Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The role of fluorine substituents in the formation of the ferroelectric nematic phase†
Ewan
Cruickshank‡
 *a,
Rebecca
Walker
*a,
Rebecca
Walker
 a,
Grant J.
Strachan
a,
Grant J.
Strachan
 b,
Ewa
Górecka
b,
Ewa
Górecka
 b,
Damian
Pociecha
b,
Damian
Pociecha
 b,
John M. D.
Storey
b,
John M. D.
Storey
 a and
Corrie T.
Imrie
a and
Corrie T.
Imrie
 a
a
aDepartment of Chemistry, University of Aberdeen, Old Aberdeen, AB24 3UE, UK. E-mail: e.cruickshank2@rgu.ac.uk
bFaculty of Chemistry, University of Warsaw, Zwirki i Wigury 101, 02-089 Warsaw, Poland
First published on 20th December 2024
Abstract
The synthesis and characterisation of a group of molecules based on the skeletal structures of 4-[(4-nitrophenoxy)carbonyl]phenyl 4-methoxybenzoate and (4-nitrophenyl)-2-methoxy-4-(4-methoxybenzoyl)oxybenzoate is reported. Fluorine substituents are added to these structures and their liquid crystalline behaviour is characterised. All eight compounds reported exhibit the ferroelectric nematic phase, NF, and in four of them the antiferroelectric phase labelled NX is observed. These include rare examples of enantiotropic NF and NX phases, and several examples of the direct NF-isotropic transition. The addition of a fluorine substituent reduces the nematic–isotropic transition temperature, and this is attributed to the reduction in both shape anisotropy and the ability of the molecules to form antiparallel dimers. The effect of the fluorine substituents on the NF and NX phases is less regular and interpreted in terms of the changes to the molecular shape and electron distribution. The stability of the NX phase follows that of the NF phase in the series without a lateral methoxy group. The addition of a lateral methoxy group reduces the stability of the NF phase and completely suppresses the NX phase.
Introduction
Following the experimental discovery of a polar nematic phase in 20171,2 and its subsequent assignment in 2020 as the ferroelectric nematic phase, NF,3 it has rapidly become the hottest topic in liquid crystal science and technology, not least due to its huge application potential. The NF phase shows very large polarisation values, strong non-linear optical responses and giant dielectric permittivity values compared to those observed for the conventional nematic, N, phase.4–30 We do note, however, that some doubt has been cast on these very large permittivity values by, for example, Erkoreka et al.31 and Clark et al.32 The phase also exhibits other interesting behaviour such as the formation of twisted structures in antiparallel rubbed cells.33 Critically it is the polar nature of the NF phase that gives rise to its fascinating fundamental behaviour and technologically valuable properties. The conventional N phase presently underpins liquid crystal display technologies34 and is the least ordered liquid crystal phase. In the N phase, the molecules show long-range orientational ordering in the absence of long-range positional ordering. Thus, the rod-like molecules align along a common direction known as the director, which is represented by the unit vector, n. The N phase possesses inversion symmetry such that n = −n, Fig. 1, and hence is non-polar. By comparison, in the NF phase, this inversion symmetry is broken such that n ≠ −n, Fig. 1, and the phase is polar. | ||
| Fig. 1 Schematic representation of the conventional nematic phase (left) and the ferroelectric nematic phase (right). | ||
The overwhelming majority of approximately 200 molecules that have been reported to exhibit the NF phase can be broadly grouped into one of two molecular templates, RM734 and DIO, Fig. 2.6–8,11,13,17,18,20,26,30,35–55 These were the first compounds reported to exhibit the NF phase2,42 and although they appear rather chemically different, both possess a large longitudinal dipole moment and structural elements promoting lateral bulk. It has become apparent that the ester links in these structures play an important role in the formation of the NF phase by disrupting the electronic distribution along the molecule such that it may be described as alternating regions of positive and negative charge density as sketched in Fig. 3.56 This representation of the electron density is similar to the longitudinal charge density waves used by Madhusudana to describe molecules and account for the formation of the NF phase.57 Specifically, the model suggests that the parallel alignment of the molecules is enhanced by minimising the amplitude of the charge density wave at either end of the molecule. Molecular dynamics simulations reported by Mandle also reveal that the molecules are displaced along their long axes to allow the favourable overlap of negative and positive regions in a parallel orientation.58,59 We recently highlighted the challenge in generating more exotic structures within this structure space through the design of highly polar dimers which, despite appearing to have the features described earlier, did not exhibit the NF phase.60
 | ||
| Fig. 3 Sketch of a rod-like molecule with regions of positive and negative charge (left) and the favoured parallel alignment of rods in the NF phase (right). | ||
We have previously reported on the role played by fluorine substituents in RM734-type compounds in the formation of the NF phase, focussing our attention on fluorine substituents on the middle aromatic ring and in the ortho position to the terminal nitro group.8,20,26,36,46,51 Here we extend our study and consider the effects on the NF phase of fluorine substituents on the 4-methoxybenzoic acid fragment. This work will look to expand on the fluorinated materials reported by Li et al.6 and this will be undertaken by adding fluorine atoms next to the terminal nitro group and removing the lateral methoxy group in the middle aromatic ring. We recently reported that adding methoxy groups to the middle aromatic ring of RM734-type compounds decreased the value of the NF transition temperatures and extinguished the conventional nematic phase. Therefore, we would expect the compounds we are reporting herein, in which the methoxy group is removed from the middle aromatic ring, to show a stabilisation of the NF phase. Here we report the synthesis and characterisation of eight compounds structurally related to RM734 each containing fluorine substituents on the 4-methoxybenzoic acid fragment rather than a lateral methoxy group and designed to exhibit the NF phase. We refer to these compounds using the code ECnF and their structures are shown in Table 1. Of these eight compounds, six are reported here for the first time. The properties of these compounds will allow us to better understand the structural factors that drive the formation of the fascinating NF phase which in turn will underpin the rational design of new materials having targeted properties.
Experimental
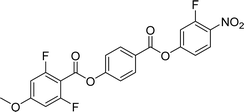
The synthetic route used to prepare EC1F, EC2F, EC5F and EC6F is shown in Scheme 1 and for EC3F, EC4F, EC7F and EC8F in Scheme 2. A detailed description of the preparation of the intermediates and final products, including their structural characterisation, is provided in the ESI.†Optical studies
Phase characterisation was performed using polarised light microscopy, using a Zeiss AxioImager A2m equipped with a Linkam THMS600 hot stage. Slides treated for planar alignment were purchased from INSTEC or AWAT with a thickness between 2.9–3.5 μm or 1.8 μm, respectively, and both sets of cells were ITO conducting.Differential scanning calorimetry
The phase behaviour of the materials was studied by differential scanning calorimetry performed using a Mettler Toledo DSC1 or DSC3 differential scanning calorimeter equipped with TSO 801RO sample robots and calibrated using indium and zinc standards. Heating and cooling rates were 10 K min−1, with a 3 min isotherm between either heating or cooling, and all samples were measured under a nitrogen atmosphere. Transition temperatures and associated enthalpy changes were extracted from the heating traces unless otherwise noted. For each sample, two aliquots were measured, and the data listed are the average of the two sets of data.Molecular modelling
The geometric parameters of the compounds of interest were obtained using quantum mechanical DFT calculations with Gaussian09 software.61 Optimisation of the molecular structures was carried out at the B3LYP/6-31G(d) level. Visualisations of electronic surfaces and ball-and-stick models were generated from the optimised geometries using the GaussView 5 software. The electronic surfaces were found using the cubegen utility in GaussView by generating a total density cube using a SCF density matrix and coarse grid, overlayed by an ESP surface map. Visualisations of the space-filling models were produced post-optimisation using the QuteMol package.62Birefringence measurements
Birefringence was measured with a setup based on a photoelastic modulator (PEM-90, Hinds) working at a modulation frequency f = 50 kHz; as a light source, a halogen lamp (Hamamatsu LC8) was used equipped with narrow bandpass filter (532 nm). The signal from a photodiode (FLC Electronics PIN-20) was deconvoluted with a lock-in amplifier (EG&G 7265) into 1f and 2f components to yield a retardation induced by the sample. Knowing the sample thickness, the retardation was recalculated into optical birefringence. Samples were prepared in 3.0 μm-thick cells with planar anchoring. The alignment quality was checked prior to measurement by inspection under the polarised light optical microscope.Dielectric spectroscopy
The complex dielectric permittivity, ε*, was studied using a Solartron 1260 impedance analyser. Measurements were conducted in the 10 Hz–1 MHz frequency (f) range, with the probe voltage of 20 mV, and it was checked by optical observations that such a voltage is below the Fredericks transition threshold. The material was placed in 9.7 μm-thick glass cells with gold electrodes and no polymer aligning layers. Regardless of the way the dielectric spectroscopy results are interpreted, when a material with a giant permittivity is tested, instead of charging, and thus measuring the capacitor with the LC sample only the surface layers are charged because they have a smaller capacitance even though they are much thinner. Hence cells without polymer aligning layers were used in order to help this somewhat. The lack of a surfactant layer resulted in the random configuration of the director in the liquid crystal phases; microscopic observations of the optical textures suggested a dominant planar orientation without the preferable direction of the long molecular axis.Second harmonic generation
The SHG activity was tested using a microscopic setup. Solid-state laser EKSPLA NL202 pulses (λ = 1064 nm, 9 ns, 10 Hz repetition rate and max. 2 mJ pulse energy) were incident onto a LC sample prepared in 10 μm-thick glass cells with planar anchoring condition. An IR pass filter was placed at the entrance to the sample and a green pass filter at the exit of the sample.X-ray diffraction
The X-ray diffraction (XRD) measurements were performed with a Bruker D8 GADDS system (CuKα line, Goebel mirror monochromator, point beam collimator, Vantec2000 area detector). The temperature of the sample was controlled with a precision of ±0.1 K. Samples were prepared as droplets on a heated surface.Results and discussion
The transitional properties of the ECnF compounds (Table 1) are listed in Table 2. The transition temperatures of EC3F and EC7F are in good agreement with those reported previously by Li et al.6 Five of these compounds, specifically EC1F, EC2F, EC3F, EC5F and EC6F, exhibited the conventional nematic (N) phase and this was assigned on the observation of characteristic textures. Thus, for samples sandwiched between untreated glass slides a schlieren texture containing two- and four-brush point singularities was observed (Fig. 4(a)) which flashed when subjected to mechanical stress, and for samples in cells treated for planar alignment, a uniform texture was seen (Fig. 4(b)). On cooling the N phase shown by EC2F, EC5F and EC6F, a chevron texture developed indicative of the NX phase (Fig. 4(c) and (d)).8,44,47,51,63 The NX phase is also referred to as the SmZA phase and consists of a regular array of polar domains arranged in an anti-ferroelectric fashion along the direction perpendicular to the director.44,53,63–65 This question of nomenclature for this phase is yet to be resolved as there is a sharp XRD signal sometimes recorded in this phase, corresponding to the periodic domain structure, which would be considered a feature of a smectic phase. Simultaneously, a diffuse XRD signal is always observed reflecting liquid-like positional correlations for neighbouring molecules, suggesting a more nematic nature to the phase.47| Name | Phase sequence | μ/D |
|---|---|---|
| a Measured using polarised light microscopy. b Represents a combined entropy change arising from overlapping peaks in the DSC trace. c Crystallisation temperature of sample bulk, mesophases observed in isolated droplets. | ||
| EC1F | Cr-207 (11.8)-N-278 (0.13)-I | 10.98 |
| I-276a-N-157a-NX-144a-NF-162c-Cr | ||
| EC2F | Cr-192 (9.85)-N-245 (0.16)-I | 12.02 |
| I-244 (0.18)-N-175 (0.003)-NX-166 (0.12)-NF-155-Cr | ||
| EC3F | Cr-169 (12.4)-N-177 (0.16)-I | 12.26 |
| I-176 (0.21)-N-156 (0.24)-NF-141-Cr | ||
| EC4F | Cr-172 (15.3)-I | 13.31 |
| I-153 (1.25)-NF-112-Cr | ||
| EC5F | Cr-186 (9.87)-N-266a-I | 11.18 |
| I-265a-N-177-NX-172 (0.09b)-NF-134-Cr | ||
| EC6F | Cr-181 (8.44)-NF-188 (0.17b)-NX-191–231 (0.16)-I | 12.16 |
| I-232 (0.16)-N-189-NX-187 (0.22b)-NF-148-Cr | ||
| EC7F | Cr-167 (13.6)-I | 12.58 |
| I-159 (1.07)-NF-110-Cr | ||
| EC8F | Cr-162 (13.0)-I | 13.64 |
| I-149 (1.44)-NF-140-Cr | ||
On cooling the chevron texture of the NX phase seen for EC2F, EC5F and EC6F, a highly birefringent, banded texture (Fig. 4(e)) or a texture showing twisted states (Fig. 4(f)) formed, characteristic of the formation of the NF phase.14,20,30,36,41,51 A similar banded texture was seen on cooling the schlieren texture of the N phase of EC3F indicating a NF–N transition, and was also observed directly from the isotropic phase for EC4F, EC7F and EC8F implying a NF-I transition. The domains apparent in these banded textures (Fig. 4(e)) are areas in which the director has differing orientations and thus the orientation of the polarisation also differs and appears to be ‘banded’ due to the differing areas of birefringence within the domains.
The proposed phase assignments are consistent with the values of the associated scaled entropy changes, ΔS/R, listed in Table 2. The values of ΔSNI/R range between 0.13 to 0.21, and these are lower than typically measured for a conventional low molar mass nematogen for which ΔSNI/R ≈ 0.3. These smaller values presumably reflect the enhanced molecular biaxiality associated with the lateral substituents that reduce the orientational order of the nematic phase and hence, reduce ΔSNI/R.66 The values of the scaled entropy change associated with the NF-I transition, ΔSNFI/R, are several times larger than ΔSNI/R and this additional entropic contribution is associated with the ordering of the dipoles in the NF phase.
On cooling the N phase shown by EC1F, crystallisation in the bulk precluded the observation of the characteristic textures for the NF and NX phases shown in Fig. 4. Instead, the N–NX and NX–NF transitions were observed only for isolated droplets. To assign these phase transitions a phase diagram (Fig. 5) was constructed for binary mixtures of EC1F and the ferroelectric nematogen, 4EC6F (Fig. 6).47 The compounds were miscible over the entire composition range, and each mixture exhibited N, NX and NF phases assigned on the observation of characteristic optical textures (Fig. 7). Adding EC1F to 4EC6F sees TNI, TNXN and TNFNX increase in essentially a linear manner. Extrapolation of the trendlines provides estimates of the virtual transition temperatures for EC1F of TNI = 266 °C, TNXN = 146 °C and TNFNX = 137 °C, with the values for the pure sample of EC1F not included. These values are slightly lower than the transition temperatures measured for isolated droplets of EC1F using polarised optical microscopy but strongly support the assignments listed in Table 1.
 | ||
| Fig. 5 Phase diagram constructed using binary mixtures of EC1F and 4EC6F.47 Squares denote TNI, circles TNXN and triangles TNFNX. The trendlines are shown for TNI, TNXN and TNFNX. | ||
X-ray diffraction was used to characterise the N, NX and NF phases and representative diffraction patterns for EC5F are shown in Fig. 8. Only diffuse scattering is seen in these patterns arising from the short-range positional ordering of the molecules in all three phases. The periodic structure of the antiferroelectric domains in the NX phase should give rise to a sharp reflection at small angles in the X-ray diffraction pattern. This signal has a very low intensity, however, showing that the related electron density modulation is particularly weak, and in practice, a strong synchrotron source is required for it to be observed.47
 | ||
| Fig. 8 X-ray diffraction patterns obtained for EC5F in (left) the N, (middle) NX and (right) NF phases. | ||
The temperature dependence of the optical birefringence, Δn, for EC2F is shown in Fig. 9. A particularly small change in Δn of just ≈0.01 is seen at the N–NX transition indicating that the NX phase is orientationally very similar to the conventional N phase. This is followed by a larger but still small step-like increase at the NX–NF transition indicative of an increase of the order parameter, S. These observations are in complete accord with our previous reports of the temperature dependence of the optical birefringence of the NX and NF phases.47,51 The polar character of the nematic phases exhibited by EC2F was tested by measuring its complex permittivity as a function of temperature and frequency (Fig. 10). On cooling through the nematic phase, a weak dielectric mode begins to develop with a relaxation frequency slightly above 104 Hz, which shows typical softening due to the increasing polar order, with ε′ increasing to ca. 100, while the frequency decreases approaching the N–NX transition. On entering the NX phase, this mode is quenched, most probably due to the antiferroelectric nature of this phase as we have previously reported.47 The NX–NF transition is marked by the abrupt increase of the measured low-frequency permittivity, by orders of magnitude, which is characteristic of the NF phase. While the proper detailed interpretation of the measured permittivity values in the NF phase is more complex than previously thought, with many recent articles suggesting a variety of interpretations of what the values should be and why,12,31,32,67–70 these considerations are beyond the scope of this paper which has a focus on the effect of structural modifications on the phase behaviour. However, the measured temperature dependence of permittivity can be treated as qualitative proof for the determined sequence of polar phases, as it is in excellent agreement with data reported for other ferroelectric nematogens.6–12,14,30 The non-centrosymmetric nature of the NF phase in compound EC2F has been also confirmed by observation of its SHG activity. Incident IR (λ = 1064 nm) radiation resulted in a strong emission of green light (Fig. 10c), proving the ferroelectric ground state of the phase.
 | ||
| Fig. 9 Temperature dependence of the optical birefringence of EC2F measured on cooling with green light (λ = 532 nm). | ||
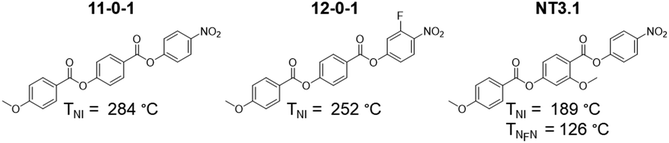
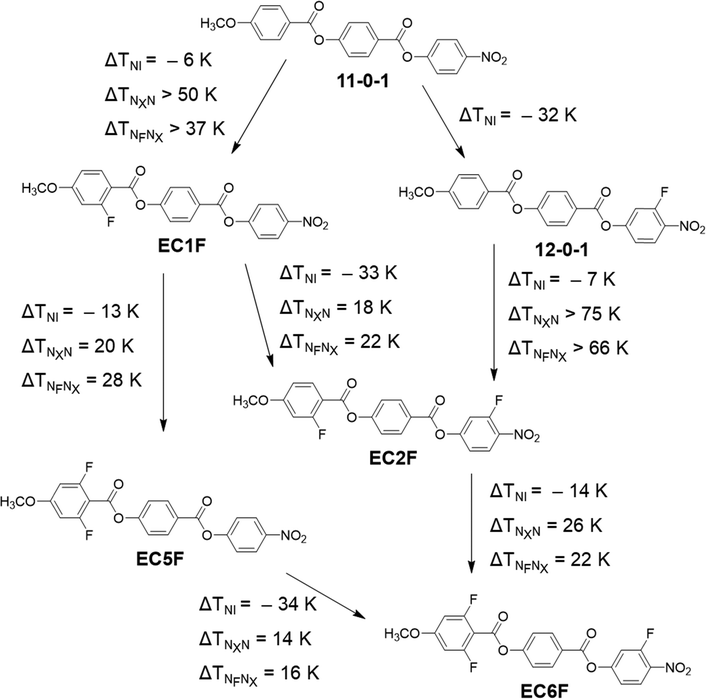
We now turn our attention to discuss the transition temperatures listed in Table 2 and how these relate to changes in molecular structure with a particular focus on the role played by fluorine substituents in driving the formation of the NF phase. With this in mind, the eight compounds listed in Table 2 may be split into two groups of four depending whether they are based on 4-[(4-nitrophenoxy)carbonyl]phenyl 4-methoxybenzoate (11-0-1)46 or (4-nitrophenyl)2-methoxy-4-(4-methoxybenzoyl)oxybenzoate (NT3.1).20 The structures and transition temperatures of 11-0-1 and NT3.1 are shown in Fig. 11. 11-0-1 has no lateral substituents and exhibits exclusively conventional N behaviour whereas NT3.1 possesses a lateral methoxy substituent on the central ring and shows both NF and N phases. We note that isolated droplets of the N phase shown by 11-0-1 may be supercooled to 107 °C prior to crystallisation but attempts to measure a virtual NF–N transition temperature were not successful.46 The addition of a fluorine substituent ortho to the nitro group in 11-0-1 gives 12-0-1 and this is also exclusively nematic, see Fig. 11.
Fig. 12 maps the changes in the three transition temperatures, TNI, TNXN and TNFNX, on increasing the number of fluorine substituents from the unsubstituted 11-0-1 to EC6F containing three fluorine atoms. EC6F is a particularly interesting compound as it exhibits enantiotropic NX and NF phases. The addition of a fluorine atom always reduces TNI but the magnitude of this decrease depends on the resulting change in structure. Thus, adding the first fluorine atom to the methoxyphenyl ring reduces TNI by around 6 K, and the second by about 13 K, irrespective of whether the nitrophenyl ring contains a fluorine atom or not. The addition of the fluorine atom ortho to the nitro group reduces TNI by about 33 K irrespective of the number of fluorine atoms on the methoxyphenyl ring. By contrast, adding fluorine atoms always increases both TNXN and TNFNX with the exception of moving from 11-0-1 to 12-0-1 for which NX and NF phases are not observed. It is clear, however, that TNFNX and TNXN must both be higher for EC1F than 12-0-1 by at least 44 K and 57 K, respectively, indicating that a fluorine atom ortho to the methoxy group is considerably more effective in promoting the NF and NX phases than if ortho to the nitro group. This is also evident in the dramatic increase in TNFNX and TNXN passing from 12-0-1 to EC2F of at least 66 K and 75 K, respectively. After the addition of the first fluorine atom to the methoxyphenyl ring, subsequent fluorine atoms have a much smaller effect, and it appears to make little difference in which order they are added. This culminates in the compound EC6F which contains three fluorines within the molecular structure and exhibits both enantiotropic NX and NF phases.
Fig. 13 shows the changes in TNFN/I and TNI on increasing the number of fluorine atoms from the unsubstituted NT3.1 to EC8F containing three fluorine atoms. Again, it is evident that the addition of a fluorine atom always decreases TNI. Broadly the trends are the same as described for the 11-0-1-based materials. The largest decreases in TNI are observed on adding a fluorine atom ortho to the nitro group. The reduction in TNI associated with the addition of the fluorine atoms is sufficiently large for the nematic phase to be extinguished and a direct NF-I transition to be observed for three of the four NT3.1-based compounds. The effect of adding a fluorine atom on TNFN/I is smaller than seen for the 11-0-1-based materials and depending on the structure may be positive or negative, which agrees with our recently published work on other ferroelectric nematic materials.46
We have seen that the addition of a fluorine atom to a compound in this collection always leads to a decrease in TNI and this effect is most pronounced when the fluorine atom is ortho to the nitro group. This reflects, at least in part, the reduction in structural anisotropy arising from the lateral substituent, see Fig. 14, and this is most pronounced for a fluorine atom ortho to the nitro group (Fig. 14(d)). This alone, however, cannot account for the observed trends given that these are similar for both sets of materials even though the relative change in shape will be smaller for the NT3.1-based materials (Fig. 14(c)). This suggests that the fluorine atom ortho to the nitro group inhibits the antiparallel association of these molecules and this will also reduce TNI. This accounts for the smaller reductions in TNI seen for additions of fluorine atoms to the methoxyphenyl ring as these will have a much smaller effect on the antiparallel association of the molecules. In addition, the effect on shape of the first fluorine atom added to the methoxyphenyl ring is reduced by the molecular structure (Fig. 14(a)). By comparison, the second fluorine atom protrudes from the excluded molecular volume (Fig. 14(b)) and gives rise to a larger reduction in TNI than seen for the first fluorine atom.
Our previous studies have suggested that a combination of electronic and shape factors drive the formation of the NF phase20,41,46 and even less is presently understood about the NX phase.47,51 For the 11-0-1-based materials a fluorine atom on the methoxyphenyl ring strongly promotes both NF and NX phases, whereas a single fluorine atom ortho to the nitro group does not. We have already noted that the change in shape arising from the addition of the first fluorine atom to the methoxyphenyl ring must be small given the associated weak change in TNI, and hence, the effect on the NF and NX phases must arise from the change in the electron distribution. The addition of the fluorine atom reduces the electron density associated with the methoxyphenyl ring (Fig. 14(a)). Within the framework of the model proposed by Madhusudana to account for the formation of the NF phase57 described earlier, minimising the amplitude of the charge density wave at either end of the molecule reduces the tendency to adopt anti-parallel structures and the NF phase is stabilised. The second fluorine atom further reduces the charge density associated with the methoxyphenyl ring and also enhances the molecular biaxiality (Fig. 14(b)) further promoting the NF and NX phases. The single fluorine atom ortho to the nitro group in 12-0-1 in itself does not promote the formation of the NF phase because the molecule does not favourably pack into parallel structures presumably due to its shape. This is despite the fluorine atom reducing the amplitude of the charge density wave at the end of the molecule and is indicative that the position of lateral substituents is a critical consideration in the phase behaviour of these materials. The shape requirement for observing the NF phase is satisfied by the addition of fluorine atoms to the methoxyphenyl ring, and the combination of the three fluorine atoms gives rise to the highest values of TNFNX and TNXN.
The effects of the fluorine atoms on the NF phases shown by the NT3.1-based materials are smaller than seen for the 11-0-1-based compounds and may either increase or decrease the transition temperatures. This cannot be accounted for entirely using Madhusudana's model, which would predict similar behaviour for both sets of compounds.57 This presumably reflects the weaker change in shape arising from the addition of the fluorine atoms in the case of the NT3.1-based compounds and suggests that some optimum molecular shape exists for the observation of the NF phase. The presence of both the methoxy and fluorine substituents may exceed this shape constraint and overcome the electronic contributions the fluorine atoms play in the formation of the NF such that a decrease in TNFN/I is observed.
Finally, we will consider the role of the methoxy lateral group and compare these two sets of molecules. As would be expected on the basis of shape, the addition of the lateral methoxy group reduces TNI by around 95 K. The smallest reduction, 82 K, is for the pair of molecules containing the highest number of fluorine substituents, EC6F and EC8F, and this reflects the smaller relative change in shape associated with the methoxy group. As we have seen, neither 11-0-1 nor 12-0-1 exhibit the NF phase and the addition of the lateral methoxy group to the central phenyl ring has a pronounced effect, increasing TNFN by at least 19 K and 42 K, respectively. The situation is more difficult to establish for the remaining pairs of compounds because differing phase transitions are being compared. It is clear, however, that for three of the pairs, EC2F/EC4F, EC5F/EC7F and EC6F (which has an enantiotropic NF-I transition)/EC8F, the stability of the NF phase is reduced on the addition of the methoxy group and for the fourth, EC1F/EC3F, there appears little change. Certainly, the addition of the methoxy group will increase the amplitude of the charge density wave in the centre of the molecule and this will promote the formation of the NF phase and hence the observed increase in transition temperatures for the 11-0-1/NT3.1 and 12-0-1/NT3F.1 pairs of molecules. Presumably for the remaining pairs, shape considerations must account for the reduced tendency to exhibit the NF phase for the methoxy-substituted materials. It is interesting to note that the methoxy substituent in the middle aromatic ring has suppressed the formation of the NX phase and the physical significance of this requires further investigation.
Conclusions
We report two sets of new ferroelectric nematogens to investigate the role played by fluorine substituents on the formation of the NF phase. The addition of fluorine substituents reduces TNI. The magnitude of this decrease depends on the position of the fluorine atom, and this is interpreted in terms of its effect on molecular shape and the tendency of the molecules to form antiparallel dimers. The effect of fluorine substituents on the stability of the NF phase is more strongly dependent on the molecular structure and TNFN/I may increase or decrease. This is interpreted in terms of a competition between changes to molecular shape and electron distribution. It appears that there is some optimum shape for the formation of the NF phase and if the addition of lateral substituents enhances the biaxiality beyond this, then the tendency to exhibit the NF phase is reduced. The contributions of the electronic effects are well accounted for in terms of the model proposed by Madhusudana.57 It is interesting to note that the stability of the NX phase with respect to changes in the molecular structure appears similar to that of the NF phase for the 11-0-1-based materials and these materials provide a rare opportunity to study this. By contrast, the addition of a lateral methoxy group extinguishes the NX phase. The physical significance of this observation is far from clear. In terms of the molecular engineering of materials, fluorine substituents clearly have an important role to play in the design of ferroelectric nematogens and here we report a rare example of a single compound exhibiting both enantiotropic NF and NX phases.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. T. I. and J. M. D. S. acknowledge the financial support from the Engineering and Physical Sciences Research Council [EP/V048775/1]. The research was also supported by the National Science Centre (Poland) under the grant no. [2021/43/B/ST5/00240]. The authors thank Dr Jadwiga Szydłowska for their help in performing the SHG experiment.References
- R. J. Mandle, S. J. Cowling and J. W. Goodby, A nematic to nematic transformation exhibited by a rod-like liquid crystal, Phys. Chem. Chem. Phys., 2017, 19, 11429–11435 RSC.
- H. Nishikawa, K. Shiroshita, H. Higuchi, Y. Okumura, Y. Haseba, S. I. Yamamoto, K. Sago and H. Kikuchi, A Fluid Liquid-Crystal Material with Highly Polar Order, Adv. Mater., 2017, 29, 1702354 CrossRef.
- X. Chen, E. Korblova, D. Dong, X. Wei, R. Shao, L. Radzihovsky, M. A. Glaser, J. E. Maclennan, D. Bedrov, D. M. Walba and N. A. Clark, First-principles experimental demonstration of ferroelectricity in a thermotropic nematic liquid crystal: polar domains and striking electro-optics, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 14021–14031 CrossRef CAS PubMed.
- F. Caimi, G. Nava, R. Barboza, N. A. Clark, E. Korblova, D. M. Walba, T. Bellini and L. Lucchetti, Surface alignment of ferroelectric nematic liquid crystals, Soft Matter, 2021, 17, 8130–8139 RSC.
- P. Rudquist, Revealing the polar nature of a ferroelectric nematic by means of circular alignment, Sci. Rep., 2021, 11, 24411 CrossRef CAS PubMed.
- J. Li, Z. Wang, M. Deng, Y. Zhu, X. Zhang, R. Xia, Y. Song, Y. Hisai, S. Aya and M. Huang, General phase-structure relationship in polar rod-shaped liquid crystals: importance of shape anisotropy and dipolar strength, Giant., 2022, 11, 100109 CrossRef CAS.
- R. Saha, P. Nepal, C. Feng, M. S. Hossain, M. Fukuto, R. Li, J. T. Gleeson, S. Sprunt, R. J. Twieg and A. Jákli, Multiple ferroelectric nematic phases of a highly polar liquid crystal compound, Liq. Cryst., 2022, 49, 1784–1796 CrossRef CAS.
- S. Brown, E. Cruickshank, J. M. D. Storey, C. T. Imrie, D. Pociecha, M. Majewska, A. Makal and E. Gorecka, Multiple Polar and Non-polar Nematic Phases, ChemPhysChem, 2021, 22, 2506–2510 CrossRef CAS PubMed.
- H. Nishikawa, K. Sano and F. Araoka, Anisotropic fluid with phototunable dielectric permittivity, Nat. Commun., 2022, 13, 1142 CrossRef CAS.
- R. J. Mandle, N. Sebastián, J. Martinez-Perdiguero and A. Mertelj, On the molecular origins of the ferroelectric splay nematic phase, Nat. Commun., 2021, 12, 4962 CrossRef CAS PubMed.
- A. Manabe, M. Bremer and M. Kraska, Ferroelectric nematic phase at and below room temperature, Liq. Cryst., 2021, 48, 1079–1086 CrossRef CAS.
- N. Vaupotič, D. Pociecha, P. Rybak, J. Matraszek, M. Čepič, J. M. Wolska and E. Gorecka, Dielectric response of a ferroelectric nematic liquid crystalline phase in thin cells, Liq. Cryst., 2023, 50, 584–595 CrossRef.
- J. Szydlowska, P. Majewski, M. Čepič, N. Vaupotič, P. Rybak, C. T. Imrie, R. Walker, E. Cruickshank, J. M. D. Storey, P. Damian and E. Gorecka, Ferroelectric Nematic–Isotropic Liquid Critical End Point, Phys. Rev. Lett., 2023, 130, 216802 CrossRef CAS.
- J. Li, R. Xia, H. Xu, J. Yang, X. Zhang, J. Kougo, H. Lei, S. Dai, H. Huang, G. Zhang, F. Cen, Y. Jiang, S. Aya and M. Huang, How Far Can We Push the Rigid Oligomers/Polymers toward Ferroelectric Nematic Liquid Crystals?, J. Am. Chem. Soc., 2021, 143, 46 CrossRef PubMed.
- H. Nishikawa and F. Araoka, A New Class of Chiral Nematic Phase with Helical Polar Order, Adv. Mater., 2021, 33, 2101305 CrossRef CAS.
- N. Sebastián, L. Cmok, R. J. Mandle, M. R. De La Fuente, I. Drevenšek Olenik, M. Čopič and A. Mertelj, Ferroelectric–Ferroelastic Phase Transition in a Nematic Liquid Crystal, Phys. Rev. Lett., 2020, 124, 037801 CrossRef PubMed.
- N. Sebastián, R. J. Mandle, A. Petelin, A. Eremin and A. Mertelj, Electrooptics of mm-scale polar domains in the ferroelectric nematic phase, Liq. Cryst., 2021, 48, 2055–2071 CrossRef.
- Y. Song, M. Deng, Z. Wang, J. Li, H. Lei, Z. Wan, R. Xia, S. Aya and M. Huang, Emerging Ferroelectric Uniaxial Lamellar (Smectic AF) Fluids for Bistable In-Plane Polarization Memory, J. Phys. Chem. Lett., 2022, 13, 9983–9990 CrossRef CAS.
- C. Feng, R. Saha, E. Korblova, D. Walba, S. N. Sprunt, A. Jákli, C. Feng, S. N. Sprunt, A. Jákli, R. Saha, E. Korblova and D. Walba, Electrically Tunable Reflection Color of Chiral Ferroelectric Nematic Liquid Crystals, Adv. Opt. Mater., 2021, 9, 2101230 CrossRef CAS.
- N. Tufaha, E. Cruickshank, D. Pociecha, E. Gorecka, J. M. D. Storey and C. T. Imrie, Molecular Shape, Electronic Factors and the Ferroelectric Nematic Phase, Chem. – Eur. J., 2023, 29, e202300073 CrossRef CAS.
- S. Abe, Y. Shibata, M. Kimura and T. Akahane, Self-Consistent Explanation of the Untwist Alignment of Ferroelectric Nematic Liquid Crystals with Decreasing Cell Thickness and Deviation of the Surface Easy Axis Experimented upon Using the Brewster Angle Reflection Method, Crystals, 2024, 14, 157 CrossRef CAS.
- H. Kamifuji, K. Nakajima, Y. Tsukamoto, M. Ozaki and H. Kikuchi, Effect of rubbing symmetry on polarization distribution in ferroelectric nematic liquid crystal cells, Appl. Phys. Express, 2023, 16, 071003 CrossRef CAS.
- F. Ye, C. Yang, X. Zhang, X. Huang, Y. Zhu, S. Aya and M. Huang, High-κ elastomer with dispersed ferroelectric nematic liquid crystal microdroplets, J. Mater. Chem. C, 2024, 12, 2738–2744 RSC.
- C. L. Folcia, J. Ortega, R. Vidal, T. Sierra and J. Etxebarria, The ferroelectric nematic phase: an optimum liquid crystal candidate for nonlinear optics, Liq. Cryst., 2022, 49, 899–906 CrossRef CAS.
- J. Ortega, C. L. Folcia, J. Etxebarria and T. Sierra, Ferroelectric chiral nematic liquid crystals: new photonic materials with multiple bandgaps controllable by low electric fields, Liq. Cryst., 2022, 49, 2128–2136 CrossRef CAS.
- D. Pociecha, R. Walker, E. Cruickshank, J. Szydlowska, P. Rybak, A. Makal, J. Matraszek, J. M. Wolska, J. M. D. Storey, C. T. Imrie and E. Gorecka, Intrinsically chiral ferronematic liquid crystals: an inversion of the helical twist sense at the chiral nematic – chiral ferronematic phase transition, J. Mol. Liq., 2021, 361, 119532 CrossRef.
- E. Zavvou, M. Klasen-Memmer, A. Manabe, M. Bremer and A. Eremin, Polarisation-driven magneto-optical and nonlinear-optical behaviour of a room-temperature ferroelectric nematic phase, Soft Matter, 2022, 18, 8804–8812 RSC.
- H. Nishikawa, K. Sano, S. Kurihara, G. Watanabe, A. Nihonyanagi, B. Dhara and F. Araoka, Nano-clustering mediates phase transitions in a diastereomerically-stabilized ferroelectric nematic system, Commun. Mater., 2022, 3, 89 CrossRef CAS.
- X. Chen, E. Korblova, M. A. Glaser, J. E. MacLennan, D. M. Walba and N. A. Clark, Polar in-plane surface orientation of a ferroelectric nematic liquid crystal: polar monodomains and twisted state electro-optics, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2104092118 CrossRef CAS.
- J. Li, H. Nishikawa, J. Kougo, J. Zhou, S. Dai, W. Tang, X. Zhao, Y. Hisai, M. Huang and S. Aya, Development of ferroelectric nematic fluids with giant-dielectricity and nonlinear optical properties, Sci. Adv., 2021, 7, eabf5047 CrossRef CAS.
- A. Erkoreka, J. Martinez-Perdiguero, R. J. Mandle, A. Mertelj and N. Sebastián, Dielectric spectroscopy of a ferroelectric nematic liquid crystal and the effect of the sample thickness, J. Mol. Liq., 2023, 387, 122566 CrossRef CAS.
- N. A. Clark, X. Chen, J. E. MacLennan and M. A. Glaser, Dielectric spectroscopy of ferroelectric nematic liquid crystals: measuring the capacitance of insulating interfacial layers, Phys. Rev. Res., 2024, 6, 013195 CrossRef CAS.
- N. Yadav, Y. P. Panarin, W. Jiang, G. H. Mehl and J. K. Vij, Spontaneous mirror symmetry breaking and chiral segregation in the achiral ferronematic compound DIO, Phys. Chem. Chem. Phys., 2023, 25, 9083–9091 RSC.
- G. W. Gray, K. J. Harrison and J. A. Nash, New family of nematic liquid crystals for displays, Electron. Lett., 1973, 9, 130–131 CrossRef CAS.
- J. Zhou, R. Xia, M. Huang and S. Aya, Stereoisomer effect on ferroelectric nematics: stabilization and phase behavior diversification, J. Mater. Chem. C, 2022, 10, 8762–8766 RSC.
- E. Cruickshank, R. Walker, J. M. D. Storey and C. T. Imrie, The effect of a lateral alkyloxy chain on the ferroelectric nematic phase, RSC Adv., 2022, 12, 29482–29490 RSC.
- H. Kikuchi, H. Matsukizono, K. Iwamatsu, S. Endo, S. Anan and Y. Okumura, Fluid Layered Ferroelectrics with Global C∞v Symmetry, Adv. Sci., 2022, 9, 2202048 CrossRef PubMed.
- R. J. Mandle, A new order of liquids: polar order in nematic liquid crystals, Soft Matter, 2022, 18, 5014–5020 RSC.
- N. Sebastián, M. Čopič and A. Mertelj, Ferroelectric nematic liquid-crystalline phases, Phys. Rev. E, 2022, 106, 021001 CrossRef PubMed.
- Y. Song, J. Li, R. Xia, H. Xu, X. Zhang, H. Lei, W. Peng, S. Dai, S. Aya and M. Huang, Development of emergent ferroelectric nematic liquid crystals with highly fluorinated and rigid mesogens, Phys. Chem. Chem. Phys., 2022, 24, 11536–11543 RSC.
- E. Cruickshank, A. Pearson, S. Brown, J. M. D. Storey, C. T. Imrie and R. Walker, The ferroelectric nematic phase: on the role of lateral alkyloxy chains, Liq. Cryst., 2023, 50, 1960–1967 CrossRef CAS.
- R. J. Mandle, S. J. Cowling and J. W. Goodby, Rational Design of Rod-Like Liquid Crystals Exhibiting Two Nematic Phases, Chem. – Eur. J., 2017, 23, 14554–14562 CrossRef CAS PubMed.
- E. Cruickshank, The Emergence of a Polar Nematic Phase: A Chemist's Insight into the Ferroelectric Nematic Phase, ChemPlusChem, 2024, e202300726 CrossRef CAS PubMed.
- P. Nacke, A. Manabe, M. Klasen-Memmer, X. Chen, V. Martinez, G. Freychet, M. Zhernenkov, J. E. Maclennan, N. A. Clark, M. Bremer and F. Giesselmann, New examples of ferroelectric nematic materials showing evidence for the antiferroelectric smectic-Z phase, Sci. Rep., 2024, 14, 1–13 CrossRef.
- H. Nishikawa, F. Araoka, D. Stéfani Teodoro Martinez, R. Furlan de Oliveira, H. Nishikawa, M. Kuwayama, A. Nihonyanagi and B. Dhara, Rapid, solvent-minimized and sustainable access to various types of ferroelectric–fluid molecules by harnessing mechano-chemical technology, J. Mater. Chem. C, 2023, 11, 12525–12542 RSC.
- E. Cruickshank, N. Tufaha, R. Walker, S. Brown, E. Gorecka, D. Pociecha, J. M. D. Storey and C. T. Imrie, The influence of molecular shape and electronic properties on the formation of the ferroelectric nematic phase, Liq. Cryst., 2024, 51, 401–415 CrossRef CAS.
- E. Cruickshank, P. Rybak, M. M. Majewska, S. Ramsay, C. Wang, C. Zhu, R. Walker, J. M. D. Storey, C. T. Imrie, E. Gorecka and D. Pociecha, To Be or Not To Be Polar: The Ferroelectric and Antiferroelectric Nematic Phases, ACS Omega, 2023, 8, 36562–36568 CrossRef CAS.
- M. Cigl, N. Podoliak, T. Landovský, D. Repček, P. Kužel and V. Novotná, Giant permittivity in dimethylamino-terminated ferroelectric nematogens, J. Mol. Liq., 2023, 385, 122360 CrossRef CAS.
- C. J. Gibb and R. J. Mandle, New RM734-like fluid ferroelectrics enabled through a simplified protecting group free synthesis, J. Mater. Chem. C, 2023, 11, 16982–16991 RSC.
- J. Karcz, N. Rychłowicz, M. Czarnecka, A. Kocot, J. Herman and P. Kula, Enantiotropic ferroelectric nematic phase in a single compound, Chem. Commun., 2023, 59, 14807–14810 RSC.
- G. Stepanafas, E. Cruickshank, S. Brown, M. M. Majewska, D. Pociecha, E. Gorecka, J. M. D. Storey and C. T. Imrie, Ferroelectric nematogens containing a methylthio group, Mater. Adv., 2023, 5, 525–538 RSC.
- X. Zhao, J. Zhou, J. Li, J. Kougo, Z. Wan, M. Huang and S. Aya, Spontaneous helielectric nematic liquid crystals: electric analog to helimagnets, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2111101118 CrossRef CAS.
- X. Chen, V. Martinez, P. Nacke, E. Korblova, A. Manabe, M. Klasen-Memmer, G. Freychet, M. Zhernenkov, M. A. Glaser, L. Radzihovsky, J. E. Maclennan, D. M. Walba, M. Bremer, F. Giesselmann, N. A. Clark, N. Abbott and P. Palffy-Muhoray, Observation of a uniaxial ferroelectric smectic A phase, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2210062119 CrossRef CAS.
- S. Nishimura, S. Masuyama, G. Shimizu, C.-Y. Chen, T. Ichibayashi and J. Watanabe, Lowering of Electrostatic Actuator Driving Voltage and Increasing Generated Force Using Spontaneous Polarization of Ferroelectric Nematic Liquid Crystals, Adv. Phys. Res., 2022, 1, 2200017 CrossRef.
- H. Matsukizono, K. Iwamatsu, S. Endo, Y. Okumura, S. Anan and H. Kikuchi, Synthesis of liquid crystals bearing 1,3-dioxane structures and characterization of their ferroelectricity in the nematic phase, J. Mater. Chem. C, 2023, 11, 6183–6190 RSC.
- A. A. Marchenko, O. L. Kapitanchuk, Y. Y. Lopatina, K. G. Nazarenko, A. I. Senenko, N. Katsonis, V. G. Nazarenko and O. D. Lavrentovich, Polar Self-Organization of Ferroelectric Nematic-Liquid-Crystal Molecules on Atomically Flat Au(111) Surface, Phys. Rev. Lett., 2024, 132, 098101 CrossRef CAS PubMed.
- N. V. Madhusudana, Simple molecular model for ferroelectric nematic liquid crystals exhibited by small rodlike mesogens, Phys. Rev. E, 2021, 104, 014704 CrossRef CAS.
- R. J. Mandle, Implementation of a cylindrical distribution function for the analysis of anisotropic molecular dynamics simulations, PLoS One, 2022, 17, e0279679 CrossRef CAS PubMed.
- R. J. Mandle, In Silico Interactome of a Room-Temperature Ferroelectric Nematic Material, Crystals, 2023, 13, 857 CrossRef CAS.
- A. Zattarin, E. Cruickshank, D. Pociecha, J. M. D. Storey, E. Gorecka and C. T. Imrie, A design approach to obtaining highly polar liquid crystal dimers, Liq. Cryst., 2024, 51, 1035–1046 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford CT, 2010 Search PubMed.
- M. Tarini, P. Cignoni and C. Montani, Ambient occlusion and edge cueing to enhance real time molecular visualization, IEEE Trans. Vis. Comput. Graphics, 2006, 12, 1237–1244 Search PubMed.
- X. Chen, V. Martinez, E. Korblova, G. Freychet, M. Zhernenkov, M. A. Glaser, C. Wang, C. Zhu, L. Radzihovsky, J. E. Maclennan, D. M. Walba and N. A. Clark, The smectic ZA phase: antiferroelectric smectic order as a prelude to the ferroelectric nematic, Proc. Natl. Acad. Sci. U. S. A., 2022, 120, e2217150120 CrossRef PubMed.
- A. V. Emelyanenko, V. Y. Rudyak, S. A. Shvetsov, F. Araoka, H. Nishikawa and K. Ishikawa, Emergence of paraelectric, improper antiferroelectric, and proper ferroelectric nematic phases in a liquid crystal composed of polar molecules, Phys. Rev. E, 2022, 105, 064701 CrossRef CAS PubMed.
- M. Mrukiewicz, M. Czerwiński, N. Podoliak, D. Repček, P. Perkowski, R. J. Mandle and D. Węgłowska, Polar nematic phases with enantiotropic ferro- and antiferroelectric behaviour, J. Mater. Chem. C, 2024, 12, 7214–7224 RSC.
- T. Donaldson, H. Staesche, Z. B. Lu, P. A. Henderson, M. F. Achard and C. T. Imrie, Symmetric and non-symmetric chiral liquid crystal dimers, Liq. Cryst., 2010, 37, 1097–1110 CrossRef CAS.
- V. Matko, E. Gorecka, D. Pociecha, J. Matraszek and N. Vaupotič, Interpretation of dielectric spectroscopy measurements of ferroelectric nematic liquid crystals, Phys. Rev. Res., 2024, 6, L042017 CrossRef.
- N. Yadav, Y. P. Panarin, J. K. Vij, W. Jiang and G. H. Mehl, Two mechanisms for the formation of the ferronematic phase studied by dielectric spectroscopy, J. Mol. Liq., 2023, 378, 121570 CrossRef CAS.
- A. Erkoreka and J. Martinez-Perdiguero, Constraining the value of the dielectric constant of the ferroelectric nematic phase, Phys. Rev. E, 2024, 110, L022701 CrossRef CAS.
- A. Adaka, M. Rajabi, N. Haputhantrige, S. Sprunt, O. D. Lavrentovich and A. Jákli, Dielectric properties of a ferroelectric nematic material: quantitative test of the polarization-capacitance Goldstone mode, Phys. Rev. Lett., 2024, 133, 038101 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc03318c |
| ‡ Present address: School of Pharmacy, Applied Sciences and Public Health, Robert Gordon University, Aberdeen, AB10 7GJ, UK. |
| This journal is © The Royal Society of Chemistry 2025 |