug delivery,170 which limits the size of drug carriers to 10–200 nm.171 The size could be tuned by the microfluidics-assisted flow rate, flow ratio, concentration, or molecular weights of the precursors.166 Carriers of varying sizes exhibit distinct targeting efficiencies, leading to different tissue distribution and localization patterns.172 For example, the smaller carriers (5–15 nm) were cleared using follicular dendritic cells within 48 hours, while the larger carriers (50–100 nm) could be retained over 5 weeks, resulting in a 175-fold increase in antigen delivery compared to the smaller ones.173 Carriers exceeding 100 nm in size demonstrate enhanced retention effects within blood vessels but exhibit limited penetration within the dense tumor matrix.174 The smaller carrier is the opposite. Therefore, delivery systems with a constant particle size face challenges in achieving both efficient “penetration” and prolonged “retention” simultaneously. To achieve efficient target delivery, delivery systems with variable particle sizes are a promising strategy for effective treatment.175 However, owing to the different targeting conditions, the efficiency of physicochemical property-mediated passive target is not always satisfactory, especially for highly sized heterogeneous human tumors.176 A meta-analysis reported that only an average of 0.7% of the injectable dose was found to be delivered to the tumor177 because physicochemical property-based passive target is not only affected by synthetic properties but also by targeting physiological characteristics. Sykes et al. studied the influence of tumor volume on passive target efficiency through experiments and Monte Carlo simulations.178 The results showed that tumor volume could selectively change the tumor uptake of drug carriers, and the retention efficiency was dominated by the frequency of interaction and Brownian motion for smaller and larger carriers, respectively. Although concerted efforts have been made to optimize the physicochemical properties of carriers, no clear trends have depended on identifying physicochemical parameters that influence targeting efficiency.179
Stimulus-mediated passive target. Additionally, target sites with specific features could be used to design stimulus-mediated passive target of carriers to increase local aggregation by incorporating responsive polymers into delivery systems. Under stimulus conditions, stimulus-mediated passive target can be divided into internal stimulus-mediated passive target and external stimulus-mediated passive target.
Internal stimulus-mediated passive target exploits distinctive characteristics of the tissue microenvironment, such as pH, redox, enzyme, and temperature. The most important microenvironment feature is pH because its differentiation exists at many specific and pathological sites in the human body, such as pH 2 in the stomach, pH 9 in the intestine, pH 6.0–6.8 at the tumor site and pH 7.0–7.4 at the normal tissue, which enable the rationale for drug target administration.85 For instance, to withstand harsh gastrointestinal conditions and prevent premature drug release, hydroxypropyl methylcellulose acetate succinate was incorporated into the middle oil phase to fabricate delivery systems.2 The microcapsules showed no leakage at pH 1.5 and 5.5 and were released rapidly at pH 6.8 and 7.4, achieving targeted treatment of inflammatory bowel disease. In addition, the fast-growing tumor regions are always accompanied by abnormal oxygen tension, and enzyme concentration, which could be treated as the signal of carrier aggregation for passive target.180,181 For instance, using the gradients of oxygen tension and acute hypoxia (less than 1.4% oxygen), hypoxia-targeted carriers were reported by Perche et al. by incorporating azobenzene with hypoxia sensitivity and specificity.182
Alternatively, the fascinating design involves not directly targeting the sites with a specific microenvironment but utilizing it as a stimulus to achieve flexible delivery. An example is stimulus-triggered size transition delivery systems for targeting acidic tumors183 (Fig. 7(a)). When circulating in the blood at a neutral pH, the carrier maintained a size of 80 nm. Upon entering the tumor with a slightly acidic microenvironment, the carriers underwent a dramatic and sharp reduction in size, transitioning to less than 10 nm. Another example involves the creation of “killer” microcapsules. As shown in Fig. 7(b), killer microcapsules are designed to selectively target and destroy targeting particles with the property of copper cations.184
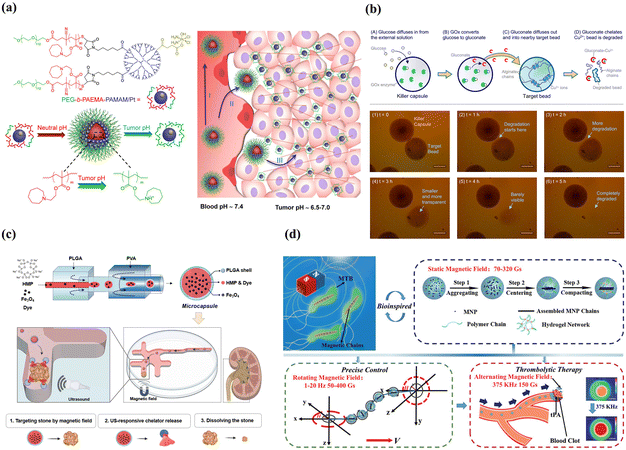 | ||
| Fig. 7 Smart microcapsules with different passive target approaches. (a) Tumor-pH-triggered size transition delivery systems could overcome biological barriers to in vivo drug delivery in poorly permeably pancreatic tumor models. Reprinted with permission from ref. 183. Copyright 2016, American Chemical Society. (b) In the presence of a glucose environment, killer microcapsules with the enzyme glucose oxidase could continuously generate gluconate ions to chelate with copper and selectively attack the particles that were cross-linked by metal ions. Reprinted with permission from ref. 184. Copyright 2016, American Chemical Society. (c) Microcapsules loaded with Fe3O4 nanoparticles and hexametaphosphate exhibited efficient magnetic mobility and targeted urolithiasis-specific sites. Reprinted with permission from ref. 185. Copyright 2023, the Royal Society of Chemistry. (d) Biomimetic spherical microrobot with magneto-collective regulation for targeted thrombolysis. The aligned magnetic nanoparticle chains played the critical role of magnetic sensitivity in the propulsion of the microrobot and amplified the thrombolysis effect in a collective motion. Reprinted with permission from ref. 186. Copyright 2020, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
External stimulus-mediated passive target is designed to respond to externally given stimuli, such as light, magnetic, and ultrasound.187 Light and ultrasound stimuli play a more significant role in triggering release than accumulation at the target site. Therefore, we highlight magnetic-mediated passive target in this section. The most important step for external magnetic-mediated passive target is the incorporation of superparamagnetic nanoparticles, which enable carriers to be manipulated in a switchable magnetic field and provide accurate and efficient target delivery. By injecting chelating solution encapsulated Fe3O4 superparamagnetic nanoparticles into the inner core (Fig. 7(c)), double emulsion templated microcapsules exhibited efficient delivery efficiency (>90%) and target delivered to specific urolithiasis sites for urolithiasis treatment.185 Besides, Fe3O4 nanoparticles could be incorporated into the capsule membrane for smart magnetic targeting.188 An advantage of this approach is that the nanoparticles embedded in the capsule membranes did not diffuse out the membranes even after repeated swelling/shrinking 20 times. Ferrofluid was also used to functionalize the polyelectrolyte microcapsules to achieve magnetic targeting.189 Although delivery systems with Fe3O4 nanoparticles can achieve site-specific targeted delivery, direction-specific delivery is restricted. By taking advantage of microfluidics, microcapsules with eccentric magnetic cores were fabricated, which enabled the microcapsules to control the specific direction via magnetic-guided rotation.190 The magnetic target combined with the responsive property enables the fabrication of multi-stimulus-responsive carriers for accurate delivery. An example was thermo-responsive PNIPAM-shell embedded superparamagnetic Fe3O4 nanoparticles and pH-responsive microcapsules, which showed magnetic-guided targeting performance, self-regulated release according to pathological sites with different pH levels, and controlled thermo-triggered release.188 It is noteworthy that micro/nanorobots driven by magnetic are the most promising systems for targeting delivery given their capacity for remote, precise, and non-invasive maneuvering.191,192 As shown in Fig. 7(d), by incorporating magnetic nanoparticles into the PEGDA and poly(ether imide) prepolymer solution, microrobots with precise magneto-collective control were fabricated.186 With the advantage of magnetic-mediated accurate positioning control (less than 4% deviation), the microrobots navigated precisely to the target sites for ultra-minimal invasive treatment.
The common ligands for active targets include antibodies,194 aptamers,195 proteins,196 peptides197 or small molecules.198 The conjugation of a suitable ligand endows the carriers with an efficient initial attachment and ensures the target delivery.199 For example, by equipping with folate ligands, microcapsules show effective cytotoxic activity for cervical cells, and growth-inhibitory activity and vitro cytotoxicity are 8 times higher than the ligand-unmodified micelle.200 Microcapsules conjugated with estrogens were fabricated to target breast cancer cells expressing estrogen receptors, and the drug uptake results were 13.9 times higher than plain drug.201 Although single-ligand-modified carriers improve the overall target and internalization ability compared with unmodified carriers, their targeting selectivity, uptake ability and transmigration are limited by complex physiological barriers.202–204 One potential solution is to incorporate different types of ligands within a single vehicle to create dual-targeting.205,206 Dual-targeting carriers could be designed to target different receptors on the same cells, thereby improving the targeting selectivity.207 For instance, by incorporating both folic acid and ABX-EGF scFv antibody to decrease off-target, microcapsules are fabricated to enhance siRNA cellular uptake and transfection efficiency. This optimized dual-ligand system exhibits 2.5- and 1.5-fold cellular activity compared to the corresponding single-modified carrier.208 Additionally, dual-ligand targeting systems enhance internalization ability by a synergistic effect for on-target delivery.204 For example, using folic acid as the targeting ligand for tumors and TAT as a penetrating peptide to reduce off-target transport, carriers with combinatorial ligands are fabricated using a microfluidics-assisted flow-focusing device.209 The results demonstrate that dual-targeting carriers achieve a synergistic targeting effect for tumors, which is 3.2 times higher in tumor cell uptake compared to single folic acid-modified carriers. Alternatively, receptor-mediated targeting was integrated with environment-mediated targeting to achieve a dual targeting design for drug delivery,210 as shown in Fig. 8(a).
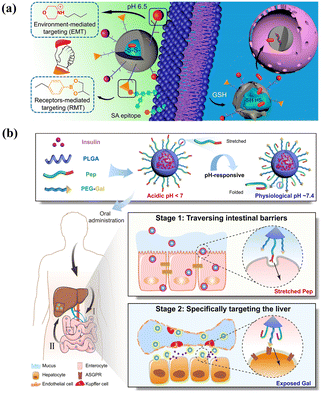 | ||
| Fig. 8 Smart microcapsules with different active targeting approaches. (a) Dual targeting delivery systems engineered with phenylboronic acid and morpholine for receptor-mediated targeting and environment-mediated targeting to enhance tumor-specific sites. Reprinted with permission from ref. 210. Copyright, 2017, American Chemical Society. (b) Ligand-switchable delivery systems with a pH-triggered stretchable cell-penetrating peptide and a hepatic targeting moiety for effective diabetes management. Reprinted with permission from ref. 211. Copyright 2022, Springer Nature. | ||
However, the effectiveness of carriers with dual-ligand targeting systems is not always enhanced owing to the influence of different formulations and the potential mutual interference between diverse ligands.212 It is critical to optimize synergetic combinations and minimize mutual interference between ligands to achieve effective active target delivery.213 One powerful strategy is to adjust varied parameters of ligands, including the density, ratio, and relative length.214 Increasing ligand density does not always result in a higher cellular association, as there exists an optimum number and minimum threshold for a better targeting outcome, which can be determined using particle counting techniques.215,216 Additionally, the ratio and relative length of ligands can influence conformation and mobility and consequently affect their targeting ability. For example, using a flow-focusing microfluidics device, Liu et al. fabricated a combinatorial library of single- and dual-ligand microcapsules to systematically study the effects of ligand targeting efficiency.217 The results showed that the dual ligand did not show a targeting effect using folic acid with a 5 K molecular weight and hyaluronic acid with either 5 K or 10 K, and the synergetic effect was enhanced when using hyaluronic acid with 7 K. An alternative strategy to enhance target selectivity is combining heterogeneous ligands, such as stimulus-responsive actuator,218 copolymer,219 and Janus structures.220 As shown in Fig. 8(b), ligand-switchable carriers were fabricated by introducing cell-penetrating peptides for pH-responsive conformational changes and the Gal moiety to target liver.211 After oral administration, in acidic environments, the peptide maintained a stretched state facilitating efficient transport in the intestine, and upon entering physiological pH, the peptide made a conformational change, which contributed to Gal exposure and promoted hepatic glycogen production by 7.2-fold for insulin therapy.
The microfluidics fluid is dominated by a laminar fluidic pattern, which limits the encounter rate between the target and recognition sites. The microfluidics platforms designed with a high surface/volume ratio, including high-aspect-ratio microchannel chip,222 micropillar array chip,223 micromixing chip,224 and 3D nanoporous chip,225 have been introduced to enhance the encounter rate. For example, a size-dictated immunocapture chip based on deterministic lateral displacement was designed using hydrodynamically optimized triangular micropillars.226 In this microchip (Fig. 9(a)), larger particles exhibited more frequent interactions with micropillars than smaller particles. This facilitated the size-based selection of circulating tumor cells, which are larger than blood cells. Additionally, a clockwise rotation of a triangular micropillar by 15° around its axis amplified adhesion force gradients and diminished hydrodynamic force gradients, which enabled the efficient enrichment of the target cells. To enhance mass diffusion, the herringbone as the microvortex-generator was introduced to design a microvortex mixing chip for efficient tumor cell aggregation (Fig. 9(b)). The herringbone chip showed a capture efficiency of 91.8% for tumor cells, achieving a 26.3% improvement compared to the control.227
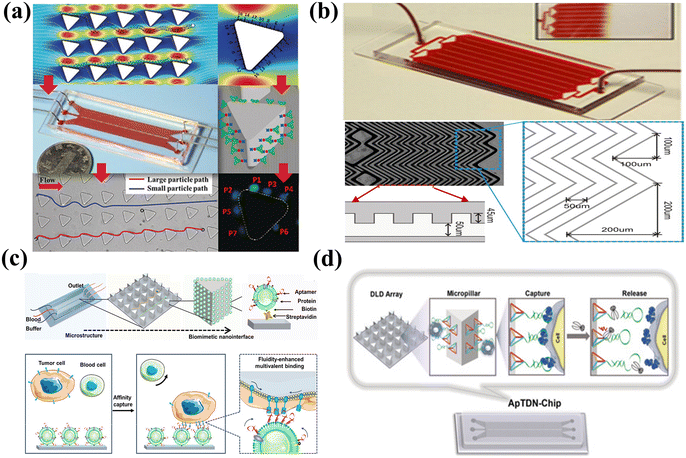 | ||
| Fig. 9 Microfluidics-assisted targeting chips. (a) Size-dictated immunocapture chip with rigorous computational analysis of various parameters and immobilized antibodies on the surface of each micropillar. Reprinted with permission from ref. 226. Copyright 2017, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Herringbone chip with herringbone-induced microvortices disrupted and increased the number of cell–surface interactions in the antibody-coated device. Reprinted with permission from ref. 227. Copyright 2010, Proceedings of the National Academy of Science of the United States of America. (c) Fluidic multivalent microfluidics chip with aptamer-functionalized leukocyte membrane nanovesicles and soft and high-affinity nanointerface for high-performance isolation of circulating tumor cells. Reprinted with permission from ref. 228. Copyright 2020, American Chemical Society. (d) DNA nanolithography in a microfluidics chip decorated with sub-10 nm three-dimensional DNA structures as frameworks with a pendant aptamer at the top vertex for effective recognition. Reprinted with permission from ref. 229. Copyright 2020, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Alternatively, the microfluidics substrate could be modified with recognition molecules to enhance the interface affinity. For instance, aptamer-functionalized leukocyte membrane nanovesicles were combined with a microfluidics chip to achieve enrichment of circulating tumor cells, as shown in Fig. 9(c). The fluidic multivalent nanointerface contributed to high affinity binding with circulating tumor cells and exhibited low absorption of background blood cells.228 Besides, the soft and flexible nanovesicles between the cell and the capture substrate acted as a cushion and could reduce cell damage caused by interfacial collisions. The modified microfluidics chip demonstrated a substantial affinity enhancement of 4 orders of magnitude and exhibited a capture efficiency that is 7 times higher than that of a chip functionalized with monovalent aptamers. Although numerous manufacturing methods have been devised for micro-scale structures, it is still a technical challenge to achieve reproducible preparation of nano-scale structures with highly precise dimensions. The “DNA nanolithography in a microfluidics chip” introduced by Zhang et al. providing an alternative approach for the fabrication and cost issues of microfluidics chips,229 as shown in Fig. 9(d). The sub-10 nm tetrahedral DNA nanostructure-17 was attached to the microfluidics substrate as the rigid framework, and aptamer SYL3C as the recognition element was assembled at the top vertex of the DNA fragments instead of directly attaching the aptamer to the microfluidics substrate. The tetrahedral DNA nanostructures facilitated the upright-oriented anchoring of recognition molecules, avoiding crowding effects and achieving an enhanced accumulation efficiency of up to 60%.
4. Microfluidics-assisted smart microcapsules for controlled release
After arriving at the designated location, the controlled release of encapsulants across the membranes is the necessary step for final delivery. The definition of controlled release provided by the European Directive (3AQ19a) is the distribution of encapsulants at a specified time interval when a particular stimulus is encountered.230 The fundamental mechanisms of controlled release are essential to be studied for designing the release profiles and release procedures. In this chapter, the release mechanisms are used as the starting point to present the controlled release procedures adjusted by the smart whole membrane and smart on/off gates and release kinetics.4.1 Release mechanisms
The release mechanism is of great importance in defining release profiles and even governs the release rate. Several release mechanisms have been introduced,230 as depicted in Table 5. It is essential to note that there is no clear independence between the different release mechanisms and often a combination of these mechanisms establishes the release of encapsulants. Based on different release mechanisms, there are different types of release profiles. According to the release time, the basic release profiles can be classified as burst release and sustained release.231 Through the flexible combination of the two basic release profiles, the programmed sequential release can be designed for controlled release.| Release mechanisms | Release properties |
|---|---|
| Diffusion | The most preponderant mechanism; the concentration gradient as the driving force; the pore size is large enough to allow the encapsulants to transport |
| Dissolution/melting | Membrane-based disintegration, easy to design; starting from outside the carriers and progressing to the inside |
| Disintegration | Membrane-based disintegration, cleavage of cross-links, triggered depolymerization, mechanical-induced degradation |
| Swelling/shrink | Membrane-based permeability alteration even breaks the shell and solvent absorption |
| Osmosis | Membrane-based permeability alteration even breaks the shell, selectively water-permeable of the carrier |
4.2 Smart microcapsules for controlled release
Under specific conditions, stimuli induce conformational transitions in the barrier shells of smart microcapsules at the microscopic level. Subsequently, the membranes amplify the conformational transitions into macroscopically measurable changes in the barrier properties.29 We summarize the common stimulus-triggered release mechanisms, as presented in Table 6. The responsive barrier shell could employ whole membranes or smart “gates” on the shell to achieve controlled release.| Structures | Key materials | Stimuli | Release mechanisms | Ref. |
|---|---|---|---|---|
| W/O/W | Phosphate buffered saline solution/toluene, chloroform, PLGA, gold nanorods/2 wt% PVA | Light | Near infrared-induced melting | 95 |
| W/O | 4 wt% biocide, 40 wt% PEGDA, 1 wt% photoinitiator, fluorescein sodium salt/98 wt% dodecane, 2 wt% EM 90 | Water | Stimulus-induced swelling | 104 |
| W/O/W | Sucrose, 3 wt% PVA /poly(ethylene glycol) divinyl ether, trimethylolpropane tris(3-mercapto propionate), dichloromethane/CaCl2, 10 wt% PVA | Osmotic pressure | Pressure-induced rupture | 133 |
| W/O/W | 5 wt% PVA/poly(phthalaldehyde), chloroform/10 wt% PVA | Fluoride | Stimulus-induced depolymerization | 232 |
| W/O/W | 5 wt% PVA /acrylic acid, methyl methacrylate, 70 vol% chloroform, 30 vol% tetrahydrofuran/10 wt% PVA, 15 wt% tetrahydrofuran | pH | Stimulus-induced dissolution | 233 |
| W/O/W | Zonyl-FSO 100/perfluorohexane Zonyl-FSO 100/10 wt% PVA | Ultrasound | Acoustic vaporization-induced rupture | 234 |
| (W/O)/W/O | Nanoparticles/1% w/v Pluronic F127, NIPAM, N,N-methylene bisacrylamide, 2,2′-azobis(2-amidinopropane) dihydrochloride/soybean oil, 8 w/v% PGPR 90 | Temperature | Stimulus-induced shrinking | 117 |
| W/O/W | 2 w/w% PVA, sodium chloride-poly(N,N-diethylacrylamide), benzophenone, chloroform-2 w/w% PVA, sodium chloride | Temperature | Stimulus-induced dissolution | 235 |
| W/O/W | Glycerol/fatty glycerides/glycerol, PVA | Temperature | Stimulus-induced melting | 114 |
| W/O/W/O | Aqueous solution containing hydrophilic actives/hexadecane/10% PEGDA, 2% PVA, and photoinitiator/2% Span 80, mineral oil | Mechanical pressure | Stimulus-induced rupture | 116 |
| W/O/W | 10 wt% PEG/chloroform, hexane, PEG-b-poly(lactic acid) (PLA), PNIPAM, PLGA, dodecyl thiol-stabilized gold nanoparticles/10 wt% PVA | Light and temperature | Stimulus-induced dissolution and melting | 157 |
| W/O/W | Graphene oxide suspensions/amodimethicone (KF 860), a diamino-modified polymeric silicone, silicone oil/80 wt% glycerol, 1 wt% PVA | Light and magnetic | Light-induced melting | 236 |
| O/W/O | Soybean oil, glutaraldehyde/chitosan, N-isopropylacrylamide, iron(III) chloride/soybean oil, glutaraldehyde | pH, temperature, magnetic | Stimulus-induced swelling and shrinking | 188 |
Chemical changes include the chemical cleavage of cross-links and trigger depolymerization. For example, microcapsules with cytosine-rich shells cross-linked by nucleic acid bridges can undergo cleavage at pH 5.0, leading to shell decomposition and pH-triggered release of encapsulants.237 Using polyphenol tannic acid as the crosslinker to fabricate supramolecular microgel, the resultant smart microgel exhibited pH-responsive burst release owing to deprotonation-induced disintegration at high pH.238 In addition, pH-enzyme-delayed colon-targeting delivery has been produced through shell degradation, in which around 20% of the protein was released in the stomach and small intestinal fluid and about 58% was released in the colon fluid containing β-glucosidase.110 Additionally, carriers with depolymerizable membranes offer tunable trigger release by depolymerizing the shell under the desired stimuli. Using poly(phthalaldehyde) as a depolymerizable polymer, fluoride-responsive microcapsules were fabricated, in which fluoride exposure led to the breaking of end-caps, rapid depolymerization from head-to-tail, and release of encapsulants.232 The nitric oxide (NO) expression increases along with intestinal inflammation and can be used to design NO-responsive delivery by embedding probiotics into poly-γ-glutamic acid microcapsules. As shown in Fig. 10(a), the dissociation of the shell was accompanied by the transformation of NO into dinitrogen trioxide and an irreversible reaction with the cross-linking agent o-phenylenediamine, enabling the rapid release of probiotics in response to NO.239
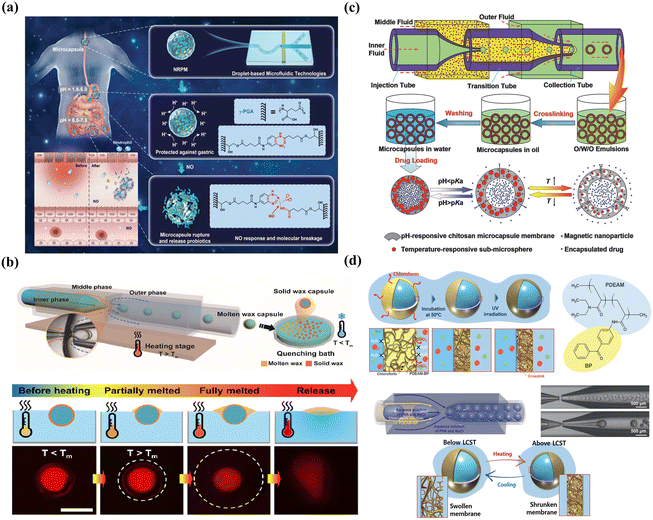 | ||
| Fig. 10 Smart microcapsules with membrane-based controlled release. (a) Smart microcapsules with NO-induced chemical dissociation for burst release. Reprinted with permission from ref. 239. Copyright 2022, Wiley-VCH GmbH. (b) Smart microcapsules with temperature-induced physical melting for burst release. Reprinted with permission from ref. 240. Copyright 2021, American Chemical Society. (c) Smart microcapsules with multiple stimuli (pH, temperature, and magnetic field) for controlled release. Reprinted with permission from ref. 188. Copyright 2014, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (d) Smart microcapsules with membrane-based thermo-responsive permeability alteration for sustained release. Reprinted with permission from ref. 235. Copyright 2021, Wiley-VCH GmbH. | ||
The physical changes, including swelling/deswelling or osmosis-induced rupture, melting/dissolution-induced decomposition, and mechanical-induced fragmentation, offer an alternative strategy for trigger release, avoiding the need for complex shell synthesis with multiple functionalities. For example, hydrogel-based shells with water-triggered release were created, in which water absorption caused the hydrogel to swell by approximately 40%, leading to the release of encapsulated biocide.104 Using the property of osmosis to increase the size of the membrane and thickness, submillimeter capsules with ultrathin shells (0.83–2.80 um) were fabricated and easily compressed to rupture, releasing the encapsulant for cosmetic applications.241 Inspired by the squirting plants ejecting seeds, microcapsules with PNIPAM-based shells were produced and achieved thermo-triggered squirting release, which could shrink and rupture at higher temperatures because of increased internal pressure.117 Using palm oil-based shells, the thermos-responsive carriers exhibited burst release of the aqueous core when the temperature was above the melting point 38 °C,240 as shown in Fig. 10(b). By incorporating shellac polymer into the shell, the microcapsule with pH-triggered release was fabricated because the carboxylic groups were ionized, and the shell eventually dissolved at alkaline pH for targeted intestinal release.67 An interesting design was using thermally induced microcracks to release active materials for fabricating amino-functionalized membranes applied in heavy metal ion removal.242
Traditional single stimulus-triggered microcapsules often exhibit burst release, which may not be suitable for certain applications, such as oral administration. It is crucial to develop microcapsules with multiple stimuli. This can be achieved by incorporating multiple stimulus-responsive materials or particles into the polymeric shell. For instance, photo- and thermo-responsive polymersomes were created by embedding photothermal gold nanoparticles into a thermosensitive polymeric membrane.157 Similarly, multi-stimulus-responsive microcapsules were fabricated by first constructing a pH-responsive chitosan crosslinked membrane and then incorporating magnetic nanoparticles and acrylamide sub-microspheres into it, as shown in Fig. 10(c).188
Directly fabricating the membrane as a diffusion barrier can affect the diffusion rate of the encapsulants and thus achieve sustained release. Notably, adjusting the shell thickness is a simple method for controlling sustained release. For example, by adjusting the concentration of monomer, the thickness was adjusted from 70 to 150 nm, and the period of sustained release was controlled from 3 to 5 months.244 Biodegradable shells, such as biodegradable materials PLA,245 PLGA,244 and paclitaxel,246 were also used to control the shell thickness and thus affected the diffusion rate of the encapsulants for sustained release. During degradation, local environmental conditions, such as pH, influence the degradation rate and need to be considered.247 It is worth mentioning that the core component can increase the diffusion path and thus prolong the release time of encapsulated substances. As reported by Kim et al., microcapsules with a hydrogel core exhibited no release for the initial few minutes, while the control group without a hydrogel core immediately released half of the encapsulants in 35 s.248 Owing to the outstanding ability of water adsorption, the hydrogel is a good candidate for the membrane material for sustained release.249 Although the inherent network structures of hydrogels with large mesh sizes are capable of molecular exchanges, hydrogel microcapsules are poorly suited for the release of small molecules for sustained release. To solve this question, Hu et al. fabricated microcapsules with two distinct layers of shells, achieving remarkable slow release for the hydrophilic small molecule rhodamine 6G.250
The combination of functional materials with stimulus-induced conformational change and permeability alteration of membrane endows carriers with the tunable cut-off threshold for size-selective permeation. The permeability could be changed by recognizing different stimuli, such as pH,251 temperature,188 and light.252 As shown in Fig. 10(d), using poly(N,N-diethylacrylamide) with volume phase transition temperature (VPTT) property as the middle phase, the degree of swelling gradually decreased as the temperature increased, which made a collapsed network with a small mesh size and low permeability.235 Quantitatively, the mesh size of the membrane was between 2.8 nm and 4.6 nm at 4 °C and 25 °C owing to the temperature-dependent change in permeability, respectively. The cutoff threshold of the membrane can be controlled by adjusting the molar mass of the functional monomer.253 In theory, factors affecting the change in VPTT could be used to control the swelling and shrinking of thermo-responsive polymers and thus control permeability. Based on this, glucose-responsive microcapsules were produced. Zhang et al. adopted 3-acrylamidophenylboronic acid acting as the glucose sensor, and the charged form was capable of stable complex formation with glucose, which changed the dissociation equilibrium and shifted the VPTT of PNIPAM to a higher temperature, resulting in glucose-induced swelling.78 Alternatively, by pH-induced protonation/deprotonation of the polyelectrolytes, swelling/shrinking changes in the membrane could be responsible for controlling permeability.254 An interesting design was to use polyacrylic acid with a pH-responsive swelling property to fabricate a booster chamber that provided a driving force for the release of the drug chamber.255
The greatest advantage of membrane-based permeability alteration is that it can avoid the irreversible change and structural damage of shells compared to membrane-based rupture, which is of great importance when designing reversibly and dynamically tunable release. As shown in Fig. 11, using thiol-ene polymerization, poly(anhydride) microcapsules were fabricated and then hydrolyzed in its aqueous environment, yielding cross-linked poly(acid) microcapsules with tethered carboxylic acids, which rendered the microcapsules with pH-responsive property. More importantly, the deprotonation at high pH of the anhydride increased the mesh size and hydrophilicity of the membrane, increasing the permeability and leading to the membrane's nondestructive and reversible swelling.256,257 In addition, the dynamic membrane could switch numerous times between impermeable and permeable even after drying in the vacuum at room temperature.
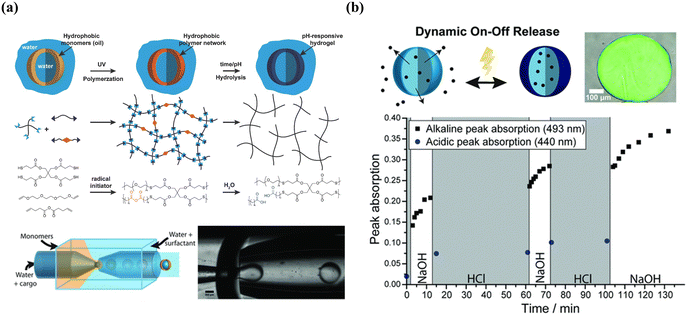 | ||
| Fig. 11 Microcapsules with reversible permeability switching for sustained release. (a) Mechanism of thiol-ene-based membrane and microfluidic-assisted production of poly(acid) microcapsules (b) illustration of dynamic on–off release and time-resolved peak absorption in acidic and alkaline conditions. Reprinted with permission from ref. 256. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Typically, programmed sequence release could be achieved by engineering smart microcapsules with a core–shell structure or multi-compartment combined with different release mechanisms. As shown in Fig. 12(a), nano-in-micron microcapsules with burst-sustained release were designed by encapsulating free drugs in the shell and drug-loaded nanoparticles in the core.2 The free drug could be rapidly released owing to the decomposition of the chitosan shell, while the drug-loaded PLGA nanoparticles provided a second and sustained release owing to PLGA degradation. Another approach to sequence release is utilizing multi-compartment microcapsules. Xu et al. developed pH- and temperature-responsive microcapsules with hydrophobic contents in the shell and hydrophilic contents in the core, achieving sequential release along with different stimuli.160 Considering the smart microcapsules with multi-compartment for programmed sequence release, capsule-in-capsule structures (outer chitosan shell and inner PEGDA shell) were fabricated and achieved the first acid-triggered burst release and followed sustainable release.66 Similarly, a polymersome-in-polymersome with a PEG-b-PLA diblock-copolymer bilayer was produced to achieve programmable release. The use of a PLA-homopolymer-loaded bilayer as the outer membrane allows for the sequential rupturing of membranes from the innermost to the outermost, controlling the release of core materials.154 Incorporating PLA homopolymers into the bilayer increases mechanical and chemical stability, preventing membrane rupture even under high osmotic shock. By the advantage of Janus microparticles created with complicated structures by phase separation, multiphasic Janus systems with different degradation properties achieved zonal drug loading and programmed release. As shown in Fig. 12(b), phase transition materials were introduced into different inlets to fabricate droplets and followed changing structures by adjusting the interfacial tension in the microfluidics system, which enabled programmed degradation and release.96
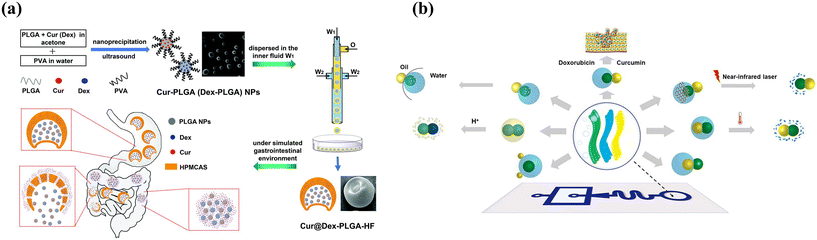 | ||
| Fig. 12 Combined release profiles for programmed sequential release. (a) Microcapsules with a core–shell structure for sequential burst-sustained drug release from different compartments. Reprinted with permission from ref. 2. Copyright 2021, Royal Society of Chemistry. (b) Multiphasic Janus microparticles fabricated by applying the microfluidics phase-separation method to complicated structures to achieve programmed degradation and release. Reprinted with permission from ref. 96. Copyright 2022, Elsevier B.V. | ||
Depending on whether the gate materials are incorporated after or during membrane formation, the fabrication approaches for smart gates can be classified into two classifications: “grafting” techniques and “blending” techniques. Although a concentrated effort has been made to highlight smart gating membranes by bulk grafting or blending,259–261 only few articles have focused on microfluidics-assisted smart gating membranes for controlled release. One point that needs to be clarified in advance is that porous microcapsules are not the focus of this section although they may be referred to as smart gating porous particles in other papers.31 Considering that the bulk blending process could be segregated in confined spaces, such as a thin middle layer of emulsion droplets fabricated by microfluidics, it would be possible to construct microcapsules with smart gates. An example is the microfluidically prepared W/O/W emulsion templated smart microcapsules with molecular polarity- and temperature-dependent permeability fabricated by Kim et al.262 By blending a ternary mixture of dodecanol, lauryl acrylate (LA), and trimethylolpropane ethoxylate triacrylate (ETPTA) as the middle oil phase, LA and ETPTA formed a polymeric framework upon photopolymerization, while continuous voids were filled with liquid dodecanol. Continuous dodecanol worked as the smart gate and selectively allowed molecular soluble in molten dodecanol to diffuse across the membrane when above the melting point of dodecanol. Similarly, using dodecanol continuous nanochannels to serve as smart gates for transmembrane transport (Fig. 13), smart microcapsules showed a high performance of photothermal heating upon near-infrared laser irradiation, attributed to polydopamine nanoparticles in the core and achieved on-demand drug release.263
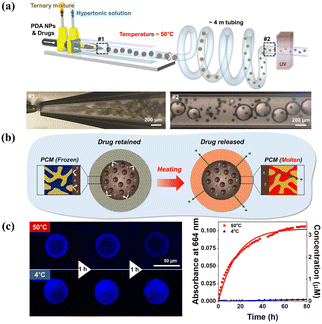 | ||
| Fig. 13 Microfluidics-assisted microcapsule membrane with smart on/off gates for controlled release. (a) Schematic illustration and microfluidics device for the synthesis of smart microcapsules with on/off gates. (b) Schematic for the smart gates for on/off state responsive to temperature to achieve controlled release. (c) Confocal laser scanning microscope images and absorbance at 664 nm of methylene blue showing the release at 50 °C and no release at 4 °C. Reprinted with permission from ref. 263. Copyright 2023, American Chemical Society. | ||
4.3 Release kinetics
By investigating the release kinetics, it is possible to control and design the optimal release of encapsulants for target delivery. Various factors influence the release kinetics of microcapsules, including production conditions and morphology. For example, different production methods, such as microfluidics and bulk fabrication, lead to distinct release kinetics. Microfluidics-made microcapsules exhibit slower initial bursts and release rates compared to conventionally made microcapsules owing to their uniform size distribution.103 Size and size distribution also play significant roles. Larger microcapsules with a fine size distribution show reduced initial bursts and longer release processes compared to smaller ones, attributed to longer diffusion routes and smaller surface-to-volume ratios.264,265 Furthermore, the structure and morphology affect the release kinetics. Core–shell microcapsules exhibit higher initial release rates compared to single-layer microparticles with the same encapsulated diameter likely owing to the larger surface area-to-volume ratio of core–shell structure.108 Different compartments within microcapsules exhibit varying release kinetics, with slower release rates observed in interior compartments compared to the outer shell, influenced by physical osmotic pressure and diffusion distance.160 Additionally, the configurations of microcapsule shells, such as the thickness and surface coverage of pores, affect release kinetics, with thicker membranes and smaller pore surface coverage exhibiting higher burst releases and shorter release times.85Mathematical models are extremely helpful for release kinetics because they can predict the release process before the target sites and measure important physical parameters, which makes them widely implemented in different target delivery kinetics.266 Various mathematical equations are used to describe the kinetic release of active materials, as shown in Table 7. Among these kinetic equations, zero-order,267 first-order,268 and Higuchi models269 are the most commonly used. For instance, alginate microcapsules encapsulating the citral exhibited a release profile well described by the first-order model, enabling sustained release.76 Biodegradable microcapsules with hydrophilic bioactives exhibited long-term release as the membranes degraded, fitted with a biexponential function.244 However, owing to the complexity and susceptibility to relevant factors, no single equation is universally accepted to accurately describe release kinetics.
5. Conclusions and outlooks
In conclusion, for a smart microcapsule, an important step is not only the selection of an encapsulation carrier for the entrapment according to final applications but also the target delivery and controlled release, including sites and modes of release. Droplet-based microfluidics provides the most effective approach for fabricating microcapsules owing to its precisely manipulating fluids on the low-energy-demand micrometer scale. In this review, we discussed microfluidics-assisted microcapsules from droplet fabrication and carrier systems to target delivery and controlled release. Despite significant progress made in exploring smart microcapsules, further efforts are needed to endow microcapsules with high throughput productivity, excellent mechanical properties, outstanding active targeting functionality, and manageable release performance.First, achieving “smart targeting” stands as a crucial aspect of microcapsule target delivery. Although considerable efforts have focused on enhancing targeting efficiency through external forces, such as magnetic or electronic fields, relatively limited emphasis has been placed on active targeting via host–guest or ligand–receptor chemistry. External stimuli, such as gradient diffusion in concentration or temperature, also hold promise for inducing target delivery. However, there are very few examples of “robot-based” delivery, leveraging intelligence for target localization and cargo delivery. Thus, the integration of artificial intelligence is imperative to streamline robot design and fortify the fabrication of robot-based delivery strategies. Moreover, machine learning stimulates the design and screen ligands to facilitate target delivery efficiently.
Second, the realm of production rate enhancement presents substantial opportunities. Despite advancements in microfluidics techniques, such as parallel or multi-layer devices, bolstering the production rate of smart microcapsules remains constrained, particularly in practical fields such as enhanced oil recovery due to extensive particle demand. Moreover, the current high throughput devices mostly increase the single emulsion templated microcapsule production rate, which is somehow limited by “smart” performance owing to shell material compromise. There are few examples in the literature of industrial applications to generate multi-compartment microcapsules or microparticles using high-throughput devices. Leveraging computer science and trainable statistical models, machine learning has emerged as a pivotal tool for prognosticating droplet generator performance and flow patterns based on design parameters. This capability curtails expensive design iterations and bridges the knowledge gap between experts and end-users. Machine learning exhibits immense potential in automating microfluidics design, optimizing operations, and facilitating the scaling up of production systems, thereby achieving high-throughput production.
Finally, to heighten loading efficiency, a promising solution involves combining droplet-based microfluidics technology with diverse self-assembly methods, such as supramolecular host–guest chemistry. Furthermore, prevailing studies on microcapsules equipped with smart gates predominantly depend on multi-step synthesis processes, which often compromise encapsulation efficiency. Alternatively, the combination of bulk nanoparticles with microfluidics-assisted emulsification has substantial potential for target delivery and quantitative release. Overall, the fabrication of smart microcapsules exhibiting active target performance, possibly crafted into artificial intelligence particles, envisages various practical applications.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors thank the National Natural Science Foundation of China Project (ZX20210340), National Talent Support Project (ZX20220351), Shandong Province Innovative Team and Talent Project (ZX20230148) and Research start-up funding projects of China University of Petroleum (East China) (20210085) for financial support. The support from the Oil and Gas Field Chemistry Institute, School of Petroleum Engineering, China University of Petroleum (East China) is also appreciated.References
- S. S. Datta, A. Abbaspourrad, E. Amstad, J. Fan, S. Kim, M. Romanowsky, H. C. Shum, B. Sun, A. S. Utada, M. Windbergs, S. Zhou and D. A. Weitz, Adv. Mater., 2014, 26, 2205–2218 CrossRef CAS PubMed.
- J. Jiang, J. Xiao, Z. Zhao, M.-S. Yuan and J. Wang, Mater. Chem. Front., 2021, 5, 6027–6040 RSC.
- T. Moragues, D. Arguijo, T. Beneyton, C. Modavi, K. Simutis, A. R. Abate, J. C. Baret, A. J. deMello, D. Densmore and A. D. Griffiths, Nat. Rev. Methods Primers, 2023, 3, 1 CrossRef.
- L. Shang, Y. Cheng and Y. Zhao, Chem. Rev., 2017, 117, 7964–8040 CrossRef CAS PubMed.
- W. Wang, B.-Y. Li, M.-J. Zhang, Y.-Y. Su, D.-W. Pan, Z. Liu, X.-J. Ju, R. Xie, Y. Faraj and L.-Y. Chu, Chem. Eng. J., 2023, 452, 139277 CrossRef CAS.
- T. Takei, Y. Yamasaki, Y. Yuji, S. Sakoguchi, Y. Ohzuno, G. Hayase and M. Yoshida, J. Colloid Interface Sci., 2019, 536, 414–423 CrossRef CAS PubMed.
- W. Wang, R. Xie, X.-J. Ju, T. Luo, L. Liu, D. A. Weitz and L.-Y. Chu, Lab Chip, 2011, 11, 1587–1592 RSC.
- A. G. Skirtach, A. M. Yashchenok and H. Möhwald, Chem. Commun., 2011, 47, 12736–12746 RSC.
- T. Li, D. Teng, R. Mao, Y. Hao, X. Wang and J. Wang, J. Biomed. Mater. Res., Part A, 2019, 107, 2371–2385 CrossRef CAS PubMed.
- J. L. de Oliveira, L. F. Fraceto, A. Bravo and R. A. Polanczyk, J. Agric. Food Chem., 2021, 69, 4564–4577 CrossRef CAS PubMed.
- G. Kowalska, J. Rosicka-Kaczmarek, K. Miśkiewicz, M. Zakłos-Szyda, S. Rohn, C. Kanzler, M. Wiktorska and J. Niewiarowska, Nutrients, 2022, 14, 2529 CrossRef CAS PubMed.
- J. S. Ribeiro and C. M. Veloso, Food Hydrocolloids, 2021, 112, 106374 CrossRef CAS.
- Z. Fang, X.-R. Cao, Y.-L. Yu and M. Li, Colloids Surf., A, 2019, 570, 282–292 CrossRef CAS.
- B. Zhou, W. Kang, H. Jiang, H. Yang, Z. Li, Z. Lv, Z. Xu, C. Ning, H. Wang and S. Xie, J. Pet. Sci. Eng., 2022, 219, 111122 CrossRef CAS.
- W. Li, L. Zhang, X. Ge, B. Xu, W. Zhang, L. Qu, C.-H. Choi, J. Xu, A. Zhang, H. Lee and D. A. Weitz, Chem. Soc. Rev., 2018, 47, 5646–5683 RSC.
- A. Gonzalez Gomez and Z. Hosseinidoust, ACS Infect. Dis., 2020, 6, 896–908 CrossRef CAS PubMed.
- P. Velmurugan, V. Ganeshan, N. F. Nishter and R. R. Jonnalagadda, Surf. Interfaces, 2017, 9, 124–132 CrossRef CAS.
- M. Mamusa, C. Sofroniou, C. Resta, S. Murgia, E. Fratini, J. Smets and P. Baglioni, ACS Appl. Mater. Interfaces, 2020, 12, 28808–28818 CrossRef CAS PubMed.
- S. Natour and R. Abu-Reziq, RSC Adv., 2014, 4, 48299–48309 RSC.
- H. O. Otor, J. B. Steiner, C. García-Sancho and A. C. Alba-Rubio, ACS Catal., 2020, 10, 7630–7656 CrossRef CAS.
- Z. Liu, F. Fontana, A. Python, J. T. Hirvonen and H. A. Santos, Small, 2019, 16, 1904673 CrossRef PubMed.
- Q. Zhang, N. F. Inagaki, H. Yoshida, M. Kamihira, Y. Sakai and T. Ito, J. Membr. Sci., 2024, 689, 122119 CrossRef CAS.
- N. Teo, C. Jin, A. Kulkarni and S. C. Jana, J. Colloid Interface Sci., 2020, 561, 772–781 CrossRef CAS PubMed.
- Y. Zhao, Z. Xie, H. Gu, L. Jin, X. Zhao, B. Wang and Z. Gu, NPG Asia Mater., 2012, 4, e25–e25 CrossRef.
- X. Huang and B. Voit, Polym. Chem., 2013, 4, 435–443 RSC.
- N. K. Preman, R. R. Barki, A. Vijayan, S. G. Sanjeeva and R. P. Johnson, Eur. J. Pharm. Biopharm., 2020, 157, 121–153 CrossRef CAS PubMed.
- G. G. Abdo, M. M. Zagho and A. Khalil, Emergent Mater., 2020, 3, 407–425 CrossRef CAS.
- A. Rezaei, F. Rafieian, S. Akbari-Alavijeh, M. S. Kharazmi and S. M. Jafari, Adv. Colloid Interface Sci., 2022, 307, 102728 CrossRef CAS PubMed.
- D. Wandera, S. R. Wickramasinghe and S. M. Husson, J. Membr. Sci., 2010, 357, 6–35 CrossRef CAS.
- X. Ju and L. Chu, R. Soc. Chem, 2019, 255–296 CAS.
- K. Thananukul, C. Kaewsaneha, P. Opaprakasit, N. Lebaz, A. Errachid and A. Elaissari, Adv. Drug Delivery Rev., 2021, 174, 425–446 CrossRef CAS PubMed.
- P. Zhu and L. Wang, Lab Chip, 2016, 17, 34–75 RSC.
- J. Guerrero, Y. Chang, A. A. Fragkopoulos and A. Fernandez-Nieves, Small, 2019, 16, e1904344 CrossRef PubMed.
- A. Kamnerdsook, E. Juntasaro, N. Khemthongcharoen, M. Chanasakulniyom, W. Sripumkhai, P. Pattamang, C. Promptmas, N. Atthi and W. Jeamsaksiri, RSC Adv., 2021, 11, 35653–35662 RSC.
- F. Fontana, M. P. A. Ferreira, A. Correia, J. Hirvonen and H. A. Santos, J. Drug Delivery Sci. Technol., 2016, 34, 76–87 CrossRef CAS.
- D. R. Link, S. L. Anna, D. A. Weitz and H. A. Stone, Phys. Rev. Lett., 2004, 92, 054503 CrossRef CAS PubMed.
- T. M. Tran, F. Lan, C. S. Thompson and A. R. Abate, J. Phys. D: Appl. Phys., 2013, 46, 114004 CrossRef.
- A. S. Utada, A. Fernandez-Nieves, H. A. Stone and D. A. Weitz, Phys. Rev. Lett., 2007, 99, 094502 CrossRef PubMed.
- W.-A. C. Bauer, M. Fischlechner, C. Abell and W. T. S. Huck, Lab Chip, 2010, 10, 1814–1819 RSC.
- D. Li, X. Li, C. Chen, Z. Zheng and H. Chang, Sens. Actuators, B, 2018, 255, 1048–1056 CrossRef CAS.
- A. M. Nightingale, S. H. Krishnadasan, D. Berhanu, X. Niu, C. Drury, R. McIntyre, E. Valsami-Jones and J. C. deMello, Lab Chip, 2011, 11, 1221–1227 RSC.
- P. N. Nge, C. I. Rogers and A. T. Woolley, Chem. Rev., 2013, 113, 2550–2583 CrossRef CAS PubMed.
- V. Narayanamurthy, Z. E. Jeroish, K. S. Bhuvaneshwari, P. Bayat, R. Premkumar, F. Samsuri and M. M. Yusoff, RSC Adv., 2020, 10, 11652–11680 RSC.
- A. Olanrewaju, M. Beaugrand, M. Yafia and D. Juncker, Lab Chip, 2018, 18, 2323–2347 RSC.
- W. J. Duncanson, T. Lin, A. R. Abate, S. Seiffert, R. K. Shah and D. A. Weitz, Lab Chip, 2012, 12, 2135–2145 RSC.
- M. P. Wolf, G. B. Salieb-Beugelaar and P. Hunziker, Prog. Polym. Sci., 2018, 83, 97–134 CrossRef CAS.
- A. Shakeri, S. Khan and T. F. Didar, Lab Chip, 2021, 21, 3053–3075 RSC.
- R. K. Shah, H. C. Shum, A. C. Rowat, D. Lee, J. J. Agresti, A. S. Utada, L.-Y. Chu, J.-W. Kim, A. Fernandez-Nieves, C. J. Martinez and D. A. Weitz, Mater. Today, 2008, 11, 18–27 CrossRef CAS.
- Q. Xu and J. Jiang, ACS Appl. Polym. Mater., 2020, 2, 3576–3586 CrossRef CAS.
- J. Zhou, D. A. Khodakov, A. V. Ellis and N. H. Voelcker, Electrophoresis, 2012, 33, 89–104 CrossRef CAS PubMed.
- S. C. Kim, D. J. Sukovich and A. R. Abate, Lab Chip, 2015, 15, 3163–3169 RSC.
- N. Bodin-Thomazo, F. Malloggi and P. Guenoun, RSC Adv., 2017, 7, 46514–46519 RSC.
- T. M. Tran, F. Lan, C. S. Thompson and A. R. Abate, J. Phys. D: Appl. Phys., 2013, 46, 114004 CrossRef.
- L. Montazeri, S. Bonakdar, M. Taghipour, P. Renaud and H. Baharvand, Lab Chip, 2016, 16, 2596–2600 RSC.
- A. R. Abate, D. Lee, T. Do, C. Holtze and D. A. Weitz, Lab Chip, 2008, 8, 516–518 RSC.
- S. Hwang, C. H. Choi and C. S. Lee, Macromol. Res., 2012, 20, 422–428 CrossRef CAS.
- D.-K. Lee, S. Y. Choi, M. S. Park and Y. H. Cho, Appl. Phys. A: Mater. Sci. Process., 2018, 124, 192 CrossRef CAS.
- D. R. Link, S. L. Anna, D. A. Weitz and H. A. Stone, Phys. Rev. Lett., 2004, 92, 054503 CrossRef CAS PubMed.
- Z. Bai, B. Wang, H. Chen and M. Wang, Sens. Actuators, B, 2015, 215, 330–336 CrossRef CAS.
- N. Leister, G. T. Vladisavljević and H. P. Karbstein, J. Colloid Interface Sci., 2022, 611, 451–461 CrossRef CAS PubMed.
- Z. Chen, Z. Lv, Z. Zhang, D. A. Weitz, H. Zhang, Y. Zhang and W. Cui, Exploration, 2021, 1, 20210036 CrossRef PubMed.
- A. R. Abate, A. Poitzsch, Y. Hwang, J. Lee, J. Czerwinska and D. A. Weitz, Phys. Rev. E, 2009, 80, 026310 CrossRef CAS PubMed.
- J. K. Nunes, S. S. H. Tsai, J. Wan and H. A. Stone, J. Phys. D: Appl. Phys., 2013, 46, 114002 CrossRef PubMed.
- S. Seiffert, J. Thiele, A. R. Abate and D. A. Weitz, J. Am. Chem. Soc., 2010, 132, 6606–6609 CrossRef CAS PubMed.
- M. Seo, C. Paquet, Z. Nie, S. Xu and E. Kumacheva, Soft Matter, 2007, 3, 986–992 RSC.
- C. Mou, W. Wang, Z. Li, X. Ju, R. Xie, N. Deng, J. Wei, Z. Liu and L. Chu, Adv. Sci., 2018, 5, 1700960 CrossRef PubMed.
- Z. Sun, C. Yang, M. Eggersdorfer, J. Cui, Y. Li, M. Hai, D. Chen and D. A. Weitz, Chin. Chem. Lett., 2020, 31, 249–252 CrossRef CAS.
- L. Zhang, L.-H. Cai, P. S. Lienemann, T. Rossow, I. Polenz, Q. Vallmajo-Martin, M. Ehrbar, H. Na, D. J. Mooney and D. A. Weitz, Angew. Chem., Int. Ed., 2016, 55, 13470–13474 CrossRef CAS PubMed.
- L. Shang, Y. Cheng, J. Wang, H. Ding, F. Rong, Y. Zhao and Z. Gu, Lab Chip, 2014, 14, 3489–3493 RSC.
- H. Zhang, L. Zhang, C. An, Y. Zhang, F. Shao, Y. Gao, Y. Zhang, H. Li, Y. Zhang, C. Ren, K. Sun, W. He, F. Cheng, H. Wang and D. A. Weitz, Biofabrication, 2022, 14, 035015 CrossRef PubMed.
- T. Femmer, A. Jans, R. Eswein, N. Anwar, M. Moeller, M. Wessling and A. J. C. Kuehne, ACS Appl. Mater. Interfaces, 2015, 7, 12635–12638 CrossRef CAS PubMed.
- D. M. Headen, J. R. García and A. J. García, Microsyst. Nanoeng., 2018, 4, 1–9 CrossRef PubMed.
- S. Shin, S. Cho, R. Song, H. Kim and J. Lee, Chem. Eng. J., 2023, 471, 144734 CrossRef CAS.
- J. H. Xu, S. W. Li, J. Tan, Y. J. Wang and G. S. Luo, Langmuir, 2006, 22, 7943–7946 CrossRef CAS PubMed.
- A. S. Utada, E. Lorenceau, D. R. Link, P. D. Kaplan, H. A. Stone and D. A. Weitz, Science, 2005, 308, 537–541 CrossRef CAS PubMed.
- W. Ma, C. Mou, S. Chen, Y. Li and H. Deng, Polymer, 2022, 14, 1165 CAS.
- G. Chen, C. H. Niu, M.-Y. Zhou, X.-J. Ju, R. Xie and L.-Y. Chu, J. Colloid Interface Sci., 2010, 343, 168–175 CrossRef CAS PubMed.
- M.-J. Zhang, W. Wang, R. Xie, X.-J. Ju, L. Liu, Y.-Y. Gu and L.-Y. Chu, Soft Matter, 2013, 9, 4150–4159 RSC.
- P. B. Umbanhowar, V. Prasad and D. A. Weitz, Langmuir, 1999, 16, 347–351 CrossRef.
- C.-L. Mou, Q.-Z. Deng, J.-X. Hu, L.-Y. Wang, H.-B. Deng, G. Xiao and Y. Zhan, J. Colloid Interface Sci., 2020, 569, 307–319 CrossRef CAS PubMed.
- Q. Xu, M. Hashimoto, T. T. Dang, T. Hoare, D. S. Kohane, G. M. Whitesides, R. Langer and D. G. Anderson, Small, 2009, 5, 1575–1581 CrossRef CAS PubMed.
- S. Takeuchi, P. Garstecki, D. B. Weibel and G. M. Whitesides, Adv. Mater., 2005, 17, 1067–1072 CrossRef CAS.
- C. Choi, H. Lee, A. Abbaspourrad, J. H. Kim, J. Fan, M. Caggioni, C. Wesner, T. Zhu and D. A. Weitz, Adv. Mater., 2016, 28, 3340–3344 CrossRef CAS PubMed.
- Y. Oh and S. Kim, J. Polym. Sci., 2021, 60, 1700–1709 CrossRef.
- B. Kim, S. Lee and S.-H. Kim, Adv. Mater. Interfaces, 2018, 5, 1701472 CrossRef.
- X.-C. Song, Y.-L. Yu, G.-Y. Yang, A.-L. Jiang, Y. Ruan and S. Fan, Colloids Surf., B, 2022, 216, 112560 CrossRef CAS PubMed.
- S. Lee, B. Che, M. Tai, W. Li and S.-H. Kim, Adv. Funct. Mater., 2021, 31, 2105477 CrossRef CAS.
- S.-H. Kim and D. A. Weitz, Angew. Chem., 2011, 123, 8890–8893 CrossRef.
- M. Brzeziński, M. Socka and B. Kost, Polym. Int., 2019, 68, 997–1014 CrossRef.
- L. Liu, N. Xiang, Z. Ni, X. Huang, J. Zheng, Y. Wang and X. Zhang, BioTechniques, 2020, 68, 114–116 CrossRef CAS PubMed.
- A. C. Daly, L. Riley, T. Segura and J. A. Burdick, Nat. Rev. Mater., 2019, 5, 20–43 CrossRef PubMed.
- Q.-W. Cai, X.-J. Ju, S.-Y. Zhang, Z.-H. Chen, J.-Q. Hu, L.-P. Zhang, R. Xie, W. Wang, Z. Liu and L.-Y. Chu, ACS Appl. Mater. Interfaces, 2019, 11, 46241–46250 CrossRef CAS PubMed.
- T. Nisisako, T. Torii, T. Takahashi and Y. Takizawa, Adv. Mater., 2006, 18, 1152–1156 CrossRef CAS.
- N. Prasad, J. Perumal, C.-H. Choi, C.-S. Lee and D.-P. Kim, Adv. Funct. Mater., 2009, 19, 1656–1662 CrossRef CAS.
- M. H. Lee, K. C. Hribar, T. Brugarolas, N. P. Kamat, J. A. Burdick and D. Lee, Adv. Funct. Mater., 2011, 22, 131–138 CrossRef.
- Z. Feng, B. Zhou, X. Su, T. Wang, S. Guo, H. Yang and X. Sun, Mater. Des., 2023, 225, 111516 CrossRef CAS.
- A. D. Bick, J. W. Khor, Y. Gai and S. K. Y. Tang, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2017822118 CrossRef CAS PubMed.
- Y. Gao, C.-X. Zhao and F. Sainsbury, J. Colloid Interface Sci., 2021, 584, 528–538 CrossRef CAS PubMed.
- M.-J. Zhang, P. Zhang, L.-D. Qiu, T. Chen, W. Wang and L.-Y. Chu, Biomicrofluidics, 2020, 14, 061501 CrossRef CAS PubMed.
- W. Wang, T. Luo, X.-J. Ju, R. Xie, L. Liu and L.-Y. Chu, Int. J. Nonlinear Sci. Numer. Simul., 2012, 13, 325–332 CrossRef CAS.
- M. Weiss, J. P. Frohnmayer, L. T. Benk, B. Haller, J.-W. Janiesch, T. Heitkamp, M. Börsch, R. B. Lira, R. Dimova, R. Lipowsky, E. Bodenschatz, J.-C. Baret, T. Vidakovic-Koch, K. Sundmacher, I. Platzman and J. P. Spatz, Nat. Mater., 2018, 17, 89–96 CrossRef CAS PubMed.
- Z.-M. Liu, Y. Yang, Y. Du and Y. Pang, Chin. J. Anal. Chem., 2017, 45, 282–296 CAS.
- Q. Xu, M. Hashimoto, T. T. Dang, T. Hoare, D. S. Kohane, G. M. Whitesides, R. Langer and D. G. Anderson, Small, 2009, 5, 1575–1581 CrossRef CAS PubMed.
- H. Pei, A. Abbaspourrad, W. Zhang, Z. Wu and D. A. Weitz, Macromol. Mater. Eng., 2019, 304, 1900156 CrossRef.
- X.-T. Mu, X.-J. Ju, L. Zhang, X.-B. Huang, Y. Faraj, Z. Liu, W. Wang, R. Xie, Y. Deng and L.-Y. Chu, J. Membr. Sci., 2019, 590, 117275 CrossRef CAS.
- J. Li, L. Jia, Y. Chen, L. Li, S. Mo, J. Wang and C. Wang, Appl. Therm. Eng., 2019, 162, 114278 CrossRef CAS.
- H. A. Son, S. K. Choi, E. S. Jeong, B. Kim, H. T. Kim, W. M. Sung and J. W. Kim, Langmuir, 2016, 32, 8909–8915 CrossRef CAS PubMed.
- Z. Luo, G. Zhao, F. Panhwar, M. F. Akbar and Z. Shu, J. Drug Delivery Sci. Technol., 2017, 39, 379–384 CrossRef CAS.
- C. Siltanen, M. Diakatou, J. Lowen, A. Haque, A. Rahimian, G. Stybayeva and A. Revzin, Acta Biomater., 2017, 50, 428–436 CrossRef CAS PubMed.
- X. Wang, M. Zhu, K. Wang, S. He, X. Shi, B. Yuan, B. Dong and Z. Wang, J. Drug Delivery Sci. Technol., 2022, 72, 103361 CrossRef CAS.
- E. Loiseau, P. A. Rühs, A. Hauser, F. Niedermair, G. Albrecht and A. R. Studart, Langmuir, 2018, 34, 205–212 CrossRef CAS PubMed.
- H. Lee, C.-H. Choi, A. Abbaspourrad, C. Wesner, M. Caggioni, T. Zhu and D. A. Weitz, ACS Appl. Mater. Interfaces, 2016, 8, 4007–4013 CrossRef CAS PubMed.
- H. Kim, S. Jo, F. Meng, Y. Guo, H. Thérien-Aubin, R. Golestanian, K. Landfester and E. Bodenschatz, Adv. Funct. Mater., 2020, 30, 2006019 CrossRef CAS.
- B. J. Sun, H. C. Shum, C. Holtze and D. A. Weitz, ACS Appl. Mater. Interfaces, 2010, 2, 3411–3416 CrossRef CAS PubMed.
- S. S. Lee, J. Park, Y. Seo and S.-H. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 17178–17185 CrossRef CAS PubMed.
- H.-S. Jeong, E. Kim, C. Nam, Y. Choi, Y.-J. Lee, D. A. Weitz, H. Lee and C.-H. Choi, Adv. Funct. Mater., 2021, 31, 2009553 CrossRef CAS.
- L. Liu, W. Wang, X.-J. Ju, R. Xie and L.-Y. Chu, Soft Matter, 2010, 6, 3759–3763 RSC.
- F. He, M. Zhang, W. Wang, Q. Cai, Y. Su, Z. Liu, Y. Faraj, X. Ju, R. Xie and L. Chu, Adv. Mater. Technol., 2019, 4, 1800687 CrossRef.
- M. Steinacher, A. Cont, H. Du, A. Persat and E. Amstad, ACS Appl. Mater. Interfaces, 2021, 13, 15601–15609 CrossRef CAS PubMed.
- K. Zhang, Y. Ren, T. Jiang and H. Jiang, Anal. Chim. Acta, 2021, 1182, 338955 CrossRef CAS PubMed.
- C.-H. Choi, S. Hwang, J.-M. Jeong, S.-M. Kang, J. Kim and C.-S. Lee, Biomed. Eng. Lett., 2012, 2, 95–99 CrossRef.
- T. Kamperman, V. D. Trikalitis, M. Karperien, C. W. Visser and J. Leijten, ACS Appl. Mater. Interfaces, 2018, 10, 23433–23438 CrossRef CAS PubMed.
- Y. Shen, L. Yuan, G. Wu, W. Yuan, Z. Cheng, J. Yan, J. Zhang, Y. Tao and Z. Yu, ACS Appl. Mater. Interfaces, 2023, 15, 591–598 CrossRef CAS PubMed.
- Q.-W. Cai, D.-W. Pan, X.-J. Ju, C. Chen, L.-P. Zhang, S.-H. Yang, R. Xie, W. Wang, Z. Liu and L.-Y. Chu, ACS Appl. Polym. Mater., 2023, 5, 5525–5536 CrossRef CAS.
- B. Haney, J. G. Werner, D. A. Weitz and S. Ramakrishnan, Soft Matter, 2020, 16, 3613–3620 RSC.
- L. Weidenbacher, A. Abrishamkar and M. Rottmar, Acta Biomater., 2017, 64, 137–147 CrossRef CAS PubMed.
- Y. Wang, J. Yuan, S. Dong and J. Hao, Langmuir, 2022, 38, 4713–4721 CrossRef CAS PubMed.
- H. Shum, J. Varnell and D. A. Weitz, Biomicrofluidics, 2012, 6, 12808–128089 CrossRef PubMed.
- R. A. de Freitas, T. Nicolai, C. Chassenieux and L. Benyahia, Langmuir, 2016, 32, 1227–1232 CrossRef CAS PubMed.
- J. Zhang, J. Hwang, M. Antonietti and B. V. K. J. Schmidt, Biomacromolecules, 2018, 20, 204–211 CrossRef PubMed.
- D. Liu, H. Zhang, B. Herranz-Blanco, E. Mäkilä, V. Lehto, J. Salonen, J. Hirvonen and H. A. Santos, Small, 2014, 10, 2029–2038 CrossRef CAS PubMed.
- Y. Du, L. Mo, X. Wang, H. Wang, X. Ge and T. Qiu, Microfluid. Nanofluid., 2020, 24, 42 CrossRef CAS.
- W. Zhang, L. Qu, H. Pei, Z. Qin, J. Didier, Z. Wu, F. Bobe, D. E. Ingber and D. A. Weitz, Small, 2019, 15, 1903087 CrossRef CAS PubMed.
- Z. Wu, J. G. Werner and D. A. Weitz, ACS Macro Lett., 2021, 10, 116–121 CrossRef CAS PubMed.
- E. Loiseau, F. Niedermair, G. Albrecht, M. Frey, A. Hauser, P. A. Rühs and A. R. Studart, Langmuir, 2017, 33, 2402–2410 CrossRef PubMed.
- H. Seo and H. Lee, Nat. Commun., 2022, 13, 5179 CrossRef CAS PubMed.
- R. Luo, S. Pashapour, O. Staufer, I. Platzman and J. P. Spatz, Adv. Funct. Mater., 2020, 30, 1908855 CrossRef CAS.
- R. L. Siegelman, E. J. Kim and J. R. Long, Nat. Mater., 2021, 20, 1060–1072 CrossRef CAS PubMed.
- G. Michielin and S. J. Maerkl, Sci. Rep., 2022, 12, 21391 CrossRef CAS PubMed.
- X. Liu, Y. Yu, D. Liu, J. Li, J. Sun, Q. Wei, Y. Zhao, S. J. Pandol and L. Li, NPG Asia Mater., 2022, 14, 1–10 CrossRef.
- W. J. Duncanson, M. Zieringer, O. Wagner, J. N. Wilking, A. Abbaspourrad, R. Haag and D. A. Weitz, Soft Matter, 2012, 8, 10636–10640 RSC.
- X. Xie, W. Zhang, A. Abbaspourrad, J. Ahn, A. Bader, S. Bose, A. Vegas, J. Lin, J. Tao, T. Hang, H. Lee, N. Iverson, G. Bisker, L. Li, M. S. Strano, D. A. Weitz and D. G. Anderson, Nano Lett., 2017, 17, 2015–2020 CrossRef CAS PubMed.
- T. Bollhorst, K. Rezwan and M. Maas, Chem. Soc. Rev., 2017, 46, 2091–2126 RSC.
- A. Laromaine, T. Tronser, I. Pini, S. Parets, P. A. Levkin and A. Roig, Soft Matter, 2018, 14, 3955–3962 RSC.
- D. Lee and D. A. Weitz, Adv. Mater., 2008, 20, 3498–3503 CrossRef CAS.
- J. S. Sander and A. R. Studart, Langmuir, 2011, 27, 3301–3307 CrossRef CAS PubMed.
- A. D. Dinsmore, M. F. Hsu, M. G. Nikolaides, M. Marquez, A. R. Bausch and D. A. Weitz, Science, 2002, 298, 1006–1009 CrossRef CAS PubMed.
- M. D. Rintoul and S. Torquato, Phys. Rev. E, 1998, 58, 532–537 CrossRef CAS.
- D. A. Olson, L. Chen and M. A. Hillmyer, Chem. Mater., 2008, 20, 869–890 CrossRef CAS.
- B. Kim, T. Y. Lee, A. Abbaspourrad and S.-H. Kim, Chem. Mater., 2014, 26, 7166–7171 CrossRef CAS.
- B. Kim, T. Y. Jeon, Y.-K. Oh and S.-H. Kim, Langmuir, 2015, 31, 6027–6034 CrossRef CAS PubMed.
- L. Chen, Y. Xiao, Z. Zhang, C.-X. Zhao, B. Guo, F. Ye and D. Chen, Front. Chem. Sci. Eng., 2022, 16, 1643–1650 CrossRef CAS.
- R. T. Rosenberg and N. R. Dan, J. Colloid Interface Sci., 2010, 349, 498–504 CrossRef CAS PubMed.
- S.-H. Kim, H. C. Shum, J. W. Kim, J.-C. Cho and D. A. Weitz, J. Am. Chem. Soc., 2011, 133, 15165–15171 CrossRef CAS PubMed.
- F. Meng, Z. Zhong and J. Feijen, Biomacromolecules, 2009, 10, 197–209 CrossRef CAS PubMed.
- I. Meerovich and A. K. Dash, in Materials for Biomedical Engineering, ed. A.-M. Holban and A. M. Grumezescu, Elsevier, 2019, pp. 269–309 Search PubMed.
- E. Amstad, S.-H. Kim and D. A. Weitz, Angew. Chem., Int. Ed., 2012, 51, 12499–12503 CrossRef CAS PubMed.
- X. Wang, J. Hu and S. Liu, Acc. Chem. Res., 2022, 55, 3404–3416 CrossRef CAS PubMed.
- Z. Gao, X. Cui and J. Cui, Supramolecular Materials, 2022, 1, 100015 CrossRef.
- W. Xu, P. A. Ledin, Z. Iatridi, C. Tsitsilianis and V. V. Tsukruk, Angew. Chem., Int. Ed., 2016, 55, 4908–4913 CrossRef CAS PubMed.
- Y. Zhao, H. C. Shum, H. Chen, L. L. A. Adams, Z. Gu and D. A. Weitz, J. Am. Chem. Soc., 2011, 133, 8790–8793 CrossRef CAS PubMed.
- N.-N. Deng, M. Yelleswarapu and W. T. S. Huck, J. Am. Chem. Soc., 2016, 138, 7584–7591 CrossRef CAS PubMed.
- J.-O. Chu, Y. Choi, D.-W. Kim, H.-S. Jeong, J. P. Park, D. A. Weitz, S.-J. Lee, H. Lee and C.-H. Choi, ACS Appl. Mater. Interfaces, 2022, 14, 2597–2604 CrossRef CAS PubMed.
- S. Lee, T. Y. Lee, E. Amstad and S. Kim, Adv. Mater. Technol., 2018, 3, 1800006 CrossRef.
- E. A. Sykes, J. Chen, G. Zheng and W. C. W. Chan, ACS Nano, 2014, 8, 5696–5706 CrossRef CAS PubMed.
- J. W. Hickey, J. L. Santos, J.-M. Williford and H.-Q. Mao, J. Controlled Release, 2015, 219, 536–547 CrossRef CAS PubMed.
- Z. Zhao, A. Ukidve, V. Krishnan and S. Mitragotri, Adv. Drug Delivery Rev., 2019, 143, 3–21 CrossRef CAS PubMed.
- D. A. Canelas, K. P. Herlihy and J. M. DeSimone, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 391–404 CAS.
- Z. Dai, M. Yu, X. Yi, Z. Wu, F. Tian, Y. Miao, W. Song, S. He, E. Ahmad, S. Guo, C. Zhu, X. Zhang, Y. Li, X. Shi, R. Wang and Y. Gan, ACS Nano, 2019, 13, 7676–7689 CrossRef CAS PubMed.
- M. T. Manzari, Y. Shamay, H. Kiguchi, N. Rosen, M. Scaltriti and D. A. Heller, Nat. Rev. Mater., 2021, 6, 351–370 CrossRef CAS PubMed.
- K. Maruyama, Adv. Drug Delivery Rev., 2011, 63, 161–169 CrossRef CAS PubMed.
- C. Kuroda, C. Mochizuki, J. Nakamura and M. Nakamura, OpenNano, 2023, 9, 100114 CrossRef.
- Y.-N. Zhang, J. Lazarovits, W. Poon, B. Ouyang, L. N. M. Nguyen, B. R. Kingston and W. C. W. Chan, Nano Lett., 2019, 19, 7226–7235 CrossRef CAS PubMed.
- L. Qin, F. Zhang, X. Lu, X. Wei, J. Wang, X. Fang, D. Si, Y. Wang, C. Zhang, R. Yang, C. Liu and W. Liang, J. Controlled Release, 2013, 171, 133–142 CrossRef CAS PubMed.
- L. Mei, J. Rao, Y. Liu, M. Li, Z. Zhang and Q. He, J. Controlled Release, 2018, 292, 67–77 CrossRef CAS PubMed.
- Q. Meng, S. Zhong, L. Xu, J. Wang, Z. Zhang, Y. Gao and X. Cui, Carbohydr. Polym., 2022, 279, 119013 CrossRef CAS PubMed.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C. W. Chan, Nat. Rev. Mater., 2016, 1, 16014 CrossRef CAS.
- E. A. Sykes, Q. Dai, C. D. Sarsons, J. Chen, J. V. Rocheleau, D. M. Hwang, G. Zheng, D. T. Cramb, K. D. Rinker and W. C. W. Chan, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E1142–E1151 CrossRef CAS PubMed.
- C. M. Dawidczyk, L. M. Russell and P. C. Searson, Front. Chem., 2014, 2, 69 Search PubMed.
- Y. Sun and E. Davis, Nanomaterials, 2021, 11, 746 CrossRef CAS PubMed.
- Q. Meng, S. Zhong, L. Xu, J. Wang, Z. Zhang, Y. Gao and X. Cui, Carbohydr. Polym., 2022, 279, 119013 CrossRef CAS PubMed.
- F. Perche, S. Biswas, T. Wang, L. Zhu and V. P. Torchilin, Angew. Chem., 2014, 126, 3430–3434 CrossRef.
- H.-J. Li, J.-Z. Du, J. Liu, X.-J. Du, S. Shen, Y.-H. Zhu, X. Wang, X. Ye, S. Nie and J. Wang, ACS Nano, 2016, 10, 6753–6761 CrossRef CAS PubMed.
- C. Arya, H. Oh and S. R. Raghavan, ACS Appl. Mater. Interfaces, 2016, 8, 29688–29695 CrossRef CAS PubMed.
- B. K. Kaang, S. Lee, J. Piao, H. J. Cho and D.-P. Kim, Lab Chip, 2023, 23, 2829–2837 RSC.
- M. Xie, W. Zhang, C. Fan, C. Wu, Q. Feng, J. Wu, Y. Li, R. Gao, Z. Li, Q. Wang, Y. Cheng and B. He, Adv. Mater., 2020, 32, 2000366 CrossRef CAS PubMed.
- Z. Li, E. Ye, David, R. Lakshminarayanan and X. J. Loh, Small, 2016, 12, 4782–4806 CrossRef CAS PubMed.
- J. Wei, X.-J. Ju, X.-Y. Zou, R. Xie, W. Wang, Y.-M. Liu and L.-Y. Chu, Adv. Funct. Mater., 2014, 24, 3312–3323 CrossRef CAS.
- M. Navi, J. Kieda and S. S. H. Tsai, Lab Chip, 2020, 20, 2851–2860 RSC.
- Y.-M. Liu, W. Wu, X.-J. Ju, W. Wang, R. Xie, C.-L. Mou, W.-C. Zheng, Z. Liu and L.-Y. Chu, RSC Adv., 2014, 4, 46568–46575 RSC.
- X. Kong, P. Gao, J. Wang, Y. Fang and K. C. Hwang, J. Hematol. Oncol., 2023, 16, 74 CrossRef PubMed.
- Z. Wang, Z. Xu, B. Zhu, Y. Zhang, J. Lin, Y. Wu and D. Wu, Nanotechnology, 2022, 33, 152001 CrossRef PubMed.
- Z. Liu, W. Wang, R. Xie, X.-J. Ju and L.-Y. Chu, Chem. Soc. Rev., 2016, 45, 460–475 RSC.
- F. B. Arslan, K. Ozturk and S. Calis, Int. J. Pharm., 2021, 596, 120268 CrossRef CAS PubMed.
- Z. Han, W. Lv, Y. Li, J. Chang, W. Zhang, C. Liu and J. Sun, ACS Appl. Bio Mater., 2020, 3, 2666–2673 CrossRef CAS PubMed.
- G. Sanità, P. Armanetti, B. Silvestri, B. Carrese, G. Calì, G. Pota, A. Pezzella, M. d'Ischia, G. Luciani, L. Menichetti and A. Lamberti, Front. Bioeng. Biotechnol., 2020, 8, 765 CrossRef PubMed.
- Y. Li, G. Yang, L. Gerstweiler, S. H. Thang and C.-X. Zhao, Adv. Funct. Mater., 2023, 33, 2210387 CrossRef CAS.
- L. Ramzy, A. A. Metwally, M. Nasr and G. A. S. Awad, Sci. Rep., 2020, 10, 10987 CrossRef CAS PubMed.
- D. E. Large, J. R. Soucy, J. Hebert and D. T. Auguste, Adv. Ther., 2018, 2, 1800091 CrossRef.
- Y. Bae, W.-D. Jang, N. Nishiyama, S. Fukushima and K. Kataoka, Mol. BioSyst., 2005, 1, 242 RSC.
- S. Rai, R. Paliwal, B. Vaidya, K. Khatri, A. K. Goyal, P. N. Gupta and S. P. Vyas, J. Drug Targeting, 2008, 16, 455–463 CrossRef CAS PubMed.
- Y. Zhang, Y. Wang, X. Li, D. Nie, C. Liu and Y. Gan, J. Controlled Release, 2022, 352, 813–832 CrossRef CAS PubMed.
- P. Mi, H. Cabral and K. Kataoka, Adv. Mater., 2020, 32, 1902604 CrossRef CAS PubMed.
- G. Sanità, P. Armanetti, B. Silvestri, B. Carrese, G. Calì, G. Pota, A. Pezzella, M. d'Ischia, G. Luciani, L. Menichetti and A. Lamberti, Front. Bioeng. Biotechnol., 2020, 8, 354–365 CrossRef PubMed.
- N. AlSawaftah, W. G. Pitt and G. A. Husseini, ACS Pharmacol. Transl. Sci., 2021, 4, 1028–1049 CrossRef CAS PubMed.
- N. Khan, Ruchika, R. K. Dhritlahre and A. Saneja, Drug Discovery Today, 2022, 27(8), 2288–2299 CrossRef CAS PubMed.
- S. Sun, H. Zou, L. Li, Q. Liu, N. Ding, L. Zeng, H. Li and S. Mao, Int. J. Pharm., 2019, 568, 118518 CrossRef CAS PubMed.
- J. Tang, C. B. Howard, S. M. Mahler, K. J. Thurecht, L. Huang and Z. P. Xu, Nanoscale, 2018, 10, 4258–4266 RSC.
- R. Ran, H. Wang, Y. Liu, Y. Hui, Q. Sun, A. Seth, D. Wibowo, D. Chen and C.-X. Zhao, Eur. J. Pharm. Biopharm., 2018, 130, 1–10 CrossRef CAS PubMed.
- J. Chen, J. Ding, W. Xu, T. Sun, H. Xiao, X. Zhuang and X. Chen, Nano Lett., 2017, 17, 4526–4533 CrossRef CAS PubMed.
- T. Yang, A. Wang, D. Nie, W. Fan, X. Jiang, M. Yu, S. Guo, C. Zhu, G. Wei and Y. Gan, Nat. Commun., 2022, 13, 6649 CrossRef CAS PubMed.
- Q. Xia, H. Ding and Y. Ma, Nanoscale, 2017, 9, 8982–8989 RSC.
- S. Maslanka Figueroa, D. Fleischmann and A. Goepferich, J. Controlled Release, 2021, 329, 552–569 CrossRef CAS PubMed.
- M. K. Prajapati, R. Pai and P. Vavia, J. Drug Delivery Sci. Technol., 2021, 66, 102809 CrossRef CAS.
- J. Shang and X. Gao, Chem. Soc. Rev., 2014, 43, 7267–7278 RSC.
- A. M. Alkilany, L. Zhu, H. Weller, A. Mews, W. J. Parak, M. Barz and N. Feliu, Adv. Drug Delivery Rev., 2019, 143, 22–36 CrossRef CAS PubMed.
- Y. Liu, Y. Hui, R. Ran, G.-Z. Yang, D. Wibowo, H.-F. Wang, A. P. J. Middelberg and C.-X. Zhao, Adv. Healthcare Mater., 2018, 7, 1800106 CrossRef PubMed.
- S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991–1003 CrossRef CAS PubMed.
- Q. Xia, T. Zhu, Z. Jiang, H. Ding and Y. Ma, Nanoscale, 2020, 12, 7804–7813 RSC.
- Y. Liu, R. Dai, Q. Wei, W. Li, G. Zhu, H. Chi, Z. Guo, L. Wang, C. Cui, J. Xu and K. Ma, ACS Appl. Mater. Interfaces, 2019, 11, 44582–44592 CrossRef CAS PubMed.
- W. Liu, Q. Wu, W. Wang, X. Xu, C. Yang and Y. Song, TrAC, Trends Anal. Chem., 2022, 157, 116827 CrossRef CAS.
- U. Dharmasiri, S. K. Njoroge, M. A. Witek, M. G. Adebiyi, J. W. Kamande, M. L. Hupert, F. Barany and S. A. Soper, Anal. Chem., 2011, 83, 2301–2309 CrossRef CAS PubMed.
- H. Wijerathne, M. A. Witek, J. M. Jackson, V. Brown, M. L. Hupert, K. Herrera, C. Kramer, A. E. Davidow, Y. Li, A. E. Baird, M. C. Murphy and S. A. Soper, Commun. Biol., 2020, 3, 613 CrossRef CAS PubMed.
- Y. Gou, Z. Chen, C. Sun, P. Wang, Z. You, Y. Yalikun, Y. Tanaka and D. Ren, Nanoscale, 2021, 13, 17765–17774 RSC.
- P. Zhang, X. Zhou, M. He, Y. Shang, A. L. Tetlow, A. K. Godwin and Y. Zeng, Nat. Biomed. Eng., 2019, 3, 438–451 CrossRef CAS PubMed.
- M. G. Ahmed, M. F. Abate, Y. Song, Z. Zhu, F. Yan, Y. Xu, X. Wang, Q. Li and C. Yang, Angew. Chem., Int. Ed., 2017, 56, 10681–10685 CrossRef CAS PubMed.
- S. L. Stott, C.-H. Hsu, D. I. Tsukrov, M. Yu, D. T. Miyamoto, B. A. Waltman, S. M. Rothenberg, A. M. Shah, M. E. Smas, G. K. Korir, F. P. Floyd, A. J. Gilman, J. B. Lord, D. Winokur, S. Springer, D. Irimia, S. Nagrath, L. V. Sequist, R. J. Lee, K. J. Isselbacher, S. Maheswaran, D. A. Haber and M. Toner, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 18392–18397 CrossRef CAS PubMed.
- L. Wu, H. Ding, X. Qu, X. Shi, J. Yang, M. Huang, J. Zhang, H. Zhang, J. Song, L. Zhu, Y. Song, Y. Ma and C. Yang, J. Am. Chem. Soc., 2020, 142, 4800–4806 CrossRef CAS PubMed.
- J. Zhang, B. Lin, L. Wu, M. Huang, X. Li, H. Zhang, J. Song, W. Wang, G. Zhao, Y. Song and C. Yang, Angew. Chem., Int. Ed., 2020, 59, 14115–14119 CrossRef CAS PubMed.
- E. Assadpour and S. M. Jafari, Release and Bioavailability of Nanoencapsulated Food Ingredients, 2020, vol. 5, pp. 1–24 Search PubMed.
- S. Boostani and S. M. Jafari, Release and Bioavailability of Nanoencapsulated Food Ingredients, 2020, vol. 5, pp. 27–78 Search PubMed.
- A. M. DiLauro, A. Abbaspourrad, D. A. Weitz and S. T. Phillips, Macromolecules, 2013, 46, 3309–3313 CrossRef CAS.
- A. Abbaspourrad, S. S. Datta and D. A. Weitz, Langmuir, 2013, 29, 12697–12702 CrossRef CAS PubMed.
- W. J. Duncanson, L. R. Arriaga, W. L. Ung, J. A. Kopechek, T. M. Porter and D. A. Weitz, Langmuir, 2014, 30, 13765–13770 CrossRef CAS PubMed.
- Y. H. Choi, J.-S. Hwang, S. H. Han, C.-S. Lee, S.-J. Jeon and S.-H. Kim, Adv. Funct. Mater., 2021, 31, 2100782 CrossRef CAS.
- G. Kaufman, K. A. Montejo, A. Michaut, P. W. Majewski and C. O. Osuji, ACS Appl. Mater. Interfaces, 2017, 9, 44192–44198 CrossRef CAS PubMed.
- F. Huang, W.-C. Liao, Y. S. Sohn, R. Nechushtai, C.-H. Lu and I. Willner, J. Am. Chem. Soc., 2016, 138, 8936–8945 CrossRef CAS PubMed.
- S.-H. Jung, S. Bulut, L. P. B. Busca Guerzoni, D. Günther, S. Braun, L. De Laporte and A. Pich, J. Colloid Interface Sci., 2022, 617, 409–421 CrossRef CAS PubMed.
- R. Wang, K. Guo, W. Zhang, Y. He, K. Yang, Q. Chen, L. Yang, Z. Di, J. Qiu, P. Lei, Y. Gu, Z. Luo, X. Xu, Z. Xu, X. Feng, S. Li, Z. Yu and H. Xu, Adv. Funct. Mater., 2022, 32, 2113034 CrossRef CAS.
- S. A. Ryu, Y.-H. Hwang, H. Oh, K. Jeon, J. H. Lee, J. Yoon, J. B. Lee and H. Lee, ACS Appl. Mater. Interfaces, 2021, 13, 36380–36387 CrossRef CAS PubMed.
- W. M. Hamonangan, S. Lee, Y. H. Choi, W. Li, M. Tai and S.-H. Kim, ACS Appl. Mater. Interfaces, 2022, 14, 18159–18169 CrossRef CAS PubMed.
- S. Cho, J.-H. Kim, K. S. Yang and M. Chang, Chem. Eng. J., 2021, 425, 130645 CrossRef CAS.
- G. Tiwari, R. Tiwari, S. Bannerjee, L. Bhati, S. Pandey, P. Pandey and B. Sriwastawa, Int. J. Pharm. Invest., 2012, 2, 2 CrossRef PubMed.
- T. Y. Lee, M. Ku, B. Kim, S. Lee, J. Yang and S.-H. Kim, Small, 2017, 13, 1700646 CrossRef PubMed.
- B. Liu, Y. Wang, F. Yang, X. Wang, H. Shen, H. Cui and D. Wu, Colloids Surf., B, 2016, 144, 38–45 CrossRef CAS PubMed.
- M. Windbergs, Y. Zhao, J. Heyman and D. A. Weitz, J. Am. Chem. Soc., 2013, 135, 7933–7937 CrossRef CAS PubMed.
- C. E. Miles, C. Gwin, K. A. V. Zubris, A. J. Gormley and J. Kohn, ACS Biomater. Sci. Eng., 2021, 7, 2580–2591 CrossRef CAS PubMed.
- S.-H. Kim, J. W. Kim, D.-H. Kim, S.-H. Han and D. A. Weitz, Small, 2013, 9, 124–131 CrossRef CAS PubMed.
- Y. H. Choi, S. Jeon and S. Kim, Adv. Mater. Interfaces, 2021, 8, 2100538 CrossRef CAS.
- Y. Hu and J. Pérez-Mercader, ACS Appl. Mater. Interfaces, 2019, 11, 41640–41648 CrossRef CAS PubMed.
- L. Jeon, Y. Kim, J. Yoon, H. Seo and H. Lee, Small Struct., 2023, 4, 2300200 CrossRef CAS.
- B. Kim, H. S. Lee, J. Kim and S.-H. Kim, Chem. Commun., 2013, 49, 1865–1867 RSC.
- J. Oh, B. Kim, S. Lee, S.-H. Kim and M. Seo, Chem. Mater., 2018, 30, 273–279 CrossRef CAS.
- J. Wei, X.-J. Ju, R. Xie, C.-L. Mou, X. Lin and L.-Y. Chu, J. Colloid Interface Sci., 2011, 357, 101–108 CrossRef CAS PubMed.
- S. Wen, X.-J. Ju, W.-Y. Liu, Y.-Q. Liu, X.-Q. Pu, Z. Liu, W. Wang, R. Xie, Y. Faraj and L.-Y. Chu, Engineering, 2023, 24, 114–125 CrossRef CAS.
- J. G. Werner, B. T. Deveney, S. Nawar and D. A. Weitz, Adv. Funct. Mater., 2018, 28, 1803385 CrossRef.
- J. G. Werner, S. Nawar, A. A. Solovev and D. A. Weitz, Macromolecules, 2018, 51, 5798–5805 CrossRef CAS.
- C. Owh, V. Ow, Q. Lin, J. H. M. Wong, D. Ho, X. J. Loh and K. Xue, Biomater. Adv., 2022, 141, 213100 CrossRef CAS PubMed.
- X.-X. Fan, R. Xie, Q. Zhao, X.-Y. Li, X.-J. Ju, W. Wang, Z. Liu and L.-Y. Chu, J. Membr. Sci., 2018, 555, 20–29 CrossRef CAS.
- H. Liu, J. Zhu, L. Hao, Y. Jiang, B. van der Bruggen, A. Sotto, C. Gao and J. Shen, J. Membr. Sci., 2019, 587, 117163 CrossRef CAS.
- X. Wang, D. Zhang, G. Wang, S. Wang, M. Si, J. Zhou, Y. Xu, G. Du, S. Yu Zheng and J. Yang, Chem. Eng. J., 2023, 472, 145185 CrossRef CAS.
- J.-W. Kim, S. S. Lee, J. Park, M. Ku, J. Yang and S.-H. Kim, Small, 2019, 15, 1900434 CrossRef PubMed.
- J.-W. Kim, S. H. Han, M. Ku, J. Yang and S.-H. Kim, Chem. Mater., 2023, 35, 4751–4760 CrossRef CAS.
- G. J. S. Dawes, L. E. Fratila-Apachitei, K. Mulia, I. Apachitei, G.-J. Witkamp and J. Duszczyk, J. Mater. Sci.: Mater. Med., 2009, 20, 1089–1094 CrossRef CAS PubMed.
- J. Wu, T. Kong, K. W. K. Yeung, H. C. Shum, K. M. C. Cheung, L. Wang and M. K. T. To, Acta Biomater., 2013, 9, 7410–7419 CrossRef CAS PubMed.
- S. Dash, P. N. Murthy, L. Nath and P. Chowdhury, Acta Pol. Pharm., 2010, 67, 217–223 CAS.
- B. J. Crielaard, S. van der Wal, H. T. Le, A. T. L. Bode, T. Lammers, W. E. Hennink, R. M. Schiffelers, M. H. A. M. Fens and G. Storm, Eur. J. Pharm. Sci., 2012, 45, 429–435 CrossRef CAS PubMed.
- Z. Dong, Y. Ma, K. Hayat, C. Jia, S. Xia and X. Zhang, J. Food Eng., 2011, 104, 455–460 CrossRef CAS.
- Y. Yang, X. Li and S. Zhang, RSC Adv., 2018, 8, 29980–29987 RSC.
| This journal is © The Royal Society of Chemistry 2024 |