Furfural production from lignocellulosic biomass: one-step and two-step strategies and techno-economic evaluation
Yuqi
Bao
a,
Zicheng
Du
a,
Xiaoying
Liu
a,
Hui
Liu
a,
Jinsong
Tang
a,
Chengrong
Qin
a,
Chen
Liang
a,
Caoxing
Huang
b and
Shuangquan
Yao
 *a
*a
aGuangxi Key Laboratory of Clean Pulp & Papermaking and Pollution Control, School of Light Industrial and Food Engineering, Guangxi University, Nanning, 530004, PR China. E-mail: yaoshuangquan@gxu.edu.cn
bJiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Nanjing Forestry University, Nanjing, 210037, PR China
First published on 12th April 2024
Abstract
Lignocellulosic biomass (LCB) is an abundant renewable energy resource. Hence, the conversion of biomass into fuel and platform chemicals has recently attracted considerable attention. Furfural is an important platform product that can be produced from xylan-rich lignocellulosic biomass. Hemicellulose undergoes two stages, hydrolysis and dehydration, resulting in the production of furfural. In this review, one-step and two-step strategies for furfural production and the subsequent techno-economic evaluation of the integration of furfural and other co-products are presented. The direct one-step production of furfural requires a one-time investment in severe pretreatment conditions or the use of more chemicals, whereas the two-step production requires the separation of additional prehydrolysates, followed by more drastic treatment conditions. In both strategies, water flow, steam flow, organic solvents, deep eutectic solvents (DESs) or ionic liquids (ILs) can be used as solvent phases to hydrolyze hemicellulose. Lewis acid and Brønsted acid catalysts can effectively convert hemicellulose-derived pentose into furfural. A biphasic solvent system can effectively prevent the degradation of furfural in water, thus increasing the yield and selectivity of furfural. The techno-economy assessment results indicate that biorefineries are transitioning to adopting an integrate model of co-production of furfural and other products. Alternative green approaches are increasingly being adopted to make the furfural industry more sustainable and profitable.
1. Introduction
The rapid depletion of non-renewable petroleum resources is driving the development of sustainable alternatives for the production of high energy density fuels and high value-added chemicals.1 Non-edible and abundant lignocellulosic biomass materials are considered among the most promising feedstocks for the production of chemicals, fuels, and materials.2 Biomass mainly consists of 40–60% cellulose, 20–40% hemicellulose, and 10–25% lignin along with minor extractives and minerals.3 Therefore, the valorization of the three polymers into chemicals is critical for the efficient utilization of biomass and has recently received significant attention.4 At least 30% of bulk chemicals is estimated to be derived from renewable biomass by 2050.5However, the cell wall of cellulosic biomass is a cross-linked layered complex. Hence, it is necessary to break the anti-degradation barrier formed by the cross-linkages between lignin, cellulose, and hemicellulose.6 Catalysts and solvents are critical for the effective depolymerization of the crosslinking bonds and for utilizing all the components. Hemicellulose, with the lowest degree of polymerization, can be separated first and hydrolyzed to form hemicellulose-derived sugars (xylo-oligosaccharides and xylose), which can be upgraded to furfural via dehydration. Hemicelluloses account for 20–30% of the total mass of three polymeric components of lignocellulosic biomass, among which hardwood, crop waste, and herbs are xylan-based polysaccharides.7 Sugarcane bagasse and corncobs are responsible for 98% of all furfural production.8 Approximately 140 billion tons of waste agricultural residues are generated annually worldwide and are potent candidates for furfural production.9Fig. 1 presents xylan-type hemicellulose resources and furfural applications.
Furfural has been identified as one of the top 12 platform chemicals and is mainly produced by the acid-catalyzed dehydration of hemicellulose-derived xylose.10 It is a water-soluble, poisonous and flammable compound used as a precursor for fuels, solvents, and polymers in the fuel, pharmaceutical and plastic industries.11 Furfural can be converted into a wide range of building blocks to replace fossil-derived chemicals, such as levulinic acid, furan dicarboxylic acid, furfuryl alcohol, tetrahydrofurfuryl alcohol, furan, tetrahydrofuran (THF), methyltetrahydrofuran (MTHF), dihydropyran, and acetylfuran.6,12 China is the largest furfural producer in the world, with annual production exceeding 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons.13 Recently, the conversion of xylan-type hemicellulose to a furfural platform has been widely reported, which can be performed by a one-step conversion process or two-step process with pretreatment and separation of the hydrolysate for subsequent upgrade.14 Well-optimized procedures usually include the appropriate selection of catalysts, solvents, equipment, and process technology upgrades; these parameters have been considered for the integrated biorefineries and highly selective furfural production. A techno-economic analysis is crucial for evaluating the overall economic feasibility of industrial-scale furfural production.15 An integrated biorefinery process for the co-production of furfural with other valuable chemicals can maximize the potential of lignocellulosic biomass residues, reduce waste and improve the overall economic viability. More stringent requirements have been proposed for modern furfural production processes based on the concept of green chemistry. Therefore, the objective of this article is to provide a systematic review of the catalytic transformation of lignocellulosic biomass, either as separate components or in entirety, into furfural using one-step and two-step strategies. In addition, techno-economic evaluations of the integrated biorefinery processes are provided and possible future outlooks and the scope for furfural production are discussed.
000 tons.13 Recently, the conversion of xylan-type hemicellulose to a furfural platform has been widely reported, which can be performed by a one-step conversion process or two-step process with pretreatment and separation of the hydrolysate for subsequent upgrade.14 Well-optimized procedures usually include the appropriate selection of catalysts, solvents, equipment, and process technology upgrades; these parameters have been considered for the integrated biorefineries and highly selective furfural production. A techno-economic analysis is crucial for evaluating the overall economic feasibility of industrial-scale furfural production.15 An integrated biorefinery process for the co-production of furfural with other valuable chemicals can maximize the potential of lignocellulosic biomass residues, reduce waste and improve the overall economic viability. More stringent requirements have been proposed for modern furfural production processes based on the concept of green chemistry. Therefore, the objective of this article is to provide a systematic review of the catalytic transformation of lignocellulosic biomass, either as separate components or in entirety, into furfural using one-step and two-step strategies. In addition, techno-economic evaluations of the integrated biorefinery processes are provided and possible future outlooks and the scope for furfural production are discussed.
2. Hemicellulose resource
2.1. Xylan-type hemicellulose and its structure
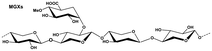
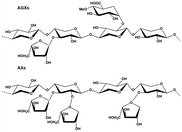
Lignocellulosic biomass resources (LBR) can be divided into agricultural residues, forest residues, as well as herbaceous and woody energy crops.16 The annual global production of LBR can reach 200 million tons.17 The various types of hemicelluloses in LBR include xylans, xyloglucans (XGs) and β-glucans with mixed linkages and mannans, with xylans being the most abundant.18 Xylan-type hemicellulose (xylans) can be divided into six structural subclasses: homoxylans (HOXs), (arabino)glucuronoxylans (AGXs), (glucurono)arabinoxylans (GAXs), glucuronoxylans (GXs), arabinoxylans (AXs), and heteroxylans (HTXs).19 Mannans are hexose and the major constituent of hemicellulose in softwoods.20 Xylans, which have higher C5 sugar contents, are the optimal choice for XOS, xylose, and furfural production. O-Acetyl-4-O-methylglucuronoxylan (MGX) is the predominant component of hardwood hemicellulose (about 15–30%).21 Most crop residues and grass seeds derived from food contain AGXs and AXs, respectively.22 Unlike grass seeds, which are used as food ingredients, crop wastes, such as bagasse, corn straw, corncobs, rice straw, nut-shells, and oil crop waste, are the least utilized resources. For example, sugarcane bagasse, which is the main by-product of industrial sugar production, contains approximately about 26.5% hemicellulose and the hemicellulose content of corn cobs and corn stalks can be as high as 35%.23 Seed shells, such as those of camellia, almonds, and walnuts, contain 25% xylan. These crop residues can provide an abundant amount of xylans for the selective preparation of functional oligosaccharides (XOS), furfural, and fermentable sugar substrates that can be used as sources of bioethanol.24–26 The hemicellulose content, type, and structure of the various raw materials are summarized in Table 1.| Classification | Lignocellulosic substrate | Hemicellulose (%) | Main type and structure | Ref. |
|---|---|---|---|---|
| Hardwood | Eucalyptus | 11–13 |
|
38 and 39 |
| Poplar | 15–21 | 40 | ||
| Birch | 20–23 | 41 and 42 | ||
| Herbaceous plants and agricultural residues | Corncob | 26–32.8 |
|
43 and 44 |
| Wheat straw | 23.8 | 45 | ||
| Corn stover | 19–22 | 46 and 47 | ||
| Switchgrass | 20–31 | 48 | ||
| Camellia oleifera shell | 27.94 | 49 | ||
| Sugarcane bagasse | 26.5 | 50 | ||
| Rice straw | 18.3 | 51 | ||
| Miscanthus | 20–23 | 52 | ||
| Miscanthus × giganteus | 22.3 | 53 | ||
| Peanuts | 13.2 | 54 | ||
| Rice husk | 18.3 | 55 | ||
| Barley husk | 28.7 | 56 | ||
| Coconut husk | 17.33 | 57 | ||
| Sweet sorghum bagasse | 18–25.5 | 58 and 59 | ||
| Empty fruit bunches | 25.3–33.8 | 60 | ||
2.2. Important furfural platform and formation mechanism
Currently, the largest producer of furfural is located in China (∼70% total production capacity).35 Other countries, such as South Africa (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t) and the Dominican Republic (32
000 t) and the Dominican Republic (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t) contribute significant furfural production.28 China and EU represent the largest and the second-largest markets with 55% and 15% of the global demand of furfural globally, respectively, which is expected to increase to 200
000 t) contribute significant furfural production.28 China and EU represent the largest and the second-largest markets with 55% and 15% of the global demand of furfural globally, respectively, which is expected to increase to 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year by 2030.36 In 2004, furfural was listed as one of the 12 most promising platform chemicals by the U.S. Department of Energy.37 Nowadays, the cost of furfural fluctuates between 800 and 1600 € per ton;30 furthermore, its global market size was valued at USD 556.74 million in 2022, which is projected to increase to 700 $ million by 2024 at a compound annual growth rate of 4.9%.27
000 tons per year by 2030.36 In 2004, furfural was listed as one of the 12 most promising platform chemicals by the U.S. Department of Energy.37 Nowadays, the cost of furfural fluctuates between 800 and 1600 € per ton;30 furthermore, its global market size was valued at USD 556.74 million in 2022, which is projected to increase to 700 $ million by 2024 at a compound annual growth rate of 4.9%.27
Glycoside bond hydrolysis of hemicellulose to form xylose. Brønsted acids (inorganic acids and organic acids with carboxylic and sulfonic functional groups) can be dissociated in water at high temperatures to release protons (H3O+); subsequently, the glycosidic linkage is initiated by protonation of either the glycosidic oxygen or ring oxygen to form a carbonium cation, which is followed by the C–O disruption of β-1,4-glycoside and hydrolysis of xylans to form XOS and xylose.62 Lewis acids also can form H3O+ in high-temperature aqueous solutions. Water can be used as a Lewis base, and Lewis acids serve as electron pair acceptors that can react with a Lewis base to form a Lewis adduct, which can be expressed as [M(H2O)x]n+ (where M is a metal element, x is the valence state, and n is a solvation number between four and nine).63 In addition to H3O+, the metal cations also are responsible for the coordination of the glycosidic oxygen to facilitate the breakage of the glycosidic linkages (C–O–C).64 Specifically, H3O+ from water autoionization at high temperatures is a co-catalyst in the additional acid hydrolysis process of hemicellulose, which synergistically accelerates the degradation of xylan into XOS and monosaccharides. Fig. 2 presents the hydrolysis mechanism of xylan-type hemicellulose by the catalysis of Brønsted acids and Lewis acids.
 | ||
| Fig. 2 Hydrolysis mechanism of xylan by acid catalysis.62,63 | ||
Xylose dehydration to form furfural. The formation of furfural from xylose with Brønsted acids catalysis involves two types of dehydration mechanisms:65,66 acyclic and cyclic dehydration. Acyclic dehydration involves the formation of 1,2-enediol intermediates via enolization or a direct dehydration to produce 2,3-(α,β-) unsaturated aldehyde via β-elimination. In cyclic dehydration, the action of the H+ on the O-2 of xypyranose results in the loss of one water molecule, forming 2,5-hydroxyfuranose via an intramolecular rearrangement, followed by two dehydration steps to produce furfural. Differently, Lewis acids catalyzed isomerization of xylose into xylulose via 1,2-hydrogen transfer, following further dehydration into furfural. The mechanism of the xylose dehydration process is shown in Fig. 3.
 | ||
| Fig. 3 Xylose dehydration for furfural formation via acyclic (a–b), cyclic dehydration (c) and 1,2-hydrogen transfer (d).65,66 | ||
3. One-step and two-step approaches for furfural production
Because the production of furfural from lignocellulose hemicellulose first requires hemicellulose hydrolysis followed by the dehydration of pentosans, the strategy for furfural production can be divided into one-step and two-step approaches.3.1. One-step approach for furfural production with homogeneous catalysts
The one-step approach involves the direct production of furfural from lignocellulose. The one-step approach for furfural production is listed in Table 3. A one-step conversion can also be conducted in a semi-batch process. Furfural can be obtained from biomass hemicellulose when a volatile stream (CO2, water vapor, and acetic acid vapor) is used as a mobile phase in a semi-batch reactor. Continuous vapor is necessary to extract furfural into the stream from the feedstock as a fixed bed, and the vapor is then cooled to obtain liquid furfural. Mao et al.67 proposed acetic acid as the volatile stream for the one-step extraction of furfural (Fig. 4a). The solid residue in the reactor was then processed via steam explosion to obtain a mixture of cellulose, soluble sugars, and lignin. Lignin can be obtained via alkali extraction and the soluble sugars can be obtained via direct water extraction. Specifically, the resulting furfural wastewater can be reused as an acetic acid source instead of a pure acetic acid stream for the next repetition, and recycled following the same procedure. The results demonstrated a higher furfural yield of 72.93% and selectivity of 73.49%; a comparable furfural yield can be obtained with reutilized acidic furfural wastewater.| Catalyst (organic solvent) | Raw material | Reactor | Pretreatment conditions (temperature–dosage–retention time) | Optimal furfural yield | Ref. |
|---|---|---|---|---|---|
| a Indicates the furfural yield can reach 70% in moderate conditions (120–170 °C, ≤90 min or 180–210 °C ≤30 min) with alternative inorganic acid catalysts. | |||||
| Acetic acid + FeCl3 + HCl | Corncob | Semi-batch tubing-bomb reactor | 190 °C–60 mM FeCl3–30 min | 72.93% | 67 |
| Sulfuric acid | Oil palm empty fruit bunch | Batch autoclave | 160 °C–0.5%–5 h | 71.4% | 68 |
| ChCl (MIBK)a | Switchgrass | Glass pressure tube | 170 °C–70 wt% ChCl + 0.6 wt% H2SO4–60 min | 84.04% | 69 |
CuCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
Poppy stalks | Autoclave | 200 °C–0.03 M–30 min | 79.86% | 70 |
| SnCl4 | Corncob | Microwave reactor | 190 °C–1%–0 min | 9.0 wt% (based on the dry weight of corncob) | 71 |
| FeCl3 (THF)a | Maple wood | Tube reactors | 170 °C–0.1 M–30 min | 95% | 72 |
| AlCl3 + [BMIM]Cla | Corncob | Microwave reactor | 170 °C–0.25 mmol AlCl3 + 2 g [BMIM]Cl–10 s | 84.8% | 73 |
| LiBr·3H2O (dichloromethane)a | Corn stalk | Batch autoclave | 120 °C–0.35 g LiBr·3H2O + 0.05 mol L−1 H2SO4–90 min | 72.5% | 74 |
| H-ZSM-5 (γ-valerolactone) | Corn cob | Batch autoclave | 190 °C–35 g L−1–60 min | 71.68% | 75 |
| H-ZSM-5 | Flax straw | Batch reactor | 250 °C–1 g–0 min | 7.6% | 76 |
| Glu-TsOH-Zr (MTHF) | Fax straw | Batch autoclave | 190 °C–0.5 g–120 min | About 22% | 77 |
| PA-FCB (GVL) | Corncob | Batch autoclave | 195 °C–0.1 g −120 min | 96.06% | 78 |
| ZnCl2 + HCl + SO42−/SnO2-diatomite (GVL) | Corncob | Batch autoclave | 170 °C–0.5 wt% HCl + 3.6 wt% SO42−/SnO2-diatomite + 15 g L−1 ZnCl2–30 min | 68.9% | 79 |
| SO42−/CX-DMSn | Corncob | Batch autoclave | 180 °C–0.32 g–5 h | 65.74% (66.83% of selectivity) | 80 |
| ImmHSO4-IL + NaCl (toluene) | Rice husk | Glass bottle | 180 °C–25 mg ImmHSO4-IL + 1.80 g NaCl–90 min | 74.6% | 81 |
| FeCl3 + NaCl (THF) | Enteromorpha prolifera | Batch autoclave | 190 °C–12.5 Mm FeCl3 + 2% NaCl–60 min | 45.0 wt% | 82 |
| SnCl4 + EMIMBr | Corn stalk | Glass vial | 130 °C–23 mg SnCl4 + 1000 mg EMIMBr–3 h | 54.5% | 83 |
| HCl | Fax shives | Microwave reactor | 180 °C–0.1 M HCl–20 min | 72.2%. | 84 |
| H-SAPO-34 (GVL)a | Eucalyptus sawdust | Batch autoclave | 210 °C–30 g L−1–20 min | 99.28% | 85 |
| p-Hydroxybenzenesulfonic acid-formaldehyde resin (GVL) | Corn stover | Batch autoclave | 190 °C–0.2 g–100 min | 50.3% | 86 |
| Al2(SO4)3·18H2O | Cannabis sativa L. shives | Bench scale reactor | 160 °C–5%–90 min | 62.7% | 87 |
| PW12/ZSM-5 + LiBr | Aspen | Pressure tube | 170 °C–25% LiBr + 10% PW12/ZSM-5–6 h | 29.9% | 88 |
| SO42−/Sn-TRP-N (DMSO) | Corn stover | Batch autoclave | 190 °C–10 wt%–3 h | 77.82% | 89 |
| AlCl3 + NaCl (MTHF) | Sugar cane bagasse | Microwave reactor | 190 °C–0.1 M AlCl3 + 10 wt% NaCl–45 min | 58.6% | 90 |
A one-step conversion is more conducted in a batch process usually operated in a high-temperature and high-pressure autoclave. Mild initial temperatures and extremely acidic environment or higher initial temperatures and mild acidic environment are required for furfural production. In a concentrated acidic system, hemicellulose tends to first degrade into xylose, and the cellulose then begins to swell and dissolve when using concentrated hydrochloric acid (>20 wt%), sulfuric acid (>70 wt%), nitric acid (>42.24 wt%), or phosphoric acid (>80 wt%) as acid catalysts.91 The separation of biomass hemicellulose and high furfural production can be achieved at mild temperatures (100–150 °C). The furfural yield of 50 mol% was obtained with concentrated formic acid (>64.40 wt%) at 150 °C.92 However, concentrated acids are highly corrosive to equipment and are not sustainable. Dilute acid treatment is alternatively considered more accessible for commercialization than the aforementioned concentrated acid pretreatment owing to its lower corrosive effect on equipment, high catalytic effectiveness, low acid catalyst cost, and reduced acidic waste production.93 Hemicellulose in dilute acid systems is mainly degraded into xylose, oligomers, and furfural according to a reported kinetic study.94,95 Higher furfural yield can be achieved at higher treatment temperatures (>150 °C) with dilute acid catalysts. Ouyang et al.68 investigated the use of dilute sulfuric acid for the one-step production of furfural from oil palm empty fruit bunches in a batch reactor (Fig. 4b). They developed a mechanism for the intermittent discharge of steam and proposed non-isothermal kinetics based on a considerable amount of xylan solubilized during the heating stage. The results demonstrated that the intermittent strategy resulted in a furfural yield of 71.4% at 160 °C for 5 h.
Homogeneous chloride-based salt solutions have been widely used for one-pot furfural production. Chen et al.69 conducted a one-pot conversion of switchgrass to furfural, ethanol, and lignin using an aqueous choline chloride/methyl isobutyl ketone (ChCl/MIBK) biphasic solvent system. An acidic ChCl solution (0.6 wt% sulfuric acid) was used in the system for the pretreatment of switchgrass and further conversion of xylose; methyl isobutyl ketone (MIBK) was used as the organic upper layer for furfural extraction. Under optimized conditions (170 °C, 60 min, 10.7 wt% solid loading), a furfural yield of 84% was achieved; high-purity lignin and highly digestible pulp were simultaneously obtained. Jiang et al.96 studied the role of ChCl as an additive in acidified water for furfural production starting from a highly concentrated xylose feed. The synthesis of choline xyloside was confirmed for the first time by HPLC-MS analysis. They proposed two possible reaction pathways: (1) 1-O-ChXyl protonation of choline xyloside, generation of oxocarbenium, and then formation of the tetrahydrofuran intermediate (Fig. 5a). (2) The generated choline xyloside intermediate undergoes ring contraction to form an oxonium ion while incorporating the choline fragment (Fig. 5b).
 | ||
| Fig. 5 Mechanisms of xylose dehydration with ChCl as catalyst: 1-O-ChXyl protonation of choline xyloside (a), ring contraction of choline xyloside to form an oxonium ion (b).96 | ||
Metal halide salts have also been widely investigated as Lewis acid catalysts for one-step production of furfural. The selectivity of five metal salt catalysts (AlCl3, CuCl2, CrCl3, FeCl3, and ZrOCl2) was evaluated for the one-step furfural production using tetrahydrofuran/water as the solvent phase.72 The pH of the salts in high-temperature aqueous solution was in the order of ZrOCl2·8H2O < FeCl3·6H2O < CuCl2·2H2O < AlCl3·6H2O < CrCl3·6H2O when xylose was used as a substrate. CrCl3·6H2O catalysts achieved both the highest furfural selectivity and conversion rate. FeCl3 reacted slower and required 20 min to reach a similar furfural selectivity of 65%. Notably, when the reaction substance was changed to maple wood, CrCl3 did not produce high furfural yields despite having high Lewis acidity, which caused xylose to rapidly dehydrate and degrade. FeCl3 demonstrated the best performance and achieved the highest furfural yield of 95%, outperforming sulfuric acid and the other four metal halides. Hoşgün et al.70 found that CuCl2 demonstrated an extreme performance and presented a maximum efficiency for furfural production (79.86%) from poppy stalks under one-pot hydrothermal reaction conditions at 180 °C. Wang et al.97 found that SnCl4, which had the lowest pH, achieved a superior furfural yield from beech xylan compared to other high-valence chloride salts (InCl3, GeCl4, AlCl3 and FeCl3). Furthermore, the 2-MTHF/water (2/5, v/v) system improved the furfural yield and selectivity. The optimal furfural yield was 78.1% in a 2-MTHF/H2O biphasic system using SnCl4 as an effective catalyst. The ratio of organic solvent/H2O is an important variable. The other optimal ratios of GVL/H2O (25%, v/v) and H2O/DMSO (5/5, v/v) were also confirmed to be desirable for promoting the formation of furfural and suppressing side reactions.98,99 However, a study indicated that the efficient synthesis of SnCl4 and MgCl2 as catalysts in a single ionic liquid, 1-ethyl-3-methylimidazolium bromide (EMIMBr), for the one-pot conversion of corn stalk to furfural was more effective than that in organic solvent/EMIMBr biphasic systems, with furfural yields as high as 54.5%.83 In a study conducted by Zhang et al., the paired CrCl3/AlCl3 catalyst demonstrated a higher furfural yield from corncobs than that of other paired salts and CrCl3 alone in the following order:73 CrCl3/AlCl3 > FeCl3·6H2O/AlCl3 > CuCl2·2H2O/AlCl3 > SnCl2·2H2O/AlCl3 > CeCl3·7H2O/AlCl3 > CrCl3. In addition, Lyu et al.100 proposed three tools (Lewis acid strength, oxidizing properties, and solubility products of the corresponding hydroxides) to quickly screen ideal catalysts for furfural production and to investigate the factors that inhibit furfural production. Metal ions with higher Lewis acid strengths are more favorable for furfural production. A higher standard reduction electrode potential (SREP) of an ion (more positive) indicates its stronger oxidizing property. High solubility products of metal hydroxides can decrease the consumption of catalysts during hydrolysis. The degradation of furfural can be observed based on the formation of humins and oxidation of the reaction substrate (CO2 and CO gas composition change). Therefore, they concluded that the ideal catalyst for furfural production should have a strong Lewis acid strength, weak oxidizing property, and high solubility product of the corresponding hydroxide; the factors that inhibit furfural production are humins and the oxidation reaction of corncobs and xylose. The order of the Lewis acid strengths of several metal ions, the standard reduction electrode potentials, and solubilities of the corresponding hydroxides are shown in Fig. 6.
 | ||
| Fig. 6 The order of the Lewis acid strengths of several metal ions, standard reduction electrode potentials and solubility of the corresponding hydroxides.100 | ||
Molten metal-salt systems can achieve higher furfural yields under mild conditions. Li et al.74 evaluated the conversion of corn stalk to glucose and furfural in a molten salt hydrate/organic solvent biphasic system (LiBr·3H2O/dichloromethane). Under the optimal reaction conditions (0.05 mol L−1 H2SO4, 120 °C, 90 min), 58.9% glucose and 72.5% furfural were yielded by the one-step process. LiCl·3H2O acted both as the solvent and catalyst for the synthesis of furfural from xylan, reported by Liu et al.101 A close furfural yield of 41.63% was achieved in a more severe condition (140 °C for 2 h) compared to that of the aforementioned study by Li et al. The GVL solvent was found to be more effective than other polar aprotic solvents (MIBK, THF, DMSO, sulfolane) used to enhance the furfural yield; an increasing yield (56.68%) was observed in the biphasic system. A maximum furfural yield of 77.22% was achieved with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mass ratio of GVL to LiCl·3H2O combined with 0.15 mmol of Al2(SO4)3 as the co-catalyst. The reaction pathway for the conversion of xylan to furfural in the GVL/LiCl·3H2O biphasic system was revealed. Xylan is initially depolymerized into xylose under the catalysis of Brønsted acid followed by isomerization, cyclization, and dehydration (Fig. 7a). The catalysts were first hydrolyzed to form the intermediates [Li(H2O)6]+ and [Al(OH)2(aq)]+, and [Al(OH)2(aq)]+ and Cl− then promoted the transfer of 1,2-hydrogen between C1 and C2 (Fig. 7b).
2 mass ratio of GVL to LiCl·3H2O combined with 0.15 mmol of Al2(SO4)3 as the co-catalyst. The reaction pathway for the conversion of xylan to furfural in the GVL/LiCl·3H2O biphasic system was revealed. Xylan is initially depolymerized into xylose under the catalysis of Brønsted acid followed by isomerization, cyclization, and dehydration (Fig. 7a). The catalysts were first hydrolyzed to form the intermediates [Li(H2O)6]+ and [Al(OH)2(aq)]+, and [Al(OH)2(aq)]+ and Cl− then promoted the transfer of 1,2-hydrogen between C1 and C2 (Fig. 7b).
 | ||
| Fig. 7 The dehydration mechanism of xylose with Al2(SO4)2 (a) and LiCl3 (b) as Lewis acid catalysts.101 | ||
The introduction of metal salts into DES systems can promote the production of furfural. An AlCl3-catalyzed ChCl-oxalic acid/MIBK biphasic system was developed for furfural production from eucalyptus, which obtained a yield of 70.3% under the optimal pretreatment conditions (at 140 °C for 90 min).102 The performance of FeCl3, AlCl3, SnCl4, and TeCl4 in an aqueous choline chloride-oxalic acid (16.4 wt% H2O) DES system was investigated for producing furfural from oil palm fronds (OPFs).103 In this co-catalyst system, Brønsted acid supplied by oxalic acid from DES is responsible for breaking the β-1,4 glycosidic bonds of hemicellulose to form xylose via hydrolysis; subsequently, the Lewis acids catalyze the isomerization of xylose to xylulose via an intramolecular 1,2-hydride shift pathway similar to that in the previous study. The χ/r2 values, denoting the electric field gradient around the cation, of four metal chlorides were found to obey the following trend: Te4+ > Sn4+ > Al3+ > Fe3+. The results demonstrated that although ADES-TeCl4-OPFsOPFs achieved a relatively lower furfural yield of 55.6% than ADES-AlCl3-OPFs (60.2%) and ADES-SnCl4-OPFs (59.4%), the lower amounts of TeCl4 (2.5%) and SnCl4 (0.25%) were required for a similar furfural yield. Therefore, they concluded that TeCl4 with a higher χ/r2 value demonstrated to be the stronger Lewis acid, thus more quickly catalyzed the degradation reaction of furfural. Furthermore, the addition of Lewis acids also enhanced the acidity of the Brønsted acid via synergistic DES–Lewis acid catalyst interactions. Fig. 8 presents the proposed mechanism of the SnCl4 and ChCl-oxalic acid interactions for furfural production.
 | ||
| Fig. 8 The proposed mechanism of SnCl4 and ChCl-oxalic acid interactions for furfural production.103 | ||
Acidic ILs can be used as catalysts and (or) solvents for the one-step production of furfural. Peleteiro et al.104 reported the production of furfural from eucalyptus wood using a Brønsted acid ionic liquid (1-butyl-3-methylimidazolium hydrogen sulfate) as both a solvent and a catalyst in the presence of a co-solvent (dioxane). An optimal furfural conversion (59.1%) was achieved at 160 °C. In addition, the reported ImmHSO4-IL catalyst exhibited a higher catalytic activity than those prepared using other immobilized imidazolium acid ionic liquid catalysts (ImmCH3SO3-IL and ImmH2PO4-IL), and achieved the highest furfural yield of 74.6% from rice husk.81
3.2. One-step approach for furfural production with heterogeneous catalysts
In addition to homogeneous acids, heterogeneous catalysts for the one-pot conversion of lignocellulose biomass have gained increasing attention owing to their simple separation after the reaction and reutilization.79 Heterogeneous catalysts can be divided into Lewis acid catalysts alone, Brønsted acid catalysts alone and the combination catalysts of two acid sites. H-ZSM-5 is an acid catalyst with only Lewis acid site. Harry et al.76 indicated that H-ZSM-5 is a high-performance zeolite catalyst with a high silica-to-alumina ratio for a high yield of furfural from flax straw in a batch reactor. Shorter reaction times minimize the formation of undesired liquid by-products, enhancing the selectivity and yield of furfural in subcritical water. The H-ZSM-5 catalyst was more favorable for the formation of furfural when HCl was not used as a co-catalyst. A furfural yield of 7.6% (based on the mass of flax straw) was achieved at an optimal condition (250 °C, 6.0 MPa, flax straw mass fraction of 5%, and 0 retention time, 1 g H-ZSM-5).Carbon-based solid acids bearing multiple Brønsted acid sites (inherent –OH, –COOH, and additional –SO3H groups after sulfonation) were also investigated, which exhibited excellent performances ascribed to the synergic effect of these groups in the catalytic production of furan derivatives.105 A phosphotungstic acid-functionalized biochar (PA-FCB) in the γ-valerolactone/subcritical water solvent demonstrated a high furfural yield of 96.06% at 468 K for 120 min.78 Satisfied furfural yields of 95% and 77.4% were obtained from corn cob and rice husk, respectively, by the one-pot transformation process with nitrogen doped carbon solid acids in γ-valerolactone as a catalyst at 170 °C for 45 min.106
Some solid acids with both Brønsted and Lewis acid sites were also reported. A sulfonated carbon-based zirconia (Glu-TsOH-Zr) demonstrated good catalytic activity, resulting in a 40% increase in the furfural yield from fax straw biomass at 190 °C after 120 min in a stainless-steel batch reactor; a recyclability investigation of the catalyst revealed only a 5% loss.107 A unique carbonized core–shell diatomite (SO42−/CX-DMSn) with Lewis and Brønsted acid sites was prepared and exhibited an excellent catalytic ability.80 The results demonstrated a furfural yield of 65.74% and a selectivity of 66.83% for corncob. Compared with SO42−/SnO2-diatomite, additional liquid Brønsted acid catalysts can enhance furfural production. HCl is inexpensive and has been found to be more effective than H3PO4, HNO3, and H2SO4 in the hydrolysis of lignocellulose biomass.108 The dilute HCl-assisted solid acid (SO42−/SnO2-diatomite) one-pot catalysis of corncob to furfural in γ-GVL–water media was investigated, confirming that Brønsted acids with lower acid dissociation constant (pKa) values (stronger hydrogen donor capacity) had higher turnover frequency (TOF) values as follows: HCl (pKa = −7.0) > H2SO4 (pKa = −3) > oxalic acid (pKa = 1.25) > fumaric acid (pKa = 3.15) > citric acid (pKa = 3.14) > D,L-malic acid (pKa = 3.49) > formic acid (pKa = 3.75) > succinic acid (pKa = 4.16) > acetic acid (pKa = 4.75).79 Furthermore, γ-GVL–water as a biphasic system exhibited a better furfural yield compared to other organic solvents (toluene, glycerol, glycol, isoamylol, dibutyl phthalate, butyl acetate, n-butanol). An appropriate volumetric ratio of γ-GVL increased the furfural yield. A high furfural yield (68.9%) was achieved from corncob under the optimal conditions (170 °C, 30 min, 0.5 wt% HCl, 3.6 wt% SO42−/SnO2-diatomite, ratio of γ-GVL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water was 6
water was 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v).
4, v/v).
Solid acid catalysts own the characteristics of good thermal stability, high reusability and chemical stability. The prepared SO42−/SiO2-Al2O3/La3+ only observed a decline of 5.28% yield of furfural after four runs.109 A dual-functional carbon-based solid acid catalyst (DFCSA) with tunable Brønsted acid sites (H3PO4 and HNO3) and Lewis acid site (Al(NO3)3) displayed excellent hydrothermal stability, with only a slight degradation in activity after 10 cycles.110 A commercially available sulfonic acid-containing heterogeneous Brønsted acids (Amberlyst 45) was found to show higher hydrothermal stability at hydrothermal flow conditions of 160 °C, with <10% loss in acidity after 48 h.111 Although a good performance of catalysts has been achieved, the deactivation and regeneration of heterogeneous catalysts still face some challenges. In fact, the activity loss of Brønsted acid-functionalized carbon-based solid acid catalysts was mainly due to the reduced number of Brønsted acid sites responsible for the recycle performance. Sulfonic-acid groups in a sulfonated activated carbon are prone to leaching in hydrothermal reaction environments because of the tendency of the C–S bond to undergo desulfonation.112 The degree of desulfonation (sulfonic acid group density) decreased from initially 0.58 mmol g−1 to 0.35, 0.30, and 0.20 mmol g−1 after treatment at 150, 200, and 225 °C in hot compressed water,113 which indicates that desulfonation increases with increasing reaction severity. Besides sulfonic acid group leaching, a hitherto unknown mode of deactivation was identified that proceeds by ion-exchange processes with cationic mineral species.112 In addition to the leaching of sulfonic acid residues, the deposition of carbonaceous residues in the hydrothermal process also result in catalyst deactivation.114 Use of a polar aprotic solvent is a successful approach to minimize fouling.111 Carbonaceous residues removal for regeneration of catalysts can be achieved via oxidation, gasification or hydrogenation.114 In conclusion, the degradation of catalysts is unavoidable due to the decrease of acid density and the increase of fouling deposition. Biocarbon-based solid acids can be prepared again by re-carbonization and sulfonation due to their low cost and ease of preparation. Commercially expensive or complex solid acids hard to prepare, such as zeolites or resins, can regenerate by coke removal to extend their life span after carbon plugging.
3.3. Two-step approach for furfural production with the same catalysts
The two-step process requires an additional separation of the hydrolysate. In the first step, the raw materials are pretreated at a mild temperature to obtain pentosan-rich hydrolysates, and cellulose is mostly retained in the solid fraction. After the separation via solid–liquid filtration, in the second step, the filtrate liquids mainly containing xylose and xylo-oligosaccharides were subjected to further reactions to generate furfural under more severe conditions. The two-step approach enables using pretreated feedstock for further utilization. The two-step process for furfural production is listed as Table 4.| Catalyst (organic solvent) | Raw material | Reactors | Pretreatment conditions (temperature–dosage–retention time) | Optimal furfural yield | Ref. |
|---|---|---|---|---|---|
| a Indicates the furfural yield can reach 70% under mild conditions (120–170 °C, ≤90 min or 180–210 °C, ≤30 min) with alternative inorganic acid catalysts. | |||||
| ChCl/P-toluenesulfonic acida | Corncob | Glass bottle | 160 °C–1.8%–80 min | 70% | 9 |
| NaCl (GVL, THF) | Pubescens | Batch autoclave | 200 °C–5%–2 h | 76.9 mol% with 82.2% selectivity | 98 |
| AlCl3 + ChCl-oxalic acid (MIBK)a | Eucalyptus | Batch autoclave | 140 °C–25.0 g DES + 95.6 mg AlCl3·6H20–90 min | 70.3% | 102 |
| Chloride-oxalic acid DES + SnCl4 | Oil palm fronds after ultrasonic irradiation | Glass beaker | 120 °C–88.0 mmol DES + 2.50 wt% SnCl4–45 min | 59.4% | 103 |
| [bmim]HSO4 (dioxane) | Eucalyptus | Batch autoclave | 160 °C–10 kg (based on 1 kg wood)–4 h | 59.1% | 104 |
| Supercritical CO2 + H2SO4 | Rice husk | Semi-batch reactor | 180 °C–1836 g CO2 + 7% H2SO4–3 h | 90% | 115 |
| Methanesulfonic acid | Bagasse | Glass ampoules | 180 °C–0.1 M–20 min | About 85 mol% | 116 |
| High-pressure CO2 (THF/MIBK) | Wheat straw | High-pressure Parr 4655 reactor | 180 °C–60 min | 43 mol% with a selectivity of 44 mol% | 117 |
| ChCl-oxalic acid | Oil palm fronds | Schott bottle | 100 °C–1.234 g L−1–135 min | 26.34% | 118 |
| Maleic acid | Corn stover | Microwave reactor | 200 °C–0.25 M–28 min | 61% | 119 |
| SO42−/SiO2−Al2O3/La3+ | Corncob | Batch reactor | 150 °C–0.01 g–150 min | 21% | 120 |
| ChCl-H2SO4 | Switchgrass | Pressure tube | 150 °C–2% AlCl3–15 min | 60% | 121 |
| MgCl2 + ChCl/HCl (2-Me-THF)a | Corn stover | Batch autoclave | 120 °C–15 g L−1 MnCl2 + 5 mL DES–60 min | 86.94% | 122 |
[C4mim]HSO4 and [C3SO3Hmim]HSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
Eucalyptus nitens wood | Microwave reactor | 180 °C–1.16 mol L−1 [C4mim]HSO4–30 min | 75.0 ± 0.71% with [C4mim]HSO4 as a catalyst and (85.6 ± 0.32%) [C3SO3Hmim]HSO4 as a catalyst | 123 |
| 180 °C–0.049 mol L−1 [C3SO3Hmim]HSO4–5 min | |||||
| H2SO4 | Corn stover | Batch autoclave | 210 °C–5 mL–20 min | 46.5% | 124 |
A high-pressure CO2 and H2O-assisted process was reported for the two-step selective conversion biomass hemicellulose into furfural.117 Subcritical water as a solvent at a temperature above its boiling point but below the critical point at a certain pressure can generate hydronium ions, which act as effective catalysts for the breakdown of hemicellulose.125 Carbonic acid formed by CO2 dissolved in water can effectively promote the hydrolysis of hemicellulose in biomass and the production of hemicellulose-derived sugars compared to the CO2-free autohydrolysis process.126,127 Hemicellulose solubilized in the liquid phase can be subjected to subsequently convert into furfural or carboxylic acids. The hemicellulose hydrolysate dehydration yielded 43 mol% of furfural with a selectivity of 44 mol% in a biphasic system with water/THF/MIBK under optimal conditions (initial CO2 pressure of 50 bar, 180 °C, 60 min). Additional dilute acids can also promote hydrothermal autocatalytic processes and reduce the reaction temperature. Cornejo et al.128 reported a two-step biorefinery process for the production of glucose and furfural from wheat straw and poplar chips. Thermochemical pretreatment with dilute H2SO4 as the catalyst was conducted for hemicellulose hydrolysis in the first step, which was followed by microwave-assisted cyclodehydration of xylose in a MIBK solvent in the second step for furfural production. Up to 12.6 kg of glucose and materials and 2.5 kg of furfural can be produced starting from 50 kg of biomass. Maleic acid has been reported to be an organic acid catalyst for selectively depolymerizing plant hemicellulose to xylose and dehydrating the resulting xylose to furfural in a microwave-assisted two-step process.119 Similar furfural yields were obtained from xylose hydrolysates of corn stover (61%) and pure xylose (67%), but in nearly half the time of the latter (Fig. 9a). SO42−/SiO2−Al2O3/La3+, as a solid acid catalyst, was applied for the two-step furfural production from corncobs, obtaining a maximum furfural yield of up to 21% at 190 °C for 60 min in the hydrothermal process.120 A multistep recycling method for enriching hemicellulose-derived sugars was also proposed. Chen et al.121 reported aqueous choline chloride as a novel solvent for the two-step furfural production from switchgrass fractionation. Under a relatively mild pretreatment condition (120 °C, 25 min), acidified ChCl solution (pH = 1.11) efficiently removed 76% of xylan. After five cycles, xylose-enriched liquor was used as the reaction substrate for furfural production, resulting in a yield of 48.22%, and 93% lignin removal from switchgrass.
 | ||
Fig. 9 Two-step process for furfural production via maleic acid (a) and ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p-TsOH (b).119,121 p-TsOH (b).119,121 | ||
ChCl-based DESs are reportedly easy to prepare and are inexpensive green liquids that are effective for lignocellulose biomass pretreatment.129 A three-constituent DES (choline chloride-oxalic acid-ethylene glycol) removed 91.09% of xylan from bamboo-based hemicellulose and the introduction of ethylene glycol into the DES can decrease the condensation of lignin.130 The pH of DES is considered an important variable for enhancing catalytic activity. p-Toluenesulfonic acid (p-TsOH) is a strong organic acid. ChCl/p-TsOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) DES was used as the solvent and catalyst for the two-step furfural production, obtaining a final optimal furfural yield of 69.8%; 97.5% of the furfural can be recycled by 1-tetradecanol and thymol based on the hydrophobic deep eutectic solvents.9 The ChCl/p-TsOH DES was found to be the best with a lower pH value of 1.0 compared to the other ChCl/oxalic acid and ChCl/levulinic acid DESs with the same 1
1 molar ratio) DES was used as the solvent and catalyst for the two-step furfural production, obtaining a final optimal furfural yield of 69.8%; 97.5% of the furfural can be recycled by 1-tetradecanol and thymol based on the hydrophobic deep eutectic solvents.9 The ChCl/p-TsOH DES was found to be the best with a lower pH value of 1.0 compared to the other ChCl/oxalic acid and ChCl/levulinic acid DESs with the same 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, and achieved a furfural yield of 85% in 1.5 h at 120 °C (ref. 131). The integrated biorefining of deep eutectic solvents (ChCl/p-TsOH) for furfural production was studied.9 First, hydrolysis of the corncob xylan was performed at 140 °C for 20 min, followed by recycling the corncob hydrolysate for 3 runs to obtain a concentrated liquor (11.6%). Subsequently the dehydration of the xylose-rich corncob hydrolysate at 200 °C and 120 min of nitrogen stripping was performed in the second step, using hydrophobic deep eutectic extraction solvents (Tdec
1 molar ratio, and achieved a furfural yield of 85% in 1.5 h at 120 °C (ref. 131). The integrated biorefining of deep eutectic solvents (ChCl/p-TsOH) for furfural production was studied.9 First, hydrolysis of the corncob xylan was performed at 140 °C for 20 min, followed by recycling the corncob hydrolysate for 3 runs to obtain a concentrated liquor (11.6%). Subsequently the dehydration of the xylose-rich corncob hydrolysate at 200 °C and 120 min of nitrogen stripping was performed in the second step, using hydrophobic deep eutectic extraction solvents (Tdec![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Thy) as an alternative to energy intensive distillation operations for the isolation and purification of furfural from the aqueous condensate obtained, finally resulting in an optimal furfural yield of 69.85% from corncob (Fig. 9b). Hu et al.122 developed an integrated strategy for the two-step hemicellulose-to-furfural conversion from corn straw in an aqueous DES (ChCl/hydrochloric acid)/2-Me-THF solvent. The aqueous DES resulted in a furfural yield of 86.94% from hydrolysate obtained after the dilute-acid pre-hydrolysis of lignocellulose, and a DES recycled yield of 85% was achieved. The lignin and cellulose were then separated from the solid residue using the same aqueous DES. They proposed that Cl− ions present in ChCl can facilitate 1,2-hydrogen transfer reactions, resulting in the formation of isomeric xylulose, which exhibits a higher reactivity compared to xylose and is more prone to dehydration and cyclization to form furfural under acidic conditions (Fig. 10).
Thy) as an alternative to energy intensive distillation operations for the isolation and purification of furfural from the aqueous condensate obtained, finally resulting in an optimal furfural yield of 69.85% from corncob (Fig. 9b). Hu et al.122 developed an integrated strategy for the two-step hemicellulose-to-furfural conversion from corn straw in an aqueous DES (ChCl/hydrochloric acid)/2-Me-THF solvent. The aqueous DES resulted in a furfural yield of 86.94% from hydrolysate obtained after the dilute-acid pre-hydrolysis of lignocellulose, and a DES recycled yield of 85% was achieved. The lignin and cellulose were then separated from the solid residue using the same aqueous DES. They proposed that Cl− ions present in ChCl can facilitate 1,2-hydrogen transfer reactions, resulting in the formation of isomeric xylulose, which exhibits a higher reactivity compared to xylose and is more prone to dehydration and cyclization to form furfural under acidic conditions (Fig. 10).
 | ||
| Fig. 10 The proposed mechanism of DES for furfural production.122 | ||
3.4. Two-step approach for furfural production with different catalysts
Both hemicellulose and lignin can be separated when the reaction is performed in an organic solvent, a DES or an IL, leaving a cellulose-rich solid residue. Lignin can regenerate in situ after the addition of a certain amount of water. Thus, hemicellulose-derived sugar hydrolysates can be selectively obtained for upgrade via a two-step process using additional catalysts. GVL is a green solvent with an excellent delignification ability. Luo et al.132 converted pubescent hemicellulose to furfural in a GVL/water co-solvent in an autoclave using a simple two-step process (Fig. 11a). First, the co-dissolution of hemicellulose (93.6 wt%) and lignin derivatives (80.2 wt%) was promoted via hydrothermal autocatalysis at 160 °C, leaving a high purity cellulose. Second, furfural was prepared by adding NaCl and THF at a higher temperature (200 °C), resulting in a yield of 76.9 mol% and selectivity of 82.2%. Moreover, the interaction mechanism of NaCl with the hemicellulose-derived oligomers and monomers was revealed. The results demonstrated that Cl− is a good hydrogen acceptor, and can form hydrogen bonds with C-OH-2,3,4 in xylose with the help of H2O. The dehydration of xylose to form furfural via the intermediate xylulose in an isomerization reaction, first occurred on the C-OH-4 of xylose and then on the C-OH-2,3 of xylose (Fig. 11b). | ||
| Fig. 11 The two-step process (a) and proposed mechanism of NaCl+THF/GVL/H2O system (b) for furfural production.132 | ||
Acidic ionic liquids have been proposed as environmental friendly catalysts for the two-step furfural production. Penín et al.123 combined hydrothermal processing with an acidic ionic liquid, 1-butyl-3-methylimidazolium hydrogen sulfate ([C4mim]HSO4), and 1-(3-sulfopropyl)-3-methylimidazolium hydrogen sulfate ([C3SO3Hmim]), as substrates in the second step in a water/methyl isobutyl ketone biphasic medium for furfural production. Under the selected operational conditions, the molar conversions of the precursors (pentosans) into furfural were 75.0 ± 0.71% with 1.16 mol [C4mim]HSO4/L as the catalyst, and 82.5 ± 0.91% with 0.049 mol [C3SO3Hmim]HSO4/L as the catalyst at 180 °C. Another acidic ionic liquid, 1-butyl-3-methylimidazolium hydrogen sulfate ([bmim]HSO4), was used as a catalyst for furfural production from wood-derived liquid streams after hydrothermal treatment in water/MIBK media.133 The results indicated that maximum furfural production was (61.6%) achieved under the optimal operational conditions (140 °C, 6 h reaction time, and catalyst loading = 0.3 g g−1 water). The recovery and cycling of ILs are necessary for cost-saving, owing to the high purchase cost. A satisfactory catalytic activity with [bmim]HSO4 as a catalyst was also observed despite undergoing eight consecutive runs (conversion range, 60.1–66.7%).
3.5. Summary
In summary, the two-step process for furfural production based on the obtained prehydrolysate has a low initial input energy, but requires an additional separation unit. To achieve a one-step production of furfural from biomass, higher-intensity treatment conditions are required to degrade the resistant structure of the cell wall and dehydrate xylose. A one-step process requires continuous vapor or water steam to extract furfural when the reaction is operated in a semi-batch reactor, and an acidic system is necessary for hemicellulose hydrolysis and xylose dehydration. Catalysts and solvents are crucial for increasing reaction rates. Cl− anions are reportedly more efficient for enhancing the formation of isomeric xylulose from xylose. Halide salts with higher pKa values and valences tend to exhibit stronger catalytic activity with lower pH values of a solution. Trivalent metal salts, such as FeCl3 and AlCl3, are better candidates for furfural production from lignocellulosic biomass. Paired cocatalysts can further improve the furfural yield. A synergistic DES–Lewis acid catalyst interaction increases the acidity of the solution. An appropriate ratio of solvent/H2O (usually 10–50%, v/v) can improve the selectivity of furfural. A decrease in furfural yield was observed when catalysts with higher catalytic activities were used for biomass pretreatment under more severe conditions.4. Techno-economy and green assessments for furfural production
A techno-economic assessment of furfural production involves considering the production technology options, total costs, and profit evaluation. Production technologies (pretreatment, extraction, and production facilities) are crucial for optimizing the production of products. The total costs consist of a fixed capital investment (cost of new-plant construction), direct costs (external battery and the total costs of purchasing and installing all process equipment), indirect costs (legal expenses, construction costs, and contractor fees), operating costs (variable and fixed costs, raw materials, and utility costs), and working capital (cost needed to start and run a plant until it generates sufficient revenue to pay off debt and purchase inventory).134 The variable operating costs (feedstocks, raw materials, waste handling, and byproduct credits) were determined based on the Aspen Plus process modeling results, and prices were obtained from prior studies.135 The fixed operating capital (employee salaries, labor burden, maintenance, and property insurance) was estimated following the assumptions of the NREL TEA report.136The economic metrics commonly used to evaluate the economic performance of a biorefinery include the internal rate of return (IRR), return on investment (ROI), net present value (NPV), earnings before interest, taxes, depreciation, amortization (EBITDA), resistance to market uncertainty (RTMU), return on capital employed (ROCE), payback period (PP), and annualized investment cost (EAC).137 Usually, a sensitivity analysis provides a better idea of the risks and RTMU of different biorefinery inputs. The reported techno-economic assessments of different biorefinery options were reviewed in detail.
4.1. Techno-economy assessment for furfural and co-product production
The traditional one-stage and proposed two-stage steam-explosion pretreatments for the production of furfural integrated with the co-production of ethanol from sugarcane lignocelluloses were evaluated.139 A sensitivity analysis showed that in terms of the product prices, the IRRs of the two-stage furfural-ethanol biorefineries were less sensitive to the furfural price fluctuations, whereas the one-step process had a higher furfural yield and lower ethanol yield, and the profitability was less dependent on the ethanol sales but more limited to higher production costs. Therefore, the total revenue from the co-production of furfural and ethanol of the two-step process was higher than that of the one-step steam-explosion (SE) process (170 °C and 0.5 wt% H2SO4). Additionally, varying severities of the steam explosion (scenarios 1, 2 and 3) were found to affect the two-step furfural revenue (Table 5). The biorefinery utilizing a pretreatment method with a medium severity level (Scenario 2) was considered more robust and economical owing to the high yields of both furfural and ethanol, demonstrated less volatile product prices, and lower investment costs owing to the reduced process intensification.
| TCI (million) | TCOP (million USS per year) | Ethanol yield (%) | IRR (%) | IRR (%) | IRR (%) without electricity credit | |
|---|---|---|---|---|---|---|
| Scenario 1 | 272 | 13.7 | 59.50 | — | 12.92 | 9.91 |
| Scenario 2 | 294 | 18.12 | — | 95.69 | 10.18 | 8.26 |
| Scenario 3 | 327 | 18.37 | 13.68 | 63.79 | 3.64 | −0.56 |
| Scenario 4 | 306 | 18.17 | 31.14 | 74.71 | 74.71 | 3.99 |
| Scenario 5 | 305 | 18.57 | 68.73 | 53.51 | 53.51 | 10.30 |
| Scenario 6 | 305 | 18.17 | 29.00 | 77.3 | 77.30 | 5.8 |
| Scenario 7 | 322 | 18.89 | 50.47 | 17.02 | 17.02 | 7.69 |
| Scenario 5* (2015 cost year) | 264 | 17.03 | 68.73 | 53.51 | 53.51 | 4.90 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579 kg h−1) can ensure a profitable system, and the MFSP can reach 945 $ per t. The techno-economic analysis results suggest that the proposed system with diverse market-driven products is cost-competitive and has a low economic risk.
579 kg h−1) can ensure a profitable system, and the MFSP can reach 945 $ per t. The techno-economic analysis results suggest that the proposed system with diverse market-driven products is cost-competitive and has a low economic risk.
| Process description | TCI (US$) | TCOP (MILLION US$ per year) | Ethanol/dry feedstock (kg t−1) | FF/dry feedstock (kg t−1) | IRR (%) |
|---|---|---|---|---|---|
| Scenario 1 (SE at 190 °C, FF reactor at 180 °C with 2.0 wt% H2SO4) | 322.58 | 20.20 | 150.2 | 78.1 | 13.11 |
| Scenario 2 (SE at 205 °C, FF reactor at 180 °C with 2.0 wt% H2SO4) | 310.23 | 19.85 | 213.8 | 90.6 | 13.59 |
| Scenario 2 (SE at 215 °C, FF reactor at 180 °C with 2.0 wt% H2SO4) | 340.46 | 20.74 | 156.3 | 46.4 | 10.07 |
| One-step FF (170 °C and 0.5 wt% H2SO4) and ethanol | 290.79 | 19.36 | 89.8 | 137.5 | 12.78 |
Overall, the integrated biorefinery for the co-production of furfural and other products would ensure robust revenues by maximizing feedstock utilization. The results of this economic analysis are encouraging for the scale-up process development.
4.2. Green evaluation
Biorefineries for furfural production should comply with the concept of “Green Chemistry”. For achieving techno-economic efficiency, green and sustainable production processes should avoid using toxic and hazardous materials, instead green solvents and catalysts should be selected to, reduce the energy input and, waste yields; furthermore, recycling technology should be applied for reusing catalysts, solvents, and waste water, and to operate waste-water treatment.| Process area and profit | Equipment installation cost (million $) | Variable operating cost (million $ per year) | Market price | |||
|---|---|---|---|---|---|---|
| Process area | Feedstock handling | 32.6 | 63.1 | |||
| Biomass fractionation | 67.0 | One-pot reactor (17.1% of TCI) | 57.7 | Feedstock cost shares (40.6% of TVC) | ||
| Distillers (3.1% of TCI) | ChCl (19.7% of TVC) | |||||
| Separators (2.5% of TCI) | MIBK and acetone (7.1% and 8.9% of TVC) | |||||
| Cellulose conversion | 61.8 | Bioreactors (8.3% of TCI) | 2.3 | |||
| Rectification columns (5.9% of TEIC) | ||||||
| Turbine and boiler | 38.4 | 1.0 | ||||
| Enzyme production | 21.7 | 13.7 | ||||
| Wastewater treatment | 38.0 | 3.0 | ||||
| Storage and utilities | 10.5 | 0.2 | ||||
| Waste disposal | 0.8 | |||||
| Grid electricity | 13.8 | |||||
| Total variable operating cost | 155.6 | |||||
| Fixed operating cost | 13.0 | |||||
| Profit | Products | Ethanol: 1.75–4.15 $ per gal | ||||
| Lignin: 250–750 $ per t | ||||||
| Furfural: around 1000 $ per t in 2017 | ||||||
| Plant scale | When it is downscaled to 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579 kg h−1 (¼ of the current design), the minimum furfural selling price increases up to 945 $ per t 579 kg h−1 (¼ of the current design), the minimum furfural selling price increases up to 945 $ per t |
|||||
| Scenario | Total capital cost (MM$) | Cost of raw material (MM$) | Cost of thermal utility (MM$) | Cost of electricity (MM$) | Total operating cost (MM$) | MFSP $ per kg |
|---|---|---|---|---|---|---|
| I | 296.1 | 187.2 | 74.4 | 1.3 | 264.6 | 2.40 |
| II | 239.9 | 187.2 | 71.3 | 0.9 | 261 | 2.26 |
| III | 113 | 174.2 | 12.6 | 11.5 | 199.9 | 1.60 |
| IV | 120.7 | 127.6 | 12.6 | 30.1 | 171.9 | 1.42 |
Similarly, Mazar et al.144 conducted a techno-economic evaluation to quantify the performance of alternative process configurations. The results showed that significant heat energy savings (99.5%) were achieved in driving the process using a heat exchanger network designed for internal heat recovery, and even the processes with the lowest performance would be profitable, robust, and capital-efficient. In addition, a process for heat integration was reported by Clauser et al. and Hernández et al. Clauser et al.145 proposed the co-production of carboxylic acids, furfural, and pellets in a pine sawdust biorefinery. Three alternatives for the conversion of residual solids were analyzed: (i) use of 100% of the residual solid for pellet production (alternative I); (ii) use of residual solid for the production of steam and 34 kg of pellets, and obtained steam for reuse (alternative II); (iii) use of residual solid for the production of steam and 105 kg of pellets with all process heat integration for reuse (alternative III). The economic results of three studied biorefinery scenarios are presented in Table 9. The results demonstrated that alternative I consumed more energy than alternative II, and alternative III consumed more energy with the heat integration process; a higher pellet yield resulted in a high IRR owing to the high market value of levulinic acid (LA) (5–9 USD per kg) and the low cost investment.
| Categories | Scenario 1 | Scenario 2 | Scenario 3 | |||
|---|---|---|---|---|---|---|
| M.USD per y | Share (%) | M.USD per y | Share (%) | M.USD per y | Share (%) | |
| Annualized production costs | ||||||
| Raw materials | 36.57 | 30.96 | 36.57 | 42.28 | 36.67 | 53.99 |
| Utilities | 71.01 | 60.12 | 33.98 | 39.30 | 11.49 | 16.92 |
| Maintenance | 2.38 | 2.02 | 3.72 | 4.30 | 4.60 | 6.77 |
| Labor | 0.51 | 0.43 | 0.51 | 0.59 | 0.67 | 0.98 |
| Fixed and general | 1.2 | 1.02 | 1.82 | 2.10 | 2.26 | 3.32 |
| Overhead | 1.52 | 1.28 | 2.21 | 2.56 | 2.75 | 4.05 |
| Capital depreciation | 4.92 | 4.17 | 7.67 | 8.87 | 9.49 | 13.97 |
| Total costs | 118.12 | 100 | 86.48 | 100 | 67.92 | 100 |
| Revenues | ||||||
| Ethanol | 48.06 | 41.33 | 48.06 | 41.33 | 48.06 | 37.87 |
| Furfural | 68.22 | 58.67 | 68.22 | 58.67 | 68.22 | 53.76 |
| Electricity | 0 | 0 | 0 | 0 | 10.61 | 8.36 |
| Total revenue | 116.28 | 100 | 116.28 | 100 | 126.89 | 100 |
| Economic metrics | ||||||
| NPV (M.USD per y) | −31.53 | 80.90 | 184.54 | |||
| Payout period (y) | Larger than project's life time | 3.41 | 2.22 | |||
Hernández et al.146 evaluated three techno-economic scenarios: (1) the base case considered power production through a direct combustion process at 850 °C and 60 bar; (2) scenario 1 considered production of xylitol, furfural, ethanol and poly-3-hydroxybutyrate PHB; (3) scenario 2 utilized the solid residues resulting from xylitol, furfural, ethanol and PHB processes for producing power and heat through a cogeneration system (Table 10). Results indicated that despite the co-production of xylitol, furfural, ethanol, and PHB integrated into a cogeneration system for producing power and heat from the solid residues increased total cost, the feasibility was positive with a profit margin of 53% and resulted in a lower contamination level regarding the environmental analysis (Table 11).
| Alternative | Option | IRR (%) | Investment (MUSDa) | Production costs | |
|---|---|---|---|---|---|
| Pellets (USD per ton) | LA (USD per kg)b | ||||
| a Millions of USD. b The production costs of LA include the production costs of FA and furfural. Alternative II-a (the use of a fraction of the pretreated sawdust for steam production); alternative II-b without any pellet production. | |||||
| I | 13.4 | 75.0 | 62.42 | 3.71 | |
| II | (a) | 16.5 | 70.5 | 132.04 | 2.57 |
| (b) | 16.6 | 70.1 | — | 2.57 | |
| III | 17.0 | 71.7 | 76.14 | 2.54 | |
| Item | Base case | Scenarios 1 | Scenarios 2 | |||
|---|---|---|---|---|---|---|
| Million US$ per year | Share (%) | Million US$ per year | Share (%) | Million US$ per year | Share (%) | |
| Depreciation of capital | 1.765 | 4 | 2.166 | 4 | 2.815 | 4 |
| Raw material | 11.4911 | 19 | 11.491 | 16 | ||
| Utilities | 20.670 | 45 | 14.789 | 24 | 18.818 | 27 |
| Operating | 23.867 | 52 | 31.916 | 53 | 36.765 | 53 |
| Total | 46.302 | 60.363 | 69.880 | |||
5. Future research outlook
5.1 Integrated biorefinery process for furfural production
An integrated strategy for furfural production has considerable potential to capitalize on all three primary components (cellulose, hemicellulose, and lignin) of lignocellulosic biomass. One- and two-step methods for the selective separation of hemicellulose and subsequent furfural production are required to ensure residue solids for further reuse. Designs for zero-waste manufacturing and waste management sectors can be obtained by reusing and recycling techniques.5.2 Cost-saving and economic sustainability
Recycling solvents and catalysts is an effective solution for achieving cost savings. Modern assisted pretreatment technologies, such as ultrasound, are novel green technologies that can effectively break down plant cells and reduce energy consumption. Liquid–liquid extraction is also energy-efficient for extraction and separation in a biphasic system. One- and two-step approaches for the separation of hemicellulose and lignin require more initial energy input than the selective separation of hemicellulose in dilute acid-catalyzed systems. Therefore, recycling and economical DES with low-cost metal salts at mild temperatures for cost-saving furfural production need to be further explored. In addition, future engineers should focus on the design of heat-capture equipment for energy collection to reduce the considerable electrical costs. Installing water recycling pipes, decreasing water consumption, and using waste water treatment systems are necessary for economic sustainability.5.3 Green technologies for improving furfural selectivity and yield
Furfural has been identified as one of the key ‘green’ chemicals produced in the so-called lignocellulosic feedstock biorefinery.147 Furfural is easily degraded into unwanted products in water. Biphasic systems with organic solvents as the extraction layer, such as MIBK/H2O and GVL/H2O systems, have been proven to enhance the furfural yield by minimizing undesired furfural degradation reactions. GVL is considered a promising green solvent and is effective for furfural production and lignin solutions. The use of traditional toxic inorganic acids as catalysts for furfural production is undesired; therefore, widely applied alternative green catalysts for large-scale integrated processes are more attractive.6. Conclusion
Agricultural waste is rich in xylan-type hemicellulose and is regarded as the best raw material for furfural production. The one-step process can directly convert biomass to furfural, whereas the two-step process requires the additional separation of pentose-rich hydrolysate, which is further converted to furfural under severe pretreatment conditions. Organic solvents and catalysts are important operating variables in the one- and two-step production of furfural. The selection of appropriate catalysts and organic solvents can effectively improve the yield and selectivity of furfural. The hydrogen ions provided by the catalyst promote the hydrolysis of hemicellulose, the presence of chloride ions accelerates the production rate of furfural, and biphasic solvent systems effectively prevent the degradation of furfural. The emerging DESs and ILs are expected to contribute to the utilization of integrated biomass resources. The industrialization of furfural requires a techno-economic evaluation of the entire process of furfural production, and the best production scheme is determined by evaluating the total cost and profit to ensure a stable profit. The integrated biorefinery strategy for the co-production of furfural and ethanol or 5-hydroxymethylfurfural is more economical than the biomass refining strategy for the production of furfural alone. Future developments will be directed towards greener and more cost-effective furfural production strategies.Author contributions
Yuqi Bao: conceptualization and writing – review and editing; Zicheng Du and Xiaoying Liu: investigation and writing – original draft; Chengrong Qin: funding acquisition and resources; Hui Liu and Jinsong Tang: validation; Caoxing Huang and Chen Liang: formal analysis; Shuangquan Yao: project administration and supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was sponsored by the National Key Research and Development Program (2022YFC2105505). This project was supported by the Guangxi Natural Science Foundation of China (2023GXNSFGA026001).References
- Y. Zhao, K. Lu, H. Xu, L. Zhu and S. Wang, Renewable Sustainable Energy Rev., 2021, 139, 110706 CrossRef CAS.
- Y. Zhuang, Z. Si, S. Pang, H. Wu, X. Zhang and P. Qin, J. Cleaner Prod., 2023, 396, 136481 CrossRef CAS.
- W. Deng, Y. Feng, J. Fu, H. Guo, Y. Guo, B. Han, Z. Jiang, L. Kong, C. Li, H. Liu, P. T. T. Nguyen, P. Ren, F. Wang, S. Wang, Y. Wang, Y. Wang, S. S. Wong, K. Yan, N. Yan, X. Yang, Y. Zhang, Z. Zhang, X. Zeng and H. Zhou, Green Energy Environ., 2023, 8, 10–114 CrossRef CAS.
- S. Zhang, M. C. Chi, J. L. Mo, T. Liu, Y. H. Liu, Q. Fu, J. L. Wang, B. Luo, Y. Qin, S. F. Wang and S. X. Nie, Nat. Commun., 2022, 13, 4168 CrossRef CAS PubMed.
- S. X. Nie, C. J. Chen and C. J. Zhu, Front. Chem. Sci. Eng., 2023, 17, 795–797 CrossRef CAS.
- C. B. T. L. Lee and T. Y. Wu, Renewable Sustainable Energy Rev., 2021, 137, 110172 CrossRef CAS.
- Y. Lu, Q. He, G. Fan, Q. Cheng and G. Song, Green Process. Synth., 2021, 10, 779–804 CrossRef CAS.
- S. G. C. Almeida, G. F. Mello, T. K. Kovacs, D. D. V. Silva, M. A. M. Costa and K. J. Dussán, Waste Biomass Valorization, 2022, 13, 4013–4025 CrossRef CAS.
- M. S. N. Sai, D. De and B. Satyavathi, J. Cleaner Prod., 2021, 327, 129467 CrossRef.
- A. Wang, N. P. Balsara and A. T. Bell, Green Chem., 2018, 20, 2903–2912 RSC.
- M. Yazdizadeh, M. R. J. Nasr and A. Safekordi, RSC Adv., 2016, 6, 55778–55785 RSC.
- M. C. Chi, S. Zhang, T. Liu, Y. H. Liu, B. Luo, J. L. Wang, C. C. Cai, X. J. Meng, S. F. Wang, Q. S. Duan and S. X. Nie, Adv. Funct. Mater., 2024, 34, 2310280 CrossRef CAS.
- V. Aristizábal-Marulanda, J. A. Poveda-Giraldo and C. A. Cardona Alzate, Sci. Total Environ., 2020, 728, 138841 CrossRef PubMed.
- B. Qiu, J. Shi, W. Hu, J. Gao, S. Li and H. Chu, Fuel, 2023, 354, 129278 CrossRef CAS.
- K. J. Yong, T. Y. Wu, C. B. T. L. Lee, Z. J. Lee, Q. Liu, J. M. Jahim, Q. Zhou and L. Zhang, Biomass Bioenergy, 2022, 161, 106458 CrossRef CAS.
- C. Feng, J. Zhu, Y. Hou, C. Qin, W. Chen, Y. Nong, Z. Liao, C. Liang, H. Bian and S. Yao, Bioresour. Technol., 2022, 348, 126793 CrossRef CAS PubMed.
- H. Zeng, B. Liu, J. Li, M. Li, M. Peng, C. Qin, C. Liang, C. Huang, X. Li and S. Yao, Bioresour. Technol., 2022, 351, 126951 CrossRef CAS PubMed.
- L. C. Nhien, N. V. D. Long and M. Lee, Energy, 2021, 14, 1152 CAS.
- T. Heinze, L. H. Skodda and M. Gericke, Carbohydr. Polym., 2023, 300, 120251 CrossRef PubMed.
- Y. Li, M. Yao, Y. Luo, J. Li, Z. Wang, C. Liang, C. Qin, C. Huang and S. Yao, ACS Appl. Mater. Interfaces, 2023, 15, 5883–5896 CrossRef CAS PubMed.
- J. Rao, Z. Lv, G. Chen and F. Peng, Prog. Polym. Sci., 2023, 140, 101675 CrossRef CAS.
- C. Huang, B. Jeuck, J. Du, Q. Yong, H. M. Chang, H. Jameel and R. Phillips, Bioresour. Technol., 2016, 219, 600–607 CrossRef CAS PubMed.
- J. Li, B. Liu, L. Liu, Y. Luo, F. Zeng, C. Qin, C. Liang, C. Huang and S. Yao, Bioresour. Technol., 2023, 376, 128855 CrossRef CAS PubMed.
- L. Liu, B. Liu, X. Li, Z. Wang, L. Mu, C. Qin, C. Liang, C. Huang and S. Yao, Ind. Crops Prod., 2023, 200, 116811 CrossRef CAS.
- C. H. Lai, Y. Jia, C. D. Yang, L. W. Chen, H. Shi and Q. Yong, ACS Sustainable Chem. Eng., 2020, 8, 1797–1804 CrossRef CAS.
- T. Zhang, W. Li, Z. Xu, Q. Liu, Q. Ma, H. Jameel, H. M. Chang and L. Ma, Bioresour. Technol., 2016, 209, 108–114 CrossRef CAS PubMed.
- E. Cousin, K. Namhaed, Y. Pérès, P. Cognet, M. Delmas, H. Hermansyah, M. Gozan, P. A. Alaba and M. K. Aroua, Sci. Total Environ., 2022, 847, 157599 CrossRef CAS PubMed.
- B. Liu, L. Liu, X. Qin, Y. Liu, R. Yang, X. Mo, C. Qin, C. Liang and S. Yao, Int. J. Mol. Sci., 2023, 24, 11809 CrossRef CAS PubMed.
- Y. Luo, B. Liu, B. Deng, X. Long, Z. Du, C. Qin, C. Liang, C. Huang and S. Yao, Ind. Crops Prod., 2024, 208, 117791 CrossRef CAS.
- R. Bielski and G. Grynkiewicz, Green Chem., 2021, 23, 7458–7487 RSC.
- Y. Lee, S. W. Lee, Y. F. Tsang, Y. T. Kim and J. Lee, Chem. Eng. J., 2020, 387, 124194 CrossRef.
- Z. Zhang, Z. Pei, H. Chen, K. Chen, Z. Hou, X. Lu, P. Ouyang and J. Fu, Ind. Eng. Chem. Res., 2018, 57, 4225–4230 CrossRef CAS.
- J. M. Guo, K. X. Huang, S. Z. Zhang and Y. Xu, J. Chem. Technol. Biotechnol., 2020, 95, 475–884 CrossRef.
- A. Kumar, A. S. Chauhan, R. Bains and P. Das, Green Chem., 2023, 25, 849–870 RSC.
- P. Y. Wen, T. Zhang, L. T. Wei, J. Y. Wang, A. J. Ragauskas, Y. Zhang, Y. Xu and J. Zhang, ACS Sustainable Chem. Eng., 2021, 9, 11361–11371 CrossRef CAS.
- R. Gérardy, D. P. Debecker, J. Estager, P. Luis and J.-C. M. Monbaliu, Chem. Rev., 2020, 120, 7219–7347 CrossRef PubMed.
- S. Takkellapati, T. Li and M. A. Gonzalez, Clean Technol. Environ. Policy, 2018, 20, 1615–1630 CrossRef CAS PubMed.
- Y. Q. Sheng, Y. Zhang, H. Z. Ma, Y. Xu and M. B. Tu, ACS Sustainable Chem. Eng., 2020, 8, 7892–7900 CrossRef CAS.
- J. Li, T. Li, Y. Wang, Z. Du, Y. Luo, C. Qin, C. Liang, C. Huang and S. Yao, Ind. Crops Prod., 2023, 205, 117453 CrossRef CAS.
- D. Fan, X. Xie, C. Li, X. Liu, J. Zhong, X. Ouyang, Q. Liu and X. Qiu, J. Agric. Food Chem., 2021, 69, 10838–10847 CrossRef CAS PubMed.
- J. L. Wen, S. L. Sun, B. L. Xue and R. C. Sun, J. Agric. Food Chem., 2013, 61, 635–645 CrossRef CAS PubMed.
- L. Fang, Y. Su, P. Wang, C. Lai, C. Huang, Z. Ling and Q. Yong, Bioresour. Technol., 2022, 348, 126795 CrossRef CAS PubMed.
- J. Han, R. Cao, X. Zhou and Y. Xu, Bioresour. Technol., 2020, 307, 123200 CrossRef CAS PubMed.
- W. J. Xu, S. P. Zhang, J. J. Lu and Q. J. Cai, Environ. Prog. Sustainable Energy, 2017, 36, 690–695 CrossRef CAS.
- P. Alvira, M. J. Negro, I. Ballesteros, A. González and M. J. B. Ballesteros, Bioethanol, 2016, 1, 66–75 Search PubMed.
- F. Wang, B. Liu, W. Cao, L. Liu, F. Zeng, C. Qin, C. Liang, C. Huang and S. Yao, Bioresour. Technol., 2023, 385, 129416 CrossRef CAS PubMed.
- Y. Hou, S. Wang, B. Deng, Y. Ma, X. Long, C. Qin, C. Liang, C. Huang and S. Yao, Int. J. Biol. Macromol., 2023, 251, 126374 CrossRef CAS PubMed.
- C. Sawatdeenarunat, K. C. Surendra, D. Takara, H. Oechsner and S. K. Khanal, Bioresour. Technol., 2015, 178, 178–186 CrossRef CAS PubMed.
- L. Zhang, W. Zhang, F. Zhang and J. Jiang, Bioresour. Technol., 2021, 341, 125897 CrossRef CAS PubMed.
- X. Zhou, J. Zhao, X. Zhang and Y. Xu, Bioresour. Technol., 2019, 289, 121755 CrossRef CAS PubMed.
- I. Kim, B. Lee, J. Y. Park, S. A. Choi and J. I. Han, Carbohydr. Polym., 2014, 99, 563–567 CrossRef CAS PubMed.
- J. Liang, B. Liu, X. Li, X. Mo, C. Qin, C. Liang, C. Huang and S. Yao, Bioresour. Technol., 2023, 384, 129328 CrossRef CAS PubMed.
- M. H. Chen, M. J. Bowman, M. A. Cotta, B. S. Dien, L. B. Iten, T. R. Whitehead, K. D. Rausch, M. E. Tumbleson and V. Singh, Carbohydr. Polym., 2016, 140, 96–103 CrossRef CAS PubMed.
- S. Ge, Y. Wu, W. Peng, C. Xia, C. Mei, L. Cai, S. Q. Shi, C. Sonne, S. S. Lam and Y. F. Tsang, Chem. Eng. J., 2020, 385, 123949 CrossRef CAS.
- M. Ebrahimi, O. B. Villaflores, E. E. Ordono and A. R. Caparanga, Bioresour. Technol., 2017, 228, 264–271 CrossRef CAS PubMed.
- B. Palmarola-Adrados, M. Galbe and G. Zacchi, J. Chem. Technol. Biotechnol., 2005, 80, 85–91 CrossRef CAS.
- S. Wang, B. Liu, J. Liang, F. Wang, Y. Bao, C. Qin, C. Liang, C. Huang and S. Yao, Bioresour. Technol., 2023, 382, 129154 CrossRef CAS PubMed.
- Q. A. Yu, X. S. Zhuang, Z. H. Yuan, W. Qi, Q. O. Wang and X. S. Tan, Bioresour. Technol., 2011, 102, 3445–3450 CrossRef CAS PubMed.
- H. Lyu, J. Zhang, J. Zhou, X. Shi, C. Lv and Z. Geng, Fuel Process. Technol., 2019, 195, 106148 CrossRef CAS.
- J. N. M. Soetedjo, C. B. Rasrendra and H. J. Heeres, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 742, 012049 CAS.
- T. A. Natsir and S. Shimazu, Fuel Process. Technol., 2020, 200, 106308 CrossRef CAS.
- H. Huang, Z. Li, Y. Ma, M. Yao and S. Yao, Carbohydr. Polym., 2023, 303, 120461 CrossRef CAS PubMed.
- Y. You, X. Zhang, P. Li, F. Lei and J. Jiang, Bioresour. Technol., 2020, 306, 123131 CrossRef CAS PubMed.
- W. Zhang, Y. You, F. Lei, P. Li and J. Jiang, Bioresour. Technol., 2018, 265, 387–393 CrossRef CAS PubMed.
- B. Danon, G. Marcotullio and W. de Jong, Green Chem., 2014, 16, 39–54 RSC.
- G. Marcotullio and W. De Jong, Green Chem., 2010, 12, 1739–1746 RSC.
- L. Y. Mao, L. Zhang, N. B. Gao and A. M. Li, Green Chem., 2013, 15, 727–737 RSC.
- D. Ouyang, T. Liu, A. A. Astimar, H. L. N. Lau, S. S. Teh, J. Nursyairah, D. Liu and X. Zhao, Bioresour. Technol., 2023, 372, 128626 CrossRef CAS PubMed.
- Z. Chen, X. Bai, A. Lusi, W. A. Jacoby and C. Wan, Bioresour. Technol., 2019, 289, 121708 CrossRef PubMed.
- E. Z. Hoşgün, Biomass Convers. Biorefin., 2021, 11, 2703–2710 CrossRef.
- J. Ren, W. Wang, Y. Yan, A. Deng, Q. Chen and L. Zhao, Cellulose, 2016, 23, 1649–1661 CrossRef CAS.
- C. M. Cai, N. Nagane, R. Kumar and C. E. Wyman, Green Chem., 2014, 16, 3819–3829 RSC.
- L. Zhang, H. Yu, P. Wang, H. Dong and X. Peng, Bioresour. Technol., 2013, 130, 110–116 CrossRef CAS PubMed.
- Y. Li, L. L. Sun, D. M. Cao, X. F. Cao and S. N. Sun, Bioresour. Technol., 2023, 386, 129520 CrossRef CAS PubMed.
- X. Li, Q. Liu, C. Si, L. Lu, C. Luo, X. Gu, W. Liu and X. Lu, Ind. Crops Prod., 2018, 120, 343–350 CrossRef CAS.
- I. Harry, H. Ibrahim, R. Thring and R. Idem, Biomass Bioenergy, 2014, 71, 381–393 CrossRef CAS.
- O. Ogundowo, G. Sadanandam and H. Ibrahim, React. Kinet., Mech. Catal., 2023, 136, 2535–2554 CrossRef CAS.
- X. Li, X. Lu, W. Hu, H. Xu, J. Chen, J. Xiong, L. Lu, Z. Yu and C. Si, Fuel Process. Technol., 2022, 229, 107178 CrossRef CAS.
- C. X. Jiang, J. H. Di, C. Su, S. Y. Yang, C. L. Ma and Y. C. He, Bioresour. Technol., 2018, 268, 315–322 CrossRef CAS PubMed.
- D. Cai, H. Chen, C. Zhang, X. Teng, X. Li, Z. Si, G. Li, S. Yang, G. Wang and P. Qin, J. Cleaner Prod., 2021, 283, 125410 CrossRef CAS.
- P. Liu, S. Shi, L. Gao and G. Xiao, React. Kinet., Mech. Catal., 2022, 135, 795–810 CrossRef CAS.
- Y. Chen, Y. Zhou, R. Zhang and C. Hu, Mol. Catal., 2020, 484, 110729 CrossRef CAS.
- Y. Bao, J. Zhu, F. Zeng, J. Li, S. Wang, C. Qin, C. Liang, C. Huang and S. Yao, Bioresour. Technol., 2022, 364, 128082 CrossRef CAS PubMed.
- O. Yemis and G. Mazza, Bioresour. Technol., 2011, 102, 7371–7378 CrossRef CAS PubMed.
- X. Li, J. Yang, R. Xu, L. Lu, F. Kong, M. Liang, L. Jiang, S. Nie and C. Si, Ind. Crops Prod., 2019, 135, 196–205 CrossRef CAS.
- T. Zhang, W. Li, S. An, F. Huang, X. Li, J. Liu, G. Pei and Q. Liu, Bioresour. Technol., 2018, 264, 261–267 CrossRef CAS PubMed.
- P. Brazdausks, A. Paze, J. Rizhikovs, M. Puke, K. Meile, N. Vedernikovs, R. Tupciauskas and M. Andzs, Biomass Bioenergy, 2016, 89, 98–104 CrossRef CAS.
- L. Zhu, X. Shao, X. Pan, Z. Sun, X. Li, X. Duan and J. Shi, Biomass Bioenergy, 2023, 171, 106734 CrossRef CAS.
- X. Teng, Z. Si, S. Li, Y. Yang, Z. Wang, G. Li, J. Zhao, D. Cai and P. Qin, Ind. Crops Prod., 2020, 151, 112481 CrossRef CAS.
- X. K. Li, Z. Fang, J. Luo and T. C. Su, ACS Sustainable Chem. Eng., 2016, 4, 5804–5813 CrossRef CAS.
- Z. Zhou, D. Liu and X. Zhao, Renewable Sustainable Energy Rev., 2021, 146, 111169 CrossRef CAS.
- K. Dussan, B. Girisuta, M. Lopes, J. J. Leahy and M. H. B. Hayes, ChemSusChem, 2015, 8, 1411–1428 CrossRef CAS PubMed.
- J. C. Solarte-Toro, J. M. Romero-García, J. C. Martínez-Patiño, E. Ruiz-Ramos, E. Castro-Galiano and C. A. Cardona-Alzate, Renewable Sustainable Energy Rev., 2019, 107, 587–601 CrossRef CAS.
- Q. Wang, X. Zhuang, W. Wang, X. Tan, Q. Yu, W. Qi and Z. Yuan, Chem. Eng. J., 2018, 334, 698–706 CrossRef CAS.
- X. Li, Q. Liu, C. Luo, X. Gu, L. Lu and X. Lu, ACS Sustainable Chem. Eng., 2017, 5, 8587–8593 CrossRef CAS.
- S. Jiang, C. Verrier, M. Ahmar, J. Lai, C. Ma, E. Muller, Y. Queneau, M. Pera-Titus, F. Jérôme and K. De Oliveira Vigier, Green Chem., 2018, 20, 5104–5110 RSC.
- W. Wang, J. Ren, H. Li, A. Deng and R. Sun, Bioresour. Technol., 2015, 183, 188–194 CrossRef CAS PubMed.
- Y. P. Luo, Z. Li, Y. N. Zuo, Z. S. Su and C. W. Hu, ACS Sustainable Chem. Eng., 2017, 5, 8137–8147 CrossRef CAS.
- Q. Qing, Q. Guo, L. Zhou, Y. Wan, Y. Xu, H. Ji, X. Gao and Y. Zhang, Bioresour. Technol., 2017, 226, 247–254 CrossRef CAS PubMed.
- X. Lyu and G. G. Botte, Chem. Eng. J., 2021, 403, 126271 CrossRef CAS.
- C. Liu, L. Wei, X. Yin, X. Pan, J. Hu, N. Li, J. Xu, J. Jiang and K. Wang, Chem. Eng. J., 2021, 425, 130608 CrossRef CAS.
- Z. K. Wang, X. J. Shen, J. J. Chen, Y. Q. Jiang, Z. Y. Hu, X. Wang and L. Liu, Int. J. Biol. Macromol., 2018, 117, 721–726 CrossRef CAS PubMed.
- C. B. T. L. Lee, T. Y. Wu, K. J. Yong, C. K. Cheng, L. F. Siow and J. M. Jahim, Sci. Total Environ., 2022, 827, 154049 CrossRef CAS PubMed.
- S. Peleteiro, V. Santos, G. Garrote and J. C. Parajó, Carbohydr. Polym., 2016, 146, 20–25 CrossRef CAS PubMed.
- L. Zhang, L. Tian, R. Sun, C. Liu, Q. Kou and H. Zuo, Bioresour. Technol., 2019, 276, 60–64 CrossRef CAS PubMed.
- J. Gao, H. Wang, X. Cao, Z. Li, H. Guo, X. Yang, W. Wang, N. Guo and Y. Ma, Mol. Catal., 2023, 535, 112890 CrossRef CAS.
- O. Ogundowo and H. Ibrahim, Biomass Convers. Biorefin., 2024, 14, 7743–7751 CrossRef CAS.
- R. Sirohi, J. P. Pandey, A. Singh, R. Sindhu, U. C. Lohani, R. Goel and A. Kumar, Ind. Crops Prod., 2020, 149, 112351 CrossRef CAS.
- H. Li, X. Wang, C. Liu, J. Ren, X. Zhao, R. Sun and A. Wu, Ind. Crops Prod., 2016, 94, 721–728 CrossRef CAS.
- S. Xiong, C. Luo, Z. Yu, N. Ji, L. Zhu and S. Wang, Green Chem., 2021, 23, 8458–8467 RSC.
- R. L. Johnson, F. A. Perras, M. P. Hanrahan, M. Mellmer, T. F. Garrison, T. Kobayashi, J. A. Dumesic, M. Pruski, A. J. Rossini and B. H. Shanks, ACS Catal., 2019, 9, 11568–11578 CrossRef CAS.
- D. Scholz, O. Kröcher and F. Vogel, ChemSusChem, 2018, 11, 2189–2201 CrossRef CAS PubMed.
- G. S. Foo, A. H. Van Pelt, D. Krötschel, B. F. Sauk, A. K. Rogers, C. R. Jolly, M. M. Yung and C. Sievers, ACS Sustainable Chem. Eng., 2015, 3, 1934–1942 CrossRef CAS.
- J. Wang, B. C. Lin, Q. X. Huang, Z. Y. Ma, Y. Chi and J. H. Yan, Energy Fuels, 2017, 31, 11681–11689 CrossRef CAS.
- W. Sangarunlert, P. Piumsomboon and S. Ngamprasertsith, Korean J. Chem. Eng., 2007, 24, 936–941 CrossRef CAS.
- D. W. Rackemann, J. P. Bartley, M. D. Harrison and W. O. S. Doherty, RSC Adv., 2016, 6, 74525–74535 RSC.
- A. R. C. Morais, M. D. D. J. Matuchaki, J. Andreaus and R. Bogel-Lukasik, Green Chem., 2016, 18, 2985–2994 RSC.
- C. B. T. L. Lee, T. Y. Wu, C. H. Ting, J. K. Tan, L. F. Siow, C. K. Cheng, J. Md. Jahim and A. W. Mohammad, Bioresour. Technol., 2019, 278, 486–489 CrossRef CAS PubMed.
- E. S. Kim, S. Liu, M. M. Abu-Omar and N. S. Mosier, Energy Fuels, 2012, 26, 1298–1304 CrossRef CAS.
- A. Deng, J. Ren, H. Li, F. Peng and R. Sun, RSC Adv., 2015, 5, 60264–60272 RSC.
- Z. Chen, W. D. Reznicek and C. Wan, ACS Sustainable Chem. Eng., 2018, 6, 6910–6919 CrossRef CAS.
- M. Hu, Y. Yu and Y. Liu, Ind. Crops Prod., 2023, 204, 117334 CrossRef CAS.
- L. Penín, M. López, V. Santos and J. C. Parajó, Molecules, 2022, 27, 4258 CrossRef PubMed.
- W. C. Li, S. J. Zhang, T. Xu, M. Q. Sun, J. Q. Zhu, C. Zhong, B. Z. Li and Y. J. Yuan, Energy, 2020, 195, 117076 CrossRef CAS.
- T. M. Santos, M. V. Alonso, M. Oliet, J. C. Domínguez, V. Rigual and F. Rodriguez, Carbohydr. Polym., 2018, 194, 285–293 CrossRef CAS PubMed.
- A. R. C. Morais, A. C. Mata and R. Bogel-Lukasik, Green Chem., 2014, 16, 4312–4322 RSC.
- S. P. M. da Silva, A. R. C. Morais and R. Bogel-Lukasik, Green Chem., 2014, 16, 238–246 RSC.
- A. Cornejo, I. Alegria-Dallo, Í. García-Yoldi, Í. Sarobe, D. Sánchez, E. Otazu, I. Funcia, M. J. Gil and V. Martínez-Merino, Bioresour. Technol., 2019, 288, 121583 CrossRef CAS PubMed.
- V. Sharma, M. L. Tsai, C. W. Chen, P. P. Sun, A. K. Patel, R. R. Singhania, P. Nargotra and C. D. Dong, Bioresour. Technol., 2022, 360, 127631 CrossRef CAS PubMed.
- N. Li, F. Meng, H. Yang, Z. Shi, P. Zhao and J. Yang, Bioresour. Technol., 2022, 346, 126639 CrossRef CAS PubMed.
- S. Arora, N. Gupta and V. Singh, ChemSusChem, 2021, 14, 3953–3958 CrossRef CAS PubMed.
- Y. P. Luo, Z. Li, Y. N. Zuo, Z. S. Su and C. W. Hu, ACS Sustainable Chem. Eng., 2017, 5, 8137–8147 CrossRef CAS.
- S. Peleteiro, V. Santos and J. C. Parajó, Carbohydr. Polym., 2016, 153, 421–428 CrossRef CAS PubMed.
- M. Z. R. Mohammed, Z. W. Ng, A. Putranto, Z. Y. Kong, J. Sunarso, M. Aziz, S. H. Zein, J. Giwangkara and I. Butar, Clean Technol. Environ. Policy, 2023, 25, 1551–1567 CrossRef CAS PubMed.
- G. Zang, A. Shah and C. Wan, J. Cleaner Prod., 2020, 260, 120837 CrossRef CAS.
- R. Gogar, S. Viamajala, P. A. Relue and S. Varanasi, ACS Sustainable Chem. Eng., 2021, 9, 3428–3438 CrossRef CAS.
- N. B. Appiah-Nkansah, J. Li, W. Rooney and D. Wang, Renewable Energy, 2019, 143, 1121–1132 CrossRef CAS.
- R. N. Ntimbani, S. Farzad and J. F. Görgens, Ind. Crops Prod., 2021, 162, 113272 CrossRef CAS.
- R. N. Ntimbani, S. Farzad and J. F. Görgens, Biofuels, Bioprod. Biorefin., 2021, 15, 1900–1911 CrossRef CAS.
- P. Halder and K. Shah, Bioresour. Technol., 2023, 371, 128587 CrossRef CAS PubMed.
- L. C. Nhien, N. V. D. Long, S. Kim and M. Lee, Biotechnol. Biofuels, 2017, 10, 81 CrossRef PubMed.
- N. Viar, I. Agirre and I. Gandarias, Chem. Eng. J., 2024, 480, 147873 CrossRef CAS.
- J. Moncada, C. A. Cardona, J. C. Higuita, J. J. Vélez and F. E. López-Suarez, Chem. Eng. Sci., 2016, 140, 309–318 CrossRef CAS.
- A. Mazar, O. Ajao, M. Benali, N. Jemaa, W. Wafa Al-Dajani and M. Paleologou, ACS Sustainable Chem. Eng., 2020, 8, 17345–17358 CrossRef CAS.
- N. M. Clauser, S. Gutiérrez, M. C. Area, F. E. Felissia and M. E. Vallejos, Biofuels, Bioprod. Biorefin., 2018, 12, 997–1012 CrossRef CAS.
- V. Hernández, J. M. Romero-García, J. A. Dávila, E. Castro and C. A. Cardona, Resour., Conserv. Recycl., 2014, 92, 145–150 CrossRef.
- M. Bariani, F. Cebreiros, M. Guigou and M. N. Cabrera, Wood Sci. Technol., 2022, 56, 1149–1173 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |