Hydrogen production from biomass by continuous fast pyrolysis and in-line steam reforming
A. Arregi,
G. Lopez,
M. Amutio *,
I. Barbarias,
J. Bilbao and
M. Olazar
*,
I. Barbarias,
J. Bilbao and
M. Olazar
Department of Chemical Engineering, University of the Basque Country UPV/EHU, P.O. Box 644, E48080 Bilbao, Spain. E-mail: maider.amutio@ehu.es
First published on 2nd March 2016
Abstract
The continuous fast pyrolysis (500 °C) of pine wood sawdust has been studied in a conical spouted bed reactor (CSBR) followed by in-line steam reforming of the pyrolysis vapours in a fluidised bed reactor on a Ni commercial catalyst. An analysis has been carried out on the effect reforming temperature in the 550–700 °C range, space time from 2.5 to 30 gcat min gvolatiles−1 and steam/biomass ratio between 2 and 5 have on the pyrolysis volatile conversion, H2 yield and gaseous stream composition. The continuous pyrolysis-reforming process has shown great potential for H2 production from biomass, with no operational problems and allowing for full conversion of pyrolysis vapours. Thus, a maximum H2 yield of 117 g per kg of biomass was obtained at 600 °C, at the highest space time studied (30 gcat min gvolatiles−1) and for a S/B ratio of 4. This yield is higher than those obtained by other alternatives, such as direct steam gasification or bio-oil reforming. Moreover, the char produced in the pyrolysis step has been continuously removed from the conical spouted bed reactor in order to be upgraded following promising valorisation alternatives.
Introduction
The promotion of H2 as an energy carrier together with its increasing demand as a fuel and raw material has generated a growing interest for the development of renewable sources for its production, in order to modify the current situation in which around 96% of H2 is produced from fossil fuels, such as natural gas, petroleum derivates or coal.1The thermochemical conversion of biomass is an alternative for the full-scale production of H2, and particularly gasification has been widely studied.2 However, biomass gasification faces several challenges related to the quality of the syngas obtained and, especially, to its tar content, which causes serious operational problems due to the blockage by fouling of process equipment3 and limits the applications of the syngas produced.4
Another alternative to produce H2 from biomass that has gained growing attention in recent years is the indirect route by bio-oil reforming.5 Bio-oil is the liquid product obtained in the biomass fast pyrolysis process, whose yield can reach 75 wt% operating under suitable conditions.6,7 This strategy has certain clear advantages compared to the direct gasification, such as the remarkably lower process temperature, which gives way to lower energy requirements and material costs,8 and the higher energy density of the bio-oil compared to biomass, leading to lower transport costs. However, the physical properties of the bio-oil pose a serious drawback for this indirect route. Thus, bio-oil is unstable and polymerizes under storage, causing an increase in viscosity and average molecular weight.9 Furthermore, the incomplete vaporization of the bio-oil also involves a great challenge due to the formation of carbonaceous deposits, and therefore a decrease in the reforming conversion efficiency.10 In order to alleviate the problems associated with bio-oil properties, most of the reforming studies in the literature have been carried out with model compounds and synthetic mixtures simulating bio-oil and tar5,8 or with the bio-oil aqueous fraction.11–13
The H2 production by biomass pyrolysis and in-line reforming of pyrolysis volatiles avoids the problems associated with bio-oil handling and vaporization. Furthermore, a fraction of the bio-oil is not discarded and all hydrocarbons of pyrolysis gases are reformed improving the potential process yield. Therefore, although it has been scarcely studied, this strategy is regarded as a feasible solution for H2 production in small scale units.14 The research group headed by Prof. Williams studied the pyrolysis and reforming of biomass and other residues in batch regime, with the unit consisting of two fixed bed reactors.15–17 The process developed by the research group headed by Prof. Tomishige is also based on two fixed bed reactors for the pyrolysis and reforming steps and, although their experimental equipment is of a relatively reduced size, it operates with continuous biomass feed.18,19 The process developed by Xiao et al.20,21 is based on a fluidised bed reactor for the pyrolysis step and a fixed bed reactor for the catalytic steam reforming operating in continuous regime. The strategy proposed by Ma et al.22 includes an intermediate char gasification step for the maximization of H2 yield. Thus, the process consists of three sequential steps: pyrolysis in a fluidised bed reactor, char gasification in an entrained flow gasifier and reforming of volatiles in a fixed bed reactor.
In a previous paper,23 a combination of a conical spouted bed reactor (CSBR) and a fixed bed reactor was used for the pyrolysis and in-line reforming of HDPE. In order to overcome the operational problems encountered when using a fixed bed in the reforming step, it has been replaced by a fluidised bed reactor.24,25 Furthermore, the CSBR has shown an excellent performance in the pyrolysis of biomass,6,26 which augurs well for a successful scale-up of the technology.27
This study pursues the development of an original continuous two-step process for the production of H2 from biomass by coupling the pyrolysis in a CSBR with the steam reforming in a fluidised bed reactor. This direct strategy is an attractive and novel alternative to the indirect bio-oil reforming process, given that it avoids the operational problems associated with bio-oil handling. Moreover, the results were obtained in an experimental unit made up of two reactors connected in series operating in continuous regime and characterized by their high heat and mass transfer rates, and therefore, under similar conditions to those of industrial reactors.
Experimental
Materials
The biomass used in this study is forest pine wood waste (Pinus insignis), which has been crushed, ground and sieved to a particle size between 1 and 2 mm in order to ease the feeding operation. This sawdust has been dried at room temperature to moisture content below 10 wt% and the main properties are summarized in Table 1. The ultimate and proximate analyses have been determined in a LECO CHNS-932 elemental analyzer and in a TGA Q5000IR thermogravimetric analyzer, respectively. The higher heating value (HHV) has been measured in a Parr 1356 isoperibolic bomb calorimeter.| Ultimate analysis (wt%) | |
| Carbon | 49.33 |
| Hydrogen | 6.06 |
| Nitrogen | 0.04 |
| Oxygen | 44.57 |
| Proximate analysis (wt%) | |
| Volatile matter | 73.4 |
| Fixed carbon | 16.7 |
| Ash | 0.5 |
| Moisture | 9.4 |
| HHV (MJ kg−1) | 19.8 |
The catalyst used in the reforming step is a commercial one for methane reforming provided by Süd Chemie (G90LDP catalyst). The metallic phase is Ni supported on Al2O3, which is doped with Ca, with the content of NiO being 14%. The original catalyst has a shape of perforated rings (19 × 16 mm), but it was ground and sieved to 0.4–0.8 mm, which is the suitable particle size to attain a stable fluidisation regime.
The physical properties of the catalyst have been determined by N2 adsorption–desorption in a Micromeritics ASAP 2010. The adsorption–desorption isotherm of this catalyst, which has been reported elsewhere,23,28 shows that it has low porosity with a BET surface area of 19 m2 g−1 and an average pore diameter of 122 Å.
In order to determine the reduction conditions required prior to use, temperature programmed reduction (TPR) of the catalyst has been carried out in an AutoChem II 2920 Micromeritics. The TPR curve (provided in previous studies23,28) showed a main peak at 550 °C associated with NiO reduction, which interacts with α-Al2O3. Moreover, another peak is observed at 700 °C, which is probably related to NiAl2O4 according to the composition given by the provider.
Thus, the catalyst has been subjected to an in situ reduction process at 710 °C for 4 h under 10 vol% H2 stream to ensure complete reduction of the metallic phase.
Equipment and reactors
The pyrolysis of biomass and in-line steam reforming of the volatiles produced has been studied in a bench scale plant whose scheme is shown in Fig. 1. The plant is provided with a CSBR for the pyrolysis step and a fluidised bed reactor for the reforming step.The CSBR has been designed and tuned in previous studies dealing with hydrodynamics, pyrolysis and gasification of different wastes, such as biomass,26,29,30 plastics31,32 and tyres.33,34
The main dimensions of the pyrolysis reactor are as follows: height of the conical section, 73 mm; diameter of the cylindrical section, 60.3 mm; angle of the conical section, 30°; diameter of the bed bottom, 12.5 mm, and diameter of the gas inlet, 7.6 mm. The reactor has a lateral outlet pipe placed above the bed surface for the removal of char particles from the bed (Fig. 1). Moreover, the reactor is provided with a gas preheater, which is filled with stainless steel pipes that increase the surface area for heat transfer. The reactor is located inside an oven of 1250 W, with this oven being controlled by two K-type thermocouples located inside the reactor, one in the bed annulus and the other one close to the wall.
The pyrolysis vapours formed in the pyrolysis process are reformed in a fluidised bed reactor. The diameter and length of this reactor are 38.1 and 440 mm, respectively. The heat required for the reforming step is provided by a 550 W radiant oven, which is controlled by a thermocouple placed in the catalyst bed.
Both the pyrolysis and the reforming reactors, together with the interconnection pipes, cyclone and filter are located inside a forced convection oven. This oven is kept at 270 °C in order to avoid the condensation of pyrolysis products and steam in the elements connecting the reaction system. The cyclone is placed downstream the pyrolysis reactor to retain the fine char particles entrained from the bed. Regarding the filter (5 μm sintered steel), its main purpose is to capture catalyst fines elutriated from the fluidised bed reactor.
The feeding system consists of a vessel equipped with a vertical shaft connected to a piston placed below the material bed. By raising the piston at the same time as the whole system is vibrated by an electric engine, the feeding system discharges the biomass through a pipe to the reactor. This pipe is cooled with tap water to avoid biomass partial degradation and blocking the system. Moreover, a very small nitrogen flow rate introduced into the vessel stops the steam entering the feeding vessel.
Experimental conditions
The operating conditions for the pyrolysis and reforming reactors were fine tuned in previous hydrodynamic studies carried out at the reaction temperatures (500 and 600 °C, respectively) in order to attain an adequate fluidisation behaviour in both steps. Therefore, not only the geometric factors of the reactors but also the size of sand particles in the pyrolysis reactor and those of sand and reforming catalyst in the reforming step have been optimized to achieve the desired fluidisation regime in each step.Thus, the conical spouted bed reactor contains 50 g of silica sand with a particle size in the 0.3–0.35 mm range. The bed in the steam reforming step is made up of a mixture of reforming catalyst and inert sand, with the total bed mass being kept constant at 25 g in all the runs. The catalyst/sand mass ratios used were chosen according to the space time studied. The particle size of the catalyst was in the 0.4–0.8 mm range and that of the inert sand in the 0.3–0.35 mm range.
The temperature in the pyrolysis step was fixed at 500 °C in all the runs performed, because this temperature was determined as the optimum one in a previous study.6 Table 2 summarizes the operating conditions used in the runs carried out for determining the influence of the parameters on the reforming step. The effect of temperature has been studied in the range from 550 to 700 °C, with steam/biomass ratio (S/B) and space time in these runs being 4 and 20 gcat min gvolatiles−1, respectively. The study of higher temperatures was discarded in order to avoid irreversible catalyst deactivation by sintering.
| a By mass unit of the biomass fed into the pyrolysis reactor.b By molar unit of the pyrolysis derived volatiles fed into the reforming step, i.e. molar flow rate of carbon contained. | |
|---|---|
| Temperature (°C) | 550, 600, 650 and 700 (space time 20, S/B 4) |
| S/B ratioa | 2, 3, 4 and 5 (600 °C, space time 20) |
| S/C ratio | 3.9, 5.8, 7.7 and 9.7 (600 °C, space time 20) |
| Space time (gcat min g−1)a | 2.1, 4.2, 8.3, 12.5, 16.7 and 25 (600 °C, S/B 4) |
| Space time (gcat min g−1)b | 2.5, 5, 10, 15, 20 and 30 (600 °C, S/B 4) |
The influence of S/B ratio has been assessed by varying this parameter between 2 and 5, with the water flow rate being constant in all the runs (3 mL min−1) in order to keep the hydrodynamic conditions in the reactors. Accordingly, the S/B ratio was adjusted by modifying the biomass feed rate in the range from 1.5 to 0.6 g min−1. Moreover, the same space time (20 gcat min gvolatiles−1) was used in these runs, which was attained by modifying the mass of catalyst for each S/B ratio. Furthermore, in order to ease comparison of the results with those in the literature, the values have been determined for the molar steam/carbon ratio (S/C) of the stream fed into reforming step (biomass pyrolysis volatile fraction), Table 2. It should be noted that a char yield around 17 wt% is obtained in the pyrolysis step. Accordingly, the carbon contained in this char is not reformed in the second step and was not considered for the S/C ratio estimation.
Finally, the effect of space time in the reforming step was studied in the 2.5–30 gcat min gvolatiles−1 range by varying the amount of catalyst, with the biomass feed rate being kept at 0.75 g min−1. In addition, the space times given by mass unit of the biomass fed into the pyrolysis reactor are also shown in Table 2.
All the runs have been performed in continuous mode for several minutes in order to ensure steady state in the process. Moreover, the runs have been repeated at least 3 times under the same conditions (with fresh catalyst) in order to guarantee reproducibility of the results.
Product analysis
The volatile stream leaving the reforming reactor has been analysed on-line by means of a GC Agilent 6890 provided with a HP-Pona column and a flame ionization detector (FID). The sample has been injected into the GC by means of a line thermostated at 280 °C, once the reforming reactor outlet stream has been diluted with an inert gas. The non-condensable gases have been analyzed on-line in a micro GC (Varian 4900) by taking the samples after the condenser and coalescence filter.Reaction indexes
In order to quantify the process results, conversion and individual product yields have been considered. The reforming conversion has been defined similarly as the carbon conversion efficiency commonly used in the gasification processes, i.e., the ratio between the moles of C recovered in the gaseous product and those fed into the reforming step.
 | (1) |
Note that the carbon contained in the biomass char is not considered for estimating conversion. Accordingly, full conversion of bio-oil compounds is attained when they are totally reformed to yield gaseous products. The bio-oil compounds yield has been determined by on-line GC analysis of the reformed product stream.
Similarly, the yield of C containing individual compounds has been based on the biomass pyrolysis volatiles stream
 | (2) |
The hydrogen yield was determined as a percentage of the maximum allowed by stoichiometry, which accounts for the hydrogen coming from the pyrolysis products and the steam. The following stoichiometry was considered:
| CnHmOk + (2n − k)H2O → nCO2 + (2n + m/2 − k)H2 | (3) |
 | (4) |
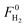 are the hydrogen molar flow rate obtained in the run and the maximum allowable by stoichiometry.
are the hydrogen molar flow rate obtained in the run and the maximum allowable by stoichiometry.
Results
Biomass pyrolysis (first step)
The pyrolysis step was carried out under steam environment, given that the steam required in the reforming step was introduced in the pyrolysis reactor, where it plays the role of a fluidising agent. A previous study revealed that steam has a negligible effect in the pyrolysis of this material, which is explained by the moderate temperature used in this process (500 °C). Moreover, it should be noted that, even operating with N2 as fluidising agent, there is a significant steam concentration in the reaction environment because the water yield in the biomass pyrolysis process is above 25 wt%.6 Thus, the results were similar to those previously reported by Amutio et al.6 in the pyrolysis of the same biomass but using N2 as fluidising agent. Kantarelis et al.35 observed certain differences in the biomass pyrolysis product yields and composition when comparing steam and N2 pyrolysis. However, the same authors stated based on a simulation study that the differences between steam and nitrogen pyrolysis are negligible in terms of heat transfer and product formation rate.36The fast pyrolysis of pine wood sawdust in a CSBR gives way to a wide distribution of products, which can be grouped into three fractions: gases, bio-oil and char. The first two fractions are the volatile products, which are driven to the fluidised bed reactor to be reformed. However, the char formed is continuously removed from the pyrolysis reactor by means of a lateral outlet pipe to avoid its accumulation in the bed. This separation is achieved in the CSBR due to the different trajectories described by char particles in this system, which has previously been used in the pyrolysis of tyres and biomass, and has been described elsewhere.26,33 Under the conditions studied, char yield is 17 wt% and its recovery is of great interest for the economy of the process, with its main applications being, amongst others, the production of adsorbent,37,38 fertilizers,39 catalyst support40–42 and soil amender.43
The main product obtained in the pyrolysis step is the liquid product or bio-oil, whose yield is 75 wt% due to the excellent features of this reactor for biomass fast pyrolysis, especially, its high heat transfer rate, short residence time and rapid char removal from the reaction environment.6 The main products of the bio-oil, which is a complex mixture of oxygenated compounds, are as follows: phenols (16.5 wt%), ketones (6.4%), saccharides (4.5%), furans (3.3%), acids (2.7%), alcohols (2.0%) and aldehydes (1.9%). Furthermore, a water yield of around 25 wt% is also obtained,6 which acts also as a reforming agent in the second reforming step.
Regarding the gaseous fraction, its yield was 7.3 wt% and is made up of CO, CO2 (similar yield for both, 3.3 wt%) and a low concentration of CH4, C2–C4 hydrocarbons and H2.6
The molecular formula corresponding to the stream of biomass pyrolysis volatiles entering the reforming reactor has been determined based on the compositions of the gas and bio-oil fractions: CH1,93O0,92.
Steam reforming (second step)
The effect of temperature, S/B ratio (or S/C ratio) and space time on the product yields and gas fraction composition have been analysed for the operating conditions summarized in Table 2. In order to ascertain the effect operating conditions have on the reaction indexes the following reactions have been considered: the steam reforming of oxygenates, eqn (3).Water gas shift (WGS):
| CO + H2O ⇔ CO2 + H2 | (5) |
Methane steam reforming:
| CH4 + H2O ⇔ CO + 3H2 | (6) |
Cracking (secondary reaction):
| CnHmOk → oxygenates + HCs + CH4 + CO + CO2 | (7) |
The effect of temperature on biomass derived volatiles conversion is shown in Fig. 2. As observed, under the conditions studied at 550 °C conversion is almost 60%, whereas at 600 °C conversion is complete. Xiao et al.20,21 studied the pyrolysis and in-line reforming of different biomasses on Ni catalysts in a fluidised-fixed bed system, and they also determined a minimum temperature of 600 °C to attain a high conversion degree of biomass tars.
 | ||
| Fig. 2 Effect of reforming temperature on conversion. Reforming conditions: space time, 20 gcat min gvolatiles−1; S/B ratio, 4. | ||
Fig. 3 shows the effect of temperature on the individual gaseous product yields (graph a) and gas composition (graph b). It should be noted that H2 yield is based on the maximum allowable by stoichiometry, but those of the other compounds are given by carbon mole unit fed into the reforming step. As observed in Fig. 3a, the effect of temperature on H2 yield is negligible once full conversion has been reached (above 600 °C), with its yield being of around 93.5% between 600 and 700 °C. Consequently, 600 °C is considered the optimum temperature from a thermodynamic point of view, because it provides the highest equilibrium concentration for H2 in the reforming of oxygenates.8 In addition, operation at this relatively low temperature avoids the irreversible catalyst deactivation by Ni sintering.
Although most of the studies in the literature dealing with the steam reforming of bio-oil report lower H2 yields based on the maximum allowable by stoichiometry than those in this study,5 certain authors have reported values as high as 90% or even slightly higher.12,13,44
Regarding H2 production, it increases from 64 g kgbiomass−1 at 550 °C to around 110 g kgbiomass−1 between 600 and 700 °C. Xiao et al.20,21 reported a yield of around 100 g kgbiomass−1 in the pyrolysis and in-line steam reforming of pine wood chips under optimum conditions in a fluidised-fixed bed system. Ma et al.22 operated in a three-step process (biomass pyrolysis in a fluidised bed reactor, gasification in an entrained flow reactor and reforming in a fixed bed) and obtained a maximum H2 yield of 76 g kgbiomass−1 at the highest reforming temperature studied, 850 °C.
Furthermore, the H2 yields reported in the biomass steam gasification process vary widely depending on the operating conditions, gasification technology, original biomass properties and, especially, on the type of catalyst used. Thus, when the gasification is performed with an inert material or a primary catalyst, such as dolomite, olivine or γ-alumina, the H2 yields are between 30 and 50 g kgbiomass−1.45–48 The yields are higher, 70–80 g kgbiomass−1, when the reforming activity is increased by improving the primary catalyst with the addition of Ni or Fe.49,50
A comparison of the H2 production results with those in the indirect route, i.e. bio-oil reforming, is complex due to the differences between these two strategies. Thus, in the pyrolysis and in-line reforming process the carbon contained in the char fraction is not reformed, but the entire volatile fraction (including gases and the whole bio-oil) is treated. Nevertheless, the bio-oil reforming strategy has hardly been applied to the whole bio-oil, with the aqueous fraction being the feed in most of the cases. Furthermore, a significant fraction of the bio-oil is lost due to its incomplete vaporization.10 Thus, problems related to phase separation of the raw bio-oil and the repolymerization of phenolic compounds, which have a great potential for H2 production, hinder the feeding of the whole bio-oil into the reforming reactor.51
Furthermore, the fact that H2 yields in the bio-oil reforming studies are referred to different basis, such as mass unit of organic compounds (without water), bio-oil aqueous fraction or whole bio-oil, also complicates comparison. Thus, the H2 production obtained by Bimbela et al.11 in the steam reforming of bio-oil aqueous phase on a Ni–Al catalyst was remarkably high, 138 g kg−1, but their yield is given by mass unit of organic compounds in the feed. The value reported by Remiro et al.52 in the reforming of raw bio-oil on a Ni/La2O3–Al2O3 catalyst is 117 g kgbio-oil−1. Salehi et al.53 obtained a maximum H2 production of 142 g kgbio-oil−1 in the reforming of raw bio-oil on a Ni–Al2O3 catalyst. The H2 yield reported by Czernik and French10 in the autho-thermal reforming of bio-oil aqueous fraction on a Pt commercial catalyst was slightly lower, between 85 and 110 g kgbio-oil−1, depending on the origin of the bio-oil tested. Accordingly, the indirect route has a lower H2 production capacity per biomass mass unit compared to the direct pyrolysis-reforming strategy, even if high bio-oil yields are obtained (65–75 wt%) in the previous biomass pyrolysis process. Table 3 summarizes a comparison of the H2 yields obtained in several biomass conversion processes.
| Reference | Strategy | Reactor | Feed | Temperature (°C) | Catalyst | H2 yield (g kg−1) | Gas yield (m3 kg−1) |
|---|---|---|---|---|---|---|---|
| a Yields per kg of biomass.b Yields per kg of organic bio-oil.c Yields per kg of bio-oil. | |||||||
| This work | Pyrolysis/reforming | Spouted bed/fluidised bed | Pine wood/pyrolysis volatiles | 500/600 | Ni commercial | 110a | 1.9a |
| 20 | Pyrolysis/reforming | Fluidised bed/fixed bed | Pine wood/pyrolysis volatiles | 650/650 | Ni–coal char | 100a | 1.9a |
| 21 | Pyrolysis/reforming | Fixed bed/fixed bed | Pine wood/pyrolysis volatiles | 700/650 | Ni–coal char | 52a | 1.12a |
| 22 | Pyrolysis/reforming | Fluidised bed/fixed bed | Timber wood/pyrolysis volatiles | 600/850 | Ni–MgO commercial | 76a | 1.69a |
| 54 | Pyrolysis/reforming | Fixed bed/fixed bed | Wood sawdust | 500/800 | Ni–Ca–AlOx | 31a | |
| 11 | Bio-oil reforming | Fixed bed | Bio-oil aqueous fraction | 850 | Ni–Al | 138b | 2.25b |
| 53 | Bio-oil reforming | Fixed bed | Raw bio-oil | 950 | Ni–Ru–Al2O3 | 142c | — |
| 52 | Bio-oil reforming | Fluidised bed | Raw bio-oil | 700 | Ni–La2O3–Al2O3 | 117c | 1.85c |
| 55 | Bio-oil reforming | Fixed bed | Raw bio-oil | 700 | Ni–Cu–Zn–Al2O3 | 102c | 1.6c |
| 44 | Bio-oil reforming | Fluidised bed | Raw bio-oil + 10% ethanol | 850 | Ni–K–Mg commercial | 129c | 2.1c |
| 10 | Oxidative bio-oil reforming | Fluidised bed | Bio-oil aqueous fraction | 850 | Pt–Al2O3 commercial | 110c | 1.9c |
| 47 | Steam gasification | Fluidised bed | Miscathus giganteus | 880 | Olivine | 49a | 1.2a |
| 45 | Steam gasification | Dual fluidised bed | Wood pellets | 850 | Olivine | 42a | 1.13a |
| 48 | Steam gasification | Spouted bed | Pine wood | 900 | Inert sand | 32a | 1a |
| 46 | Steam gasification | Updraft | Wood chips | 700–900 | — | 36a | 1a |
| 50 | Steam gasification | Fluidised bed | Miscathus giganteus | 900 | Ni–olivine | 73a | 1.6a |
| 49 | Steam gasification | Fluidised bed | Almond shell | 830 | Fe–olivine | 65a | 1.4a |
It should be pointed out that both conversion and H2 yield are strongly influenced by bio-oil composition, given that the reactivities of the compounds in the bio-oil are different. Most reforming studies in the literature focus on studying the effect of operating conditions, although certain authors conducted studies dealing with the reactivity of different model compounds in the bio-oil. Thus, Remón et al.56 studied the catalytic reforming of acetic acid, phenol, furfural, guaiacol and levoglucosan on a Ni–Co/Al–Mg catalyst at 650 °C, and the H2 yield obtained with the different model compounds followed this order: phenol > furfural > acetic acid > guaiacol > levoglucosan. The lower H2 yields of guaiacol and levoglucosan were explained by the high amount of carbon converted into coke. 2-Methylfuran, furfural and guaiacol steam reforming were studied by Trane-Restrup and Jensen57 and the highest temperature (780 °C) needed for complete conversion in the reforming of guaiacol was reported, whereas full conversion was achieved at 700 °C in the reforming of 2-methylfuran and furfural. Moreover, the highest carbon deposition was observed for guaiacol followed by furfural and 2-methylfuran. Wang et al.58 investigated the steam reforming of phenol, acetic acid and hydroxyacetone at 700 °C on a Ni/nano-Al2O3 catalyst and the conversion and H2 yield decrease as follows: hydroxyacetone > acetic acid > phenol. Some model compounds of bio-oil were also studied by Hu and Lu59 at temperatures below 500 °C for the steam reforming of acetic acid, ethylene glycol and acetone, while higher temperatures were needed for the reforming of ethyl acetate and m-xylene.
Nevertheless, the reactivity of these compounds is different when they are reformed alone or in a mixture of different organic compounds. These interactions were studied by Remón et al.56 and a different reactivity was reported for the acetic acid depending on the medium. Thus, 100% conversion was achieved for the reforming of an aqueous solution of acetic acid, whereas 87% of the acetic acid was converted in the reforming of the aqueous fraction of the bio-oil. Wu et al.60 studied the difference between two simulated aqueous fractions of bio-oil, a light fraction (methanol, ethanol, acetic acid and acetone mixture) and a heavy fraction (furfural, phenol, catechol and m-cresol mixture), and they report that higher temperatures are needed for reforming the heavy fraction, with coke deposition being more significant. Consequently, the difficulty is evident in studying the reactivity of the compounds of biomass pyrolysis volatiles, due to the high amount of species contained and the interactions between them.
Carbon monoxide and dioxide yields are enhanced by increasing reforming temperature from 550 to 600 °C (Fig. 3a). However, above 600 °C, an increase in CO is observed at the expense of decreasing CO2, with this trend being related to the exothermic nature of the WGS reaction (eqn (5)), which is hindered by temperature.
The effect of temperature on the gaseous fraction composition is not so remarkable (Fig. 3b). In fact, H2 concentration takes values of around 66 vol% in the range studied. A slight effect on CO2 and, especially, on CO concentration is observed, with their evolution being explained by the effect of temperature on the WGS reaction equilibrium. The slight differences between the gas composition obtained at 550 and 600 °C, but great differences in conversion, are explained by the fact that almost all the gases are produced by reforming. Thus, the formation of gases by secondary reactions involving pyrolysis products, i.e., cracking, decarboxylation, decarbonilation and so on, are of minor significance.
An increase in space time enhances both steam reforming (eqn (3)) and WGS (eqn (5)) reactions, and therefore the formation of H2, CO2 and CO is favoured, as shown in Fig. 5a. It should be noted that, for the highest space time studied (30 gcat min gvolatiles−1), CO yield decreases due to the displacement of the WGS reaction. This fact, together with the intensification of CH4 and other hydrocarbon reforming, gives way to an increase in H2 yield, reaching a value of 95.8% of the maximum allowable by stoichiometry. A qualitatively similar effect of space time on product yields has been observed by several authors in the steam reforming of bio-oil.52,61,62
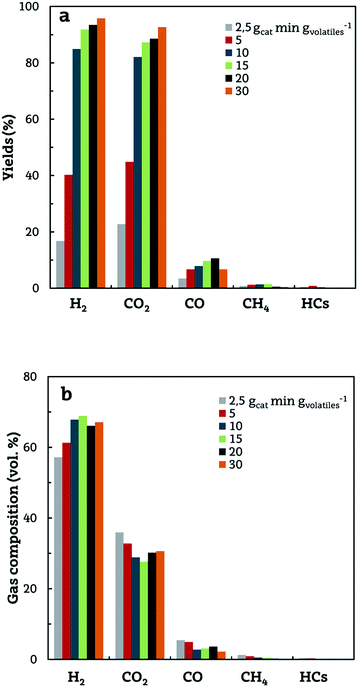 | ||
| Fig. 5 Effect of reforming space time on the yields of gaseous products (a) and their concentrations (on a dry basis) (b). Reforming conditions: 600 °C; S/B ratio, 4. | ||
Furthermore, hydrogen mass balance (considering the hydrogen content in the volatiles, the water fed into the reaction medium and the H2 produced) allows verifying that the water reacted increases when space time is increased. This value is negative for 2.5 gcat min gvolatiles−1 because the inner biomass moisture is not consumed, whereas for 30 gcat min gvolatiles−1 the water reacted accounts for 522 g kgbiomass−1 and the H2 produced for 117 g kgbiomass−1.
The influence of space time on gas fraction composition is shown in Fig. 5b. As observed, the main effect of increasing space time is an increase in H2 and CO2 concentration and a decrease in that of CO. In addition, a remarkable improvement of CH4 conversion is also observed.
 | ||
| Fig. 6 Effect of S/B ratio on conversion. Reforming conditions: 600 °C; space time, 20 gcat min gvolatiles−1. | ||
Furthermore, as observed in Fig. 7a, the effect of S/B ratio on the yields of the individual compounds is more noticeable. Thus, an increase in S/B ratio causes a steady increase in H2 and CO2 yields and a reduction in those of CO and CH4. An increase in the steam partial pressure in the reaction environment enhances steam reforming reaction kinetics (eqn (3) and (6)), as well as the displacement of the WGS reaction equilibrium (eqn (5)). Thus, H2 yield increases from 89.2% to 94.2% when S/B ratio is raised from 2 to 5, although a similar conversion is attained in both experiments. In the same line, the yield of CO2 increases from 84.0 to 90.1%, whereas the opposite trend is observed for CO, i.e., a decrease from 14.4 to 8.9%. The same effect of S/B ratio on product yields has been reported by other authors in the reforming of bio-oil and pyrolysis vapours20,52,61 and in the biomass steam gasification.48,63
 | ||
| Fig. 7 Effect of S/B ratio on the yields of the gaseous products (a) and their concentrations (on a dry basis) (b). Reforming conditions: 600 °C; space time, 20 gcat min gvolatiles−1. | ||
Although process conversion efficiency improves for high S/B ratios and reduces coke formation,5 this parameter should be carefully optimized bearing in mind energy efficiency,20 i.e., high S/B ratios require high amounts of steam to be produced and unreacted steam to be condensed at the outlet of the reformer. The optimum S/B ratios determined for biomass steam gasification are in the 0.6–0.85 range,64 but those for the pyrolysis-reforming strategy should be higher due to the higher steam consumption.
As observed in Fig. 7b, the effect of S/B ratio on the concentration of gaseous products is qualitatively similar to the effect on product yields. H2 concentration increases with S/B ratio to a value of 66.1 vol% for an S/B value of 5. However, CO content in the gas decreases from 5.3 to 3.0 vol% in the S/B ratio range studied. The CO2 concentration does not follow a clear trend, with its concentration being between 30 and 32 vol%. The content of CH4 and other gaseous hydrocarbons is very low due to the relatively high space time used.
Conclusions
The pyrolysis of biomass in a CSBR and in-line reforming in a fluidised bed reactor has proven to be a feasible alternative for the production of H2. The separation of pyrolysis and reforming steps has several practical advantages, both from an operational point of view and from the catalytic reforming performance. Moreover, steam atmosphere in the pyrolysis has a limited effect on product distribution, with the results being similar to those previously obtained using N2 as fluidising agent.The Ni commercial catalyst is highly active for the reforming of biomass pyrolysis volatiles. A minimum temperature of 600 °C and a space time of 20 gcat min gvolatiles−1 are required to attain complete conversion with an S/B ratio of 4. Once this temperature has been reached, a further increase to 700 °C showed a limited effect on product yield and composition, with H2 production being 110 g kgbiomass−1 in this temperature range.
An increase in space time enhances both the reforming and the WGS reaction, leading to an increase in the yields of H2 and CO2. Thus, at the highest space time studied, a H2 yield of 95.8% of the maximum allowable by stoichiometry was obtained. Similarly, the main effect of increasing the S/B ratio is the shifting of the reforming and WGS reactions, thereby improving H2 yield. However, an increase in this ratio involves higher heating requirements in the process.
Acknowledgements
This work was carried out with financial support from the Ministry of Economy and Competitiveness of the Spanish Government (CTQ2013-45105 R and CTQ2014-59574-JIN), the EDRF funds, the Basque Government (IT748-13) and the University of the Basque Country (UFI 11/39).References
- H. Balat and E. Kirtay, Hydrogen from biomass - Present scenario and future prospects, Int. J. Hydrogen Energy, 2010, 35, 7416–7426 CrossRef CAS.
- A. A. Ahmad, N. A. Zawawi, F. H. Kasim, A. Inayat and A. Khasri, Assessing the gasification performance of biomass: A review on biomass gasification process conditions, optimization and economic evaluation, Renewable Sustainable Energy Rev., 2016, 53, 1333–1347 CrossRef CAS.
- S. Anis and Z. A. Zainal, Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review, Renewable Sustainable Energy Rev., 2011, 15, 2355–2377 CrossRef CAS.
- P. J. Woolcock and R. C. Brown, A review of cleaning technologies for biomass-derived syngas, Biomass Bioenergy, 2013, 52, 54–84 CrossRef CAS.
- R. Trane, S. Dahl, M. S. Skjoth-Rasmussen and A. D. Jensen, Catalytic steam reforming of bio-oil, Int. J. Hydrogen Energy, 2012, 37, 6447–6472 CrossRef CAS.
- M. Amutio, G. Lopez, M. Artetxe, G. Elordi, M. Olazar and J. Bilbao, Influence of temperature on biomass pyrolysis in a conical spouted bed reactor, Resour., Conserv. Recycl., 2012, 59, 23–31 CrossRef.
- Z. Ma, L. Wei, W. Zhou, L. Jia, B. Hou, D. Li and Y. Zhao, Overview of catalyst application in petroleum refinery for biomass catalytic pyrolysis and bio-oil upgrading, RSC Adv., 2015, 5, 88287–88297 RSC.
- A. A. Lemonidou, P. Kechagiopoulos, E. Heracleous and S. Voutetakis, Steam Reforming of Bio-oils to Hydrogen, The Role of Catalyst for the Sustainaible Production of Bio-Fuels and Bio-Chemicals, 2013, pp. 467–493 Search PubMed.
- A. Demirbas, Competitive liquid biofuels from biomass, Appl. Energy, 2011, 88, 17–28 CrossRef CAS.
- S. Czernik and R. French, Distributed production of hydrogen by auto-thermal reforming of fast pyrolysis bio-oil, Int. J. Hydrogen Energy, 2014, 39, 744–750 CrossRef CAS.
- F. Bimbela, M. Oliva, J. Ruiz, L. García and J. Arauzo, Hydrogen production via catalytic steam reforming of the aqueous fraction of bio-oil using nickel-based coprecipitated catalysts, Int. J. Hydrogen Energy, 2013, 38, 14476–14487 CrossRef CAS.
- B. Valle, A. Remiro, A. T. Aguayo, J. Bilbao and A. G. Gayubo, Catalysts of Ni/Al2O3 and Ni/La2O3-Al2O3 for hydrogen production by steam reforming of bio-oil aqueous fraction with pyrolytic lignin retention, Int. J. Hydrogen Energy, 2013, 38, 1307–1318 CrossRef CAS.
- P. N. Kechagiopoulos, S. S. Voutetakis, A. A. Lemonidou and I. A. Vasalos, Hydrogen production via reforming of the aqueous phase of bio-oil over Ni/olivine catalysts in a spouted bed reactor, Ind. Eng. Chem. Res., 2009, 48, 1400–1408 CrossRef CAS.
- T. Namioka, A. Saito, Y. Inoue, Y. Park, T. j. Min, S. a. Roh and K. Yoshikawa, Hydrogen-rich gas production from waste plastics by pyrolysis and low-temperature steam reforming over a ruthenium catalyst, Appl. Energy, 2011, 88, 2019–2026 CrossRef CAS.
- J. Alvarez, S. Kumagai, C. Wu, T. Yoshioka, J. Bilbao and M. Olazar, Hydrogen production from biomass and plastic mixtures by pyrolysis-gasification, Int. J. Hydrogen Energy, 2014, 39, 10883–10891 CrossRef CAS.
- M. A. Nahil, X. Wang, C. Wu, H. Yang, H. Chen and P. T. Williams, Novel bi-functional Ni-Mg-Al-CaO catalyst for catalytic gasification of biomass for hydrogen production with in situ CO2 adsorption, RSC Adv., 2013, 3, 5583–5590 RSC.
- A. K. Olaleye, K. J. Adedayo, C. Wu, M. A. Nahil, M. Wang and P. T. Williams, Experimental study, dynamic modelling, validation and analysis of hydrogen production from biomass pyrolysis/gasification of biomass in a two-stage fixed bed reaction system, Fuel, 2014, 137, 364–374 CrossRef CAS.
- M. Koike, C. Ishikawa, D. Li, L. Wang, Y. Nakagawa and K. Tomishige, Catalytic performance of manganese-promoted nickel catalysts for the steam reforming of tar from biomass pyrolysis to synthesis gas, Fuel, 2013, 103, 122–129 CrossRef CAS.
- L. Wang, D. Li, M. Koike, S. Koso, Y. Nakagawa, Y. Xu and K. Tomishige, Catalytic performance and characterization of Ni-Fe catalysts for the steam reforming of tar from biomass pyrolysis to synthesis gas, Appl. Catal., A, 2011, 392, 248–255 CrossRef CAS.
- X. Xiao, X. Meng, D. D. Le and T. Takarada, Two-stage steam gasification of waste biomass in fluidized bed at low temperature: Parametric investigations and performance optimization, Bioresour. Technol., 2011, 102, 1975–1981 CrossRef CAS PubMed.
- X. Xiao, J. Cao, X. Meng, D. D. Le, L. Li, Y. Ogawa, K. Sato and T. Takarada, Synthesis gas production from catalytic gasification of waste biomass using nickel-loaded brown coal char, Fuel, 2013, 103, 135–140 CrossRef CAS.
- Z. Ma, S. Zhang, D. Xie and Y. Yan, A novel integrated process for hydrogen production from biomass, Int. J. Hydrogen Energy, 2014, 39, 1274–1279 CrossRef CAS.
- A. Erkiaga, G. Lopez, I. Barbarias, M. Artetxe, M. Amutio, J. Bilbao and M. Olazar, HDPE pyrolysis-steam reforming in a tandem spouted bed-fixed bed reactor for H2 production, J. Anal. Appl. Pyrolysis, 2015, 116, 34–41 CrossRef CAS.
- A. Remiro, B. Valle, B. Aramburu, A. T. Aguayo, J. Bilbao and A. G. Gayubo, Steam reforming of the bio-oil aqueous fraction in a fluidized bed reactor with in situ CO2 capture, Ind. Eng. Chem. Res., 2013, 52, 17087–17098 CrossRef CAS.
- P. Lan, Q. Xu, M. Zhou, L. Lan, S. Zhang and Y. Yan, Catalytic steam reforming of fast pyrolysis bio-oil in fixed bed and fluidized bed reactors, Chem. Eng. Technol., 2010, 33, 2021–2028 CrossRef CAS.
- M. Amutio, G. Lopez, R. Aguado, J. Bilbao and M. Olazar, Biomass Oxidative Flash Pyrolysis: Autothermal Operation, Yields and Product Properties, Energy Fuels, 2012, 26, 1353–1362 CrossRef CAS.
- J. Makibar, A. R. Fernandez-Akarregi, M. Amutio, G. Lopez and M. Olazar, Performance of a conical spouted bed pilot plant for bio-oil production by poplar flash pyrolysis, Fuel Process. Technol., 2015, 137, 283–289 CrossRef CAS.
- G. Lopez, A. Erkiaga, M. Artetxe, M. Amutio, J. Bilbao and M. Olazar, Hydrogen Production by High Density Polyethylene Steam Gasification and In-Line Volatile Reforming, Ind. Eng. Chem. Res., 2015, 54, 9536–9544 CrossRef CAS.
- J. Alvarez, M. Amutio, G. Lopez, I. Barbarias, J. Bilbao and M. Olazar, Sewage sludge valorization by flash pyrolysis in a conical spouted bed reactor, Chem. Eng. J., 2015, 273, 173–183 CrossRef CAS.
- A. Erkiaga, G. Lopez, M. Amutio, J. Bilbao and M. Olazar, Steam gasification of biomass in a conical spouted bed reactor with olivine and γ-alumina as primary catalysts, Fuel Process. Technol., 2013, 116, 292–299 CrossRef CAS.
- A. Erkiaga, G. Lopez, M. Amutio, J. Bilbao and M. Olazar, Syngas from steam gasification of polyethylene in a conical spouted bed reactor, Fuel, 2013, 109, 461–469 CrossRef CAS.
- M. Artetxe, G. Lopez, G. Elordi, M. Amutio, J. Bilbao and M. Olazar, Production of Light Olefins from Polyethylene in a Two-Step Process: Pyrolysis in a Conical Spouted Bed and Downstream High-Temperature Thermal Cracking, Ind. Eng. Chem. Res., 2012, 51, 13915–13923 CrossRef CAS.
- G. Lopez, M. Olazar, M. Amutio, R. Aguado and J. Bilbao, Influence of Tire Formulation on the Products of Continuous Pyrolysis in a Conical Spouted Bed Reactor, Energy Fuels, 2009, 23, 5423–5431 CrossRef CAS.
- G. Lopez, M. Olazar, R. Aguado, G. Elordi, M. Amutio, M. Artetxe and J. Bilbao, Vacuum Pyrolysis of Waste Tires by Continuously Feeding into a Conical Spouted Bed Reactor, Ind. Eng. Chem. Res., 2010, 49, 8990–8997 CrossRef CAS.
- E. Kantarelis, W. Yang and W. Blasiak, Production of liquid feedstock from biomass via steam pyrolysis in a fluidized bed reactor, Energy Fuels, 2013, 27, 4748–4759 CrossRef CAS.
- P. Mellin, E. Kantarelis, C. Zhou and W. Yang, Simulation of bed dynamics and primary products from fast pyrolysis of biomass: Steam compared to nitrogen as a fluidizing agent, Ind. Eng. Chem. Res., 2014, 53, 12129–12142 CrossRef CAS.
- J. Alvarez, G. Lopez, M. Amutio, J. Bilbao and M. Olazar, Upgrading the rice husk char obtained by flash pyrolysis for the production of amorphous silica and high quality activated carbon, Bioresour. Technol., 2014, 170, 132–137 CrossRef CAS PubMed.
- S. Wang, B. Gao, Y. Li, Y. Wan and A. E. Creamer, Sorption of arsenate onto magnetic iron-manganese (Fe-Mn) biochar composites, RSC Adv., 2015, 5, 67971–67978 RSC.
- M. Uchimiya, S. Hiradate and M. J. Antal, Dissolved Phosphorus Speciation of Flash Carbonization, Slow Pyrolysis, and Fast Pyrolysis Biochars, ACS Sustainable Chem. Eng., 2015, 3, 1642–1649 CrossRef CAS.
- C. M. Dominguez, P. Ocon, A. Quintanilla, J. A. Casas and J. J. Rodriguez, Highly efficient application of activated carbon as catalyst for wet peroxide oxidation, Appl. Catal., B, 2013, 140–141, 663–670 CrossRef CAS.
- S. Ren, H. Lei, L. Wang, Q. Bu, S. Chen and J. Wu, Hydrocarbon and hydrogen-rich syngas production by biomass catalytic pyrolysis and bio-oil upgrading over biochar catalysts, RSC Adv., 2014, 4, 10731–10737 RSC.
- Y. Shen, C. Areeprasert, B. Prabowo, F. Takahashi and K. Yoshikawa, Metal nickel nanoparticles in situ generated in rice husk char for catalytic reformation of tar and syngas from biomass pyrolytic gasification, RSC Adv., 2014, 4, 40651–40664 RSC.
- C. R. Smith, E. M. Buzan and J. W. Lee, Potential impact of biochar water-extractable substances on environmental sustainability, ACS Sustainable Chem. Eng., 2013, 1, 118–126 CAS.
- S. Czernik, R. Evans and R. French, Hydrogen from biomass-production by steam reforming of biomass pyrolysis oil, Catal. Today, 2007, 129, 265–268 CrossRef CAS.
- S. Koppatz, C. Pfeifer and H. Hofbauer, Comparison of the performance behaviour of silica sand and olivine in a dual fluidised bed reactor system for steam gasification of biomass at pilot plant scale, Chem. Eng. J., 2011, 175, 468–483 CrossRef CAS.
- K. Umeki, K. Yamamoto, T. Namioka and K. Yoshikawa, High temperature steam-only gasification of woody biomass, Appl. Energy, 2010, 87, 791–798 CrossRef CAS.
- R. Michel, S. Rapagna, P. Burg, d. C. Mazziotti, C. Courson, T. Zimny and R. Gruber, Steam gasification of Miscanthus X Giganteus with olivine as catalyst production of syngas and analysis of tars (IR, NMR and GC/MS), Biomass Bioenergy, 2011, 35, 2650–2658 CrossRef CAS.
- A. Erkiaga, G. Lopez, M. Amutio, J. Bilbao and M. Olazar, Influence of operating conditions on the steam gasification of biomass in a conical spouted bed reactor, Chem. Eng. J., 2014, 237, 259–267 CrossRef CAS.
- S. Rapagna, M. Virginie, K. Gallucci, C. Courson, M. Di Marcello, A. Kiennemann and P. U. Foscolo, Fe/olivine catalyst for biomass steam gasification: Preparation, characterization and testing at real process conditions, Catal. Today, 2011, 176, 163–168 CrossRef CAS.
- R. Michel, S. Rapagna, M. Di Marcello, P. Burg, M. Matt, C. Courson and R. Gruber, Catalytic steam gasification of Miscanthus X giganteus in fluidised bed reactor on olivine based catalysts, Fuel Process. Technol., 2011, 92, 1169–1177 CrossRef CAS.
- C. Rioche, S. Kulkarni, F. C. Meunier, J. P. Breen and R. Burch, Steam reforming of model compounds and fast pyrolysis bio-oil on supported noble metal catalysts, Appl. Catal., B, 2005, 61, 130–139 CrossRef CAS.
- A. Remiro, B. Valle, A. T. Aguayo, J. Bilbao and A. G. Gayubo, Steam reforming of raw bio-oil in a fluidized bed reactor with prior separation of pyrolytic lignin, Energy Fuels, 2013, 27, 7549–7559 CrossRef CAS.
- E. Salehi, F. S. Azad, T. Harding and J. Abedi, Production of hydrogen by steam reforming of bio-oil over Ni/Al2O3 catalysts: Effect of addition of promoter and preparation procedure, Fuel Process. Technol., 2011, 92, 2203–2210 CrossRef CAS.
- F. Chen, C. Wu, L. Dong, A. Vassallo, P. T. Williams and J. Huang, Characteristics and catalytic properties of Ni/CaAlOx catalyst for hydrogen-enriched syngas production from pyrolysis-steam reforming of biomass sawdust, Appl. Catal., B, 2016, 183, 168–175 CrossRef CAS.
- T. Kan, J. Xiong, X. Li, T. Ye, L. Yuan, Y. Torimoto, M. Yamamoto and Q. Li, High efficient production of hydrogen from crude bio-oil via an integrative process between gasification and current-enhanced catalytic steam reforming, Int. J. Hydrogen Energy, 2010, 35, 518–532 CrossRef CAS.
- J. Remón, F. Broust, G. Volle, L. García and J. Arauzo, Hydrogen production from pine and poplar bio-oils by catalytic steam reforming. Influence of the bio-oil composition on the process, Int. J. Hydrogen Energy, 2015, 40, 5593–5608 CrossRef.
- R. Trane-Restrup and A. D. Jensen, Steam reforming of cyclic model compounds of bio-oil over Ni-based catalysts: Product distribution and carbon formation, Appl. Catal., B, 2015, 165, 117–127 CrossRef CAS.
- S. Wang, Q. Cai, F. Zhang, X. Li, L. Zhang and Z. Luo, Hydrogen production via catalytic reforming of the bio-oil model compounds: Acetic acid, phenol and hydroxyacetone, Int. J. Hydrogen Energy, 2014, 39, 18675–18687 CrossRef CAS.
- X. Hu and G. Lu, Investigation of the steam reforming of a series of model compounds derived from bio-oil for hydrogen production, Appl. Catal., B, 2009, 88, 376–385 CrossRef CAS.
- C. Wu, M. Sui and Y. Yan, A comparison of steam reforming of two model bio-oil fractions, Chem. Eng. Technol., 2008, 31, 1748–1753 CrossRef.
- P. Fu, W. Yi, Z. Li, X. Bai, A. Zhang, Y. Li and Z. Li, Investigation on hydrogen production by catalytic steam reforming of maize stalk fast pyrolysis bio-oil, Int. J. Hydrogen Energy, 2014, 39, 13962–13971 CrossRef CAS.
- F. Seyedeyn-Azad, E. Salehi, J. Abedi and T. Harding, Biomass to hydrogen via catalytic steam reforming of bio-oil over Ni-supported alumina catalysts, Fuel Process. Technol., 2011, 92, 563–569 CrossRef CAS.
- K. Goransson, U. Soderlind and W. Zhang, Experimental test on a novel dual fluidised bed biomass gasifier for synthetic fuel production, Fuel, 2011, 90, 1340–1349 CrossRef.
- P. Kaushal and R. Tyagi, Steam assisted biomass gasification-an overview, Can. J. Chem. Eng., 2012, 90, 1043–1058 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |



