Easy and versatile coating approach for long-living white hybrid light-emitting diodes†
Lukas
Niklaus
a,
Haider
Dakhil
bc,
Monika
Kostrzewa
b,
Pedro B.
Coto
d,
Uwe
Sonnewald
e,
Andreas
Wierschem
b and
Rubén D.
Costa
*a
aDepartment of Chemistry & Pharmacy, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Egerlandstr. 3, D-91058 Erlangen, Germany. E-mail: ruben.costa@fau.de
bInstitute of Fluid Mechanics, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Cauerstr. 4, D-91058 Erlangen, Germany
cFaculty of Engineering, University of Kufa, Kufa P.O. Box 21, 00964 Najaf, Iraq
dInstitut für Theoretische Physik, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Staudtstr. 7/B2, D-91058 Erlangen, Germany
eDepartment of Biology, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Staudtstr. 5, D-91058 Erlangen, Germany
First published on 25th April 2016
Abstract
Herein, we provide a new easy-to-do protocol for preparing luminescent rubber-like materials based on a wide palette of compounds, such as small-molecules, quantum dots, polymers, and coordination complexes. The combination of this new protocol with that for preparing similar rubbers based on fluorescent proteins states the universal character of our approach. This is further assessed by using comprehensive spectroscopic and rheological investigations. Furthermore, the novel luminescent rubbers are applied as down-converting packing systems to develop white hybrid light-emitting diodes (WHLEDs), which are heralded as a solid alternative to achieve energy-saving, solid-state, and white-emitting sources in the coming future. As such, the current work also provides a clear prospect of this emerging lighting technology by means of a direct comparison among WHLEDs fabricated with all the above-mentioned down-converting systems. Here, the use of rubbers based on coordination complexes outperforms the others in terms of both luminous efficiency and colour quality with an unprecedented stability superior to 1000 h under continuous operation conditions. This represents an order of magnitude enhancement compared to the state-of-the-art WHLEDs, while keeping luminous efficiencies of around 100 lm W−1.
Conceptual insightsWhite hybrid light-emitting diodes (WHLEDs) combine an inorganic LED with organic down-converting packings, which are easy-to-prepare and eco-friendly. Currently, the bottleneck of WHLEDs is to find a universal packing matrix and the best organic down-converting compounds in order to maximize the device performance. Herein, we demonstrate a versatile and easy-to-do approach for rubber-like packings, in which – for the first time – a wide variety of down-converting materials like small-molecules, quantum dots, polymers, and coordination complexes can be implemented and directly compared in WHLEDs. This protocol is not based on UV- or thermal-curable crosslinking approaches or adsorption procedures into the matrix as reported so far. As such, WHLEDs with unprecedented stabilities of more than 1000 h – extrapolated 4000 h – with luminous efficiencies of 100 lm W−1 and no colour degradation can be obtained. |
Introduction
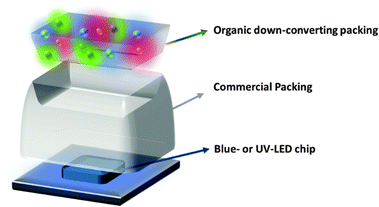
The development of efficient and stable white solid-state lighting sources is one of the key technological research forefronts, as incandescent light bulbs and fluorescent lamps have reached their limit in terms of balancing luminous efficiency, stability, and environmental/recycling issues.1 Two main alternatives are almost ready-to-go towards the next generation of sustainable bulbs. On one hand, organic light-emitting diodes (OLEDs) are a potential technology to provide flexible and thin lighting sources for screens and in-door luminaires.2 However, despite efforts during the last 20 years, white OLEDs still show a clear trade-off in terms of low-cost production and high performance.2 On the other hand, inorganic white light-emitting diodes (WLEDs) have been strongly developed by both industry and scientific communities since the pioneering works on blue-LEDs by Akasaki, Amano, and Nakamura et al. in the early 90's.3 Generally speaking, the chip of the blue- or UV-emitting LEDs is coated with inorganic down-converting phosphors based on rare-earth elements like the archetypal Y3Al5O12; YAG:Ce and its derivatives.4 As a result, the combination of the LED emission and that of the down-converting coating leads to WLEDs featuring high luminous efficiencies and stabilities when the packing system is optimized. Their main drawbacks are (i) the high production cost due to the use of rare earth crust materials, (ii) the scarcity of efficient deep-red emitters that compromises the colour quality of the white emission, and (iii) the lack of efficient protocols to recycle these materials. Thus, this strategy barely addresses the basis of green economics in terms of ecological sustainability in concert with a low-cost production.1As an alternative, recent research has explored the possibility of using eco-friendly organic down-converting materials in the so-called white hybrid inorganic/organic LEDs (WHLEDs).5–8 Similar to WLEDs, the architecture of WHLEDs consists of a standard inorganic blue- or UV-LED, in which the encapsulation system is replaced by an organic-based down-converting material, which upon continuous excitation features a broad low-energy emission band – Fig. 1 and 2. This architecture has recently led to WHLEDs with high colour quality – i.e., commission international de l'Éclairage (CIE) coordinates of 0.30–3/0.30–3, colour rendering index (CRI) above 90, and correlated colour temperature (CCT) between 2500–6500 K, but still with low stabilities of around 100 h due to degradation of the luminescent material, the matrix, or both upon continuous excitation under ambient conditions.5–8
Up to date, there are four different approaches to develop down-converting coatings. Firstly, thin films, which consist of a mixture of organic materials with UV- or thermal-curable sealing reagents, are typically deposited either onto a glass substrate or on top of the packing of the LED – Fig. 2.5 Several authors have shown that both the degradation of the down-converting materials under continuous excitation and a phase separation in the morphology of the coating upon preparation are common factors that limit the stability of the WHLEDs up to values of a few hours.5i,k,l However, strategies based on pre-encapsulating the luminescent material or increasing the gap between the LED and the down-converting coating provide stabilities of approximately hundred hours.5d,o,q Secondly, an interesting alternative approach was reported by Li and Su et al. in 2013.6a The authors proposed the use of metal organic frameworks (MOFs) that show high-energy emission features and pores of tunable sizes, in which one or a mixture of several down-converting materials can be embedded – Fig. 2.6 As such, the inorganic LED excites either both the MOF and the adsorbed organic moiety or only the MOF that further transfers the energy to the organic moiety. More interesting, several groups have started to show that the MOF-based approach is compatible with quantum dots, coordination complexes, and small-molecules.6 Thus, it bears a great potential for commercial purposes. As state-of-the-art WHLEDs with the MOF encapsulation, white devices featuring CRI from 70 to 90 and luminous efficiency beyond 50 lm W−1 have been achieved, but still their stability has not been studied in depth. Thirdly, a new coating based on a cellulose derivative – Fig. 2, in which, for example, inorganic and graphitic quantum dots have been embedded, has led to new WHLEDs spanning the whole visible spectrum.7 Here, the devices have shown CIE coordinates of 0.33/0.37 and efficiency values up to 31.6 lm W−1, but the stability has not been reported yet. Lastly, we have recently developed a new down-converting coating method based on the mixture of fluorescent proteins with a combination of branched and linear polymers in water. The latter form a gel that is further transformed into a luminescent rubber-like material that is easily applied as a packing system to fabricate bio-WHLEDs.8 They show similar CRI and luminous efficiencies to those noted for the other approaches, along with an encouraging stability of less than 10% loss of efficiency after 120 h. Here, the instability of the bio-WHLEDs was solely attributed to the degradation of the red-emitting proteins. In this contribution, we demonstrate that our new rubber-like encapsulation method can be easily modified to implement a wide variety of down-converting materials that span small-molecules, quantum dots, polymers, and coordination complexes. This is further supported by spectroscopic studies to determine the changes of the photoluminescence features of the down-converting materials embedded in the rubbers and by rheological assays to elucidate the mechanical properties of both the gels and rubbers. Finally, this work provides a roadmap for future developments, since a direct comparison between WHLEDs based on the above-mentioned rubbers is provided.
As the most remarkable result, the use of coordination complexes stands up among the others, featuring unprecedented stabilities of more than 1000 h with a slight loss of luminous efficiency and no colour degradation. The latter is further extrapolated to around 4000 h, representing more than one order of magnitude enhancement in stability compared to the state-of-the-art WHLEDs. Hence, we strongly believe that the combination of high luminous efficiency (100 lm W−1) and stabilities of thousands of hours highlight the versatility and potentiality of our approach for the development of WHLEDs for low- and mid-power applications.
Results and discussion
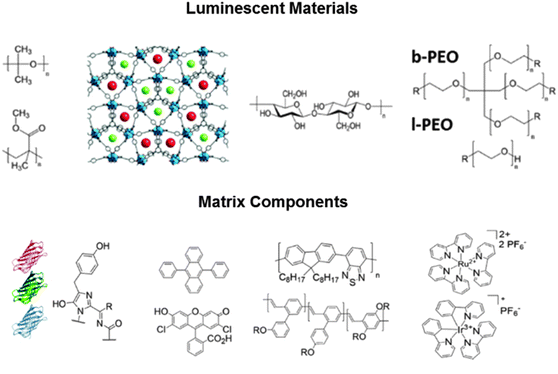
As previously reported for bio-WHLEDs, the composition of the matrix – i.e., as shown in Fig. 2, branched and linear poly(ethylene oxide) compounds (b- and l-PEO, respectively) in different mass ratios – was optimized to form gels and rubber materials after mixing them with fluorescent proteins diluted in an aqueous media.8 Without the addition of water, neither the gel nor the rubber are formed. This could limit the versatility of this concept, as only compounds soluble in water could be applied. To challenge this statement, several solvents ranging from polar protic, to polar aprotic, and to nonpolar were used for the preparation of both gels and rubbers. Here, the gels were formed by mixing b- and l-PEO with different amounts of solvents. Upon strong stirring (750 or 1500 rpm) under ambient conditions over night, this mixture becomes a gel. The mass ratio is optimized for the formation of a gel-like material that allows the further film forming via doctor-blading onto any kind of substrate like, for example, glass slides. Independently of the amount of solvent employed, the composition of the b- and l-PEO mixtures, and the stirring conditions, only acetonitrile and water turned out to be suitable for forming homogenous gels – Table S1 and Fig. S1 (ESI†). Similar to water-based gels,8 the viscous properties of the acetonitrile-based gels allow an excellent handling for coating purposes. Indeed, the viscosity can be controlled by modifying the amount of the l-PEO – Fig. S2 (ESI†). The mixture b-PEO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) l-PEO = 6
l-PEO = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt with 150 μL of acetonitrile was chosen for the preparation of soft-films onto glass slides by means of a doctor-blading technique. Upon a drying process – i.e., a solvent loss of <1 wt% – under gentle vacuum conditions the soft-films transform into a rubber material – see ESI.† The final materials are best described as rubbers, which are easily peeled off from the substrate with a tweezer and can be transferred to another substrate. Thicknesses of up to the millimetre regime with a low average roughness value (<10%) are easily achieved by sequential repetition of doctor-blading and drying processes – Fig. S3 (ESI†). Rheology assays show that both water- and acetonitrile-based rubbers feature similar values for the storage (G′) and loss (G′′) moduli, which quantify the elastic and the viscous material behaviour, respectively. The only exception are the rubbers with the highest l-PEO content (3
1 wt with 150 μL of acetonitrile was chosen for the preparation of soft-films onto glass slides by means of a doctor-blading technique. Upon a drying process – i.e., a solvent loss of <1 wt% – under gentle vacuum conditions the soft-films transform into a rubber material – see ESI.† The final materials are best described as rubbers, which are easily peeled off from the substrate with a tweezer and can be transferred to another substrate. Thicknesses of up to the millimetre regime with a low average roughness value (<10%) are easily achieved by sequential repetition of doctor-blading and drying processes – Fig. S3 (ESI†). Rheology assays show that both water- and acetonitrile-based rubbers feature similar values for the storage (G′) and loss (G′′) moduli, which quantify the elastic and the viscous material behaviour, respectively. The only exception are the rubbers with the highest l-PEO content (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt), where the water-based rubbers show a higher mechanical stability than the acetonitrile-based ones – Fig. S4 (ESI†).
1 wt), where the water-based rubbers show a higher mechanical stability than the acetonitrile-based ones – Fig. S4 (ESI†).
The linear viscoelastic regime of both rubbers is restricted to less than 1% strain. Here, G′ is always larger than G′′, but of the same order of magnitude. Frequency sweeps in the linear viscoelastic range revealed only a small frequency dependence, as it is typical for rubber-like materials. In general, the increase of the l-PEO content leads to an enhancement of G′ and G′′ values – Fig. S4 (ESI†). Finally, the refractive index of both rubbers was superior to 1.8, which is close to the ideal one for encapsulation materials used in LEDs like silicone.9 Thus, the new acetonitrile-based rubber is also suitable for encapsulation purposes in the preparation of WHLEDs.
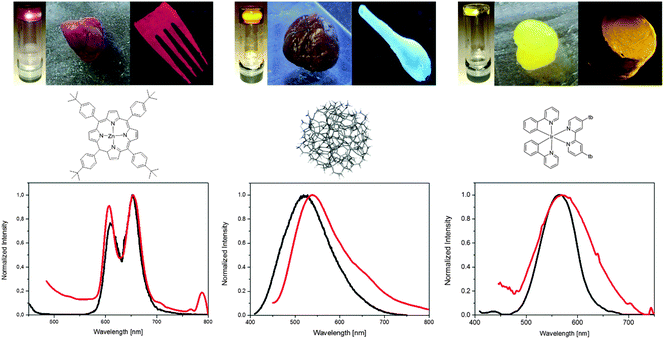
Next, we exploit the possibility to use both water and acetonitrile solvents to integrate a wide palette of luminescent materials into the rubbers. To this end, we selected commercial luminescent materials with different emission wavelength (λem) – see ESI,† namely (i) small-molecules like Coumarin 334 (1, λem = 496 nm), Fluorescein 27 (2, λem = 544 nm), zinc-tetraphenylporphyrin (ZnTPP) (3, λem = 609, 624, 652 nm), (ii) water soluble yellowish orange emitting carbon-based quantum dots (4, λem = 450 nm (λexc = 310 nm); λem = 519 nm (λexc = 390 nm); 549 nm (λexc = 450 nm)),10 (iii) an emitting polymer like super yellow (5, λem = 550 nm), and (iv) coordination complexes such as [Ir(ppy)2(acac)] (6, λem = 470, 490 nm) and [Ir(ppy)2(tb-bpy)][PF6] (7, λem = 570 nm) where ppy is 2-phenylpyridine, acac is acetylacetone, and tb-bpy is 4,4-di-tert-butyl-2,2-dipyridyl. Their chemical structures are shown in Fig. 3 and Fig. S5 (ESI†).
The gels were formed by mixing b-PEO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) l-PEO in a ratio of 6
l-PEO in a ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt and 1 mg of each luminescent materials with 150 μL of acetonitrile for 1–3/5–7 and water for 4. Fig. 3 and Fig. S6 (ESI†) display the comparison of the normalized absorption and emission spectra in both solutions and rubbers, indicating that, in general, there is no degradation of the compounds upon rubber formation. However, a strong interaction between the small-molecules and graphitic quantum dots with the matrix in the rubber is highlighted by the red shifted (5–15 nm) absorption and emission spectra, as well as the decrease of the excited-state lifetimes, pointing to a quenching of the emission – Fig. S6 and Table S2 (ESI†). On the contrary, the encapsulation of polymers and coordination complexes into the rubber matrix increases their excited-state lifetimes in minor and major fashions, respectively – Table S2 (ESI†). This is quite likely related to the effective encapsulation of compounds into the matrix preventing the well-known emission quenching of ambient oxygen. As such, the matrix seems to be more suited for the luminescent polymers and coordination complexes. The addition of the luminescent materials does not have a major impact on both the formation and characteristics of the rubber materials in terms of the refractive index (>1.8) and the rheological parameters – i.e., G′ and G′′ as shown in Fig. S7 (ESI†). Finally, we investigate the stability of the rubbers by monitoring the absorption features of the organic compounds under different scenarios, such as room conditions – i.e., storage stability, upon irradiation with a UV lamp (310 nm, 8 W) under ambient conditions – i.e., irradiation stability, and under a heating ramp ranging from room temperature to 120 °C with 20 °C steps under ambient conditions – i.e., thermal stability. As shown in Fig. S8–S10 (ESI†), all rubbers show excellent storage stabilities over 40 days, while the irradiation stability is also sound for the rubbers based on the coordination complexes and quantum dots, but not for those containing small-molecules and polymers, which degrade after a few hours. Moreover, the changes in the absorption features upon heating clearly indicate that all rubbers are stable up to temperatures of 100 °C.
1 wt and 1 mg of each luminescent materials with 150 μL of acetonitrile for 1–3/5–7 and water for 4. Fig. 3 and Fig. S6 (ESI†) display the comparison of the normalized absorption and emission spectra in both solutions and rubbers, indicating that, in general, there is no degradation of the compounds upon rubber formation. However, a strong interaction between the small-molecules and graphitic quantum dots with the matrix in the rubber is highlighted by the red shifted (5–15 nm) absorption and emission spectra, as well as the decrease of the excited-state lifetimes, pointing to a quenching of the emission – Fig. S6 and Table S2 (ESI†). On the contrary, the encapsulation of polymers and coordination complexes into the rubber matrix increases their excited-state lifetimes in minor and major fashions, respectively – Table S2 (ESI†). This is quite likely related to the effective encapsulation of compounds into the matrix preventing the well-known emission quenching of ambient oxygen. As such, the matrix seems to be more suited for the luminescent polymers and coordination complexes. The addition of the luminescent materials does not have a major impact on both the formation and characteristics of the rubber materials in terms of the refractive index (>1.8) and the rheological parameters – i.e., G′ and G′′ as shown in Fig. S7 (ESI†). Finally, we investigate the stability of the rubbers by monitoring the absorption features of the organic compounds under different scenarios, such as room conditions – i.e., storage stability, upon irradiation with a UV lamp (310 nm, 8 W) under ambient conditions – i.e., irradiation stability, and under a heating ramp ranging from room temperature to 120 °C with 20 °C steps under ambient conditions – i.e., thermal stability. As shown in Fig. S8–S10 (ESI†), all rubbers show excellent storage stabilities over 40 days, while the irradiation stability is also sound for the rubbers based on the coordination complexes and quantum dots, but not for those containing small-molecules and polymers, which degrade after a few hours. Moreover, the changes in the absorption features upon heating clearly indicate that all rubbers are stable up to temperatures of 100 °C.
Finally, a direct comparison of the stability of the luminescent compounds between the solutions and the rubbers under UV irradiation is shown in Fig. S11 (ESI†), indicating that the matrix further stabilizes all the compounds. This is clearly noted for the small-molecules and polymers, while especially the carbon-based quantum dots and the coordination complexes show a sound irradiation stability in both solutions and rubbers. Hence, the interaction between the rubber components and the down-converting materials is beneficial in terms of stability and photophysical features.
Taking these findings into account, we fabricated white-emitting HLEDs combining a blue-LED – i.e., maximum 450 nm – with a cascade coating system combining rubbers with small-molecules (SM-WHLEDs), quantum dots (QDs-WHLEDs), polymers (P-WHLEDs), and coordination complexes (CC-WHLEDs) – see ESI.† The preparation of the WHLEDs concerns a two-step procedure. Firstly, the gels are deposited onto the LED wetting the complete surface. Secondly, the coated LED is transferred to the vacuum chamber under 1–10 mbar for less than 1 h. This procedure is repeated to enhance the light down-conversion efficiency of the WHLED. Independently of the thickness of the coating, it can be easily peeled off from the LED surface for a further analysis. Noteworthy, the optimization of the thickness of the luminescent down-converting coatings was realized to obtain the right balance between white colour quality and high luminous efficiency as shown in Fig. S12 (ESI†) and discussed below. As starting conditions, the performance of the WHLEDs was measured under dried N2 atmosphere and the most stable devices were afterwards measured under ambient conditions.
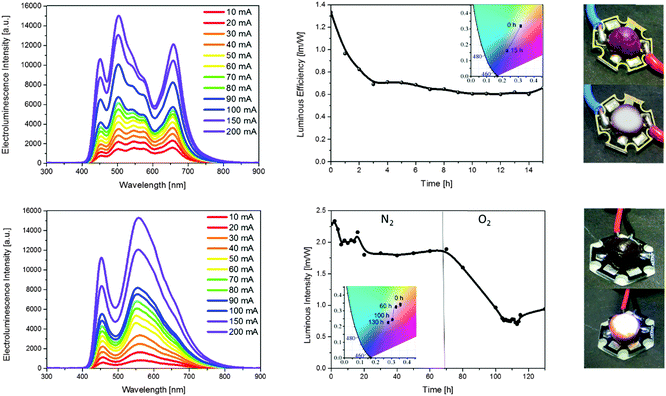
SM-WHLEDs feature an architecture of a blue-LED with a top coating based on 1 (0.14 mm)/2 (0.06 mm)/3 (0.10 mm). Upon increasing the driving current from 10 to 250 mA, the electroluminescence spectra clearly show distinguishable peaks for all small-molecules with a stable white colour with, for example, CIE coordinates of 0.35/0.35 to 0.28/0.28, CRI values of 93 and 78, and CCT of 4776 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 851 K for 10 and 250 mA, respectively – Fig. 4. Importantly, this is independent of the visual angle (0, 30, 45, 90°) as the coating homogenously covers the whole packing – Fig. 4. Next, the long-term stability of the device was studied under a driving current of 10 mA. As shown in Fig. 4 and Fig. S13 (ESI†), even at this mild operation condition and under inert atmosphere, the initial white light with CIE coordinates of 0.31/0.32 changes in a few hours towards the blue region with CIE of 0.23/0.16. Moreover, the CRI decreases from 77 to 64 and the CCT decreases from 6600 to 5900 K. Finally, the luminous efficiency significantly reduces due to changes in the emission spectrum – i.e., ca. 1 h with a loss >30%. This result is expected as the small-molecules show a sound photo-assisted degradation in both solutions and rubbers – Fig. S9 and S11 (ESI†). Thus, no further experiments were performed with this family of luminescent compounds.
851 K for 10 and 250 mA, respectively – Fig. 4. Importantly, this is independent of the visual angle (0, 30, 45, 90°) as the coating homogenously covers the whole packing – Fig. 4. Next, the long-term stability of the device was studied under a driving current of 10 mA. As shown in Fig. 4 and Fig. S13 (ESI†), even at this mild operation condition and under inert atmosphere, the initial white light with CIE coordinates of 0.31/0.32 changes in a few hours towards the blue region with CIE of 0.23/0.16. Moreover, the CRI decreases from 77 to 64 and the CCT decreases from 6600 to 5900 K. Finally, the luminous efficiency significantly reduces due to changes in the emission spectrum – i.e., ca. 1 h with a loss >30%. This result is expected as the small-molecules show a sound photo-assisted degradation in both solutions and rubbers – Fig. S9 and S11 (ESI†). Thus, no further experiments were performed with this family of luminescent compounds.
Not being able to obtain stable white lighting sources with small-molecules, we turned to investigate the QDs-WHLEDs with blue-LED/4 (0.1 mm). Similar to the SM-WHLEDs, white devices were easily achieved independently of the applied driving currents – Fig. 4. More interesting, the quality of the white colour was monitored over time under operation conditions and inert atmosphere, showing CIE coordinates of 0.33–2/0.32–0, CRI value of ∼90–95, and a CCT of 5500–6200 K for a time of ca. 20 h – Fig. 4 and Fig. S13 (ESI†). Beyond this operation time, the red contribution of the electroluminescence spectrum gets more prominent, changing the white quality with CIE coordinates of 0.36/0.32, CRI values of 90, and CCT values of 4100 K, as well as slightly reducing the luminous efficiency.
Although the changes of the photoluminescence behaviour of this type of materials under different excitations and environmental conditions – i.e., temperature, pH, irradiation, etc. – is still under debate,10 this might be related either to interactions of the outer substituents with the matrix that promote emission from trapping states or to a release of the peripheral substituents changing the core of the QDs. At this point, the QD-WHLED was probed under ambient conditions.
During the first 30 h, the electroluminescence spectra quickly evolved until a more balanced contribution in the yellow and red parts – Fig. 4 and Fig. S13 (ESI†), but with a more prominent blue component – i.e., CIE of 0.33/0.32, CRI of 94, and CCT of 5800 K. This also affects the luminous efficiency that further reduces – Fig. 4. After this point, the electroluminescence spectrum is constant. Thus, there are two downsides of using this type of material, namely the initial low luminous efficiency of around 2 lm W−1 that is related to the poor photoluminescence quantum yields and the changes of the electroluminescence spectrum over long periods of time. It is important to note that a proper design of graphitic quantum dots with, for example, organosilane outer substituents and/or encapsulated carbon QDs might solve both problems as very promising results have been recently shown.5q
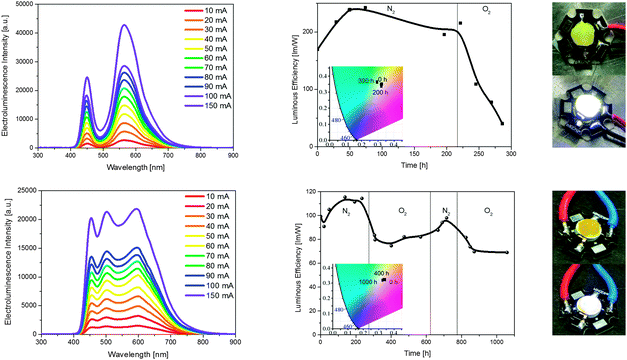
Next, we investigated the use of well-known emitting polymers for the development of P-WHLEDs. The optimized device was a blue-LED/5 (0.1 mm), which independently of the applied current shows a broad electroluminescence spectrum with two maxima at 450 and 560 nm, corresponding to the blue-LED and the polymer, respectively – Fig. 5. The quality of the white light is highlighted by almost no change in CIE coordinates from 0.32/0.34 along with CRI values slightly superior to 70 with a CCT of 6150–5900 K. The moderate CRI is attributed to a low electroluminescence intensity in the red region of the visible spectrum. More striking is the high luminous efficiency of around 200 lm W−1 that is stable over about 200 h under inert atmosphere. The efficiency value is similar to the best all-inorganic white-emitting LEDs. Thus, the same P-WHLED was subsequently probed under ambient conditions – Fig. 5 and Fig. S14 (ESI†). Unfortunately, the emission of the polymer is immediately damaged by the well-known photo-assisted oxidation process,5d,11 as both the colour quality and the luminous efficiency declined – Fig. 5 and Fig. S14 (ESI†). This finding is not surprising, since thin-film lighting devices based on this luminescent polymer have demonstrated stabilities of thousands of hours under inert atmosphere, but need of a rigorous encapsulation when it is tested out of the glove-box.11b,c Indeed, the irradiation stability of 5 is also moderate in solution and rubbers compared to the other luminescent compounds – Fig. S9 and S11 (ESI†). Thus, a further encapsulation system will be necessary for improving the lifespan of the P-WHLEDs with the shortcoming of a less user-friendly fabrication process.
Finally, we probed the CC-WHLEDs with the optimized architecture blue-LED/6 (0.1 mm)/7 (0.1 mm) – Fig. 5 and Fig. S14 (ESI†). Upon applying different driving currents from 10 to 200 mA, clearly distinguishable emission peaks for the blue-LED, 6, and 7 were observed. As expected, CIE coordinates of 0.33–0/0.32–0, CRI values superior to 80, and CCT of 6000–8500 K were achieved. For comparison, the stability study of the CC-WHLEDs was carried out monitoring the changes of the electroluminescence spectrum and the luminous efficiency over time under inert atmosphere – Fig. 5 and Fig. S14 (ESI†). Similar to the P-WHLEDs, the CC-WHLEDs show an excellent stability in terms of both colour quality – i.e., CIE: 0.32/0.34; CRI: 85; CCT: ∼5500–6000 K – and luminous efficiency (100 lm W−1) for around 200 h. But, in stark contrast to the P-WHLEDs, a remarkable stability in terms of colour and efficiency over more than 1000 hours under ambient operation conditions was noted. This is expected as these rubbers show an excellent stability independently of the environmental conditions under both irradiation and heating treatments, as well as an enhancement of the luminescence features in the rubber material – vide supra.12 Interestingly, while the colour quality is stable over this long period of time, the luminous efficiency is immediately reduced or increased when transferring the CC-WHLED from N2 to ambient conditions and vice versa as shown in Fig. 5. This is related to the well-known phosphorescence quenching by oxygen, which can also be circumvented by using a top isolating coating as that proposed for the P-WHLEDs. Taking the dependency of the luminous efficiency with the environment into account, this device shows extrapolated lifetimes of around 4000 h until reaching the half of its starting maximum under ambient conditions – Fig. S15 (ESI†). As such, although there is no need for encapsulation in terms of stability – vide supra, it might be advantageous for fabricating more efficient CC-WHLEDs. The latter turns more encouraging when comparing the stability of our WHLEDs with the state-of-art stability that is around a few hundreds of hours.5i,k,l,o,q,8
Conclusions
This manuscript provides two major thrusts in the field of WHLEDs. On one hand, we demonstrate the ease of preparation and application of luminescent rubbers for down-converting lighting schemes. Here, important assets of our approach are (i) the in situ preparation of the rubbers without using any cross-linking and UV- or thermal-curing methods, and (ii) its versatility in terms of using any kind of luminescent materials, such as fluorescent proteins,8 small-molecules, carbon quantum dots, polymers, and coordination complexes. Here, we have ensured that the amount of the compounds is kept constant, but a further enhancement of the luminous efficiency should be possible if the concentration of the compounds is increased, as it will reduce the coating thickness. In this regard, the latter has been optimized to obtain a high quality white emission as shown in Fig. S12 (ESI†). In addition, the luminous efficiency linearly decreases upon increasing the coating thickness – Fig. S12 (ESI†). On the other hand, we provide for the first time a direct comparison of different down-converting materials, showing that under the same working conditions, WHLEDs fabricated with a down-converting rubber encapsulation based on coordination complexes bear a great potential for future breakthroughs. This is demonstrated by the unprecedented stability in terms of colour quality (CRI > 80) and luminous efficiency (>100 lm W−1) of more than 1000 h (extrapolated 4000 h) independently of the environmental conditions. Equally important is the potential prospect of carbon quantum dots if the photoluminescence quantum yields are enhanced. Noteworthy, it would be interesting to determine the stability of the device under outdoor conditions – i.e., 50–70 °C and 80% moisture, however due to the sound thermal stability of all the compounds we do not envisage any important change to the results presented. More importantly, since the irradiation stability of all the down-converting compounds is slightly enhanced in the rubbers when compared to that in solution, it is safe to postulate that the stability differences between devices might be related to the intrinsic instability of the compounds.It is important to point out that although all-inorganic white LEDs feature much higher stabilities than the WHLEDs, our CC-WHLED shows similar CRI and luminous efficiencies to those of all-inorganic white LEDs, while its stability represents a one order of magnitude enhancement compared to the state-of-the-art hybrid white LEDs. As such, we strongly believe that this work could be a landmark for future breakthroughs in the field of WHLEDs. In this regard, a future challenge is the development of down-converting encapsulation systems for high-powerful LED arrays, which hold high operation temperatures. Currently, we are working on different strategies to develop thermally stable organic-based coatings.
Acknowledgements
R. D. C. and L. N. acknowledge the support of the ‘Fonds der Chemischen Industrie’ (FCI) in the Liebig grant framework and the EAM Starting Grant of the Cluster of Excellence ‘Engineering of Advanced Materials’ (EAM) promoted by the German Research Foundation (DFG) within the framework of its ‘Excellence Initiative’. U.S. was supported by the Emerging Field Initiative (EFI) of the University of Erlangen-Nuremberg (Germany) (Project: SynBio). Johannes Margraf and Volker Strauß are heartily acknowledged for providing the carbon quantum dots.Notes and references
- (a) Global LED Display Industry Report, 2015; (b) McKinsey&Company: Lighting the Way – Perspectives on the Global Lighting Market, 2012; (c) US Department of Energy: Manufacturing Roadmap Solid-State Lighting Research and Development, 2014.
- (a) D. Volz, M. Wallesch, C. Fléchon, M. Danz, A. Verma, J. M. Navarro, D. M. Zink, S. Bräse and T. Baumann, Green Chem., 2015, 17, 1988 RSC; (b) S. Reineke, M. Thomschke, B. Lüssem and K. Leo, Rev. Mod. Phys., 2013, 85, 1245 CrossRef CAS.
- (a) http://www.nobelprize.org/nobel_prizes/physics/laureates/2014/press.html ; (b) K. Itoh, T. Kawamoto, H. Amano, K. Hiramatsu and I. Akasaki, Jpn. J. Appl. Phys., 1991, 30, 1924 CrossRef CAS; (c) I. Akasaki, H. Amano, K. Itoh, N. Koide and K. Manabe, Int. Phys. Conf. Ser., 1992, 129, 851 Search PubMed; (d) S. Nakamura, M. Senoh and T. Mukai, Jpn. J. Appl. Phys., 1993, 32, L8 CrossRef CAS; (e) S. Nakamura, M. Mukai, T. Senoh, S.-I. Nagahama and N. Iwasa, J. Appl. Phys., 1993, 74, 3911 CrossRef CAS; (f) S. Nakamura, T. Mukai and M. Senoh, Appl. Phys. Lett., 1994, 64, 1687 CrossRef CAS; (g) I. Akasaki, H. Amano, S. Sota, H. Sakai, T. Tanaka and M. Koike, Jpn. J. Appl. Phys., 1995, 34, L1517 CrossRef CAS; (h) S. Nakamura and G. Fasol, The Blue Laser Diode, Springer, 1997 Search PubMed.
- (a) J. K. Park, C. H. Kim, S. H. Park, H. D. Park and S. Y. Choi, Appl. Phys. Lett., 2004, 84, 1647 CrossRef CAS; (b) P. Pust, V. Weiler, C. Hecht, A. Tücks, A. S. Wochnik, A.-K. Henß, D. Wiechert, C. Scheu, P. J. Schmidt and W. Schnick, Nat. Mater., 2014, 13, 891 CrossRef CAS PubMed; (c) T. M. Tolhurst, T. D. Boyko, P. Pust, N. W. Johnson, W. Schnick and A. Moewes, Adv. Opt. Mater., 2015, 3, 546 CrossRef CAS; (d) R.-J. Xie, Y. Q. Li, N. Hirosaki and H. Yamamoto, Nitride Phosphors and Solid-State Lighting, CRC Press, 2011 Search PubMed; (e) C. Che and R.-S. Liu, J. Phys. Chem. Lett., 2011, 2, 1268 CrossRef PubMed; (f) R. Zhang, H. Lin, Y. Yu, D. Chen, J. Xu and Y. Wang, Laser Photonics Rev., 2014, 8, 158 CrossRef CAS.
- (a) G. Heliotis, P. N. Stavrinou, D. D. C. Bradley, E. Gu, C. Griffin, C. W. Jeon and M. D. Dawson, Appl. Phys. Lett., 2005, 87, 103505 CrossRef; (b) G. Heliotis, G. Itskos, R. Murray, M. D. Dawson, I. M. Watson and D. D. C. Bradley, Adv. Mater., 2006, 18, 334 CrossRef CAS; (c) E. Gu, H. X. Zhang, H. D. Sun, M. D. Dawson, A. R. Mackintosh, A. J. C. Kuehne, R. A. Pethrick, C. Belton and D. D. C. Bradley, Appl. Phys. Lett., 2007, 90, 031116 CrossRef; (d) I. O. Huyal, U. Koldemir, T. Ozel, H. V. Demir and D. Tuncel, J. Mater. Chem., 2008, 18, 3568 RSC; (e) O. Kim, S. Ha, J. I. Kim and J. Lee, ACS Nano, 2010, 4, 3397 CrossRef CAS PubMed; (f) M. Stupca, O. M. Nayfeh, T. Hoang, M. H. Nayfeh, B. Alhreish, J. Boparai, A. Al Dwayyan and M. Al Salhi, J. Appl. Phys., 2012, 112, 074313 CrossRef; (g) D. Ban, J. Chen, J. Tao, M. G. Helander, Z. Wang, J. Qiu and Z. Lu, Phys. Status Solidi, 2012, 9, 2594 CrossRef CAS; (h) W.-S. Song and H. Yang, Chem. Mater., 2012, 24, 1961 CrossRef CAS; (i) E.-P. Jang, W.-S. Song, K.-H. Lee and H. Yang, Nanotechnology, 2013, 24, 045607 CrossRef PubMed; (j) N. J. Findlay, C. Orofino-Peña, J. S. Bruckbauer, E. T. Elmasly, S. Arumugam, A. R. Inigo, A. L. Kanibolotsky, R. W. Martin and P. J. Skabara, J. Mater. Chem. C, 2013, 1, 2249 RSC; (k) C.-F. Lai, C.-J. Chang, C.-L. Hsieh, Y.-L. Chen and C.-S. Tuan, Opt. Lett., 2013, 38, 4082 CrossRef CAS PubMed; (l) D. D. Martino, L. Beverina, M. Sassi, S. Brovelli, R. Tubino and F. Meinardi, Sci. Rep., 2014, 4, 4400 Search PubMed; (m) P.-C. Shen, M.-S. Lin and C.-F. Lin, Sci. Rep., 2014, 4, 5307 CAS; (n) J. Chen, W. Liu, L.-H. Mao, Y.-J. Yin, C.-F. Wang and S. Chen, J. Mater. Sci., 2014, 49, 7391 CrossRef CAS; (o) N. J. Findlay, J. Bruckbauer, A. R. Inigo, B. Breig, S. Arumugam, D. J. Wallis, R. W. Martin and P. J. Skabara, Adv. Mater., 2014, 26, 7290 CrossRef CAS PubMed; (p) C. Sun, Y. Zhang, K. Sun, C. Reckmeier, T. Zhang, X. Y. Zhang, J. Zhao, C. Wu, W. W. Yua and A. L. Rogach, Nanoscale, 2015, 7, 12045 RSC; (q) Y. Hyein, S. J. Ho, L. Kwangyeol and W. Kyoungja, Nanoscale, 2015, 7, 12860 RSC.
- (a) C.-Y. Sun, X.-L. Wang, X. Zhang, C. Qin, P. Li, Z.-M. Su, D.-X. Zhu, G.-G. Shan, K.-Z. Shao, H. Wu and J. Li, Nat. Commun., 2013, 4, 2717 Search PubMed; (b) Q. Gong, Z. Hu, B. J. Deibert, T. J. Emge, S. J. Teat, D. Banerjee, B. Mussman, N. D. Rudd and J. Li, J. Am. Chem. Soc., 2014, 136, 16724 CrossRef CAS PubMed; (c) Y. Lu and B. Yan, Chem. Commun., 2014, 50, 15443 RSC; (d) Y. Cui, T. Song, J. Yu, Y. Yang, Z. Wang and G. Qian, Adv. Funct. Mater., 2015, 25, 4796 CrossRef CAS.
- (a) H. Tetsuka, A. Nagoya and R. Asahi, J. Mater. Chem. C, 2015, 3, 3536 RSC; (b) D. Zhou, H. Zou, M. Liu, K. Zhang, Y. Sheng, J. Cui, H. Zhang and B. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 15830 CrossRef CAS PubMed.
- M. D. Weber, L. Niklaus, M. Pröschel, P. B. Coto, U. Sonnewald and R. D. Costa, Adv. Mater., 2015, 27, 5493 CrossRef CAS PubMed.
- M. Ma, F. W. Mont, X. Yan, J. Cho, E. F. Schubert, G. B. Kim and C. Sone, Opt. Express, 2011, 19, A11352011 Search PubMed.
- V. Strauss, J. T. Margraf, C. Dolle, B. Butz, T. J. Nacken, J. Walter, W. Bauer, W. Peukert, E. Spiecker, T. Clark and D. M. Guldi, J. Am. Chem. Soc., 2014, 136, 17308 CrossRef CAS PubMed.
- (a) Z. Yu, L. Li, H. Gao and Q. Pei, Sci. China: Chem., 2013, 56, 1075 CrossRef CAS; (b) J. Fang, P. Matyba and L. Edman, Adv. Funct. Mater., 2009, 19, 2671 CrossRef CAS; (c) A. Asadpoordarvish, A. Sandström and L. Edman, Adv. Eng. Mater., 2016, 18, 105 CrossRef CAS.
- R. D. Costa, F. Monti, G. Accorsi, A. Barbieri, H. J. Bolink, E. Ortí and N. Armaroli, Inorg. Chem., 2011, 50, 7229 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details for the preparation and characterization of the gel- and rubber-like materials, as well as device preparation and characterization are provided. See DOI: 10.1039/c6mh00038j |
| This journal is © The Royal Society of Chemistry 2016 |