One-step preparation of fluorescent inorganic–organic hybrid material used for explosive sensing†
Xin-Ming
Hu
ab,
Qi
Chen
a,
Ding
Zhou
a,
Jie
Cao
*c,
Yu-Jian
He
*b and
Bao-Hang
Han
*a
aNational Center for Nanoscience and Technology, Beijing, 100190, China. E-mail: hanbh@nanoctr.cn; Fax: +86-10-82545576
bCollege of Chemistry and Chemical Engineering, Graduate University of Chinese Academy of Sciences, Beijing, 100049, China. E-mail: heyujian@gucas.ac.cn
cDepartment of Chemistry, Beijing Institute of Technology, Beijing, 100081, China. E-mail: jcao@bit.edu.cn
First published on 21st February 2011
Abstract
As a fluorescent inorganic–organic hybrid polymer, cross-linked poly(tetraphenylethylene-co-cyclotriphosphazene) (TPE-CP) is prepared through one-step polycondensation and well characterized. Owing to the aggregation-induced emission feature of the tetraphenylethylene residue, TPE-CP exhibits a remarkable fluorescent emission property in the suspension and solid-state. Both 2,4,6-trinitrotoluene (TNT) and 2,4,6-trinitrophenol (picric acid, PA) can cause a significant fluorescence quenching of TPE-CP due to the electron transfer from TPE-CP to TNT or PA. Additional energy transfer makes PA more sensitive to TPE-CP than TNT in the fluorescence quenching detection.
Introduction
The recent rise in concerns over global terror threats and anti-terrorism activities has made an urgent demand for reliable detection of trace explosive materials and their precursors in security screening processes. Various analytical techniques have been applied into explosive detection, such as gas chromatography,1mass spectrometry,2Raman spectroscopy,3ion mobility spectrometry4 and so on. However, the expensive and complicated manipulation is the bottle-neck for popularization of these methods. As an alternative detecting approach, fluorescence-based sensing has drawn much attention from academic to industry, because it is highly sensitive, convenient, and cost-effective. More and more fluorescent materials have been prepared for development of sensitive explosive-detecting methods,5–9 which take advantage of both human visual processing ability and instrumental evaluation. Nevertheless, as for most of fluorescent materials, the synthetic work is laborious, time-consuming, and inefficient, which leads to difficulties in up-scale and high-throughput screening format and practical application. Additionally, aggregation-caused fluorescence quenching of traditional dyes often takes place when dispersed in solvent or interacted with other molecules,10 resulting in drastically negative effects on efficiencies and sensitivities of the sensors or probes. To overcome these problems, herein, a facile one-step preparation of fluorescent inorganic–organic hybrid polymer based on aggregation-induced emission (AIE) feature is developed and used for explosive sensing.Recently, the molecules with AIE characteristics provide a unique platform for exploiting novel optical materials11–17 and sensors,18–24 due to their enhanced emission in the aggregate form or the solid-state. The AIE effect can significantly improve the fluorescence quantum yield of the molecules by up to three orders of magnitude, enhancing the photoluminescence intensity from faint luminophores into strong emitters.9 Especially, since Tang's group reported the facile preparation of tetraphenylethylene (TPE)-based luminophores, TPE-based AIE active materials have already shown potential applications in OLEDs,11 chemosensors,9,25 and bioprobes.26–31
A hybrid of inorganic and organic molecules gives rise to versatile materials with interesting properties. Over the past decade, the utilization of cyclophosphazene (CP) as a polymer modifier has attracted considerable attention, in which CP is not only an excellent building block for synthesis of cyclomatrix polymers, cyclolinear polymers, dendrimers, and star-shaped polymers,32–35 but also plays an important role in material modification to improve flame retardancy, to enhance mechanical properties, or to favor the formation of compatible blends.36–38 Therefore, we expect that incorporation of CP with TPE can offer a novel fluorescent polymer material with an exceptional feature and promising applications.
Herein we report a fluorescent cross-linked inorganic–organic hybrid polymer, poly(tetraphenylethylene-co-cyclotriphosphazene) (TPE-CP), which was prepared through one-step polycondensation. TPE-CP exhibits remarkable fluorescent emission properties in the suspension or solid-state, due to the AIE feature of the TPE residue. Both 2,4,6-trinitrotoluene (TNT) and 2,4,6-trinitrophenol (picric acid, PA) can cause a significant fluorescence quenching of TPE-CP, owing to the electron transfer from TPE-CP to TNT or PA. In addition, energy transfer makes PA more sensitive to TPE-CP than TNT in the fluorescence quenching detection. Therefore, TPE-CP shows a promising application in detection of explosives.
Experimental section
Materials and measurement
All chemical reagents were commercially available and used as received unless otherwise stated. Deionized water was obtained with a Millipore purification system (Milli-Q water). Tetrahydrofuran (THF) was purified by distillation from sodium under nitrogen immediately prior to use.The 1H and 13C NMR spectra were recorded on a Bruker DMX400NMR spectrometer. Solid-state magic angle spinning (MAS) 13C and 31P NMR were recorded on an AVANCE III 400 NMR spectrometer. Ultraviolet-visible (UV-Vis) spectra were measured using a Perkin-Elmer Lamda 950 UV-Vis spectrometer and quartzcells with 1 cm path length. The fluorescence spectra were measured in a conventional cell with 1 cm path length using a Perkin-Elmer LS 55 luminescence spectrometer. The infrared (IR) spectra were recorded using a Perkin-Elmer Spectrum One Fourier transform infrared spectrometer. Thermogravimetric analyses were performed on a Pyris Diamond thermogravimetric/differential thermal analyzer (10 °C min−1, nitrogen).
Synthesis of dimethoxytetraphenylethylene (DMTPE)
To a mixture of benzophenone (2.01 g, 11.0 mmol) and 4,4′-dimethoxybenzophenone (2.66 g, 11.0 mmol) in dry THF (120 mL) were added zinc dust (7.38 g) and TiCl3/AlCl3 (5.48 g). After refluxing under nitrogen atmosphere for 20 h, the mixture was cooled to room temperature and filtered. The solvent was evaporated under vacuum and the residue was purified by a silica gel column using dichloromethane–petroleum ether (v/v from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 1
4 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford a white solid (2.24 g, 52%). 1H NMR (400 MHz, CDCl3): δ 7.14–7.09 (m, 6H), 7.06–7.04 (m, 4H), 6.97 (d, J = 8.8 Hz, 4H), 6.66 (d, J = 8.8 Hz, 4H), 3.75 (s, 6H). 13C NMR (100 MHz, CDCl3): δ 158.2, 144.4, 140.2, 139.4, 136.5, 132.7, 131.5, 127.8, 126.2, 113.1, 55.2.
2) to afford a white solid (2.24 g, 52%). 1H NMR (400 MHz, CDCl3): δ 7.14–7.09 (m, 6H), 7.06–7.04 (m, 4H), 6.97 (d, J = 8.8 Hz, 4H), 6.66 (d, J = 8.8 Hz, 4H), 3.75 (s, 6H). 13C NMR (100 MHz, CDCl3): δ 158.2, 144.4, 140.2, 139.4, 136.5, 132.7, 131.5, 127.8, 126.2, 113.1, 55.2.
Synthesis of dihydroxytetraphenylethylene (DHTPE)
In a 100 mL flask, DMTPE (2.24 g, 5.7 mmol) was dissolved in 30 mL of anhydrous dichloromethane, and then the solution was cooled to −45 °C under nitrogen atmosphere. BBr3 (2.3 mL, 24.4 mmol) was added with vigorous stirring. After the addition, the reaction was stirred at room temperature for another 6 h and then the mixture was poured into cold water giving a white precipitate. After filtration, DHTPE was obtained as a white solid in 96% yield (1.99 g) without further purification. 1H NMR (400 MHz, DMSO-d6): δ 7.12–7.03 (m, 6H), 6.93 (d, J = 6.8 Hz, 4H), 6.74 (d, J = 8.4 Hz, 4H), 6.49 (d, J = 8.8 Hz, 4H). 13C NMR (100 MHz, DMSO-d6): δ 155.9, 144.2, 140.7, 137.8, 134.2, 132.1, 130.8, 127.8, 126.0, 114.6.Preparation of TPE-CP
To a solution of DHTPE (500 mg, 1.37 mmol) in anhydrous THF (20 mL) was added sodium hydride (60%, 165 mg, 4.13 mmol) at room temperature. After stirring for 30 min, a solution of hexachlorocyclotriphosphazene (HCCP, 160 mg, 0.457 mmol) in THF (10 mL) was added dropwise to the reaction mixture. The resulting suspension was refluxed under nitrogen protection for 36 h and then poured into ice water. The precipitated product was filtered, washed three times with ultrapure water and methanol, and then dried under vacuum to yield TPE-CP as a yellowish powder (460 mg).Results and discussion
One-step preparation of the fluorescent inorganic–organic hybrid polymer, TPE-CP, is shown in Scheme 1. TPE-CP was efficiently synthesized by polycondensation between DHTPE and HCCP under basic conditions, in which DHTPE can be obtained through an improved method.26 Based on this reproducible procedure, TPE-CP can be efficiently obtained on a large scale.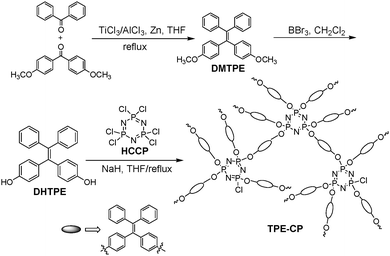 | ||
| Scheme 1 Synthetic route to fluorescent TPE-CP. | ||
TPE-CP is characterized using Fourier transform infrared spectroscopy (FT-IR), solid-state magic angle spinning (MAS) 13C and 31P NMR. As shown in the FT-IR spectra in Fig. 1, the absorption at 1601 and 1502 cm−1 corresponds to the phenyl absorption of TPE units. A new absorption peak at 950 cm−1 is assigned to the P–O–(Ph) bond. A broad strong absorption peak of the phenolic hydroxylgroup of DHTPE ranging from 2990 to 3510 cm−1 disappears after the polycondensation. The MAS 13C NMR spectrum (Fig. S1 in the ESI†) shows signal peaks ranging from 123.8 to 151.8 ppm, which indicate the presence of the TPE structural unit. Formation of P–O–(Ph) bond was also confirmed by the MAS 31P NMR spectrum (Fig. S2 in the ESI†), in which the peak of P–O (δ = 8.56 ppm) in TPE-CP appears clearly. In addition, the thermal stability of TPE-CP was examined by thermogravimetric analysis (TGA), which shows the onset of the thermal degradation temperature (Td) at around 341 °C (Fig. 2). Compared with the two monomers, TPE-CP possesses a better thermal stability, probably due to the cross-linked structures.
 | ||
| Fig. 1 FT-IR spectra of DHTPE, HCCP, and TPE-CP. | ||
 | ||
| Fig. 2 Thermogravimetric analysis of TPE-CP. | ||
The inorganic–organic hybrid polymer, TPE-CP, is insoluble in water and common organic solvents such as THF, acetonitrile, chloroform, and methanol. TPE-CP exhibits a remarkable fluorescent emission feature in both suspension and solid-state, due to the AIE feature of TPE residue (Fig. 3). The suspension of TPE-CP in water–THF (50 μg mL−1, water/THF = 9/1) is prepared by ultrasonication and the formed dispersion displays a strong fluorescent emission with an emission peak at 462 nm. The resultant suspension is stable enough, so that it can be applied for later experiments on detection of explosives. Similar fluorescent property was also found in other solvents (Fig. S3 in the ESI†). The fluorescence spectrum of TPE-CP in solid-state shows an emission peak at 485 nm. The obvious bathochromic/red shift in emission spectra for TPE-CP could be attributed to the enhanced planar conformation or aggregation of TPE units in solid-state. Moreover, fluorescence quantum yield of TPE-CP suspension in water–THF (0.35 mg mL−1) was estimated to be 9.9% using quinine sulfate in 0.1 N H2SO4 as standard. The quantum yield of TPE-CP in solid-state is supposed to be higher than its suspension state owing to the AIE feature.24
 | ||
| Fig. 3 Fluorescence spectra of TPE-CP in suspension (dashed line) and solid-state (solid line); the insets display the photos of the corresponding TPE-CP under UV light (365 nm) illumination. | ||
Due to the efficient photoluminescence properties of TPE-CP in the aggregate state, fluorescence detection of explosive chemicals was performed through a series of spectrofluorometric titrations using the TPE-CP suspension in water–THF as the probe. Nitroaromatics such as TNT and PA were used as analytes. Fluorescence quenching detection takes advantage of the relatively low LUMO energies of these explosive materials, which can accept an excited state electron from the fluorophore.5 As shown in Fig. 4, a significant fluorescence quenching for TPE-CP was observed after adding TNT ([TNT] = 0–290 μg mL−1) in water–THF for 5 min. The emission quenching is observed at a concentration as low as 1 μg mL−1. When treated with PA ([PA] = 0–40 μg mL−1), a more significant fluorescence quenching for TPE-CP was detected. The emission quenching is observed at a concentration as low as 0.1 μg mL−1. When the concentration of PA is up to 40 μg mL−1, almost complete fluorescence quenching of TPE-CP can be detected.
 | ||
| Fig. 4 Fluorescence spectra of TPE-CP (suspension in water–THF, 50 μg mL−1) in the presence of different amounts of (a) TNT (0–290 μg mL−1) and (b) PA (0–40 μg mL−1); the insets display the photos of the corresponding suspension of TPE-CP in the absence (A) and presence (B) of TNT (290 μg mL−1) under UV light (365 nm) illumination. | ||
The AIE feature of TPE-CP inspires us to detect explosive materials in solid-state. As shown in Fig. 5, TPE-CP (25 μg) exhibits a strong fluorescent emission when treated with TNT (100 ppm) by solvent-assisted exposure, the luminescence of TPE-CP becomes faint after solvent volatilization. However, upon exposure to PA (50 ppm), the fluorescent emission of TPE-CP is completely quenched.
 | ||
| Fig. 5 The photos of TPE-CP solid (25 μg) in the absence (A), in the presence of TNT (100 ppm) (B) and PA (50 ppm) (C) under UV light (365 nm) illumination. | ||
It is well-known that nitroaromatics are categorized as electron-deficient chemicals. The main mechanism of fluorescence quenching is electron transfer from the excited electron-rich polymer (TPE-CP) to the electron-deficient analytes (nitroaromatics). In addition, further insight into the absorption spectrum of PA indicates a spectral overlap between the emission of TPE-CP aggregates and PA absorption in wavelength ranging from 350 to 480 nm (Fig. 6), which prompts the energy transfer from the excited state of the polymer to the ground state of PA, resulting in the efficient fluorescence quenching. As for TNT, however, no similar spectral overlap is observed. Due to the intermolecular absorption properties, PA is more sensitive to TPE-CP than TNT in the fluorescence quenching detection.
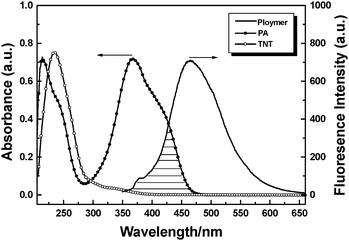 | ||
| Fig. 6 Absorption spectra of PA and TNT in THF solution and fluorescence spectrum of TPE-CP suspension in water–THF. | ||
Conclusion
In conclusion, cross-linked poly(tetraphenylethylene-co-cyclotriphosphazene), TPE-CP, is prepared efficiently through a one-step polycondensation and well characterized. As an inorganic–organic hybrid polymer, TPE-CP exhibits remarkable fluorescent emission properties in the suspension or solid-state, due to the AIE feature of TPE residue. Meanwhile, the cross-linked structure provides TPE-CP with a good thermal stability. Considering the nitroaromatics (TNT and PA) possessing relatively low LUMO energies can accept an excited state electron from the fluorophore, fluorescence detection of explosive chemicals (TNT and PA) was performed through a series of spectrofluorometric titration using the suspension of TPE-CP as the probe. Both TNT and PA can cause a significant fluorescence quenching of TPE-CP. The spectral overlap between the emission of TPE-CP aggregate and PA absorption makes PA more sensitive to TPE-CP than TNT in the fluorescence quenching detection. Facile preparation and rapid response to explosives make TPE-CP a promising fluorescent sensing material.Acknowledgements
This work was supported by the Ministry of Science and Technology of China (National Basic Research Program, Grant 2007CB808000) and the National Science Foundation of China (Grants 20972035 and 21002017).Notes and references
- R. Hodyss and J. L. Beauchamp, Anal. Chem., 2005, 77, 3607–3610 CrossRef CAS.
- I. A. Popov, H. Chen, O. N. Kharybin, E. N. Nikolaev and R. G. Cooks, Chem. Commun., 2005, 1953–1955 RSC.
- J. M. Sylvia, J. A. Janni, J. D. Klein and K. M. Spencer, Anal. Chem., 2000, 72, 5834–5840 CrossRef CAS.
- R. G. Ewing, D. A. Atkinson, G. A. Eiceman and G. J. Ewing, Talanta, 2001, 54, 515–529 CrossRef CAS.
- J. C. Sanchez, A. G. DiPasquale, A. L. Rheingold and W. C. Trogler, Chem. Mater., 2007, 19, 6459–6470 CrossRef CAS.
- J.-S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 11864–11873 CrossRef CAS.
- Y. Liu, R. C. Mills, J. M. Boncella and K. S. Schanze, Langmuir, 2001, 17, 7452–7455 CrossRef CAS.
- J. C. Sanchez, S. A. Urbas, S. J. Toal, A. G. DiPasquale, A. L. Rheingold and W. C. Trogler, Macromolecules, 2008, 41, 1237–1245 CrossRef CAS.
- A. Qin, J. W. Y. Lam, L. Tang, C. K. W. Jim, H. Zhao, J. Sun and B. Z. Tang, Macromolecules, 2009, 42, 1421–1424 CrossRef CAS.
- J. B. Birks, Photophysics of Aromatic Molecules, Wiley, London, 1970 Search PubMed.
- Y. Dong, J. W. Y. Lam, A. Qin, J. Liu, Z. Li, B. Z. Tang, J. Sun and H. S. Kwok, Appl. Phys. Lett., 2007, 91, 11111 CrossRef.
- K. H. Cheng, Y. Zhong, B. Y. Xie, Y. Q. Dong, Y. Hong, J. Z. Sun, B. Z. Tang and K. S. Wong, J. Phys. Chem. C, 2008, 112, 17507–17511 CrossRef CAS.
- S.-J. Lim, B.-K. An and S. Y. Park, Macromolecules, 2005, 38, 6236–6239 CrossRef CAS.
- Y. Liu, X. Tao, F. Wang, X. Dang, D. Zou, Y. Ren and M. Jiang, J. Phys. Chem. C, 2008, 112, 3975–3981 CrossRef CAS.
- Z. Zhao, S. Chen, J. W. Y. Lam, P. Lu, Y. Zhong, K. S. Wong, H. S. Kwok and B. Z. Tang, Chem. Commun., 2010, 46, 2221–2223 RSC.
- V. S. Vyas and R. Rathore, Chem. Commun., 2010, 46, 1065–1067 RSC.
- Z. Zhao, S. Chen, X. Shen, F. Mahtab, Y. Yu, P. Lu, J. W. Y. Lam, H. S. Kwok and B. Z. Tang, Chem. Commun., 2010, 46, 686–688 RSC.
- T. Sanji, K. Shiraishi and M. Tanaka, ACS Appl. Mater. Interfaces, 2009, 1, 270–273 CrossRef CAS.
- T. Sanji, K. Shiraishi, M. Nakamura and M. Tanaka, Chem.–Asian J., 2010, 5, 817–824 CrossRef CAS.
- M. C. Zhao, M. Wang, H. J. Liu, D. S. Liu, G. X. Zhang, D. Q. Zhang and D. B. Zhu, Langmuir, 2009, 25, 676–678 CrossRef CAS.
- M. Wang, D. Q. Zhang, G. X. Zhang, Y. L. Tang, S. Wang and D. B. Zhu, Anal. Chem., 2008, 80, 6443–6448 CrossRef CAS.
- M. Wang, D. Q. Zhang, G. X. Zhang and D. B. Zhu, Chem. Commun., 2008, 4469–4471 RSC.
- H. Tong, Y. N. Hong, Y. Q. Dong, M. Haussler, J. W. Y. Lam, Z. Li, Z. F. Guo, Z. H. Guo and B. Z. Tang, Chem. Commun., 2006, 3705–3707 RSC.
- J. Liu, Y. Zhong, P. Lu, Y. Hong, J. W. Y. Lam, M. Faisal, Y. Yu, K. S. Wong and B. Z. Tang, Polym. Chem., 2010, 1, 426–429 RSC.
- L. Liu, G. Zhang, J. Xiang, D. Zhang and D. Zhu, Org. Lett., 2008, 10, 4581–4584 CrossRef CAS.
- H. Tong, Y. N. Hong, Y. Q. Dong, M. Haeussler, Z. Li, J. W. Y. Lam, Y. P. Dong, H. H. Y. Sung, I. D. Williams and B. Z. Tang, J. Phys. Chem. B, 2007, 111, 11817–11823 CrossRef CAS.
- Y. N. Hong, M. Haussler, J. W. Y. Lam, Z. Li, K. K. Sin, Y. Q. Dong, H. Tong, J. Z. Liu, A. J. Qin, R. Renneberg and B. Z. Tang, Chem.–Eur. J., 2008, 14, 6428–6437 CrossRef CAS.
- M. Wang, X. Gu, G. Zhang, D. Zhang and D. Zhu, Anal. Chem., 2009, 81, 4444–4449 CrossRef CAS.
- T. Kato, A. Kawaguchi, K. Nagata and K. Hatanaka, Biochem. Biophys. Res. Commun., 2010, 394, 200–204 CrossRef CAS.
- F. Mahtab, Y. Hong, J. Liu, Y. Yu, J. W. Y. Lam, A. Qin, P. Lu and B. Z. Tang, Chem.–Eur. J., 2010, 16, 4266–4272 CrossRef.
- Q. Chen, N. Bian, C. Cao, X.-L. Qiu, A.-D. Qi and B.-H. Han, Chem. Commun., 2010, 46, 4067–4069 RSC.
- R. De Jaeger and M. Gleria, Prog. Polym. Sci., 1998, 23, 179–276 CrossRef CAS.
- W. Wei, X. Huang, X. Zhao, P. Zhang and X. Tang, Chem. Commun., 2010, 46, 487–489 RSC.
- J. Barberá, M. Bardají, J. Jiménez, A. Laguna, M. P. Martínez, L. Oriol, J. L. Serrano and I. Zaragozano, J. Am. Chem. Soc., 2005, 127, 8994–9002 CrossRef CAS.
- M. Touaibia and R. Roy, J. Org. Chem., 2008, 73, 9292–9302 CrossRef CAS.
- C. W. Allen, J. Fire Sci., 1993, 11, 320–328 Search PubMed.
- C. W. Allen, J. C. Shaw and D. E. Brown, Macromolecules, 1988, 21, 2653–2657 CrossRef CAS.
- K. Miyata, Y. Watanabe, T. Itaya, T. Tanigaki and K. Inoue, Macromolecules, 1996, 29, 3694–3700 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra of DHTPE and its precursor; MAS 13C and 31P NMR spectra of TPE-CP; fluorescence spectra of TPE-CP suspension in different solvents. See DOI: 10.1039/c1py00012h |
| This journal is © The Royal Society of Chemistry 2011 |
