A polymer/clay nanocomposite gel via chlorinated paraffin solvent initiated photopolymerization with electrorheological performance†
Yuemei Ye and
Qigang Wang*
Department of Chemistry, and Advanced Research Institute, Tongji University, Shanghai, 200092, China. E-mail: wangqg66@tongji.edu.cn
First published on 19th December 2014
Abstract
This communication reports a mild preparation of a polymer/clay nanocomposite gel of chlorinated paraffin with an electrorheological response to an external direct-current (DC) voltage due to the alignment of the clay within the gel networks.
Electrorheological (ER) materials are emerging as promising smart materials due to their adjustable rheological properties under an applied electrical field.1–3 The mechanism of ER materials is the alignment of inorganic particles within the matrix under an electrical field. As a pioneering example, an electrorheological fluid can turn from liquid to gel under an external electrical field and return to liquid after the removal of the electrical field, which has been applied in various industrial fields.4–8 ER elastomers are smart materials due to their improved mechanical strength and stability.9 Shiga10 and Liu's groups11 have reported silicone elastomers with inorganic particles, whose mechanical properties were dependent on the polarization of charged particles under the electrical field. The scattering observation also proved that the clay/gelatin ER elastomers have anisotropic chains along with the direction of the electrical field.12 The disadvantage of these ER elastomers is the unchangeable mechanical property when the electric field was re-applied.13,14 The reason is the inorganic particles were fixed within the matrix during the solidification.
Hence, it is highly desirable to prepare elastic materials where the immobilized inorganic particles can be aligned under an external electrical field. Gels with a three-dimensional porous network have attracted significant attentions as the platform for immobilization of substance from molecules to particles. The semi-solid structure of gels due to the occupation of more than 80% solvents can make the entrapped particle easy to move and be aligned under external stimuli. Therefore, stimuli-responsive gels15–18 are promising soft materials because of their various applications in chemical sensors,14,19 controlled drug releasing,20–22 dampers,23–25 and artificial muscles.26,27
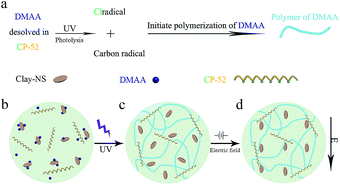
Herein, we present a photo-polymerization approach to prepare a polymer/clay nanocomposite gel with ER response, whose mechanical strength under electrical field could be enhanced due to the non-covalent effect. The solvent chlorinated paraffin 52 °C (CP-52) is also the photo-initiator, which produces chlorine free radicals to initiate the polymerization of monomers in precursor under UV irradiation.28 The non-covalent interaction within gel makes inorganic particles easy to be aligned under electrical field. The detailed preparation of gels was shown in Fig. 1. Our system consists of three components, chlorinated paraffin 52 °C (CP-52), N,N-dimethylacrylamide (DMAA), and clay nanosheets (Clay-NS). At first, the precursor was prepared by mixing Clay-NS and DMAA (5–20% weight) within CP-52. The maximum concentration of Clay-NS in gel is 2% due to the dispersed issue. Then the dispersion transformed to yellow and transparent solution after 5 minutes stirring at room temperature. At last, the preparation of the gel consists of Clay-NS and DMAA within CP-52 (Clay-CP-Gel) was realized by UV irradiation for 3 hours with average of 22.4 mW cm−2 intensity at 365 nm. The single gel which consists of DMAA and CP-52 (CP-Gel) was formed at the same condition.
The formation of Clay-CP-Gel is realized by a self-initiated mechanism. The solvent CP-52 has many chlorine atoms on its molecule chains. UV irradiation can lead to cleavage of carbon chlorine bonds of CP-52 and generating chlorine and carbon radicals (Fig. 1a). The generated radicals will initiate polymerization of DMAA by forming the propagating long-lived carbon radicals. The in situ formed PDMAA can fabricate three dimensional gel networks via the interaction between polymer chains and paraffin molecules (Fig. 1b and c). The addition of Clay-NS can obtain the Clay-CP-Gel with significantly improved properties relative to the CP-Gel. The Clay-NS in Clay-CP-Gel would like to be orientated in the direction of the electric field (Fig. 1d) due to the weak interaction of clay with polymer chains in the paraffin gels. The further evidence of photo-initiated radical polymerization is shown in Fig. 2a. The electron paramagnetic resonance (EPR) spectroscopy of pure CP-52 with alpha-phenyl-tert-butyl-nitrone (PBN) under UV irradiation exhibits three obvious twin peaks in magnetic field from 320.3 MT to 320.6 MT, which are the typical peaks of chlorine free radicals.29 The real happen of polymerization is confirmed by the long-lived propagating radicals observed in the EPR spectrum of the UV irradiated precursors (Fig. S1, ESI†). Furthermore, the time-dependent 1HNMR analysis also shows approximately 96% DMAA conversion rate of the Clay-CP-Gel-20 (with 20% DMAA and 2% Clay-NS) after 180 min of irradiation under UV (Fig. 2b).
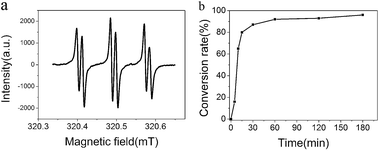 | ||
| Fig. 2 (a) EPR of CP-52, adding alpha-phenyl-tert-butyl-nitrone (PBN), UV irradiation. (b) The monomer conversation rate in Clay-CP-Gel-20 via the NMR analysis along with the UV irradiation time. | ||
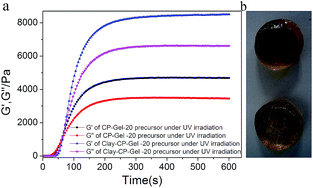
The photo-initiated gelation of the gel can be observed by an in situ rheological measurement under ultraviolet irradiation (Fig. 3). The time sweep measurements can monitor the storage modulus (G′) and loss modulus (G′′), as a function of time. The gelation time of CP-Gel-20 (with 20% DMAA) calculated by the crossing point of G′ and G′′ curve is 59 s, which is quicker than the 71 s of Clay-CP-Gel-20 and it also takes less time to reach equilibration than Clay-CP-Gel-20. The Clay-NS in the gel can partly shield UV light and prolong the gelation time. After the completed gelation, the G′ of CP-Gel-20 and Clay-CP-Gel-20 are both larger than the according G′′, which indicates the formation of the elastic gels. As shown in Table 1, the rheological strength of these nanocomposite gels are highly relevant to the amount of DMAA and Clay-NS. For the CP-Gel, the value of G′ increases from 33.8 Pa to 4684.0 Pa as the amount of DMAA increases from 5 wt% to 20 wt%. For the Clay-CP-Gel, the value of G′ also increases from 39.7 Pa to 8548.0 Pa as the amount of DMAA increases from 5 wt% to 20 wt%. It is clear that the addition of Clay-NS can lead to the enhancement of G′ even the change from solution to weak gel. It is interesting that the Clay-CP-Gel-20 exhibits a very rapid recovery of mechanical property under large amplitude oscillatory break-down. The recovery can be fully repeated with four cycles of strain from small oscillation force (γ = 1.35%) to large one (γ = 267%) in parallel plate geometry with 0.4 mm gap (Fig. S2, ESI†). The non-covalent interactions endow Clay-CP-Gel with self-recovering property.30 The compression tests also reveal that the incorporation of Clay-NS efficiently enhances the compressive properties of the gel (Fig. 4). The compressive strength of Clay-CP-Gel-20 at 95% strain are 1347.37 kPa, which are 1.81 times that of CP-Gel-20.
| Sample | DMAA (w/w) | Clay (w/w) | G′ (Pa) | G′′ (Pa) | Situation |
|---|---|---|---|---|---|
| 1 | 5% | 0 | 33.8 | 137.6 | Sol |
| 2 | 11% | 0 | 943.4 | 1109.0 | Sol |
| 3 | 20% | 0 | 4684.0 | 3449.0 | Gel |
| 4 | 5% | 2% | 39.7 | 142.5 | Sol |
| 5 | 11% | 2% | 1342.6 | 1137.2 | Gel |
| 6 | 20% | 2% | 8548.0 | 6618.6 | Gel |
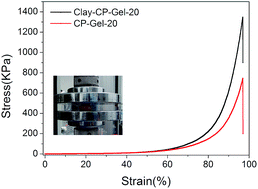 | ||
| Fig. 4 Comparison of the mechanical behavior of CP-Gel-20 and Clay-CP-Gel-20 during compression measurements. | ||
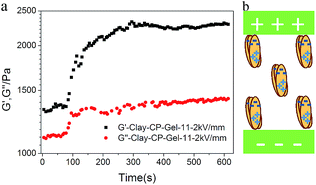
Furthermore, it is supposed that the Clay-NS can be rearranged under certain external force, which can change the mechanical properties of the gel. As shown in Fig. 5a, the Clay-CP-Gel with 11% DMAA and 2% Clay-NS (Clay-CP-Gel-11) has a fast response to the direct electric field. However, without external electric field, the G′ and G′′ of the Clay-CP-Gel were roughly stable (Fig. S4, ESI†). Here, the solvent CP-52 acts as insulate medium when a high direct-current voltage as much as 2 kV mm−1 was applied on the gel without short circuiting. The G′ of Clay-CP-Gel-11 enhanced as much as 70% during 225 s under 2 kV mm−1 electrical field. The mechanism in Fig. 5b indicates that DC electrical field can induce the polarization of the Clay-NS and their aggregates, which are forced to align under the direction of electrical field and thus enhance the final mechanical strength.31 As the evidence, the dispersed Clay-NS in the CP-52 gel can be found in SEM image of the supercritical dried Clay-CP-Gel-11 (Fig. S3, ESI†).
In summary, we have prepared a type of polymer/clay nanocomposite gel via the CP-52 solvent initiated photo-polymerization without additional cross-linkers. The final gels have a high mechanical strength and self-recovering behavior due to the non-covalent effect. The chlorinated paraffin gel exhibits quick response to direct electrical field, whose strength can be instantly improved under external direct electrical field. These ER materials similar to ER elastomers and ER fluids are really significant for the development of new smart material in vibration control and bionic intelligent filed.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21274111 and 51473123), the Program for New Century Excellent Talents in University of Ministry of Education of China (NECT-11-0386), and the Recruitment Program of Global Experts and China Postdoctoral Science Foundation (2014M550245).Notes and references
- D. B. Amabilino and J. Puigmartí-Luis, Soft Matter, 2010, 6, 1605–1612 RSC.
- F. Li, K. Lania, X. L. Wang, G. Xue and H. H. Winter, Soft Matter, 2010, 6, 2442–2448 RSC.
- J. Marins, B. Soares, A. Silva and S. Livi, RSC Adv., 2014, 4, 50925–50931 RSC.
- A. Allahverdizadeh, M. J. Mahjoob, N. Nasrollahzadeh and I. Eshraghi, J. Vib. Contr., 2014, 20, 1855–1868 CrossRef PubMed.
- W. Qiu, Y. Y. Hong, Y. Mizuno, W. J. Wen and K. Nakamura, Sens. Actuators, A, 2014, 217, 124–128 CrossRef CAS PubMed.
- Y. D. Liu and H. J. Choi, Soft Matter, 2012, 8, 11961–11978 RSC.
- Y. G. Ko and U. S. Choi, Soft Matter, 2012, 8, 253–259 RSC.
- T. Plachy, M. Sedlacik, V. Pavlinek, M. Trchová, Z. Morávková and J. Stejskal, Chem. Eng. J., 2014, 256, 398–406 CrossRef CAS PubMed.
- Y. D. Liu, X. Quan, B. Hwang, Y. K. Kwon and H. J. Choi, Langmuir, 2014, 30, 1729–1734 CrossRef CAS PubMed.
- T. Shiga, A. Okada and T. Kurauchi, Macromolecules, 1993, 26, 6958–6963 CrossRef CAS.
- B. Liu and M. T. Shaw, J. Rheol., 2001, 45, 641–657 CrossRef CAS.
- B. Wang, Z. Rozynek, M. Zhou and J. O. Fossum, J. Phys.: Conf. Ser., 2009, 149, 012032 CrossRef.
- L. M. Hao, Z. H. Shi and X. P. Zhao, React. Funct. Polym., 2009, 69, 165–169 CrossRef CAS PubMed.
- P. Ludeelerd, S. Niamlang, R. Kunaruksapong and A. Sirivat, J. Phys. Chem. Solids, 2010, 71, 1243–1250 CrossRef CAS PubMed.
- D. Roy, J. N. Cambre and B. S. Sumerlin, Prog. Polym. Sci., 2010, 35, 278–301 CrossRef CAS PubMed.
- Q. Wang, J. L. Mynar, M. Yoshida, E. Lee, M. Lee, K. Okuro, K. Kinbara and T. Aida, Nature, 2010, 463, 339–343 CrossRef CAS PubMed.
- A. Okada and A. Usuki, Macromol. Mater. Eng., 2006, 291, 1449–1476 CrossRef CAS.
- Y. Liu, D. Wu, Y. Ma, G. Tang, S. Wang, C. He, T. Chung and S. Goh, Chem. Commun., 2003, 2630–2631 RSC.
- Z. Tang, L. Gao, Y. H. Wu, T. Su, Q. Wu, X. H. Liu, W. J. Li and Q. G. Wang, J. Mater. Chem. B, 2013, 1, 5393–5397 RSC.
- I. I. Slowing, B. G. Trewyn, S. Giri and V. S.-Y. Lin, Adv. Funct. Mater., 2007, 17, 1225–1236 CrossRef CAS.
- H. M. Wang, A. T. Han, Y. B. Cai, Y. Xie, H. Zhou, J. F. Long and Z. M. Yang, Chem. Commun., 2013, 49, 7448–7450 RSC.
- D. Yuan, R. Zhou, J. F. Shi, X. W. Du, X. M. Li and B. Xu, RSC Adv., 2014, 4, 26487–26490 RSC.
- K. X. Wei, G. Meng, S. Zhou and J. W. Liu, J. Sound Vib., 2006, 298, 1150–1158 CrossRef PubMed.
- R. Stanway, J. L. Sproston and A. K. El-Wahed, Smart Mater. Struct., 1996, 5, 464–482 CrossRef CAS.
- M. Bassil, J. Davenas and M. EL Tahchi, Sens. Actuators, B, 2008, 134, 496–501 CrossRef CAS PubMed.
- X. Lu, Z. T. Zhang, H. P. Li, X. M. Sun and H. S. Peng, J. Mater. Chem. A, 2014, 2, 17272–17280 CAS.
- F. Vogel, S. Goktepe, P. Steinmann and E. Kuhl, Eur. J. Mech. A. Solids, 2014, 48, 112–128 CrossRef PubMed.
- X. H. Liu, Z. B. Wen, D. B. Wu, H. L. Wang, J. H. Yang and Q. G. Wang, J. Mater. Chem. A, 2014, 2, 11569–11573 CAS.
- E. G. Janzen and B. J. Blackburn, J. Am. Chem. Soc., 1968, 90, 5909–5910 CrossRef CAS.
- X. H. Liu, D. B. Wu, H. L. Wang and Q. G. Wang, Adv. Mater., 2014, 26, 4370–4375 CrossRef CAS PubMed.
- B. X. Wang and X. P. Zhao, J. Mater. Chem., 2002, 12, 1865–1869 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization and SEM figures. See DOI: 10.1039/c4ra14275f |
| This journal is © The Royal Society of Chemistry 2015 |