Role of carbon content in qualifying Li3V2(PO4)3/C as a high capacity anode for high rate lithium battery applications
V. Mani,
K. Nathiya and
N. Kalaiselvi*
Central Electrochemical Research Institute, Karaikudi-630 006, Tamilnadu, India. E-mail: kalaiselvicecri@gmail.com
First published on 19th March 2014
Abstract
Nanocrystalline Li3V2(PO4)3 has been prepared by the oxalic dihydrazide assisted combustion (ODHAC) method, and the corresponding Li3V2(PO4)3/C composites containing different concentrations of super P carbon, viz. 10, 20 and 30 wt% have been explored individually as anodes for lithium batteries. Among the chosen composites, Li3V2(PO4)3/C-20 exhibits superior electrochemical properties, thus recommending a carbon content of 20 wt% as an optimum amount required to improve the electrochemical properties significantly. An initial capacity of 500 mA h g−1 and a progressive capacity of ∼400 mA h g−1 up to 100 cycles have been delivered by a Li3V2(PO4)3/C-20 anode with an admissible capacity fade of 20%, especially when cycled at a current density of 100 mA g−1. The optimized Li3V2(PO4)3/C-20 composite thus demonstrates itself as a high capacity anode that is suitable for high rate applications by way of exhibiting appreciable capacity values of 450, 350, 302 and 220 mA h g−1, under the influence of 100, 200, 300 and 400 mA g−1 current density.
1. Introduction
Lithium-ion batteries are widely studied energy storage devices for application in portable electronic devices, electric vehicles (EV) and hybrid electric vehicles (HEV).1–3 The selection of suitable electrodes gains importance when considering how to improve the power and energy density of lithium-ion batteries. Graphite is the most widely used anode material, especially after the commercialization of Sony's lithium-ion battery in 2001. Driven by the low theoretical capacity and sloping voltage plateau of graphite,4 certain lithium alloying metal and tin oxides, viz., Mn3O4,5 Co3O4,6 SnO2,7 CuO,8 Fe2O3 (ref. 9) and Fe3O4 (ref. 10) have been extensively studied along with the zero strain Li4Ti5O12 anode.11 However, issues such as larger irreversible capacity loss, volume expansion and higher intercalation potential of such anodes pose the necessity to identify and explore newer electrode materials as alternative anodes for lithium-ion batteries.Transition metal phosphates as cathodes are popularly known for their high stability, rate capability and safety. On the other hand, Kalaiselvi et al.12 in 2004 reported on the anodic behaviour of LiFePO4 for lithium battery applications, and LiVOPO4 is another cathode material reported recently for its application as an anode material.13
Towards this direction, monoclinic Li3V2(PO4)3/C, an upcoming cathode with high energy density, rate capability and safety, has intrigued recent researchers to explore the possibility of using it as an anode. This idea gets validated by the possible existence of vanadium in at least three different oxidation states and its ability to undergo insertion and/or alloying reaction with lithium in a wide potential range of 0.05–4.8 V. In this regard, X. H. Rui et al.14 reported on the performance of a Li3V2(PO4)3/C anode in the potential range of 3.0–0.0 V to obtain a capacity of 203 mA h g−1 versus Li+/Li, and W. F. Mao et al. discussed the possible existence of a Li3V2(PO4)3 (cathode)‖Li3V2(PO4)3 (anode) assembly.15 Herein, the open structure of Li3V2(PO4)3 allows the easy entry/departure of lithium ions into/from the electrode without causing structural change.
Quite different from such scarcely available reports on the Li3V2(PO4)3 anode, our present work that deals with the investigation of combustion synthesized Li3V2(PO4)3/C as a high capacity and high rate anode material is the first of its kind to report on the possibility of extracting a high specific capacity of ∼400 mA h g−1 under 100 mA g−1, wherein the suitability of the Li3V2(PO4)3/C anode for high rate (400 mA g−1) applications has also been demonstrated. The synergistic effect of the oxalic dihydrazide assisted combustion method (ODHAC) and the optimized addition of super P carbon (20 wt%) is believed to be responsible for the excellent electrochemical properties of the Li3V2(PO4)3/C anode.
2. Experimental procedure
2.1 Material synthesis of Li3V2(PO4)3/C
Li3V2(PO4)3/C was synthesized by the solution assisted combustion (SAC) method using oxalic dihydrazide (ODH), and the details of the ODHAC method are reported elsewhere.16 Li3V2(PO4)3 thus obtained was treated with different compositions (10, 20 and 30 wt%) of super P carbon and ball milled for 5 h at 300 rpm. Subsequently, the mixture was heated in a furnace at 700 °C for 2 h to ensure better adherence of carbon to the surface of the Li3V2(PO4)3 compound. Carbon coated Li3V2(PO4)3 samples thus prepared were investigated further for their performance as anodes in lithium-ion cell assembly.2.2 Physical and electrochemical characterization
The structure and phase purity of the synthesized compound were examined with Bruker D8 Advance X-ray diffraction (XRD) using Ni-filtered Cu Kα radiation (λ = 1.5406 Å). Particle size, carbon coating and surface morphology of the synthesized active material were investigated using Tecnai 20 G2 (FEI make) Transmission Electron Microscopy (TEM) and Gemini Field Emission Scanning Electron Microscopy (FESEM). TG/DTA studies were performed using a TA Instruments SDT Q600 thermogravimetric analyzer. Cyclic voltammetry (CV) was carried out using a VMP3 multichannel potentiostat–galvanostat system (Biologic Science Instrument). Charge–discharge studies were carried out using an ARBIN charge–discharge cycler.2.3 Electrode preparation and coin cell fabrication
Electrochemical characterization was carried out using CR2032 coin cells. Preparation of the electrode and fabrication of coin cells are similar to our earlier reports,12 and the fabricated cells were subjected to charge–discharge studies galvanostatically in the voltage range of 3.0–0.05 V versus Li+/Li at room temperature. Cyclic voltammetry (CV) measurements were performed at a scan rate of 0.2 mV s−1 between 0.05 and 3.5 V for the cells containing Li3V2(PO4)3/C anode vs. Li metal separated by Celgard separator soaked in the electrolyte consisting of 1 M LiPF6 dissolved in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC (1
DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v).
1 v/v).
3. Results and discussion
3.1 Structural characterization – X-ray diffraction, TEM, FE-SEM and elemental mapping
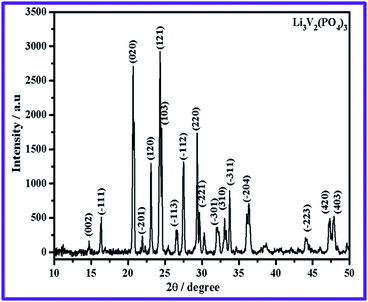
Fig. 1 shows the XRD pattern of Li3V2(PO4)3 synthesized at 850 °C. The position and intensity of all peaks indicate the formation of polycrystalline Li3V2(PO4)3 with a monoclinic structure and P21/n space group. Indexing of miller indices (hkl) of respective diffraction peaks of Li3V2(PO4)3 evidences the absence of impurity peaks. The calculated lattice parameter values, viz., a = 8.59 Å, b = 8.57 Å, c = 12.02 Å and β = 90.5° are in agreement with the literature report.17 Hence, it is understood that the ODHAC method produces a Li3V2(PO4)3 compound with the desired purity and crystallinity. Particle size and the presence of carbon on Li3V2(PO4)3/C have been investigated by TEM studies (Fig. 2), and the corresponding SAED pattern is appended as the inset of Fig. 2a. The TEM image shows the presence of particles of ∼100 nm size (Fig. 2a). Further, TEM evidences the presence of a carbon coating on Li3V2(PO4)3 particles (Fig. 2b), and the thickness of such a continuous carbon coating is 15 nm (Fig. 2c). SAED pattern (inset of Fig. 2a) confirms the polycrystalline nature of the pristine Li3V2(PO4)3 product obtained from the ODHAC method. Further, the SAED pattern recorded for the carbon present in the Li3V2(PO4)3/C composite evidences the amorphous nature of added super P carbon (Fig. 2d).The presence of the carbon layer effectively suppresses the growth of Li3V2(PO4)3 particles during high calcination processes, and the carbon layer is expected to improve the electronic conductivity of pristine Li3V2(PO4)3, which is of great importance in improving its electrochemical properties. Further, the flaky appearance of the combustion synthesised Li3V2(PO4)3 compound and the stoichiometry of the Li3V2(PO4)3/C composite are confirmed from FE-SEM and EDX analysis, respectively (Fig. 3a and b).
Elemental mapping (Fig. 3c–f) of the Li3V2(PO4)3/C composite, with a special reference to cumulative elemental mapping (inset of Fig. 3c), confirms the presence of V, P, O and C in the individual particles. The total carbon content of the title composite has been calculated using TG/DTA and is found to be 20 wt% (Fig. 4).
3.2 Cyclic voltammetry studies
Typical cyclic voltammetry (CV) behaviour of the Li3V2(PO4)3/C composite anode is shown in Fig. 5. The first cycle CV is quite different from the following cycles due to the formation of the SEI layer and is not unusual. Insertion/de-insertion of lithium occurs at different voltages, viz., 1.95/1.72, 1.86/1.77, 1.74/1.90, 1.65/2.02 V, corresponding to the formation of two phase (>1.6 V) and single phase (≤1.6 V) lithium insertion related intermediate compounds with varying lithium content,14 which is evident from CV cycles. However, a slight shift in peak position of the first and successive CV curves is observed, which is due to the SEI formation-driven unavoidable irreversible capacity loss with respect to the initial cycle. Interestingly, the following 2nd and 3rd cycles also show the presence of four redox pairs in the respective anodic region, thus confirming the reversibility and structural stability of the currently synthesized Li3V2(PO4)3/C anode.3.3 Charge–discharge studies
Charge–discharge behaviour of Li3V2(PO4)3/C anodes consisting of 10, 20 and 30 wt% of super P carbon under a current density of 100 mA g−1 is shown in Fig. 6. The initial charge capacities corresponding to Li3V2(PO4)3/C composites containing 10, 20 and 30 wt% of carbon are found to be 414, 500 and 418 mA h g−1. After 50 cycles, a nominal specific capacity of 156, 400 and 201 mA h g−1 with a respective capacity retention of 38, 80 and 48% has been exhibited by Li3V2(PO4)3/C anodes containing 10, 20 and 30 wt% of carbon. From this observation, it is understood that 20 wt% of super P carbon exhibits better cycling performance compared with those of 10 and 30 wt% of super P carbon.Even though it appears to be straightforward that an increase in carbon content would improve the electrochemical behaviour in a linear manner, the observed improvement in the electrochemical performance is not linear with the increasing carbon content.
The probable reason may be understood as follows: it is evident from Fig. 7 that the Rct value of Li3V2(PO4)3/C-20 is lower than those of Li3V2(PO4)3/C-10 and Li3V2(PO4)3/C-30 anodes, especially upon cycling. Similarly, a higher peak current (ip) has been exhibited upon cycling by the Li3V2(PO4)3/C-20 anode in comparison with the Li3V2(PO4)3/C-10 and Li3V2(PO4)3/C-30 anodes, thus substantiating the advantageous role of 20 wt% carbon in improving the electrochemical behaviour of the Li3V2(PO4)3/C anode by offering a desired conducting network to facilitate faster lithium diffusion kinetics.
 | ||
| Fig. 7 Impedance spectra (a and b) and cyclic voltammetry (c and d) of Li3V2(PO4)3/C composite anodes containing 10, 20 and 30 wt% carbon. | ||
In addition, the gradually reducing diffraction spots (Fig. 8) observed in the SAED pattern with the increasing carbon content clearly evidences the fact that the intensity of the carbon cloud is adversely high in the Li3V2(PO4)3/C-30 composite, thus impeding the faster diffusion of lithium ions. As a result, Li3V2(PO4)3/C-20 exhibits better electrochemical performance compared with those of Li3V2(PO4)3/C-10 and Li3V2(PO4)3/C-30 anodes.
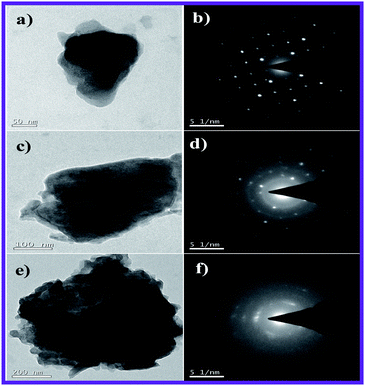 | ||
| Fig. 8 TEM images and SAED pattern of Li3V2(PO4)3/C composites containing (a and b) 10, (c and d) 20 and (e and f) 30 wt% super P carbon. | ||
Based on the above mentioned reasons, further studies such as extended charge–discharge studies and a rate capability test were restricted to Li3V2(PO4)3/C containing 20 wt% carbon. The voltage profiles of the Li3V2(PO4)3/C-20 anode corresponding to 1, 10, 20, 30, 40, 50 and 100 cycles are shown in Fig. 9, wherein consistent appearance of voltage plateaus at the respective positions is observed up to 100 cycles, with the exception of the initial discharge curve. The appreciable capacity retention and the admissible fade (∼20%) in capacity of the Li3V2(PO4)3/C-20 anode, as evident from Fig. 10, are in favour of considering it as a potential anode for lithium battery applications. In other words, a reasonable capacity of 400 mA h g−1 that has been observed up to 100 cycles under the influence of 100 mA g−1 current density substantiates the superiority of the currently synthesized Li3V2(PO4)3/C-20 anode over conventional graphite.
 | ||
| Fig. 10 Charge–discharge behavior of the Li3V2(PO4)3/C-20 anode upon extended cycling (100 mA g−1 current density). | ||
In addition, the currently observed capacity of 400 mA h g−1 under the influence of 100 mA g−1 current density is found to be superior than the capacity value reported for the Li3V2(PO4)3/C anode, and the irreversible capacity loss of 20 mA h g−1 is also nominal compared with the literature report.14
Hence, it is understood that the amount of carbon plays a crucial role in deciding the electrochemical performance, and 20 wt% carbon has been identified as the optimum concentration to prepare Li3V2(PO4)3/C anodes with appreciable specific capacity behaviour.
3.4 Rate capability test
The rate capability behavior of the Li3V2(PO4)3/C-20 anode was investigated upon progressive cycling as a function of different current densities such as 100, 200, 300 and 400 mA g−1 (Fig. 11).Li3V2(PO4)3/C-20 anodes exhibit acceptable capacity values of 450, 350, 302 and 220 mA h g−1 corresponding to a current density of 100, 200, 300 and 400 mA g−1, respectively. Interestingly, the Li3V2(PO4)3/C-20 anode is found to resume the initial capacity of ∼ 400 mA h g−1 even after subjecting it to higher current densities such as 200, 300 and 400 mA g−1, which is in favour of its suitability and structural stability for high rate applications.
4. Conclusions
The ODHAC method, by virtue of offering a phase pure Li3V2(PO4)3 compound and its synergy with the addition of the optimized amount of 20 wt% of super P carbon, results in the formation of a Li3V2(PO4)3/C-20 anode, which in turn is responsible for the improved electrochemical behaviour. Accordingly, a Li3V2(PO4)3/C-20 anode delivers an appreciable specific capacity of ∼400 mA h g−1 up to 100 cycles under the influence of 100 mA g−1 current density. Inferior electrochemical properties observed with 10 and 30 wt% super P carbon emphasizes the crucial role of the addition of 20 wt% carbon in offering a desirable conducting network to facilitate facile lithium diffusion kinetics and to improve the electrochemical behaviour. The study recommends the suitability of high capacity Li3V2(PO4)3/C-20 anodes for high rate applications, as evident from the extraction of a nominal capacity of ∼220 mA h g−1 under a 400 mA g−1 condition.Acknowledgements
Among the authors, V. Mani is thankful to DST, New Delhi and K. Nathiya to CSIR, New Delhi for financial support through CSIR-EMPOWER Project. N. Kalaiselvi is thankful to CSIR, New Delhi and DST, New Delhi for support through CSIR-EMPOWER and Grant-in-aid Project (GAP) respectively.Notes and references
- J. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- C. M. Park, J. H. Kim, H. Kim and H. J. Sohn, Chem. Soc. Rev., 2010, 39, 3115–3141 RSC.
- H. Nozakia, K. Nagaokab, K. Hoshib, N. Ohtaa and M. Inagaki, J. Power Sources, 2009, 194, 486–493 CrossRef PubMed.
- H. Wang, L. F. Cui, Y. Yang, H. S. Casalongue, J. T. Robinson, Y. Liang, Y. Cui and H. Dai, J. Am. Chem. Soc., 2010, 132, 13978–13980 CrossRef CAS PubMed.
- Y. M. Kang, M. S. Song, J. H Kim, H. S. Kim, M. S. Park, J. Y. Lee, H. K. Liu and S. X. Dou, Electrochim. Acta, 2005, 50, 3667–3673 CrossRef CAS PubMed.
- N. Li, C. R. Martin and B. Scrosati, Electrochem. Solid-State Lett., 2000, 3, 316–318 CrossRef CAS PubMed.
- J. Y. Xiang, J. P. Tu, L. Zhang, Y. Zhou, X. L. Wang and S. J. Shi, J. Power Sources, 2010, 195, 313–319 CrossRef CAS PubMed.
- H. Liu, G. Wang, J. Park, J. Wang, H. Liu and C. Zhang, Electrochim. Acta, 2009, 54, 1733–1736 CrossRef CAS PubMed.
- S. Ito, K. Nakaoka, M. Kawamura, K. Ui, K. Fujimoto and N. Koura, J. Power Sources, 2005, 146, 319–322 CrossRef CAS PubMed.
- L. Aldon, P. Kubiak, M. Womes, J. C. Jumas, J. O. Fourcade, J. L. Tirado, J. I. Corredor and C. P. Vicente, Chem. Mater., 2004, 16, 5721–5725 CrossRef CAS.
- N. Kalaiselvi, C. H. Doh, C. W. Park, S. I. Moon and M. S. Yun, Electrochem. Commun., 2004, 6, 1110–1113 CrossRef CAS PubMed.
- M. M. Ren, Z. Zhou and X. P. Gao, J. App. Electrochem., 2010, 40, 209–213 CrossRef CAS PubMed.
- X. H. Rui, N. Yesibolati and C. H. Chen, J. Power Sources, 2011, 196, 2279–2282 CrossRef CAS PubMed.
- W.-F. Mao, H.-Q. Tang, Z.-Y. Tang, J. Yan and Q. Xu, ECS Electrochem. Lett., 2013, 2(7), A69–A71 CrossRef CAS PubMed.
- K. Nathiya, D. Bhuvaneswari, Ganguli babu, D. Nirmala and N. Kalaiselvi, RSC Adv., 2012, 2, 6885–6889 RSC.
- Y. Li, L. Honga, J. Suna, F. Wua and S. Chen, Electrochim. Acta, 2012, 85, 110–115 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |