Organocatalysis by p-sulfonic acid calix[4]arene: a convenient and efficient route to 2,3-dihydroquinazolin-4(1H)-ones in water†
Matiur Rahmana,
Irene Linga,
Norbani Abdullaha,
Rauzah Hashim*a and
Alakananda Hajra*b
aDepartment of Chemistry, University of Malaya, 50603 Kuala Lumpur, Malaysia. E-mail: rauzah@um.edu.my
bDepartment of Chemistry, Visva-Bharati (A Central University), Santiniketan 731235, India. E-mail: alakananda.hajra@visva-bharati.ac.in
First published on 22nd December 2014
Abstract
An efficient and eco-friendly method is reported for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones from the direct cyclocondensation of anthranilamide with aldehydes using p-sulfonic acid calix[4]arene (p-SAC) as a recyclable organocatalyst in excellent yields in water at room temperature. The catalyst was reusable without significant loss of catalytic efficiency. Operational simplicity, the compatibility with various functional groups, non-chromatographic purification technique, high yields and mild reaction conditions are the notable advantages of this procedure. Large scale reaction demonstrated the practical applicability of this methodology.
Introduction
Catalysis has played a crucial role in the success of chemistry in the twentieth century.1 Nowadays organocatalysis is one of the hot research topics in advanced organic chemistry. In the past decade, organocatalysis has opened a new window for carrying out organic transformations and has become a powerful tool in the synthesis of biologically active and structurally complex compounds.2 Compared with transition-metal catalysts, the cost and toxicity of organocatalysts are low, thus making organocatalysts beneficial for the production of pharmaceutical intermediates.2,3 Moreover, organocatalysts are tolerant of water and air, are usually easy to use and finally avoid expensive metal reagents or catalysts.4The development of eco-friendly methodologies that are environmentally benign and waste-free, as well as being able to produce the desired products in high purity, have received much attention in recent years because of the increasing tendency of the chemical industry towards greener processes.5 The major drive towards this idea is the replacement of volatile organic solvents by green solvents,6 as organic solvents are the major contributors to environmental pollution. In this view, water is the most ideal solvent7 and the use of water in organic reactions as solvent has received much attention.8 Water is a safe, harmless and environmentally benign solvent in comparison with a large number of harmful organic solvents; the unique physicochemical properties of water can even accelerate some reactions.9 However, the poor solubility of most organic compounds in water often makes an unfavorable impact on water mediated organic synthesis. Therefore, the development of water-tolerant catalysts that allow organic reactions to be carried out in aqueous media is a challenging task in the field of green catalysis. Aqueous biphasic reaction systems using water-soluble catalysts have the practical advantages that the catalysts can be reused after simple decantation or extraction of the water-insoluble products and the catalysts show high catalytic activity in water.10 The thought of environmental factor (E-factor) and atom economy have gradually become incorporated into conventional organic synthesis in both industry and academia. Solvents are the main reason for an insufficient E-factor, especially in synthesis of fine chemicals and pharmaceutical industries.11
The calixarenes are a class of cyclooligomers formed via condensation between para substituted phenols and formaldehyde.12 Calixarenes are good receptors for the complexation with various kinds of guests such as anions, cations and neutral organic/inorganic molecules.13 Calixarenes have been widely used in sensors, enzyme-mimics, ion carriers, solid-phase support materials, ion selective electrodes, drug-delivery agents etc.14 The discovery of water-soluble calixarenes as catalyst in organic reaction has attracted the attention of researchers with the objective of faster reaction and use of lower amount of catalyst.15 In this sense, water-soluble calix[n]arenes could be used as surfactant-type Brønsted acid catalyst to facilitate the reactions through the formation of a host–guest complex, with a nucleophile component in the organic–aqueous interfacial layer.10 So the use of calix[n]arenes as catalyst in water is in demand from the aspect of green chemistry. However the use of calixarenes as catalyst in water is very rare.15a
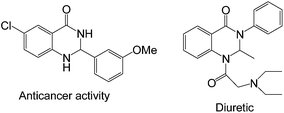
The N-containing heterocyclic compounds play a crucial role in the field of drug scaffolds, synthetic organic chemistry, medicinal chemistry as well as material sciences.16 2,3-Dihydroquinazolinone derivatives act as important intermediates in the synthesis of drug molecules, and natural products.17 These also exhibit various activities such as antitumor, anti-cancer, antibiotic, antidefibrillatory, antipyretic, analgesic, antihypertonic, diuretic, antihistamine, antidepressant, and vasodilating agents18 (Fig. 1).
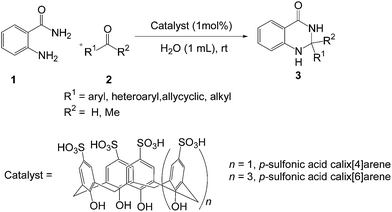
In view of their significant uses, green methodologies for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones should be benefited in the synthetic and medicinal chemistry. Although numerous protocols have been developed for the synthesis of 2,3-dihydroquinazolinones,19 regardless their efficiency and reliability, most of these methods suffer from one or more of these disadvantages, such as the use of hazardous organic solvents, requires higher temperature, low yields, strongly acidic conditions, expensive moisture sensitive catalysts, and tedious work-up procedure. Therefore, it is important to develop economically and environmentally more viable procedure for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Very recently, we have developed an environmentally benign greener strategy using recyclable nano indium oxide as catalyst for the one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones by a three-component condensation of isatoic anhydride with primary amines or ammonium salts and aromatic aldehydes in ethanol.19o As a part of our ongoing research for the synthesis of heterocycles through a greener way,19o,20 herein we report a simple and practical metal-free method for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones by the cyclocondensation of anthranilamide with aldehydes using p-sulfonic acid calix[4]arene (p-SAC) as a recyclable organocatalyst in water at room temperature (Scheme 1).
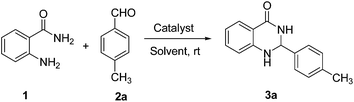
We started our study with preparing p-sulfonic acid calix[n]arenes in our laboratory according to the literature procedure.21 Next the catalytic activity of these organocatalyst were investigated for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones. In order to find out the optimum reaction conditions, we have selected the reaction of anthranilamide 1 and 4-methylbenzaldehyde 2a as the model reaction (Table 1). To our delight, the desired product 3a was obtained in 92% yield in presence of 1 mol% p-sulfonic acid calix[4]arene (p-SAC) in water at room temperature for 20 min (Table 1, entry 1). No further improvement was noticed by increasing the reaction time (Table 1, entry 2). Increasing the amount of catalyst (5 mol%) did not improve the yield noticeably (Table 1, entry 3) whereas decreasing the amount of catalyst (0.5 mol%) decreased the yield (Table 1, entry 4). To clarify the role of the catalyst (p-SAC) in this process, the same reaction was carried out without p-SAC (Table 1, entry 5). However the reaction did not proceed at all without the catalyst after 20 min of reaction. Water appeared to be the best choice as solvent among the common solvents like MeOH, MeCN, toluene, DMF, 1,2-DCE (Table 1, entries 6–10). The reaction did not proceed well in absence of any solvent (Table 1, entry 11). The chain length of calixarenes also played a significant role. By increasing the chain length of 4 to 6 (n = 1 to 3) the yield of the desired product decreased noticeably (Table 1, entry 12). To explore the role of p-sulfonic acid calix[4]arene (p-SAC) we used p-hydroxy benzenesulfonic acid (p-HSA) as a catalyst. It was observed that p-HSA was less efficient than p-SAC (Table 1, entry 13). This indicates that the sulfonyl and phenolic groups in calixarene moiety are not solely responsible for fast and efficient reaction. On the other hand, p-TSA was not also effective for this conversion (Table 1, entry 14). Phenol was less efficient than p-SAC (Table 1, entry 15). Other Brønsted acid catalyst like p-dodecylbenzenesulfonic acid (DBSA, Kobayashi's catalyst2m) was also tested, but no improvement of the yield was observed (Table 1, entries 16). Thus, optimal reaction conditions were obtained using anthranilamide (1, 1 mmol), 4-methylbenzaldehyde (2a, 1 mmol) in presence of 1 mol% of p-sulfonic acid calix[4]arene (p-SAC) in water (1 mL) at room temperature for 20 min (Table 1, entry 1).
| Entry | Catalyst (mol%) | Solvent (1 mL) | Time (min) | Yieldsb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 mmol of 1 and 1 mmol of 2a in the presence of catalyst and solvent (1 mL) at room temperature.b Isolated yields.c No reaction. p-SAC = p-sulfonic acid calix[4]arene. p-HSA = p-hydroxybenzenesulfonic acid. p-TSA = p-toluenesulfonic acid. TBAB = tetrabutyl ammonium bromide. DBSA = p-dodecylbenzenesulfonic acid. | ||||
| 1 | p-SAC (1) | H2O | 20 | 92 |
| 2 | p-SAC (1) | H2O | 60 | 92 |
| 3 | p-SAC (5) | H2O | 20 | 93 |
| 4 | p-SAC (0.5) | H2O | 20 | 84 |
| 5 | — | H2O | 20 | n. r.c |
| 6 | p-SAC (1) | MeOH | 20 | 80 |
| 7 | p-SAC (1) | MeCN | 20 | 72 |
| 8 | p-SAC (1) | Toluene | 20 | 38 |
| 9 | p-SAC (1) | DMF | 20 | 75 |
| 10 | p-SAC (1) | DCE | 20 | 32 |
| 11 | p-SAC (1) | — | 20 | 20 |
| 12 | p-Sulfonic acid calix[6]arene [n = 3] (1) | H2O | 20 | 67 |
| 13 | p-HSA (1) | H2O | 20 | 60 |
| 14 | p-TSA (1) | H2O | 20 | 57 |
| 15 | Phenol (1) | H2O | 20 | 18 |
| 16 | DBSA (5) | H2O | 20 | 58 |
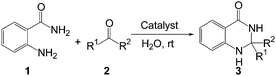
Next, we studied the scope and limitation of this reaction by employing a wide range of aldehydes (2) under optimized reaction conditions. The results are summarized in Table 2. To our delight, the corresponding dihydroquinazolin-4(1H)-ones (3) were obtained with good to excellent yields in all cases. Aromatic aldehydes with electron-donating as well as electron-withdrawing substituents reacted very well, affording good to excellent yields of 2,3-dihydroquinazolinones. The chloro- and bromo-substituted benzaldehydes afforded the corresponding products 3d and 3e in 87% and 86% yields respectively. Substituent at the ortho position of the phenyl ring also afforded the corresponding products with excellent yields (3j and 3k). Heteroaryl aldehyde like 2-thiophenecarboxaldehyde was also compatible under this reaction conditions to give the desired product (3p) without polymerization. Gratifyingly, the current methodology is also applicable for the aliphatic aldehydes. Cyclohexane carboxaldehyde gave the desired product (3q) with high yield. Butyraldehyde also afforded the corresponding 2,3-dihydroquinazolinone (3r) with good yield however longer reaction time is required. Moreover, ketone like acetone also afforded the desired product (3s) with moderate yield. The all synthesized compounds have been characterized by spectral and analytical data. Results in Table 2 clearly show that the present protocol is indeed superior to several reported methods in terms of product yields, reaction time, avoiding the use of volatile organic solvents, and reaction temperature. Moreover, we have developed a greener reaction conditions bearing lower E-factor11,22 of 0.17 and 0.15 in the cases of synthesizing 3a and 3b respectively (see ESI†).
| Entry | R1 | R2 | Products | Time (min) | Yieldsb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (1 mmol), 2 (1 mmol) in water (1 mL) at room temperature in presence of 1 mol% of p-SAC.b Isolated yields.c 1 (20 mmol), 2a (20 mmol) in water (20 mL) at room temperature in presence of 1 mol% of p-SAC. | |||||
| 1 | 4-Me-C6H4 | H | 3a | 20 | 92, 86c |
| 2 | Ph | H | 3b | 18 | 94 |
| 3 | 4-MeO-C6H4 | H | 3c | 20 | 90 |
| 4 | 4-Cl-C6H4 | H | 3d | 22 | 87 |
| 5 | 4-Br-C6H4 | H | 3e | 22 | 86 |
| 6 | 4-NO2-C6H4 | H | 3f | 25 | 82 |
| 7 | 4-MeS-C6H4 | H | 3g | 20 | 88 |
| 8 | 4-CN-C6H4 | H | 3h | 22 | 86 |
| 9 | 4-OH-C6H4 | H | 3i | 24 | 82 |
| 10 | 2-Cl-C6H4 | H | 3j | 20 | 90 |
| 11 | 2-OH-C6H4 | H | 3k | 22 | 88 |
| 12 | 4-OH-3,5-(OMe)2-C6H2 | H | 3l | 24 | 84 |
| 13 | 5-Br-2-OH-C6H3 | H | 3m | 22 | 85 |
| 14 | 3-NO2-C6H4 | H | 3n | 24 | 88 |
| 15 | 1-Pyrenyl | H | 3o | 26 | 82 |
| 16 | 2-Thiophenyl | H | 3p | 22 | 86 |
| 17 | Cyclohexyl | H | 3q | 30 | 82 |
| 18 | C3H7 | H | 3r | 40 | 68 |
| 19 | CH3 | CH3 | 3s | 90 | 64 |
The dihydroquinazolinones were generally precipitated from the reaction mixtures and the solid precipitation was filtered off and recrystallized from hot ethanol to obtain pure products. This methodology is also applicable on a gram-scale synthesis. We have successfully synthesized the dihydroquinazolinone 3a in 86% yield by the reaction of anthranilamide (1, 20 mmol) and 4-methylbenzaldehyde (2a, 20 mmol) (Table 2, entry 1).
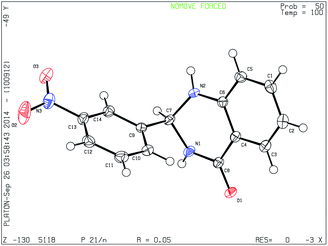
Finally the single crystal X-ray diffraction analysis of 3n was carried out for further confirmation of the structure of product (Fig. 2).23 In crystalline state the compound shows the 2,3-dihydroquinazoline moiety is planar. The pyrimidine ring is in a flattened half-chair conformation. The nitro-substituted benzene ring forms dihedral angle of 85.91°, with the benzene ring of the dihydroquinazoline group. In the crystal, molecules are arranged in crisscross manner along a-axis, with N–O⋯H hydrogen bonds linking molecules at 2.451 to 2.708 Å in the extended network. Short contacts of hydrogen bonding interaction related to carbonyl oxygen atoms with neighboring hydrogen atoms is observed with O⋯H distances ranging from 2.080 to 2.636 Å.
Next, we turned our attention towards the recovery and reusability of the catalyst. For this purpose, we have chosen the reaction of anthranilamide (1) with 4-methylbenzaldehyde (2a) in presence of 1 mol% p-sulfonic acid calix[4]arene (p-SAC) in water at room temperature as the model reaction. After completion of the reaction distilled water was added to the reaction mixture. The reaction mixture was then filtered off and the catalyst was recovered by evaporating the water. Pure product was obtained by recrystallizing the residue from hot ethanol. The recovered catalyst was reused for a subsequent fresh batch of the reaction. The catalytic activity was checked upto fourth cycle and it showed similar (Table 3).
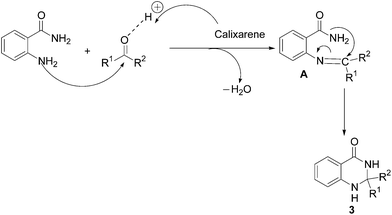
Based on the literature reports,19g a plausible mechanistic pathway to 2,3-dihydroquinazolin-4(1H)-ones (3) is illustrated in Scheme 2. In the initial step, condensation of anthranilamide (1) with the aldehyde (2) gives imine A, which upon intramolecular cyclization afforded the final product 3. The catalyst might activate the aldehyde through the co-ordination with the oxygen of the corresponding carbonyl group which facilitates the subsequent nucleophilic attack by the nitrogen atom on the carbonyl carbon.
Conclusions
In summary, we have demonstrated a remarkable catalytic activity of p-sulfonic acid calix[4]arene (p-SAC) as an organocatalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones by the condensation between various aldehydes and anthranilamide in water at room temperature. This protocol is also applicable on a gram-scale synthesis. Clean reaction, ease of product isolation, short reaction time, mild reaction conditions (room temperature), low catalyst loading, low E-factor, and use of water as solvent are the notable advantages of the present methodology and these features make this procedure to be a green synthetic protocol. We believe that our novel procedure will open up a new practical and convenient route for the synthesis of biologically important 2,3-dihydroquinazolin-4(1H)-ones.Experimental section
General
Melting points were determined on a glass disk with an electric hot plate and are uncorrected. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were run in CDCl3 & DMSO-d6 solutions (Bruker Avance 400). Chemical shift were recorded as δ values in parts per million (ppm), and the signals were reported as s (singlet), d (doublet), t (triplet), m (multiplet) and coupling constants J were given in Hz. IR spectra were taken in a Perkin Elmer FTIR-Spectrum 400. Commercially available substrates were freshly distilled before the reaction. Solvents, reagents and chemicals were purchased from Sigma Aldrich and Merck.General procedure for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones
p-Sulfonic acid calix[4]arene (1 mol%) was added to a solution of anthranilamide (1 mmol) and aldehyde/ketone (1 mmol) in water (1 mL) and the mixture was stirred at room temperature for a specified period of time. After completion of the reaction, cold distilled water (5 mL) was added to the reaction mixture. Then the product was filtered off and the catalyst was recovered by evaporating the water. The recovered catalyst was reused for a subsequent fresh batch of the reaction after reactivation. The crude product was recrystallized from hot ethanol to afford the pure product.Acknowledgements
We thank the University of Malaya and the Malaysian Ministry of Higher Education High Impact Research Grant UM.C/625/1/HIR/MOHE/05 for financial support.Notes and references
- (a) R. A. Sheldon, CHEMTECH, 1994, 25, 38–47 Search PubMed; (b) Fine Chemicals Through Heterogeneous Catalysis, ed. R. A. Sheldon and H. van Bekkum, Wiley-VCH, Weinheim, Germany, 2001 Search PubMed.
- (a) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2001, 40, 3726–3748 CrossRef CAS; (b) B. List, J. Am. Chem. Soc., 2000, 122, 9336–9337 CrossRef CAS; (c) J. Seayad and B. List, Org. Biomol. Chem., 2005, 3, 719–724 RSC; (d) J. H. Kim, I. Coric, S. Vellalath and B. List, Angew. Chem., Int. Ed., 2013, 52, 4474–4477 CrossRef CAS PubMed; (e) B. List, Synlett, 2001, 1675–1686 CrossRef CAS; (f) D. W. C. MacMillan, Nature, 2008, 455, 304–308 CrossRef CAS PubMed; (g) D. Cantillo, B. Gutmann and C. O. Kappe, J. Am. Chem. Soc., 2011, 133, 4465–4475 CrossRef CAS PubMed; (h) A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis Jr, J. Am. Chem. Soc., 2002, 124, 5961–5963 Search PubMed; (i) L. Myles, N. Gathergood and S. J. Connon, Chem. Commun., 2013, 49, 5316–5318 RSC; (j) S. Bertelsen and K. A. Jørgensen, Chem. Soc. Rev., 2009, 38, 2178–2189 RSC; (k) M. Gruttadauria, F. Giacalone and R. Noto, Chem. Soc. Rev., 2008, 37, 1666–1688 RSC; (l) L. W. Xu, L. Li and Z. H. Shi, Adv. Synth. Catal., 2010, 352, 243–279 CrossRef CAS; (m) K. Manabe, Y. Mori and S. Kobayashi, Tetrahedron, 2001, 57, 2537–2544 CrossRef CAS.
- (a) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138–5175 CrossRef CAS PubMed; (b) B. List, Chem. Commun., 2006, 819–824 RSC; (c) A. Dondoni and A. Massi, Angew. Chem., Int. Ed., 2008, 47, 4638–4660 CrossRef CAS PubMed; (d) E. N. Jacobsen and D. W. C. MacMillan, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20618–20619 CrossRef CAS PubMed; (e) J. G. Hernandez and E. Juaristi, Chem. Commun., 2012, 48, 5396–5409 RSC.
- (a) Organic Catalysis, Wiley-VCH, Weinheim, Germany, 2004, pp. 1007–1249 Search PubMed; (b) Organocatalysis, American Chemical Society, Washington, DC, USA, 2007, pp. 5413–5883 Search PubMed.
- (a) P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed; (b) S. Shimizu, S. Shirakawa, Y. Sasaki and C. Hirai, Angew. Chem., Int. Ed., 2000, 39, 1256–1259 CrossRef CAS; (c) F. Zhang and H. Li, Chem. Sci., 2014, 5, 3695–3707 RSC; (d) R. A. Sheldon, I. Arends and U. Hanefeld, Green Chemistry and Catalysis, Wiley-VCH, Weinheim, 2007 Search PubMed; (e) R. T. Baker and W. Tumas, Science, 1999, 284, 1477–1479 CrossRef CAS; (f) C.-J. Li, Chem. Rev., 2005, 105, 3095–3166 CrossRef CAS PubMed; (g) U. K. Lindstrom, Chem. Rev., 2002, 102, 2751–2772 CrossRef PubMed; (h) A. Lubineau, J. Augé and Y. Queneau, Synthesis, 1994, 741–760 CrossRef CAS PubMed; (i) G. Fioroni, F. Fringuelli, F. Pizzo and L. Vaccaro, Green Chem., 2003, 5, 425–428 RSC; (j) B. C. Ranu and K. Chattopadhyay, in Eco-friendly Processes and Procedures for the Synthesis of Fine Chemicals, ed. R. Ballini, Royal Society of Chemistry, Cambridge, UK, 2009, pp. 186–215 Search PubMed.
- (a) C. Capello, U. Fischer and K. Hungerbuhler, Green Chem., 2007, 9, 927–934 RSC; (b) D. Kumar, K. Seth, D. N. Kommi, S. Bhagat and A. K. Chakraborti, RSC Adv., 2013, 3, 15157–15168 RSC; (c) R. B. Nasir-Baig and R. S. Varma, Chem. Soc. Rev., 2012, 41, 1559–1584 RSC.
- (a) K. Shanab, C. Neudorfer, E. Schirmer and H. Spreitzer, Curr. Org. Chem., 2013, 17, 1179–1187 CrossRef CAS; (b) K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 3–10 RSC; (c) H. C. Hailes, Org. Process Res. Dev., 2007, 11, 114–120 CrossRef CAS.
- (a) R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302–6337 CrossRef CAS PubMed; (b) S. Minakata and M. Komatsu, Chem. Rev., 2009, 109, 711–724 CrossRef CAS PubMed; (c) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725–748 CrossRef CAS PubMed; (d) M. Chen, F. Zhang and H. Li, Curr. Org. Chem., 2013, 17, 1051–1057 CrossRef CAS; (e) J.-N. Tan, H. Li and Y. Gu, Green Chem., 2010, 12, 1772–1782 RSC; (f) Y. Gu, R. De Sousa, G. Frapper, C. Bachmann, J. Barrault and F. Jérôme, Green Chem., 2009, 11, 1968–1972 RSC.
- (a) L. Liu and D. Wang, “On Water” for Green Chemistry in Handbook of Green Chemistry 5: Reaction in Water, ed. P. T. Anastas and C.-J. Li, Wiley-VCH, Weinbeim, 2010, pp. 207–228 Search PubMed; (b) J. Mlynarski and J. Paradowska, Chem. Soc. Rev., 2008, 37, 1502–1511 RSC; (c) F. Fringuelli, O. Piermatti, F. Pizzo and L. Vaccaro, Eur. J. Org. Chem., 2001, 3, 439–455 CrossRef; (d) A. Manna and A. Kumar, J. Phys. Chem. A, 2013, 117, 2446–2454 CrossRef CAS PubMed; (e) A. K. Chakraborti, S. Rudrawar, K. B. Jadhav, G. Kaur and S. V. Chankeshwara, Green Chem., 2007, 9, 1335–1340 RSC; (f) G. L. Khatik, R. Kumar and A. K. Chakraborti, Org. Lett., 2006, 8, 2433–2436 CrossRef CAS PubMed.
- S. Shimizu, N. Shimada and Y. Sasaki, Green Chem., 2006, 8, 608–614 RSC.
- R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC.
- (a) S. Sayin and M. Yilmaz, RSC Adv., 2014, 4, 2219–2225 RSC; (b) A. Casnati, Chem. Commun., 2013, 49, 6827–6830 RSC; (c) D. M. Homden and C. Redshaw, Chem. Rev., 2008, 108, 5086–5130 CrossRef CAS PubMed.
- (a) F. Cardona, G. Isoldi, F. Sansone, A. Casnati and A. Goti, J. Org. Chem., 2012, 77, 6980–6988 CrossRef CAS PubMed; (b) S. Sayin, E. Yilmaz and M. Yilmaz, Org. Biomol. Chem., 2011, 9, 4021–4024 RSC; (c) A. A. Bhatti, M. A. Kamboh, I. B. Solangi and S. Memon, J. Appl. Polym. Sci., 2013, 130, 776–785 CrossRef CAS.
- (a) S. J. Dalgarno, K. M. Claudio-Bosque, J. E. Warren, T. E. Glass and J. L. Atwood, Chem. Commun., 2008, 1410–1412 RSC; (b) B. M. Rambo, S. K. Kim, J. S. Kim, C. W. Bielawski and J. L. Sessler, Chem. Sci., 2010, 1, 716–722 RSC; (c) J. L. Atwood, S. J. Dalgarno, M. J. Hardie and C. L. Raston, Chem. Commun., 2005, 337–339 RSC; (d) C. D. Gutsche and K. C. Nam, J. Am. Chem. Soc., 1988, 110, 6153–6162 CrossRef CAS PubMed.
- (a) L. Liu, Y. L. Wang, Y. C. Han and Y. J. Chen, Green Chem., 2008, 10, 635–640 RSC; (b) D. L. da Silva, S. A. Fernandes, A. A. Sabino and A. de Fátima, Tetrahedron Lett., 2011, 52, 6328–6330 CrossRef CAS PubMed; (c) S. A. Fernandes, R. Natalino, P. A. R. Gazolla, M. J. da Silva and G. N. Jham, Tetrahedron Lett., 2012, 53, 1630–1633 CrossRef CAS PubMed.
- (a) A. M. Farghaly, R. Soliman, M. A. Khalil, A. A. Bekhit, A. El-Din and A. Bekhit, Boll. Chim. Farm., 2002, 141, 372–378 CAS; (b) H. J. Yale and M. Kalkstein, J. Med. Chem., 1967, 10, 334–336 CrossRef CAS PubMed; (c) M. Hour, L. Huang, S. Kuo, Y. Xia, K. Bastow, Y. Nakanishi, E. Hamel and K. Lee, J. Med. Chem., 2000, 43, 4479–4487 CrossRef CAS PubMed; (d) M. Rahman, A. K. Bagdi, S. Mishra and A. Hajra, Chem. Commun., 2014, 50, 2951–2953 RSC.
- R. P. Maskey, M. Shaaban, I. Grun-Wollnu and H. Laatsch, J. Nat. Prod., 2004, 67, 1131–1134 CrossRef CAS PubMed.
- (a) Y. S. Sadanadam, R. M. Reddy and A. Bhaskar, Eur. J. Med. Chem., 1987, 22, 169–173 CrossRef; (b) J. I. Levin, P. S. Chan, T. Bailey, A. S. Katocs and A. M. Venkatesan, Bioorg. Med. Chem. Lett., 1994, 4, 1141–1146 CrossRef CAS; (c) G. L. Neil, L. H. Li, H. H. Buskirk and T. E. Moxley, Cancer Chemother. Rep., 1972, 56, 163–173 CAS.
- (a) P. VNS Murthy, D. Rambabu, G. R. Krishna, C. M. Reddy, K. R. S. Prasad, M. V. B. Rao and M. Pal, Tetrahedron Lett., 2012, 53, 863–867 CrossRef PubMed; (b) N. B. Darvatkar, S. V. Bhilare, A. R. Deorukhkar, D. G. Raut and M. M. Salunkhe, Green Chem. Lett. Rev., 2010, 3, 301–306 CrossRef CAS; (c) S. Rostamizadeh, A. M. Amani, G. H. Mahdavinia, H. Sepehrian and S. Ebrahimi, Synthesis, 2010, 1356–1360 CrossRef CAS PubMed; (d) X. Cheng, S. Vellalath, R. Goddard and B. List, J. Am. Chem. Soc., 2008, 130, 15786–15787 CrossRef CAS PubMed; (e) Z.-H. Zhang, H.-Y. Lu, S.-H. Yang and J.-W. Gao, J. Comb. Chem., 2010, 12, 643–646 CrossRef CAS PubMed; (f) P. Salehi, M. Dabiri, M. A. Zolfigol and M. Baghbanzadeh, Synlett, 2005, 1155–1157 CrossRef CAS PubMed; (g) F. Li, Y. Feng, Q. Meng, W. Li, Z. Li, Q. Wang and F. Tao, ARKIVOC, 2007, (i), 40–50 CrossRef; (h) M. Desroses, M. Scobie and T. Helleday, New J. Chem., 2013, 37, 3595–3597 RSC; (i) A. Davoodnia, S. Allameh, A. R. Fakhari and N. Tavakoli-Hoseini, Chin. Chem. Lett., 2010, 21, 550–553 CrossRef CAS PubMed; (j) M. Sharma, S. Pandey, K. Chauhan, D. Sharma, B. Kumar and P. M. S. Chauhan, J. Org. Chem., 2012, 77, 929–937 CrossRef CAS PubMed; (k) M. Wang, T. T. Zhang and Z. G. Song, Chin. Chem. Lett., 2011, 22, 427–430 CrossRef CAS PubMed; (l) J. Safari and S. Gandomi-Ravandi, J. Mol. Catal. A: Chem., 2014, 390, 1–6 CrossRef CAS PubMed; (m) K. Ramesh, K. Karnakar, G. Satish, K. H. V. Reddy and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 53, 6095–6099 CrossRef CAS PubMed; (n) D. Huang, X. Li, F. Xu, L. Li and X. Lin, ACS Catal., 2013, 3, 2244–2247 CrossRef CAS; (o) S. Santra, M. Rahman, A. Roy, A. Majee and A. Hajra, Catal. Commun., 2014, 49, 52–57 CrossRef CAS PubMed; (p) Y. Zheng, M. Bian, X.-Q. Deng, S.-B. Wang and Z.-S. Quan, Arch. Pharm. Chem. Life Sci., 2013, 346, 119–126 CrossRef CAS PubMed; (q) M. Abdollahi-Alibeik and E. Shabani, J. Iran. Chem. Soc., 2014, 11, 351–359 CrossRef CAS PubMed; (r) S. Vasudhevan and R. J. Karunakaran, Int. J. ChemTech Res., 2013, 5, 2844–2853 CAS; (s) J. Wang, Y. Zong, R. Fu, Y. Niu, G. Yue, Z. Quan, X. Wang and Y. Pan, Ultrason. Sonochem., 2014, 21, 29–34 CrossRef CAS PubMed; (t) V. B. Labade, P. V. Shinde and M. S. Shingare, Tetrahedron Lett., 2013, 54, 5778–5780 CrossRef CAS PubMed; (u) G. A. N. K. Durgareddy, R. Ravikumar, S. Ravi and S. R. Adapa, J. Chem. Sci., 2013, 125, 175–182 CrossRef CAS PubMed; (v) X. Liu, D.-H. Hu and H. Shen, Asian J. Chem., 2012, 24, 1365–1367 CAS.
- (a) S. Santra, A. K. Bagdi, A. Majee and A. Hajra, RSC Adv., 2013, 3, 24931–24935 RSC; (b) S. Santra, A. Majee and A. Hajra, Tetrahedron Lett., 2012, 53, 1974–1977 CrossRef CAS PubMed; (c) M. Rahman, D. Kundu, A. Hajra and A. Majee, Tetrahedron Lett., 2010, 51, 2896–2899 CrossRef CAS PubMed.
- (a) S. Shinkai, Pure Appl. Chem., 1986, 58, 1523–1528 CrossRef CAS; (b) S. Shinkai, K. Araki, T. Tsubaki, T. Arimura and O. Manabe, J. Chem. Soc., Perkin Trans. 1, 1987, 11, 2297–2299 RSC; (c) M. Makha and C. L. Raston, Tetrahedron Lett., 2001, 42, 6215–6217 CrossRef CAS; (d) M. Makha and C. L. Raston, Chem. Commun., 2001, 2470–2471 RSC.
- A. Kamal, V. Srinivasulu, B. N. Seshadri, N. Markandeya, A. Alarifi and N. Shankaraiah, Green Chem., 2012, 14, 2513–2522 RSC.
- ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C NMR data and Spectra, crystallographic data, E-factor calculations. CCDC 1026285. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra16374e |
| This journal is © The Royal Society of Chemistry 2015 |