Microwave assisted non-solvothermal synthesis of metal–organic frameworks†
Abstract
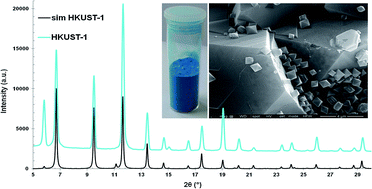
A new method for the preparation of HKUST-1 using a microwave-assisted non-solvothermal synthesis is presented. The influence of the reaction parameters (concentration of reactant mixtures, solvent, temperature, reaction time and microwave power) on the material’s textural properties and yields has been investigated and the synthetic method was optimized. By exposing the reaction mixture to microwaves for up to 10 minutes HKUST-1 with a surface area and pore volume close to the theoretical values and a yield of about 70% was obtained. In addition, yields could reach around 90% if the product formed in the mother liquor is counted.

 Please wait while we load your content...
Please wait while we load your content...