Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fast and simple analysis of the content of Zn, Mg, Ca, Na, and K in selected beverages widely consumed by athletes by flowing liquid cathode atmospheric pressure glow discharge optical emission spectrometry†
Monika
Gorska
 *,
Joanna
Weiss
and
Pawel
Pohl
*,
Joanna
Weiss
and
Pawel
Pohl

Wroclaw University of Science and Technology, Faculty of Chemistry, Division of Analytical Chemistry and Chemical Metallurgy, Wybrzeze Stanislawa Wyspianskiego 27, 50-370 Wroclaw, Poland. E-mail: monika.gorska@pwr.edu.pl
First published on 29th March 2023
Abstract
An atmospheric pressure glow discharge (APGD) system, generated between a flowing liquid cathode (FLC) and a gas (He) jet anode, was applied for the determination of Zn, Mg, Ca, Na, and K in selected beverages commonly chosen by athletes (namely Coca-Cola Zero, energy and vitamin drinks, pre-workout, branched-chain amino acids, almond drink, and whey protein) by optical emission spectrometry (OES). In some cases (i.e., Coca-Cola, energy drink, and almond drink), sugared and sugar-free versions of the beverages were analyzed with the purpose of establishing the impact of added sugar on the analyte signal intensities. The analysis was performed after a simplified sample preparation procedure, which involved only their dilution and acidification with HNO3 to a concentration of 0.2 mol L−1. To determine the most suitable conditions for performing the analysis, optimization of the crucial operating parameters and sample dilution was carried out. Under the compromise conditions, the instrumental detection limits (DLs) were established and found to be 21, 0.91, 20, 0.062, and 0.14 μg L−1 for Zn, Mg, Ca, Na, and K, respectively. Due to the relatively low detection limits, the analyte content could be determined for a fairly high dilution, being concurrently the same for all analytes, which further simplified the whole procedure. It was found that the vast majority of samples could be determined using external calibration with simple standard solutions. The standard addition technique used for calibration was only required for the determination of Mg in three samples. The analysis results were consistent (in the majority of cases the recovery values were in the range of 88–111%) with the values obtained for the reference method (inductively coupled plasma optical emission spectrometry, ICP-OES), which proved the reliability of the results obtained from the developed FLC-APGD-OES system.
1. Introduction
Over the last few years, an increasing number of various types of supplements that are supposed to enhance the training performance of participants or have a beneficial impact on overall health has been observed in the fitness industry.1,2 Nowadays, the number of supplements that are available in this market is very high. They include whey, casein, and vegan protein powders, branched-chain amino acids (BCAAs), beta-alanine, glutamine, arginine, different chemical forms of creatine, citrulline malate, turkesterone, dehydroepiandrosterone (DHEA), ashwagandha, pre-workouts, and multivitamins. However, according to the data available in the related literature, they are generally either not needed or the scientific consensus is ambiguous, and thus, more research is needed to confirm or refute their usefulness to athletes. For instance, BCAA supplements contain three of the essential amino acids (EAAs), namely leucine, isoleucine, and valine. Given that the EAAs cannot be synthesized by humans at a fast enough rate, they have to come from an appropriately balanced diet. Concurrently, these three amino acids, particularly leucine, are key components for the synthesis of muscle protein, which is obviously important for body-builders.3 However, not all dietary proteins contain all these amino acids, and it has also been shown that all EAAs are needed for effective muscle protein stimulation.3–5 Therefore, supplementation with BCAAs makes sense only when ingested with a meal that contains a proper amount of other EAAs, and even then it is still less effective than the case of an equal amount of a high-quality dietary protein source.6,7 Another group of supplements that are readily used by athletes are pre-workouts. They are meant to be taken prior to exercise and usually contain a blend of different ingredients, e.g., amino acids, beta-alanine, caffeine, carbohydrates, creatine, and others, which as a whole are supposed to boost the exercise performance and subsequent recovery.2,8 However, there are a few issues related to this particular group of supplements. Firstly, many of the ingredients are found in real food, e.g., amino acids and carbohydrates. Secondly, unlike the pre-workout itself, some of its ingredients, such as amino acids and creatine, are intended to be taken daily to observe significant effects.9 Moreover, the actual composition of several pre-workout supplements are often not clear due to the common habit of displaying “proprietary blend” on the supplement facts label.2,10,11 Additionally, many of the actual ingredients of pre-workouts are underdosed.10 Another type of widely consumed supplements is multivitamins, which not only contain vitamins but a wide variety of minerals and are expected to prevent and treat vitamin and mineral deficiencies from their insufficient intake from real food.11 However, given that the manufacturers of minerals and vitamins are not obligated to submit the thorough documentation regarding their safety and effectiveness, their intake is related to many potential harmful effects, usually resulting from overdosing. Moreover, they can be contaminated with doping substances.11–13 Accordingly, the only supplements that seem to be worthwhile are protein powder and creatine, which are usually the only supplements recommended by coaches, especially for beginners. Nevertheless, protein powder is still not needed in the majority of cases if enough protein is consumed with a well-balanced diet. Moreover, some of these supplements may contain heavy metals, such as Cd, Hg, and Pb.14,15Unfortunately, there are very few papers regarding the mineral content in these supplements and when they appear, they focus on heavy metals.14–16 Generally, to assess the elemental content of selected metals in the above-mentioned supplements, graphite furnace atomic absorption spectrometry (GF-AAS), inductively coupled plasma optical emission spectrometry (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS) are applied.14 Nevertheless, the analysis performed using the above-mentioned instruments may be challenging due to certain issues related to their practical application. Firstly, the necessity of performing the time-consuming digestion of samples that contain significant amounts of organic matrix, high viscosity or dissolved solid particles given that all these factors negatively impact (decrease) the signal intensity of the analyte. Moreover, the presence of solid particles in the analyzed solutions may clog the solution delivery capillaries in the instruments. Thus, the digestion procedure is performed with relatively high amounts of concentrated reagents (mostly acids), high temperature, and sometimes decreased pressure. Another disadvantage is the complex and bulky setup required to perform the analyses. Moreover, the costs associated with the use of the apparatus is very high due to its high power and gas consumption. All of this translates to high purchasing and operating costs related to the practical application of these instruments.
Thus, to address this issue, many researchers have devoted their efforts to developing alternative instruments that can performing the analysis with equal reliability, similar or better DLs of elements, and high accuracy and precision of measurements but at significantly reduced costs of both developing the necessary setup and applying it for the analysis of real samples. Currently, instruments in which a discharge is generated upon contact with liquids are being widely developed. Furthermore, various excitation sources have been proposed, including electrolyte jet cathode glow discharge (EJC-GD),17 liquid electrode plasma (LEP),18 discharge on boiling in a channel (DBC),19 electrolyte cathode discharge (ELCAD),20 liquid sampling-atmospheric pressure glow discharge (LS-APGD),21 flowing liquid cathode-atmospheric pressure glow discharge (FLC-APGD),21 solution cathode glow discharge (SCGD),22 hanging drop electrode-atmospheric pressure glow discharge (HDE-APGD),23 flowing liquid anode-atmospheric pressure glow discharge (FLA-APGD),24 solution anode glow discharge (SAGD),25 and liquid drop anode-atmospheric pressure glow discharge (LDA-APGD).26 Among them, FLC-APGD seems to be one of the most promising sources. This is due to several factors, which are mentioned below, that make the FLC-APGD system suitable for real samples analysis. Although the DLs of elements obtained with this system are not the highest (the FLA-APGD system offers significantly lower DLs but for a limited group of elements, i.e., Ag, Bi, Cd, Hg, In, Pb, Tl, and Zn), they are still comparable to or better than that offered by atomic absorption spectrometry (AAS) and ICP-OES.27–29 Alternatively, in the FLA-APGD-OES method, matrix effects are not often observed and even if they are present, their extent is acceptably low.30,31 Moreover, similar to ICP-OES, FLC-APGD-OES enables the analysis of the content of many different elements in samples.32–35 Owing to all these advantages, the FLC-APGD-OES method has been successfully applied for the analysis of different samples with complex matrices, such as blood,36 honey,37 human hair,38 fruit juices,28 soils,33 and wines29 as well as samples with simpler matrices such as different types of water.39–45
The costs involved in the application of large-scale commercial instrumentation such as ICP-OES vary depending on several factors, including the specific instrument being used, the cost of consumables (e.g., plasma gas, nebulizer, and pump tubing), and the cost of electricity. Additionally, maintenance and repair costs should be considered. Due to the complexity of these instruments, the overall purchasing costs are very high, whereas the developed microplasma systems are comprised of cheap elements, such as graphite tubes and PTFE reservoirs. Also, many of the large-scale setup elements need to be replaced every few months. Routine maintenance, such as cleaning and calibration, can cost several hundred dollars a year. Repairs can cost several thousand dollars, depending on the extent of the damage. Regarding the operating costs, compared to the currently developed microplasma sources, the plasma gas (argon) usage is typically several dozen higher for ICP-OES. However, many microplasma systems can be operated without the use of any gas. Moreover, the microplasma sources do not require any nebulizer for transporting the analytes into the plasma. Also, the power consumption is several dozen times lower in the case of microplasma excitation sources. All these facts translate to a significant reduction in operating costs when applying microplasma sources instead of the currently used large-scale instrumentation.
Therefore, the aim of this work was to show the suitability of the miniaturized FLC-APGD excitation source for the OES determination of different elements in various types of beverages commonly chosen by athletes. This was done using a simplified sample preparation procedure that completely avoided any type of sample pre-digestion and included only sample dilution and acidification. To the best of our knowledge, the analysis of such samples is rarely done using conventional instrumentation and has never been carried out with the aid of any type of microplasma source. The selected matrices, with particular emphasis on the protein matrix, have never been analyzed thus far, using the above-mentioned APGD sources. Additionally, compared to our previous works,28 the design of the studied FLC-APGD system was modified by replacing the tungsten rod with a tungsten tube, which improved the discharge stability by using an He jet anode and cooling the tube with the He gas flux. To achieve the goal of this work, the optimization of the most crucial operating parameters was performed initially, followed by the optimization of the sample dilutions to find the most suitable one. Subsequently, the DLs of the analytes (Zn, Mg, Ca, Na, and K) were established under the compromise conditions. Finally, the developed method was applied for the analysis of 13 different beverages for their content of Zn, Mg, Ca, Na, and K. The obtained results were compared with that obtained from ICP-OES measurements of the analyzed samples, after their wet-digestion, and discussed.
2. Experimental
2.1. Instrumentation
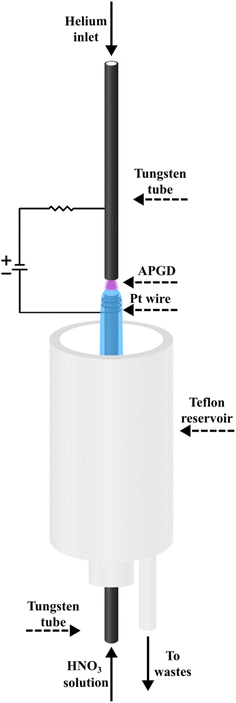
A schematic image of the studied FLC-APGD system is shown in Fig. 1. The discharge was generated in an open-to-air chamber between two tungsten tubes (OD/ID 3/2 mm, length 50 mm), serving as the solution and He delivery tubes. This is the difference to our previous FLC-APGD setup,28 which was intended to enhance the stability of the measurements of samples comprised of highly complex matrix components. The distance between the tubes (so-called the discharge gap) was constant throughout the experiments and set to approximately 1 mm. The liquid cathode solutions acidified with HNO3 were introduced in the bottom tungsten tube with the aid of a 3-channel REGLO ICC peristaltic pump (Ismatec, USA) at various flow rates in the range of 2.0–4.0 mL min−1. As the solution flowed down to a PTFE reservoir, it was pumped out from it by means of the previously mentioned peristaltic pump. The He gas was introduced through the upper tube at various flow rates in the range of 50–350 mL min−1. The electrical contact was provided directly in the case of the upper gas delivery tungsten tube and with a platinum spiral wrapped around the bottom solution delivery tube. The voltage was applied to both tubes by means of an HV dc power supply (model DP50H-024PH, DSC-Electronics, Germany) and its value changed in the range of 800–1200 V, depending on the applied discharge current, acid concentration and the gas and sample flow rates. A 6.9 kΩ ballast resistor was connected to the electrical circuit to achieve the discharge stabilization.The radiation emitted by the discharge was imaged (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on the entrance slit (10 μm) of a Shamrock 500i imaging spectrometer (Andor, UK), using an achromatic quartz lens (f = 80). The spectrometer setup consisted of a holographic grating (1800 lines per mm) and a Newton DU-920P-OE UV-Vis CCD camera (Andor, UK). The integration time was 10 s for all experiments and the traced emission lines of the studied elements were as follows: Zn (213.9 nm), Mg (285.2 nm), Ca (422.7 nm), Na (589.0 nm), and K (766.5 nm). Unless stated otherwise, each spectrum was recorded three times and the average results were given.
1) on the entrance slit (10 μm) of a Shamrock 500i imaging spectrometer (Andor, UK), using an achromatic quartz lens (f = 80). The spectrometer setup consisted of a holographic grating (1800 lines per mm) and a Newton DU-920P-OE UV-Vis CCD camera (Andor, UK). The integration time was 10 s for all experiments and the traced emission lines of the studied elements were as follows: Zn (213.9 nm), Mg (285.2 nm), Ca (422.7 nm), Na (589.0 nm), and K (766.5 nm). Unless stated otherwise, each spectrum was recorded three times and the average results were given.
To obtain the reference values of the concentration of Zn, Mg, Ca, Na, and K in the analyzed samples, the samples were wet-digested and subjected to analysis by means of ICP-OES using an Agilent 5110 Synchronous Vertical Dual View (SVDV) instrument. The spectrometer was equipped with an easy-to-fit quartz torch with a standard 1.8 mm injector and a sample introduction system, which consisted of a Seaspray nebulizer and a double-pass glass cyclonic spray chamber. The operating conditions used to for the ICP-OES instrument are presented in Table SI1.†
2.2. Reagents and sample preparation
Deionized water (18.2 MΩ cm) was obtained from an Polwater water purification system (Labopol-Polwater, Poland) and was used during the whole study. High-purity He (99.999%) was provided by Air Products (Poland). The purity of all chemicals was at least of analytical grade. Stock standard solutions of Zn, Mg, Ca, Na, and K (1000 mg L−1) were supplied by Sigma-Aldrich (Germany) and used to prepare all working standard solutions. For the acidification of the FLC solutions, a Suprapur® grade concentrated HNO3 (65% m m−1) solution from Merck (Germany) was applied. A Suprapur® 30% H2O2 solution (Merck, Germany) along with the mentioned concentrated HNO3 solution were employed for the sample digestion before the ICP-OES analysis.Various products willingly chosen by athletes were employed to perform this study, including Coca-Cola Zero, energy drinks, pre-workout, BCAAs, almond drink, and protein powders. For comparison, sugared and sugar-free versions of Coca-Cola, black energy drink, and almond drink were studied. All the mentioned samples were purchased at local stores, with the exception of the protein powders, which were purchased from an online shop. Henceforth, the following abbreviations will be used for the simplification: CCZ (Coca-Cola Zero), CCO (Coca-Cola Original, sugared), VMg (multi-vitamin energy drink with added Mg), VZn (multi-vitamin energy drink with added Zn), BSF (Black, Sugar-Free), BS (Black, Sugared), PW (pre-workout), BCAA-1, BCAA-2 (two different samples of BCAAs varying only in taste), ADSF (almond drink, sugar-free), ADS (almond drink, sugared), WPC (whey protein, chocolate flavor), and WPS (whey protein, strawberry flavor). At this point, it is worth briefly mentioning that almond drink is better known as almond milk, however, the term “milk” should be avoided given that according to the European Court of Justice statement, this term means only the natural secretion of mammals, not plants.46 Therefore, the term “drink” will be used herein.
The almond drink and whey protein samples had to be filtered prior to both FLC-APGD-OES and ICP-OES analysis. To achieve this, they were filtered through 0.45 μm syringe filters, given that the formation of protein precipitates occurred after the addition of HNO3. To prepare the sample solutions for the FLC-APGD-OES measurements, appropriate amounts of each of the studied samples were transferred to twist-cup containers and acidified with concentrated HNO3 to the final concentration of 0.2 mol L−1. Subsequently, the solutions were filled with deionized water to reach final dilutions that were 50-, 100-, 500-, or 5000-fold (depending on the sample). The prepared solutions were analyzed in regard to the external calibration curves with simple standards solutions, and – if needed – using the standard addition technique. In the latter case, all the mentioned sample preparation steps were repeated but after the sample acidification, each sample was divided into three separate parts and two standard additions of Mg were added to reach the concentration of 50 and 100 μg L−1, respectively. Subsequently, all the samples were filled with deionized water to reach the final dilution of 100- or 500-fold.
For the ICP-OES analysis, all the studied samples had to be wet-digested prior to the actual analysis, with the purpose of destroying any type of organic matrix present in them. To achieve this, aliquots of 5.0 g of each studied sample (or filtrates of almond drink and whey protein) along with 5.0 mL of concentrated HNO3 were transferred to a DigiPrep tube and heated in a DigiPrep block digestion system for 180 min at 120 °C. Subsequently, the resulting sample digests were cooled and 5.0 mL of H2O2 (30%) was added. Then, the digested samples were further heated for 60 min. After cooling, the final digested samples were filled with deionized water to the mass of 50.0 g. Subsequently, appropriate amounts of digested samples were transferred to twist-cup containers and filled with deionized water up to 30.0 g. The final dilutions (on the weight basis) of the samples were 10- (CCZ and CCO), 100- (VMg, VZn, BSF, BS, PW, BCAA-1, BCAA-2, ADSF, and ADS) or 1000-fold (WPC and WPS).
All the analyzed samples were prepared in triplicate along with the appropriate procedural blanks that were considered in the final results. The analytes were determined in regard to the external calibration curves with simple standards solutions.
3. Results and discussion
3.1. Optimization of discharge operating conditions
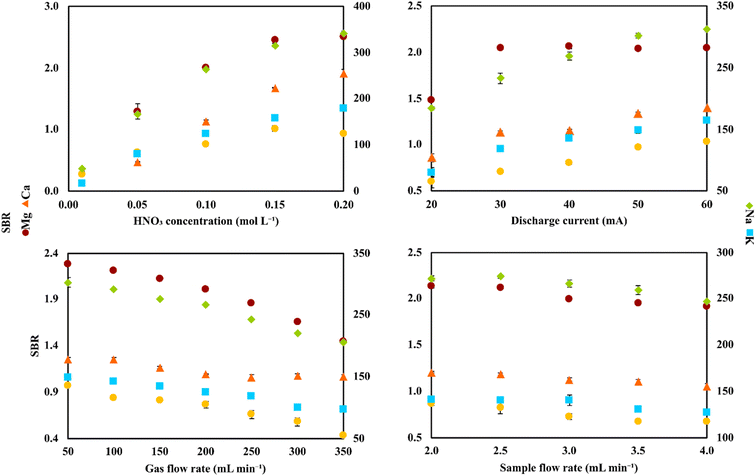
Initially, to establish which elements were actually present in the studied samples, a preliminary study was carried out. To achieve this, samples of different dilutions varying in the 10–100-fold range were introduced in the FLC-APGD discharge and the emission spectra in the range of 200–900 nm were recorded for each of them. The analytical lines of Mg, Ca, Na, and K were detected in each of the samples. Additionally, a relatively high signal of Zn was detected in the VZn sample. Besides these elements, no other elements were found in any of these samples, indicating that they were likely present at concentrations lower than the respective detection limits (DLs) achievable with FLC-APGD-OES. It was noted that the signals of the analytes differed between the samples in terms of their intensity, which was obviously expected. Nevertheless, in the case of the WPC and WPS samples, the analyte signal intensities were significantly higher (compared to the other samples) in the 100-fold diluted samples. Thus, it was decided to apply higher dilutions of these two samples for each subsequent research step. Consequently, unless stated otherwise, the samples dilutions applied in this study were 10- (CCZ and CCO), 20- (VMg, VZn, BSF, BS, PW, BCAA-1, BCAA-2, ADSF, and ADS) or 1000-fold (WPC and WPS) and the operating conditions were as follows: acid concentration of 0.1 mol L−1, discharge current of 40 mA, He gas flow rate of 200 mL min−1, and sample flow rate of 3.0 mL min−1.Initially, to successfully apply the developed FLC-APGD system for the determination of Zn, Mg, Ca, Na, and K in the studied samples, optimization of the most crucial operating parameters was carried out. The following parameters were found to have a significant impact on the analyte signals and were included in the optimization step: the acid concentration (0.01–0.20 mol L−1) used for the acidification of the sample solutions, the discharge current (20–60 mA), the gas flow rate (50–350 mL min−1) in the case of the He jet anode, and the sample flow rate (2.0–4.0 mL min−1) in the case of the FLC solution. The measured response of the analytes was the signal-to-background ratio (SBR) of their analytical lines.
Initially, the optimization was performed on a solution containing only the studied analytes and acidified with HNO3. The analyte concentrations were 200, 200, 600, 100, and 100 μg L−1 for Zn, Mg, Ca, Na, and K, respectively. The results are shown in Fig. 2, which indicated that the SBRs for the atomic emission lines of the studied analytes increased with an increase in the acid concentration and discharge current, but decreased with an increase in the He gas flow rate and sample flow rate. These results are consistent with the related literature data26,47,48 for similar systems. Although the analyte response was similar for each investigated parameter, some differences for the impact of these parameters between themselves on the analyte signals were noted. For example, an increase of HNO3 concentration had a greater impact on the enhancement of the SBR values than an increase of the discharge current, while an increase of the He gas flow rate caused a more noticeable drop in the SBRs than an increase of the sample flow rate. Considering that the discharge stability was impaired for some values of the studied parameters (e.g., the discharge current of 60 mA or the sample flow rate of 2.0 mL min−1), this observation was important for choosing the compromise operating conditions.
The reasons for the observed impact of each of the investigated parameters on the SBR values are not fully clear, however, some assumptions have been made on this matter in the previous studies. Regarding the acid concentration, the growing tendency of the analytical signals, as a result of its increasing values, are usually explained by higher solution conductivity and lower water evaporation observed for higher acid concentrations.27 For the discharge current, its increasing values caused improved atomization and excitation conditions due to the higher population of high-energy electrons present in the discharge.27,28,45 The decreasing tendency of the analyte signals for the increasing He gas flow rate in the FLC-APGD system with the He jet as the anode is likely due to a narrowed diffusion of the analyte atoms in the discharge zone as a result of the stronger gas flux.45,49 Finally, the impact of the sample flow rate on the intensity of the atomic lines is not as straightforward as expected. Intuitively, it was assumed that the analyte signals would increase with an increase of the sample flow rate due to the higher amounts of analyte atoms being released into the discharge per unit time, which was actually observed in many previous works.25,50,51 Alternatively, at higher sample flow rates, higher amounts of water are also present in the interfacial zone as a result of the higher water evaporation per unit time. This can cause impaired atomization and excitation conditions in the discharge, yielding decreased analyte signals. This was also the case in many related papers.47,52,53 Therefore, it can be concluded that the obtained intensity or SBR values for the analytical lines of Zn, Mg, Ca, Na, and K are the result of these two effects taking place simultaneously. However, it should be noted that different results obtained by various research groups are likely related to the different system setups used and varying operating conditions, which can co-influence the sputtering efficiency and water evaporation of the system.
Nevertheless, for the elemental analysis of real samples, the impact of the sample matrix on the overall signal behavior should be considered. Thus, in this part of the study, the optimization was repeated for the studied samples to observe any possible differences in the signal behavior between samples with and without the matrix. Consequently, not only the optimal conditions for the actual samples could be established but some insight into the potential matrix effects could be gained. Therefore, the studied samples were diluted 10- (CCZ and CCO), 20- (VMg, VZn, BSF, BS, PW, BCAA-1, BCAA-2, ADSF, and ADS) or 1000-fold (WPC and WPS) and the optimization of these samples was performed. The obtained results are shown in Fig. SI1–SI7.† As it can be seen, the general tendencies in terms of the impact of a given parameter on the SBRs of the analytical lines were similar to that obtained for the sample solutions without any matrix (just standard). This finding suggests that the behavior of the analyte signals was indeed dependent on the atomization and excitation conditions in the discharge, as mentioned previously, and the sample matrices had nothing to do with these tendencies. Therefore, it can be assumed at this stage that either there were no matrix effects observed under these conditions or their contribution was minor. Although the latter conclusion cannot be stated with absolute certainty, it would be expected that the presence of some matrix effects would impact the signal sensitivity and give different results regarding the impact of these parameters on the SBR values. This is because the sample matrix can impact the atomization and excitation conditions in the discharge, leading to different signal behaviors under the same conditions. Alternatively, the matrix of real samples certainly influenced the stability of the atomic signals, which was noted while performing the measurements and it was elucidated on the figures by the error bars. This led to the conclusion that the sample dilution should be further decreased for the actual analysis.
Nevertheless, it can be concluded that the most favorable conditions in terms of the SBR values of the analytical lines of Zn, Mg, Ca, Na, and K were acid concentration of 0.2 mol L−1, discharge current of 60 mA, He gas flow rate of 50 mL min−1, and sample flow rate of 2.0 mL min−1. However, based on our previous experience,28,54 it was expected that the combination of all these parameters would result in significant instability of the discharge operation. This assumption is supported by the observations made herein, indicating lower discharge stability for the combination of just one of the above-mentioned parameter values with the other ones, being in the middle of the studied ranges. Thus, we decided to choose the most favorable conditions only in the case of the parameters that influenced the analyte signals the most and less suitable conditions for the rest of them. Moreover, the impact of each of the studied parameters on the discharge stability was also considered. For example, both the discharge current and sample flow rate influenced the SBRs of the analytical lines to a lower extent (than the other studied parameters), but higher discharge current values caused a lower discharge stability than lower sample flow rates. Consequently, the compromise conditions applied for all further experiments were as follows: acid concentration of 0.2 mol L−1, discharge current of 40 mA, He gas flow rate of 50 mL min−1, and sample flow rate of 2.5 mL min−1.
3.2. Analytical performance
To establish the most suitable dilutions of the studied samples for their analysis by FLC-APGD-OES for the content of Zn, Mg, Ca, Na, and K, the analytical characteristics of the developed method were determined under the compromise conditions. For this purpose, the DLs of Zn, Mg, Ca, Na, and K, and the upper linearity ranges (ULRs) of the respective calibration curves were determined. The precision (expressed as RSD) was omitted in this study given that it was assumed that this parameter would strongly depend on both the sample dilution as well the sample composition with particular emphasis on the organic matrix. Therefore, the measured RSD values for the solutions containing only the analytes would not probably elucidate well the actual precision in the real samples. The DL values were calculated as 3σ/a, with “σ” representing 3 times the standard deviation of 10 consecutive measurements of an acidified blank solution and “a” the sensitivity of a corresponding calibration curve. The upper linearity range was determined using 8 standard solutions in varying concentration ranges for different elements including 0.2–2 mg L−1 for Zn and Ca, 0.1–1 mg L−1 for Mg, and 0.05–0.5 mg L−1 for Na and K. The obtained results are shown in Table SI2.†The DLs of the elements in this study for the developed FLC-APGD-OES method were 21, 0.91, 20, 0.062, and 0.14 μg L−1 for Zn, Mg, Ca, Na, and K, respectively. These values are similar or slightly worse compared to that obtained by our group in previous studies.28,29 The small differences between the DLs obtained in these three works likely resulted from the slightly different operating conditions (which affected not only the signal intensities but also the background level in the vicinity of the analytical lines) as well as tubes of different diameters applied in this work, which we know from our expercience to slightly affect the signal intensities. Comparing the obtained DLs of Zn, Mg, Ca, Na, and K with the values reported by other researchers for similar FLC-APGD systems (see Table 1), it can be noticed that the DLs of the elements obtained herein are on average 1 order of magnitude better than those published in the majority of recently published papers. In this case, it can be expected that these low DLs of Zn, Mg, Ca, Na and K will enable the determination of the studied analytes in relatively highly diluted samples.
| Element | System | DL (μg L−1) | Reference |
|---|---|---|---|
| a Compared to the listed excitation sources, an He gaseous jet anode was used in the place of a pin metallic anode. FLC-APGD – flowing liquid cathode atmospheric pressure glow discharge, LCGD – liquid cathode glow discharge, HA-SCGD – hollow anode solution cathode glow discharge, HDE-APGD – hanging drop electrode atmospheric pressure glow discharge, FIA-SCGD – flow injection analysis solution cathode glow discharge. | |||
| Zn | FLC-APGDa | 21 | This work |
| LCGD | 50 | 48 | |
| HA-SCGD | 201 | 47 | |
| HDE-APGD | 6 | 24 | |
| Mg | FLC-APGDa | 0.91 | This work |
| LCGD | 40 | 48 | |
| HA-SCGD | 21.6 | 47 | |
| FIA-SCGD | 5.5 | 41 | |
| SCGD | 54.9 | 40 | |
| LCGD | 10 | 55 | |
| Ca | FLC-APGDa | 20 | This work |
| LCGD | 190 | 48 | |
| HA-SCGD | 240 | 47 | |
| FIA-SCGD | 11 | 41 | |
| SCGD | 131 | 40 | |
| LCGD | 10 | 55 | |
| Na | FLC-APGDa | 0.062 | This work |
| LCGD | 40 | 48 | |
| HA-SCGD | 0.22 | 47 | |
| FIA-SCGD | 0.14 | 41 | |
| SCGD | 1.51 | 40 | |
| LCGD | 20 | 55 | |
| K | FLC-APGDa | 0.14 | This work |
| LCGD | 20 | 48 | |
| HA-SCGD | 1.33 | 47 | |
| FIA-SCGD | 0.49 | 41 | |
| SCGD | 4.13 | 40 | |
| LCGD | 200 | 55 | |
Regarding the calibration curves, they were found to be linear (R2 > 0.999) over the whole investigated concentration range. This was especially beneficial given that we attempted to determine all the analytes at one dilution of each sample. This is because the intensities of the Na and K emission lines were significantly higher compared to that of the Zn, Mg, and Ca emission lines, which would require a dynamic linearity range to be able to determine the concentration of these elements simultaneously.
3.3. Optimization of sample dilution
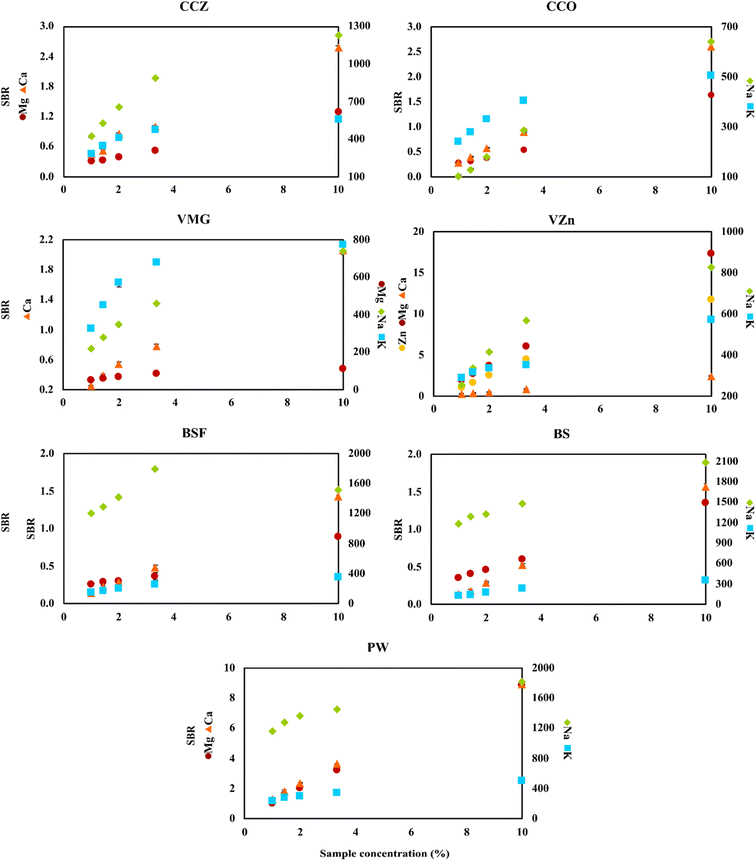
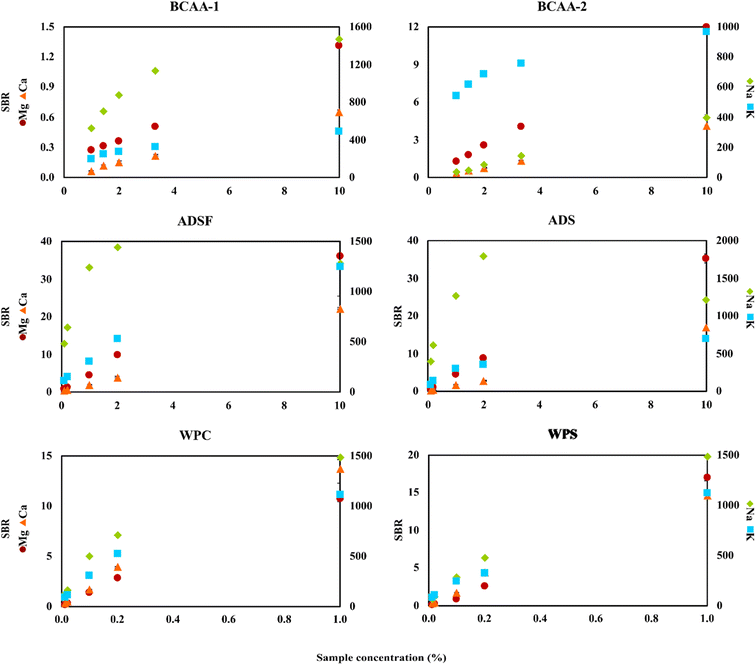
To perform the real sample analysis after dilution and acidification, two aspects were initially considered. One was the FLC-APGD stability during its operation, given that it is known that this discharge can be successfully operated only up to a certain threshold of the FLC solution conductivity.28,29,54 This was particularly the case herein, given that the chosen acid concentration was 0.2 mol L−1, which is higher than the concentration commonly used in the majority of works focusing on FLC-APGD or similar discharge systems. Thus, it was expected that the discharge stability would be impaired for lower dilutions of certain samples, which was actually observed during the previous optimization step. The second aspect was the interference effects coming from both the organic and inorganic matrix, which are usually expected to occur in the case of lower sample dilutions. In this case, the inorganic matrix was not expected to be an issue since, firstly, the studied samples did not contain much of it, and secondly the FLC-APGD system was repeatedly proven to be relatively resistant to it.30,31,50,54,55 However, the samples analyzed in this study contained more of the organic matrix (depending on the sample), which differed between them. Considering all these facts, it was established optimization of the sample dilution is needed to evaluate its impact on both the discharge stability and the linearity of the analyte signal response. Therefore, different dilutions of each studied sample, in the range of 10–100 (CCZ, CCO, VMg, VZn, BSF, BS, PW, BCAA-1, and BCAA-2), 10–1000 (ADSF and ADS) or 100–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (WPC and WPS), were tested. For a better overview of the linearity of the analyte signal response, the results were presented as a function of the percent sample concentration (in the final sample solution), which corresponded to 1–10% for CCZ, CCO, VMg, VZn, BSF, BS, PW, BCAA-1, and BCAA-2, 0.1–10% for ADSF and ADS, and 0.01–1% for WPC and WPS. The measured response was the SBR value and the results are shown in Fig. 3 and 4.
000 (WPC and WPS), were tested. For a better overview of the linearity of the analyte signal response, the results were presented as a function of the percent sample concentration (in the final sample solution), which corresponded to 1–10% for CCZ, CCO, VMg, VZn, BSF, BS, PW, BCAA-1, and BCAA-2, 0.1–10% for ADSF and ADS, and 0.01–1% for WPC and WPS. The measured response was the SBR value and the results are shown in Fig. 3 and 4.
As it can be seen, the intensity of the atomic emission lines of Mg and Ca changed linearly in the whole studied range for all the samples, whereas the response of the atomic emission lines of Na and K varied, depending on the sample. For some of them (e.g., WPS), this response for both analytes was still linear, for others (e.g., CCO, BS, BCAA-2, and ADSF) only one of the elements gave a linear response, whereas for the majority, both analytes presented some deviation from linearity starting from a given dilution. This observation can be easily clarified if considering the intensity of the signals. For the majority of samples, the SBRs of Mg and Ca did not exceed 3.0, whereas the SBRs of Na and K were at least a couple of hundred. Thus, it was to be expected that the signals of Na and K would be over the upper linearity range, causing them to decrease starting from a given dilution. This finding suggests that the cause of the decline in signal intensity for the lower sample dilutions was not the matrix effects but the limited upper linearity ranges. Balancing this finding against the linear response from Mg and Ca, it led to the further assumption of the matrix effects not being the case for all samples at the studied dilution ranges. However, it is worth noting that in some cases the drop in the Na emission line was observed at a given SBR value, whereas for other samples this drop was not observed even though the SBR values were similar or even higher, e.g., BSF and BS. Therefore, it seems like the matrix effects could have some impact on the linearity range shifts. In the case of Zn, it was present only in the VZn sample; however, the behavior of its emission line in this particular sample was observed to be similar to the Mg and Ca lines in the other samples.
In terms of the discharge stability, it was established based on visual observations of its behavior during the measurements of the optimized sample dilutions. As expected, it was found that the lower the dilution, the worse the robustness of the discharge. Particularly, this was the case for the samples that were both measured up to the 10-fold dilution and contained a relatively rich organic matrix, e.g., the samples of BCAAs and almond drink. Alternatively, relatively good discharge stability was noted for WPC and WPS in the whole measured range of the sample dilutions. This was due to lower samples dilutions in this case as well as the filtering process carried out before the measurements of these samples. Accordingly, much better discharge stability was observed for the samples containing less organic matrix, e.g., the cola and black soda samples.
Considering all the above-mentioned findings, it can be concluded that almost any studied dilution factor can be applied in this work, as long as the discharge can be stably operated, given that the matrix effects were not an issue herein. Nevertheless, higher samples dilutions seemed to be favorable for the determination of Na and K because they exceeded the upper linearity range observed for these elements at lower sample dilutions. Hence, considering the relatively low DLs of the elements in this study and to prove the ability of the investigated system to determine these elements present in the samples at both low (Mg and Ca) and high (Zn, Na, and K) concentration ranges, we decided to choose the following dilutions of the analyzed samples for their analysis: 50-fold for CCZ, CCO, BBC, BZC, PW, BCAA-1, and BCAA-2, 100-fold for VMg and VZn, 500-fold for ADSF and ADS, and 5000-fold for WPC and WPS.
3.4. Sample analysis
To show the credibility of the developed method to analyze real samples, the content of Zn, Mg, Ca, Na, and K was determined in all the studied samples and compared with the results obtained for the ICP-OES measurements. Obviously, all the analyzed samples needed to be wet-digested before the ICP-OES analysis. After performing the wet-digestion procedure, the samples were diluted 10- (CCZ and CCO), 100- (VMg, VZn, BSF, BS, PW, BCAA-1, BCAA-2, ADSF, and ADS) or 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold (WPC and WPS) and the resultant sample acidification was ∼1.4 mol L−1. The measurements were performed using the external calibration curves. In the case of the FLC-APGD-OES system, the samples were only diluted to the appropriate factors (being the same in most cases, except the WPC and WPS samples, which were highly diluted, i.e., 5000-fold) and acidified with HNO3 up to 0.2 mol L−1. The samples of ADSF and ADS were also filtered after their preparation using syringe filters. In the case of the WPC and WPS samples, we attempted to determine the analyte content in these samples without the filtration process. According to our past experience,28,56 this system is resistant to the presence of some suspensions or even precipitates, as long as their abundance in the sample is fairly low. Thus, given that the dilution of the WPC and WPS samples was as high as 5000-fold, it was assumed the protein precipitates that formed after the acidification would neither deteriorate the analyte signals nor the discharge stability to a significant extent.
000-fold (WPC and WPS) and the resultant sample acidification was ∼1.4 mol L−1. The measurements were performed using the external calibration curves. In the case of the FLC-APGD-OES system, the samples were only diluted to the appropriate factors (being the same in most cases, except the WPC and WPS samples, which were highly diluted, i.e., 5000-fold) and acidified with HNO3 up to 0.2 mol L−1. The samples of ADSF and ADS were also filtered after their preparation using syringe filters. In the case of the WPC and WPS samples, we attempted to determine the analyte content in these samples without the filtration process. According to our past experience,28,56 this system is resistant to the presence of some suspensions or even precipitates, as long as their abundance in the sample is fairly low. Thus, given that the dilution of the WPC and WPS samples was as high as 5000-fold, it was assumed the protein precipitates that formed after the acidification would neither deteriorate the analyte signals nor the discharge stability to a significant extent.
Initially, the external calibration curve (in the concentration range of 0.1–10 mg L−1) was used for the determination of Zn, Mg, Ca, Na, and K in the prepared solutions by FLC-APGD-OES. The results are presented in Table 2, which clearly show that almost all the analytes could be successfully determined with the aid of external calibration curves with simple standards solutions. The recovery values (calculated as the percent value of measured with FLC-APGD-OES with reference to ICP-OES) in the majority of cases were in the range of 88–111%, further confirming the lack of the matrix effects. Only the results obtained for the determination of Mg in VMg, ADSF, and ADS led to the recoveries being significantly lower, i.e., around 57%, 66%, and 75%, respectively. This was likely due to the presence of certain compounds in these samples that formed complexes and/or sediments with Mg, which could not be fully atomized in the discharge. Some additional reactions taking place between Mg and other samples compounds with the aid of the discharge constituents could also occur. In this case, the concentration of Mg in these samples was determined once more, this time using the standard addition technique, which resulted in the recovery values being the range of 95–105%. Moreover, the determination of BSF and BS was challenging due to high amounts of Na present in these samples. Due to the relatively low concentrations of the other elements (particularly Mg and Ca), BSF and BS could not be further diluted; however, in this case, the intensity of the Na atomic emission lines exceeded the measured range of the linearity of this element. Nevertheless, the recoveries of Na in these samples was around 94%, suggesting that the actual upper linearity range was not exceeded. Therefore, to be certain, the analysis of these samples for the content of Na was repeated in a more diluted sample (namely 500-fold) and the obtained results agreed well with that obtained for a 50-fold diluted sample. It is also worth noting that there were no differences between the results obtained for the sugared and sugar-free samples of the same type. This was interesting considering previous studies reporting the observed matrix effects coming from the presence of sugar in a similar system.36 Nevertheless, the lack of matrix effects observed in this study was most likely due to the relatively low sugar amounts (around a few grams per 100 mL), which apparently was not high enough to cause any serious matrix effects. Regarding the measurement precision (expressed as RSD), it varied in the range of 0.05–8.60% range for ICP-OES and 0.02–8.31% for the FLC-APGD-OES method. The average RSD values were 1.28% and 3.12% for ICP- and FLC-APGD-OES, respectively. This indicated that the measurement precision was slightly worse for the FLC-APGD-OES method; nevertheless, most of the results still fitted below the acceptable level of 5%. Moreover, given that almost all the RSD values obtained for whey protein fell in the range of 0.50–4.57%, it was proven that at this high dilution the samples could be reliably analyzed even without the filtration process.
| Element | Sample | Concentration (mg L−1) | Recovery (%) | RDAa (%) | |
|---|---|---|---|---|---|
| ICP-OES (RSD, %) | FLC-APGD-OES (RSD, %) | ||||
| a Per portion, meaning 300 mL for CCZ, CCO, VMg, VZn, BSF, BS, BCAA-1, BCAA-2, ADSF, and ADS; 80 mL (one shot) for PW, and 30 g (one scoop) for WPC and WPC. b Using the standard addition technique. c For the dilution factor of 500. | |||||
| Zn | VZn | 31.15 ± 0.17 (0.55) | 29.23 ± 1.01 (3.46) | 93.8 | 62.6 |
| Mg | CCZ | 1.10 ± 0.01 (0.91) | 1.14 ± 0.07 (6.14) | 103.6 | 0.1 |
| CCO | 0.98 ± 0.01 (1.02) | 0.97 ± 0.03 (3.09) | 99.0 | 0.1 | |
| VMg | 641.66 ± 1.67 (0.26) | 370.35 ± 3.11 (0.84) | 57.7 | 57.6 | |
| 671.63 ± 3.61b (0.54) | 104.7 | ||||
| VZn | 15.49 ± 0.12 (0.77) | 15.89 ± 1.32 (8.31) | 102.6 | 1.4 | |
| BSF | 0.70 ± 0.01 (1.43) | 0.62 ± 0.04 (6.45) | 88.6 | 0.1 | |
| BS | 0.60 ± 0.01 (1.67) | 0.55 ± 0.04 (7.27) | 91.7 | 0.1 | |
| PW | 6.57 ± 0.01 (0.15) | 6.44 ± 0.07 (1.09) | 98.0 | 0.2 | |
| BCAA-1 | 1.07 ± 0.01 (0.93) | 1.06 ± 0.02 (1.89) | 99.1 | 0.1 | |
| BCAA-2 | 10.87 ± 0.20 (1.84) | 9.63 ± 0.04 (0.42) | 88.6 | 0.8 | |
| ADSF | 64.75 ± 0.21 (0.32) | 42.66 ± 0.01 (0.02) | 65.9 | 5.3 | |
| 61.82 ± 3.44b (5.56) | 95.5 | ||||
| ADS | 64.04 ± 0.51 (0.80) | 48.33 ± 2.22 (4.59) | 75.5 | 5.6 | |
| 65.60 ± 3.42b (5.21) | 102.4 | ||||
| WPC | 181.25 ± 1.76 (0.97) | 170.01 ± 4.26 (2.51) | 93.8 | 9.7 | |
| WPS | 98.78 ± 4.22 (4.27) | 91.28 ± 2.22 (2.43) | 92.4 | 5.2 | |
| Ca | CCZ | 8.06 ± 0.15 (1.86) | 7.68 ± 0.41 (5.34) | 95.3 | 0.3 |
| CCO | 7.38 ± 0.02 (0.27) | 7.29 ± 0.39 (5.35) | 98.8 | 0.2 | |
| VMg | 9.93 ± 0.36 (3.63) | 9.26 ± 0.22 (2.38) | 93.3 | 0.3 | |
| VZn | 8.18 ± 0.22 (2.69) | 9.13 ± 0.13 (1.42) | 111.6 | 0.3 | |
| BSF | 5.73 ± 0.02 (0.35) | 5.37 ± 0.13 (2.42) | 93.7 | 0.2 | |
| BS | 5.67 ± 0.06 (1.06) | 5.57 ± 0.16 (2.87) | 98.2 | 0.2 | |
| PW | 41.59 ± 0.15 (0.36) | 40.06 ± 0.81 (2.02) | 96.3 | 0.4 | |
| BCAA-1 | 3.18 ± 0.09 (2.83) | 3.37 ± 0.19 (5.64) | 106.0 | 0.1 | |
| BCAA-2 | 15.85 ± 0.24 (1.51) | 15.07 ± 0.15 (1.00) | 95.1 | 0.5 | |
| ADSF | 96.27 ± 0.55 (0.57) | 101.52 ± 2.79 (2.75) | 105.5 | 3.4 | |
| ADS | 103.48 ± 0.95 (0.92) | 96.90 ± 5.30 (5.47) | 93.6 | 3.2 | |
| WPC | 695.09 ± 3.46 (0.50) | 771.98 ± 20.47 (2.65) | 111.1 | 17.2 | |
| WPS | 715.65 ± 29.80 (4.16) | 785.92 ± 22.30 (2.84) | 109.8 | 17.5 | |
| Na | CCZ | 69.75 ± 0.44 (0.63) | 63.71 ± 2.20 (3.45) | 91.3 | 3.3 |
| CCO | 6.69 ± 0.03 (0.45) | 6.13 ± 0.28 (4.57) | 91.6 | 0.3 | |
| VMg | 25.52 ± 0.21 (0.82) | 26.38 ± 0.75 (2.84) | 103.4 | 1.4 | |
| VZn | 25.88 ± 0.13 (0.50) | 25.75 ± 0.18 (0.70) | 99.5 | 1.3 | |
| BSF | 711.21 ± 1.89 (0.27) | 672.29 ± 10.80 (1.61) | 94.5 | 34.0 | |
| 652.47 ± 4.57c (0.70) | 91.7 | ||||
| BS | 715.73 ± 2.39 (0.33) | 672.09 ± 9.98 (1.48) | 93.9 | 35.4 | |
| 678.30 ± 19.41c (2.86) | 94.8 | ||||
| PW | 367.64 ± 1.05 (0.29) | 365.26 ± 2.07 (0.57) | 99.4 | 5.1 | |
| BCAA-1 | 99.28 ± 1.00 (1.01) | 90.53 ± 2.34 (2.58) | 91.2 | 4.7 | |
| BCAA-2 | 4.16 ± 0.09 (2.16) | 4.48 ± 0.20 (4.46) | 107.7 | 0.2 | |
| ADSF | 495.94 ± 1.06 (0.21) | 493.95 ± 38.66 (7.83) | 99.6 | 25.8 | |
| ADS | 496.52 ± 2.01 (0.40) | 446.21 ± 20.63 (4.62) | 89.9 | 23.3 | |
| WPC | 431.25 ± 8.31 (1.93) | 388.60 ± 8.65 (2.23) | 90.1 | 13.5 | |
| WPS | 274.35 ± 12.55 (4.57) | 272.43 ± 8.94 (3.28) | 99.3 | 9.5 | |
| K | CCZ | 57.33 ± 0.29 (0.51) | 57.82 ± 1.75 (3.03) | 100.9 | 0.5 |
| CCO | 33.18 ± 0.25 (0.75) | 36.99 ± 0.87 (2.35) | 111.5 | 0.3 | |
| VMg | 231.82 ± 1.60 (0.69) | 233.82 ± 4.43 (1.89) | 100.9 | 2.0 | |
| VZn | 230.37 ± 0.61 (0.26) | 227.72 ± 3.41 (1.50) | 98.9 | 2.0 | |
| BSF | 27.37 ± 0.61 (2.23) | 27.39 ± 0.80 (2.92) | 100.1 | 0.2 | |
| BS | 13.39 ± 0.17 (1.27) | 14.31 ± 0.64 (4.47) | 106.9 | 0.1 | |
| PW | 55.19 ± 0.59 (1.07) | 58.59 ± 1.20 (2.05) | 106.2 | 0.1 | |
| BCAA-1 | 79.47 ± 0.44 (0.55) | 75.86 ± 3.55 (4.68) | 95.5 | 0.7 | |
| BCAA-2 | 221.66 ± 0.10 (0.05) | 213.86 ± 2.63 (1.23) | 96.5 | 1.8 | |
| ADSF | 123.93 ± 1.26 (1.02) | 132.86 ± 4.23 (3.18) | 107.2 | 1.1 | |
| ADS | 124.68 ± 0.59 (0.47) | 134.21 ± 6.40 (4.77) | 107.6 | 1.2 | |
| WPC | 1150.07 ± 3.92 (0.34) | 1048.36 ± 25.46 (2.43) | 91.2 | 6.0 | |
| WPS | 606.82 ± 52.19 (8.60) | 564.51 ± 4.14 (0.73) | 93.0 | 3.2 | |
Therefore, in summary, based on the results obtained at this stage of research, it can be stated that the developed FLC-APGD-OES method gave reliable results and can be effectively applied for the determination of this type of sample. Additionally, in contrast to the application of ICP-OES, the samples did not need to be digested. FLC-APGD-OES also requires a significantly lower acid concentration and has the advantage of being resistant to the presence of suspensions or sediments, which allowed the filtration step to be omitted once the sample was significantly diluted. This, together with the fairly low DLs of the studied elements make this method a very promising alternative for the currently applied methods with the bulky instrumentation such as FAAS, ICP-OES or ICP-MS.
3.5. Evaluation of nutritional quality
To evaluate the nutritional quality of the studied beverages, the concentrations of the determined elements were correlated with the percent of the recommended daily allowance (RDA), which is 14, 350, 900, 575, and 3500 mg day−1, for Zn, Mg, Ca, Na, and K, respectively57 (see Table 2). The percent of RDA was calculated from the concentrations obtained for the FLC-APGD-OES analysis and with reference to one portion. Given that most of the drinks were of portions of around 300 mL, this value was considered to be a portion for the majority of the samples (including almond drink, which was supposed to be used for comparison purposes). The only exceptions were PW given that it was sold as one shot (80 mL) and the whey protein samples, in which the manufacturer recommended one scoop (around 30 g) per a portion. As can be seen from the table, Zn was only present in one sample (VZn); however, one portion of this beverage covered over 60% of its RDA. In the case of Mg, most of the analyzed drinks (except VMg, ADSF, ADS, WPC, and WPS) turned out to be a very poor source of this element given that the RDA was only in the range of 0.05–1.36%. Even worse results were obtained for Ca given that almost all the studied samples (except ADSF, ADS, WPC, and WPS) contained this element at concentrations equal to or below 0.5% of its RDA. Much better outcomes were observed for Na given that many of the analyzed samples (namely BSF, BS, ADSF, ADS, WPC, and WPS) contained Na at concentrations covering over 9% of its RDA and the percentage of the RDA varied for the other samples in the range of 0.23–5.08%. This was most likely due to the presence of Na-based salts, which are commonly used as preservatives. Regarding the K content, it was rather low and varied in the range of 0.12–6.00% of its RDA, depending on the sample.A fairly high coverage of the RDAs for the studied elements was observed for the almond drink and whey protein samples. Nevertheless, it is worth mentioning that the concentration of Mg, Ca, and K in the almond drink was still noticeably lower than that in the animal-based milk, whereas the concentration of Na was similar.58 Nevertheless, given that the concentration differences in Mg, Ca, and Na between the animal-based milk and almond drink were not very high, it can be stated that almond drink is still an acceptably good source of these elements. The highest coverage of all determined elements (except Zn) was found in the whey protein samples, which means that whey protein is not only a very good source of high-quality protein but also significantly supplements the studied elements. Therefore, compared to the BCAA samples, it can be concluded that whey protein is superior not only in terms of its amino acid profile but also in terms of the mineral content. When comparing energy drinks to the pre-workout sample (which are used for similar purposes), it can be noticed that even though none of these samples were a good source of the analyzed minerals, pre-workout contained significantly higher amounts of Mg, Ca, and K, which makes it a superior supplement. Nevertheless, it should be noted that the content of these elements (with particular emphasis on Mg, Ca, and K) is noticeably higher in regular coffee (assuming preparing the coffee beverage from 10 g of dry coffee).59–61 Thus, according to all the above-mentioned observations, it can be stated that most of the studied samples are not a decent source of minerals and should be replaced with regular food that contains not only the necessary elements but in most cases, also high amounts of antioxidants. Alternatively, almond drink seems to be an interesting alternative for vegetarians as well as a part of a low-calorie diet, whereas whey protein is a fair supplement for athletes struggling with meeting their daily protein intake. However, it should be considered that the mineral bioavailability from plant-based beverages, including almond drink, is usually quite poor.62
4. Conclusions
The FLC-APGD system along with an He jet anode was applied to determine the content of certain elements (namely Zn, Mg, Ca, Na, and K) in samples of selected drinks commonly chosen by athletes, using OES detection. To achieve this, optimization of the crucial operating parameters and different samples solution was carried out. It was found that higher acid concentrations and discharge currents as well as lower He gas and sample flow rates favored better analyte detectability (in terms of the signal intensities of the analytes). It was established that the most suitable operating conditions had to be a compromise due to the discharge stability issues. Therefore, the conditions that resulted in the highest signal intensity enhancement (namely the acid concentration and sample flow rate) were set to the most suitable values, whereas the values of the other parameters (the discharge current and the He gas flow rate) were lowered to the value that ensured good discharge stability. Regarding the sample dilutions, it was found that even for the highest studied dilution, the signals of all the elements could be detected. Moreover, the investigation of the sample dilution showed an almost linear response for the analytes signals. The only exceptions were the signals of Na and K, which due to their high sensitivity likely exceeded the upper linearity range. This finding suggested the lack of matrix effects, which could affect the sample analysis. This assumption was further confirmed during the actual analysis, given that for the majority of cases the obtained results agreed well with that obtained previously for the ICP-OES measurements. Only the content of Mg in certain samples (namely VMg, ADSF, and ADS) could not be determined using the external calibration curve. Nevertheless, the standard addition technique solved this issue.Considering all the above-mentioned results, it was concluded that the developed FLC-APGD-OES method can be a reliable alternative to the ICP-OES method, as well as other methods that use commercially available bulky instrumentation such as FAAS or AFS. The proposed method not only assured similar DLs of Zn, Mg, Ca, Na, and K but also allowed the sample analysis to be performed without any sample pre-digestion, which was not the case for ICP-OES. It was also shown that for higher dilutions even the filtering process could be omitted given that the FLC-APGD system was fairly resistant to the presence of some suspensions and sediments in the sample. Notably, this enabled us to simplify the sample preparation procedure, reducing the cost because we avoided the use of concentrated reagents and a heating block or another digestion unit. Moreover, it was found that in almost all cases, the analyte content could be determined using only the external calibration with simple standard solutions. Additionally, the developed FLC-APGD-OES method had the advantages of small size, which is promising for the future miniaturization of the whole system, requiring significantly lower acid concentration and gas flow rate (as compared to ICP-OES), and thus noticeably reducing the time and cost of the performed analysis. Therefore, the added value of the present work is the simplicity of the measurement system and extremely low costs associated with its construction, operation and sample preparation.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.References
- R. Crowley and L. Harvey FitzGerald, The impact of cGMP compliance on consumer confidence in dietary supplement products, Toxicology, 2006, 221, 9–16 CrossRef CAS PubMed.
- P. S. Harty, H. A. Zabriskie, J. L. Erickson, P. E. Molling, C. M. Kerksick and A. R. Jagim, Multi-ingredient pre-workout supplements, safety implications, and performance outcomes: a brief review, J. Int. Soc. Sports Nutr., 2018, 15, 41–69 CrossRef PubMed.
- R. R. Wolfe, Branched-chain amino acids and muscle protein synthesis in humans: myth or reality?, J. Int. Soc. Sports Nutr., 2017, 14, 30–37 CrossRef PubMed.
- E. Volpi, H. Kobayashi, M. Sheffield-Moore, B. Mittendorfer and R. R. Wolfe, Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults, Am. J. Clin. Nutr., 2003, 78, 250–258 CrossRef CAS PubMed.
- S. R. Jackman, O. C. Witard, A. Philp, G. A. Wallis, K. Baar and K. D. Tipton, Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans, Front. Physiol., 2017, 8, 390–402 CrossRef PubMed.
- T. A. Churchward-Venne, N. A. Burd, C. J. Mitchell, D. W. D. West, A. Philp, G. R. Marcotte, S. K. Baker, K. Baar and S. M. Phillips, Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men, J. Physiol., 2012, 590, 2751–2765 CrossRef CAS PubMed.
- D. L. Plotkin, K. Delcastillo, D. W. van Every, K. D. Tipton, A. A. Aragon and B. J. Schoenfeld, Isolated Leucine and Branched-Chain Amino Acid Supplementation for Enhancing Muscular Strength and Hypertrophy: A Narrative Review, Int. J. Sport Nutr. Exercise Metab., 2021, 31, 292–301 CAS.
- A. William Kedia, J. E. Hofheins, S. M. Habowski, A. A. Ferrando, M. David Gothard and H. L. Lopez, Effects of a pre-workout supplement on lean mass, muscular performance, subjective workout experience and biomarkers of safety, Int. J. Med. Sci., 2014, 11, 116–126 CrossRef PubMed.
- J. R. Poortmans and M. Francaux, Adverse effects of creatine supplementation: fact or fiction?, Sports Med., 2000, 30, 155–170 CrossRef CAS PubMed.
- A. R. Jagim, P. S. Harty and C. L. Camic, Common Ingredient Profiles of Multi-Ingredient Pre-Workout Supplements, Nutrients, 2019, 11, 254–262 CrossRef CAS PubMed.
- I. Garthe, Dietary supplements and elite athletes: when nature becomes high risk, Curr. Opin. Endocr. Metab. Res., 2019, 9, 66–73 CrossRef.
- H. Geyer, M. Kristina Parr, K. Koehler, U. Mareck, W. Schänzer and M. Thevis, Nutritional supplements cross-contaminated and faked with doping substances, J. Mass Spectrom., 2008, 43, 892–902 CrossRef CAS PubMed.
- G. Moses, The safety of commonly used vitamins and minerals, Aust. Prescr., 2021, 44, 119–123 CrossRef PubMed.
- S. M. Elgammal, M. A. Khorshed and E. H. Ismail, Determination of heavy metal content in whey protein samples from markets in Giza, Egypt, using inductively coupled plasma optical emission spectrometry and graphite furnace atomic absorption spectrometry: A probabilistic risk assessment study, J. Food Compos. Anal., 2019, 84, 103300 CrossRef CAS.
- S. Moret, A. Prevarin and F. Tubaro, Levels of creatine, organic contaminants and heavy metals in creatine dietary supplements, Food Chem., 2011, 126, 1232–1238 CrossRef CAS.
- B. Ruiz Brandao Da Costa, R. Rocha Roiffe and M. Nogueira Silva de La Cruz, Quality Control of Protein Supplements: A Review, Int. J. Sport Nutr. Exercise Metab., 2021, 31, 369–379 Search PubMed.
- V. V. Yagov, M. L. Getsina and B. K. Zuev, Use of Electrolyte Jet Cathode Glow Discharges as Sources of Emission Spectra for Atomic Emission Detectors in Flow-Injection Analysis, J. Anal. Chem., 2004, 59, 1037–1041 CrossRef CAS.
- A. Kitano, A. Iiduka, T. Yamamoto, Y. Ukita, E. Tamiya and Y. Takamura, Highly sensitive elemental analysis for Cd and Pb by liquid electrode plasma atomic emission spectrometry with quartz glass chip and sample flow, Anal. Chem., 2011, 83, 9424–9430 CrossRef CAS PubMed.
- B. K. Zuev, V. V. Yagov, M. L. Getsina and B. A. Rudenko, Discharge on Boiling in a Channel as a New Atomization and Excitation Source for the Flow Determination of Metals by Atomic Emission Spectrometry, J. Anal. Chem., 2002, 57, 907–911 CrossRef CAS.
- T. Cserfalvi, P. Mezei and P. Apai, Emission studies on a glow discharge in atmospheric pressure air using water as a cathode, J. Phys. D: Appl. Phys., 1993, 26, 2184–2188 CrossRef CAS.
- P. Jamroz and W. Zyrnicki, Spectroscopic Characterization of Miniaturized Atmospheric-Pressure dc Glow Discharge Generated in Contact with Flowing Small Size Liquid Cathode, Plasma Chem. Plasma Process., 2011, 31, 681–696 CrossRef CAS.
- M. R. Webb, F. J. Andrade, G. Gamez, R. McCrindle and G. M. Hieftje, Spectroscopic and electrical studies of a solution-cathode glow discharge, J. Anal. At. Spectrom., 2005, 20, 1218–1225 RSC.
- K. Swiderski, P. Pohl and P. Jamroz, A miniaturized atmospheric pressure glow microdischarge system generated in contact with a hanging drop electrode – a new approach to spectrochemical analysis of liquid microsamples, J. Anal. At. Spectrom., 2019, 34, 1287–1293 RSC.
- K. Greda, K. Swiderski, P. Jamroz and P. Pohl, Flowing Liquid Anode Atmospheric Pressure Glow Discharge as an Excitation Source for Optical Emission Spectrometry with the Improved Detectability of Ag, Cd, Hg, Pb, Tl, and Zn, Anal. Chem., 2016, 88, 8812–8820 CrossRef CAS PubMed.
- X. Liu, Z. Zhu, D. He, H. Zheng, Y. Gan, N. Stanley Belshaw, S. Hu and Y. Wang, Highly sensitive elemental analysis of Cd and Zn by solution anode glow discharge atomic emission spectrometry, J. Anal. At. Spectrom., 2016, 31, 1089–1096 RSC.
- P. Jamroz, K. Greda, A. Dzimitrowicz, K. Swiderski and P. Pohl, Sensitive Determination of Cd in Small-Volume Samples by Miniaturized Liquid Drop Anode Atmospheric Pressure Glow Discharge Optical Emission Spectrometry, Anal. Chem., 2017, 89, 5729–5733 CrossRef CAS PubMed.
- P. Pohl, P. Jamroz, K. Greda, M. Gorska, A. Dzimitrowicz, M. Welna and A. Szymczycha-Madeja, Five years of innovations in development of glow discharges generated in contact with liquids for spectrochemical elemental analysis by optical emission spectrometry, Anal. Chim. Acta, 2021, 1169, 338399 CrossRef CAS PubMed.
- M. Gorska and P. Pohl, Simplified and rapid determination of Ca, K, Mg, and Na in fruit juices by flowing liquid cathode atmospheric glow discharge optical emission spectrometry, J. Anal. At. Spectrom., 2021, 36, 1455–1465 RSC.
- K. Greda and P. Pohl, Direct analysis of wines from the province of Lower Silesia (Poland) by microplasma source optical emission spectrometry, Food Chem., 2022, 371, 131178 CrossRef CAS PubMed.
- Q. Lu, F. Feng, J. Yu, L. Yin, Y. Kang, H. Luo, D. Sun and W. Yang, Determination of trace cadmium in zinc concentrate by liquid cathode glow discharge with a modified sampling system and addition of chemical modifiers for improved sensitivity, Microchem. J., 2020, 152, 104308 CrossRef CAS.
- J. Yu, Y. Kang, Q. Lu, H. Luo, Z. Lu, L. Cui and J. Li, Improvement of analytical performance of liquid cathode glow discharge for the determination of bismuth using formic acid as a matrix modifier, Microchem. J., 2020, 159, 105507 CrossRef CAS.
- R. Manjusha, M. A. Reddy, R. Shekhar and S. Jaikumar, Determination of major to trace level elements in Zircaloys by electrolyte cathode discharge atomic emission spectrometry using formic acid, J. Anal. At. Spectrom., 2013, 28, 1932–1939 RSC.
- K. Greda, P. Jamroz and P. Pohl, The improvement of the analytical performance of direct current atmospheric pressure glow discharge generated in contact with the small-sized liquid cathode after the addition of non-ionic surfactants to electrolyte solutions, Talanta, 2013, 108, 74–82 CrossRef CAS PubMed.
- L. Bencs, N. Laczai, P. Mezei and T. Cserfalvi, Detection of some industrially relevant elements in water by electrolyte cathode atmospheric glow discharge optical emission spectrometry, Spectrochim. Acta, Part B, 2015, 107, 139–145 CrossRef CAS.
- M. Gorska and P. Pohl, Application of atmospheric pressure glow discharge generated in contact with liquids for determination of chloride and bromide in water and juice samples by optical emission spectrometry, Talanta, 2022, 237, 122921 CrossRef CAS PubMed.
- J. Yu, X. Zhang, Q. Lu, X. Wang, D. Sun, Y. Wang and W. Yang, Determination of calcium and zinc in gluconates oral solution and blood samples by liquid cathode glow discharge-atomic emission spectrometry, Talanta, 2017, 175, 150–157 CrossRef CAS PubMed.
- K. Greda, P. Jamroz, A. Dzimitrowicz and P. Pohl, Direct elemental analysis of honeys by atmospheric pressure glow discharge generated in contact with a flowing liquid cathode, J. Anal. At. Spectrom., 2015, 30, 154–161 RSC.
- Z. Zhang, Z. Wang, Q. Li, H. Zou and Y. Shi, Determination of trace heavy metals in environmental and biological samples by solution cathode glow discharge-atomic emission spectrometry and addition of ionic surfactants for improved sensitivity, Talanta, 2014, 119, 613–619 CrossRef CAS PubMed.
- P. Zheng, Y. Gong, J. Wang and X. Zeng, Elemental Analysis of Mineral Water by Solution-Cathode Glow Discharge–Atomic Emission Spectrometry, Anal. Lett., 2017, 50, 1512–1520 CrossRef CAS.
- C. Yang, L. Wang, Z. Zhu, L. Jin, H. Zheng, N. Stanley Belshaw and S. Hu, Evaluation of flow injection-solution cathode glow discharge-atomic emission spectrometry for the determination of major elements in brines, Talanta, 2016, 155, 314–320 CrossRef CAS PubMed.
- R. Shekhar, K. Madhavi, N. N. Meeravali and S. Jai Kumar, Determination of thallium at trace levels by electrolyte cathode discharge atomic emission spectrometry with improved sensitivity, Anal. Methods, 2014, 6, 732–740 RSC.
- J. Mo, L. Zhou, X. Li, Q. Li, L. Wang and Z. Wang, On-line separation and pre-concentration on a mesoporous silica-grafted graphene oxide adsorbent coupled with solution cathode glow discharge-atomic emission spectrometry for the determination of lead, Microchem. J., 2017, 130, 353–359 CrossRef CAS.
- C. Huang, Q. Li, J. Mo and Z. Wang, Ultratrace Determination of Tin, Germanium, and Selenium by Hydride Generation Coupled with a Novel Solution-Cathode Glow Discharge-Atomic Emission Spectrometry Method, Anal. Chem., 2016, 88, 11559–11567 CrossRef CAS PubMed.
- P. Jamroz, P. Pohl and W. Zyrnicki, An analytical performance of atmospheric pressure glow discharge generated in contact with flowing small size liquid cathode, J. Anal. At. Spectrom., 2012, 27, 1032–1037 RSC.
- M. Gorska, K. Greda and P. Pohl, Determination of bismuth by optical emission spectrometry with liquid anode/cathode atmospheric pressure glow discharge, J. Anal. At. Spectrom., 2021, 36, 165–177 RSC.
- K. E. Scholz-Ahrens, F. Ahrens and C. A. Barth, Nutritional and health attributes of milk and milk imitations, Eur. J. Nutr., 2020, 59, 19–34 CrossRef PubMed.
- P. Zheng, W. Li, J. Wang, N. Wang, C. Zhong, Y. Luo, X. Wang, X. Mao and C. Lai, Analytical Performance of Hollow Anode-Solution Cathode Glow Discharge-Atomic Emission Spectrometry, Anal. Lett., 2020, 53, 693–704 CrossRef CAS.
- J. Yu, S. Yang, D. Sun, Q. Lu, J. Zheng, X. Zhang and X. Wang, Simultaneously determination of multi metal elements in water samples by liquid cathode glow discharge-atomic emission spectrometry, Microchem. J., 2016, 128, 325–330 CrossRef CAS.
- K. Greda, P. Jamroz and P. Pohl, Comparison of the performance of direct current atmospheric pressure glow microdischarges operated between a small sized flowing liquid cathode and miniature argon or helium flow microjets, J. Anal. At. Spectrom., 2013, 28, 1233–1241 RSC.
- J. Yu, L. Yin, Q. Lu, F. Feng, Y. Kang and H. Luo, Highly sensitive determination of mercury by improved liquid cathode glow discharge with the addition of chemical modifiers, Anal. Chim. Acta, 2020, 1131, 25–34 CrossRef CAS PubMed.
- J. Yu, S. Zhu, Q. Lu, Z. Zhang, D. Sun, X. Zhang, X. Wang and W. Yang, Liquid Cathode Glow Discharge as a Microplasma Excitation Source for Atomic Emission Spectrometry for the Determination of Trace Heavy Metals in Ore Samples, Anal. Lett., 2018, 51, 2128–2140 CrossRef CAS.
- P. Zheng, X. Zhai, J. Wang and P. Tang, Analytical Characterization of a Solution Cathode Glow Discharge with an Interference Filter Wheel for Spectral Discrimination, Anal. Lett., 2018, 51, 2304–2315 CrossRef CAS.
- P. Zheng, Y. Chen, J. Wang and S. Xue, A pulsed atmospheric-pressure discharge generated in contact with flowing electrolyte solutions for metal element analysis by optical emission spectrometry, J. Anal. At. Spectrom., 2016, 31, 2037–2044 RSC.
- M. Gorska and P. Pohl, Comparison of the performance of atmospheric pressure glow discharges operated between a flowing liquid cathode and either a pin-type anode or a helium jet anode for the Ga and In determination by the optical emission spectrometry, Talanta, 2021, 226, 122155 CrossRef CAS PubMed.
- J. Yu, X. Zhang, Q. Lu, L. Yin, F. Feng, H. Luo and Y. Kang, Liquid Cathode Glow Discharge as an Excitation Source for the Analysis of Complex Water Samples with Atomic Emission Spectrometry, ACS Omega, 2020, 5, 19541–19547 CrossRef CAS PubMed.
- M. Gorska and P. Pohl, The application of tetramethylammonium hydroxide for generating atmospheric pressure glow discharge in contact with alkalized flowing liquid cathode solutions – evaluation of the analytical performance, J. Anal. At. Spectrom., 2021, 36, 1768–1781 RSC.
- M. Grembecka, E. Malinowska and P. Szefer, Differentiation of market coffee and its infusions in view of their mineral composition, Sci. Total Environ., 2007, 383, 59–69 CrossRef CAS PubMed.
- T. J. M. Jeurnink and K. G. de Kruif, Calcium concentration in milk in relation to heat stability and fouling, Neth. Milk Dairy J., 1995, 1995, 151–165 Search PubMed.
- I. Gogoasa, A. Pirvu, L. M. Alda, A. Velciov, M. Rada, D. M. Bordean, D. Moigradean, A. Simion and I. Gergen, The Mineral Content of Different Coffee Brands, Journal of Horticulture, Forestry and Biotechnology, 2013, 2013, 68–71 Search PubMed.
- P. Pohl, E. Stelmach, M. Welna and A. Szymczycha-Madeja, Determination of the Elemental Composition of Coffee Using Instrumental Methods, Food Anal. Methods, 2013, 6, 598–613 CrossRef.
- P. Pohl, M. Welna, A. Szymczycha-Madeja, K. Greda, P. Jamroz and A. Dzimitrowicz, Response surface methodology assisted development of a simplified sample preparation procedure for the multielement (Ba, Ca, Cu, Fe, K, Mg, Mn, Na, Sr and Zn) analysis of different coffee brews by means of inductively coupled plasma optical emission spectrometry, Talanta, 2022, 241, 123215 CrossRef CAS PubMed.
- E. Feyza Aydar, S. Tutuncu and B. Ozcelik, Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects, J. Funct. Foods, 2020, 70, 103975 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ay00092c |
| This journal is © The Royal Society of Chemistry 2023 |