Effects of ionic liquids on the reaction kinetics of a laccase–mediator system†
Nora Harwardta,
Natascha Striplinga,
Simon Rothb,
Haifeng Liub,
Ulrich Schwanebergbc and
Antje C. Spiess*ac
aAachener Verfahrenstechnik - Enzyme Process Technology, RWTH Aachen University, Worringerweg 1, 52074 Aachen, Germany. E-mail: antje.spiess@avt.rwth-aachen.de; Fax: +49 2418023301; Tel: +49 2418023309
bLehrstuhl für Biotechnologie, RWTH Aachen University, Worringerweg 1, 52074 Aachen, Germany
cDWI - Leibniz-Institute for Interactive Materials, Forckenbeckstr. 50, 52074 Aachen, Germany
First published on 1st April 2014
Abstract
Lignocellulosic biomass can potentially be transformed into a wide variety of products ranging from biofuels and bulk chemicals to high value products. Through enzymatic hydrolysis, cellulose and hemicellulose can be utilized. However, lignin remains difficult to degrade which is partially due to its insolubility in aqueous solutions. Ionic liquids (IL) such as [EMIM][EtSO4] and [EMIM][Ac] increase lignin solubility and thereby, enzymatic access to lignin. Therefore, the reaction kinetics of the laccase–mediator system for the oxidation of lignin model compounds in IL solutions was investigated. Laccase in buffer solution had a higher activity compared to laccase in 5, 15, and 30% (v/v) [EMIM][EtSO4] and [EMIM][Ac]. However, the presence of 15% (v/v) IL helped to stabilize laccase activity over time. Cyclic voltammetry was used to investigate the reaction kinetics between the mediator ABTS and the lignin model compound veratryl alcohol in different ILs and IL concentrations. The increased conductivity at low IL concentrations expectedly lead to a rising reaction rate of mediator with substrate, whereas further increasing IL concentrations lead to higher viscosities and correspondingly, lower reaction rates. As a result, the investigation of the laccase–mediator system's reaction kinetics in buffer and ionic liquids provides a basis for evaluating and optimizing the lignocellulosic biomass degradation process.
Introduction
Diminishing fossil fuel resources and global warming have made the production of biofuels from renewable lignocellulosic biomass an appealing and sustainable alternative. Lignocellulosic biomass, composed of cellulose, hemicellulose, and lignin can potentially be transformed into a wide range of products from biofuels and bulk chemicals to high value products. Therefore, biomass pretreatment and lignin depolymerization methods are becoming increasingly important. Cellulose and hemicellulose have become accessible through enzymatic and chemical hydrolysis. However, lignin, one of the most abundant sources of renewable aromatic polymers, is less utilized because it is difficult to degrade and insoluble in aqueous solutions.1–3To make lignocellulosic biomass accessible, it is important to develop robust processes for pretreatment of the biopolymers.4 Current biomass pretreatments include physical and chemical pretreatments, and solvent fractionation.1 Some of these methods require the use of harsh chemicals or large amounts of energy.5 Therefore, improved methods of pretreatment are being investigated, among them the use of ionic liquids which facilitate the dissolution of lignocellulosic biomass.6,7 Ionic liquids are liquids at temperatures less than 100 °C with low vapor pressure and high thermal stability. They consist of mostly organic cations and anions which give them tunable properties.8–11 However, ionic liquids tend to have high viscosities which may reduce diffusion rate of a solute in solution.12,13 They typically have higher conductivities than organic solvents and do not require supporting electrolytes, which can facilitate electrochemical processes like oxidation of lignin.14 Although complete dissolution of lignin is challenging, (and complete dissolution of lignin in untreated wooden biomass has not been observed), the presence of ionic liquids, such as 1-ethyl-3-methylimidazolium ethylsulfate ([EMIM][EtSO4]), increases lignin solubility and the potential for degradation of lignin.5,15
In nature, white-rot fungi are able to degrade lignin by utilizing enzymes such as laccase, lignin peroxidase, manganese peroxidase, and cellobiose dehydrogenase.16 Among the different lignin degrading enzymes, laccases have the ability to oxidize a broad range of substrates.16,17 Unlike other lignin degrading enzymes, laccases (p-diphenol: dioxygen oxidoreductases EC 1.10.3.2) are able to use oxygen instead of hydrogen peroxide as the electron acceptor for the enzyme reaction. Laccase from the white-rot fungus Trametes versicolor is a blue multi-copper oxidase containing four copper ions, divided into one mononuclear (T1) and one trinuclear (T2, T3) cluster.16–18 The substrate binds to the T1 copper which coordinates electron transfer from the substrate to the oxygen in the T2, T3 trinuclear cluster.19 Due to steric hindrance, lignin cannot directly enter the active site of laccase. Therefore, small mediator molecules can be used to shuffle the electrons between laccase and lignin.20–22 The oxidized mediator then oxidizes the substrates, facilitating degradation into smaller compounds.23–25 Mediators also increase the redox potential range of the laccase such that the laccase–mediator system can oxidize a broader range of substrates. Laccases are able to oxidize a range of phenolic and non-phenolic compounds and potentially degrade lignin with the help of mediators.26,27 Kinetic models of laccase in buffer and ionic liquids have previously been investigated.28–31
Using ionic liquids for solvent pretreatment, small amounts of ionic liquids (10–15% (v/v)) will potentially remain in the reaction media prior to recycling.1 The kinetics of the redox reaction between the mediator and lignin may be affected by the ionic liquid. Likewise, it is necessary to understand how the enzymes are affected by the presence of ionic liquids in order to improve their tolerance.1,30 Rehmann et al. developed a method to quantify laccase activity in 63 different ionic liquids.33 Not only does laccase activity vary in different ionic liquids with currently limited predictability based on properties, they are also influenced by concentration. Enzymatic activity decreases with increasing ionic liquid concentrations. However, low concentrations of ionic liquids actually facilitate enzyme stability.30,31 Rodriguez et al. measured laccase stability at 0–50% (v/v) ionic liquid concentration at varying pH-values and observed half lives in a range of ionic liquids including [EMIM][EtSO4] between 2 and 6 days, with improved stability at higher pH-values.7,8 The same group also measured the ABTS activity of laccase incubated in ionic liquids. The maximum reaction rate in this solvent was slightly below the one in 0.05 mM citrate/0.1 mM phosphate buffer pH 4.5. Dominguez et al. showed that laccase activity decreased in higher concentrations of ionic liquids.30 Ionic liquid anions with longer chain lengths stabilized laccase activity compared to anions with shorter chain lengths.30 Generally, laccase stability increased in ionic liquid solutions with shorter alkyl chains in the methylimidazolium rings.30,31 Liu et al. showed that laccase (residual) activity decreased in the presence of 0 to 20% (v/v) [EMIM][EtSO4], which could be improved through modification of the enzyme by directed evolution.32
The aim of this study is to quantify the effects of ionic liquids and their properties, in particular viscosity and conductivity, on the kinetics of the laccase–mediator system. Enzymatic activity and stability were determined in buffer and ionic liquid solutions. The reaction rate between the mediator and the lignin model substrate veratryl alcohol was determined using cyclic voltammetry. The effects of conductivity and viscosity were briefly investigated using ionic liquid-equivalent solutions prepared using sodium chloride and polyethylene glycol (PEG400) to determine their effects on the laccase–mediator system.
Experimental section
Materials
All chemicals were of analytical reagent grade or higher and purchased from Sigma Aldrich (Hamburg, Germany) or Carl Roth (Karlsruhe, Germany). T. versicolor laccase powder (≥10 U mg−1) (catalogue no. 51639) and veratryl alcohol (catalogue no. D133000) were purchased from Sigma Aldrich. Pure 1-ethyl-3-methylimidazolium ethylsulfate ([EMIM][EtSO4]) was purchased from IoLiTec (Heilbronn, Germany). 1-Ethyl-3-methylimidazolium acetate ([EMIM][Ac]) was kindly provided by BASF (Ludwigshafen, Germany).Measurement of laccase activity and stability
Laccase activity was determined spectrophotometrically at 420 nm by measuring the increase in absorbance of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radicals (ABTS˙+) at 30 °C in a Synergy 4 microplate reader (Biotek, Winooski, VT, USA) at 1 minute intervals for 60 minutes. The amount of enzyme that converts one micromole of substrate per minute is defined as one unit (U). The reaction was initiated by the addition of laccase (60 μL, 0.1 U mL−1) to the ABTS solution (140 μL, 0.5 mM ABTS, 0.1 M sodium acetate buffer, pH 4.5) in 96-well microtiter plates (ε420 = 3.6 × 104 M−1 cm−1). The initial reaction rate was determined from the linear slope of the progress curves. Laccase activity was also measured in 5, 15 and 30% (v/v) ionic liquids.Kinetic parameters of laccase in buffer and ionic liquid solutions were calculated using Matlab (Nonlinear Least Squares Minimization) based on the Michaelis–Menten model V = Vmax[S]/([S] + Km) where Vmax is the maximum velocity of the enzyme reaction, [S] is the ABTS concentration, varied between 0.1 and 10 mM and Km is the Michaelis–Menten constant. The rate of reaction V was expressed in mM min−1.33
To determine laccase stability, 0.1 U mL−1 laccase was incubated in [EMIM][EtSO4] and [EMIM][Ac] at concentrations of 0, 10, 20, 30, and 40% (v/v) ionic liquid in 0.1 M sodium acetate buffer, pH 4.5. Laccase activities at each ionic liquid concentration were determined for up to 7 days.
Viscosity and conductivity measurements
The viscosity of 0–100% (v/v) polyethylene glycol 400 (PEG400) and ionic liquid solutions were measured with a MCR 301 rheometer (Anton Paar, Graz, Austria). A correlation between the viscosity and PEG400 concentrations was determined so that a buffer solution with a specified viscosity could be produced. PEG400 was used to adjust the viscosity of the buffer solution to equal the viscosity of the ionic liquid solutions, independent of the other IL physical properties, e.g. ionic strength. Conductivities of 0.1 M sodium acetate buffer, pH 4.5, and of sodium chloride and ionic liquid solutions were measured with a Mettler Toledo FiveEasy conductivity meter (Gießen, Germany). Sodium chloride was used to adjust the conductivities of the buffer solution to equal the ionic liquid conductivities. The viscosity and conductivity of buffer solutions (0.1 M sodium acetate buffer, pH 4.5) were then adjusted using PEG400 and NaCl to obtain solutions corresponding in viscosity and/or conductivity to ionic liquid solutions at concentrations of 5, 15, and 30% (v/v) in buffer. The conductivities and viscosities of the ionic liquids and the resulting ionic liquid equivalent solutions are presented in Table A in the ESI.†Cyclic voltammetry
Cyclic voltammetry was used to experimentally determine the electrochemical potentials and currents during the non-enzymatic redox reaction between the ABTS mediator and the lignin model compound veratryl alcohol. Electro-analytical experiments were performed in buffer and ionic liquid solutions using a BAS CV-100 B/W Voltammetric Analyzer (Bioanalytical Systems, Indianan, USA) and a C3 Cell Stand (Bioanalytical Systems, Indiana, USA) with a glass cell vial containing 15 mL solution. The setup of the electrochemical cell consisted of an Ag/AgCl reference electrode, a platinum wire counter electrode, and a glassy carbon working electrode all purchased from Bioanalytical Systems. Solutions were degassed with nitrogen prior to measuring. All experiments were performed at room temperature. Potential was periodically varied from 0 to 1200 mV at scan rates of 10–80 mV s−1. The current responses were measured in buffer, each 5, 15, 30% (v/v) [EMIM][EtSO4] and [EMIM][Ac] solutions in buffer, and the corresponding ionic liquid equivalent solutions.From the experimentally obtained cyclic voltammograms peak currents were determined with the BAS 100 software. The catalytic peak currents were measured using solutions containing both, the mediator ABTS and the substrate veratryl alcohol. The diffusion peak currents were obtained using solutions containing only ABTS. The reaction rate constant, k, was calculated using the same approach as Bourbonnais.34 According to Nicholson and Shain,35 the electrode reaction model results in a boundary value problem which is solved numerically to calculate peak currents. The calculated peak currents for electrochemical (diffusive) and catalytic reaction set-ups are used to formulate working curves.34,36 These working curves correlate the ratio of the catalytic and diffusion peak currents ik/id of the voltammograms to the non-dimensional kinetic parameter (kf/a)1/2, where kf is the heterogeneous electron transfer rate constant and a = nFv/RT with n – number of transferred electrons, F = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485.3 C per mol Faraday constant, v – scan rate, R = 8.314 J mol−1 K−1 gas constant, and T – temperature. For each varying substrate concentration, the kinetic parameters kf/a were obtained from the working curves by reading the experimentally obtained ratio of the catalytic and diffusion peak current ik/id and were plotted against the inverse scan rates (1/v) to obtain the pseudo-first order rate constants kf from the linear regression of the slope of the resulting diagrams. The pseudo-first order rate constants kf obtained were then plotted against veratryl alcohol substrate concentrations to obtain the second-order homogenous rate constants k by regression.35 Example figures obtained for calculating the rate constant, k, are shown in Fig. A–D in the ESI.†
485.3 C per mol Faraday constant, v – scan rate, R = 8.314 J mol−1 K−1 gas constant, and T – temperature. For each varying substrate concentration, the kinetic parameters kf/a were obtained from the working curves by reading the experimentally obtained ratio of the catalytic and diffusion peak current ik/id and were plotted against the inverse scan rates (1/v) to obtain the pseudo-first order rate constants kf from the linear regression of the slope of the resulting diagrams. The pseudo-first order rate constants kf obtained were then plotted against veratryl alcohol substrate concentrations to obtain the second-order homogenous rate constants k by regression.35 Example figures obtained for calculating the rate constant, k, are shown in Fig. A–D in the ESI.†
Results and discussion
Characterization of ionic liquids viscosity and conductivity at varying concentrations
Viscosities and conductivities at 0–100% (v/v) [EMIM][Ac] and [EMIM][EtSO4] in buffer solutions were measured (Fig. 1). These ionic liquids were chosen for their ability to improve lignin solubility.7,8,32 Due to its high buffer capacity, a 0.05 M citric acid/0.1 M Na2HPO4 buffer was used to perform initial experiments with a large ionic liquid concentration range. Subsequent reaction experiments with 0–40% (v/v) ionic liquid were performed in sodium acetate buffer with a targeted buffer range of pH 4 to 6, since the optimal pH for laccase is 4.5. | ||
| Fig. 1 Viscosity and conductivity at 0–100% (v/v) [EMIM][Ac], [EMIM][EtSO4] in 0.05 M citric acid, 0.1 M Na2HPO4 buffer pH = 4.5, T = 22 °C. | ||
Viscosity increases with increasing ionic liquid concentration. The viscosity of the buffer solution is 1.24 mPa s while pure ionic liquids show viscosities of 148 mPa s for [EMIM][Ac] and 105 mPa s for [EMIM][EtSO4]. Solutions containing [EMIM][Ac] were generally more viscous than those with [EMIM][EtSO4]. Seddon et al. also showed similar increasing viscosity at increasing ionic liquid concentration.37
The conductivity of the 0.05 M citric acid/0.1 M Na2HPO4 buffer system at pH 4.5 is 12.18 mS cm−1 and the conductivities of the pure ionic liquids are less than 5 mS cm−1 as shown in Fig. 1. [EMIM][Ac] reaches a maximum conductivity of 52.9 mS cm−1 at 30% (v/v) ionic liquid and [EMIM][EtSO4] reaches one of 40.4 mS cm−1 at 40% (v/v). The increase in conductivity is expected due to increased ion concentration with higher concentrations of the molten salt. However, above a certain concentration, viscosity effects overlay the further increased ion concentration by reduced mobility of the ions in solution. This is also reflected by the Stokes–Einstein equation where the viscosity is inversely related to the diffusion coefficient.38
Kinetic characterization of laccase in buffer and ionic liquid
A laccase activity assay was performed at varying mediator concentrations to determine the Michaelis–Menten kinetic parameters of laccase in buffer and varying ionic liquid concentrations. Since solutions with increased concentrations of ionic liquids are known to have higher viscosities and conductivities which may adversely affect laccase activity, buffer solutions were adjusted to similar viscosities and conductivities as the ionic liquid solutions and tested in parallel. Fig. 2 shows the maximal activity Vmax, the Michaelis–Menten constant Km, and the catalytic efficiency Vmax/Km of laccase in buffer and 5, 15, 30% (v/v) ionic liquid and the corresponding IL equivalent solutions.Laccase activity was highest in buffer (0.147 mM min−1), and decreased with increasing concentrations in ionic liquid and IL equivalent solutions, down to 0.0004 mM min−1 at 30% (v/v) [EMIM][Ac] and 0.0054 mM min−1 at 30% (v/v) [EMIM][EtSO4]. The Km value increases with increasing ionic liquid and IL equivalent solution concentration, however less significantly. The Km value of laccase in pure buffer solution is 0.191 mM and rises 3- to 5-fold for 30% (v/v) [EMIM][Ac] and 30% (v/v) [EMIM][EtSO4], respectively. The catalytic efficiency, Vmax/Km, can be calculated from the kinetic constants. Solutions with higher ionic liquid concentration have lower catalytic efficiency due to lower reaction rates and higher Km values. The catalytic efficiency for laccase in the buffer solution is the highest at 0.077 min−1 compared to 0.0281 min−1 in 30% (v/v) [EMIM][Ac] and 0.0456 min−1 in 30% (v/v) [EMIM][EtSO4].
The observed trends of the kinetic constants may indicate the effects of higher viscosities of the ionic liquids since similar trends can be seen in the ionic liquid equivalent solutions. A decrease in maximal reaction rate has been observed by Engel et al. for cellulase activity in ionic liquid solutions. This decrease could be partially related to increasing viscosity and partially to ionic strength.1 However, ionic liquids seem to positively influence the laccase-catalyzed reaction compared to ionic liquid equivalent solutions. Laccase in ionic liquid solutions tends to have higher reaction rates and catalytic efficiency than laccase in ionic liquid equivalent solutions. The reaction rate of 5% (v/v) [EMIM][Ac] and 5% (v/v) [EMIM][EtSO4] is 0.007 mM min−1 and 0.0134 mM min−1 respectively, compared to only 0.0027 mM min−1 for the 5% (v/v) ionic liquid equivalent solution. The corresponding conductivities and viscosities of the ILs and IL equivalent solutions are shown in Table A in the ESI.†
Stability of laccase in the presence of buffer and ionic liquid
For the economic use of laccase its stability in ionic liquids is relevant. As shown above, the presence of ionic liquids lowers enzyme activity. In some cases IL even decrease enzyme stability at high concentrations.8 Residual activity of laccase incubated in buffer and ionic liquids was measured over a period of 7 days using the laccase activity assay. Residual activity was calculated based on laccase activity at day 0 (Fig. 3).As shown in Fig. 3, residual enzyme activity, i.e. the activity after several days of incubation relative to the activity of the same enzyme preparation at time zero, decreases over time. The half-life of laccase in 15% (v/v) [EMIM][EtSO4] and [EMIM][Ac] amounts to a couple of months and 9.8 days, respectively, whereas the half-life in buffer is only 2.4 days. Apparently, the presence of ionic liquids helps to stabilize laccase activity. Laccase in [EMIM][EtSO4] and [EMIM][Ac] had, at all times, a higher residual activity compared to laccase in buffer. After the first day, laccase incubated in buffer has a residual activity of 0.55 compared to the residual activity of nearly 1 in both ionic liquids. After 7 days, there is a 37.5% decrease in activity in 15% (v/v) [EMIM][EtSO4] and an 11% decrease in 15% (v/v) [EMIM][Ac] after 7 days compared to a more than 80% activity loss in buffer. In summary, the decrease in residual enzyme activity over time is much faster in buffer compared to that in both ionic liquids.
Rodriguez et al. observed that laccase activity decreased 26.7% over seven days in 10% (v/v) [EMIM][EtSO4] (pH = 5) compared to 35.9% in 0.05 mM citrate/0.1 mM phosphate buffer pH 5. They also observed that enzyme stability after 7 days was slightly higher at 25 and 50% (v/v) ionic liquid with activity losses of 23.2% and 24.9%.8 The same trend was observed by Carneiro et al. where peroxidases retained their activity after being incubated in aqueous mixtures of 10% (v/v) [EMIM][MDEGSO4] and 10% (v/v) [EMIM][EtSO4] over seven days despite an initial decrease from the initial activity to day 1.38 Rodriguez et al. explain that hydrophilic, water-miscible ionic liquids are able to improve laccase stability because the structure of the enzyme is retained by the water molecules surrounding it.8
In summary, the tested ionic liquids [EMIM][Ac] and [EMIM][EtSO4] adversely affect laccase activity, but improve stability. The ionic liquid properties relevant for this effect on the enzyme, viscosity and conductivity (via ionic strength) are expected to also influence the electrochemical reaction between mediator and lignin model substrate which is investigated below.
Kinetic characterization of the mediator–substrate reaction in buffer and ionic liquid
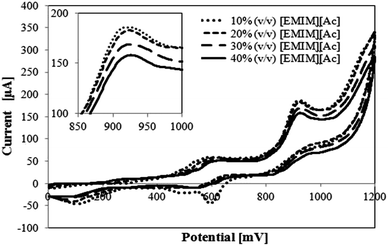
The electrochemical reaction kinetics between the mediator ABTS and the substrate veratryl alcohol were analyzed using cyclic voltammetry. First, cyclic voltammograms for 2 mM ABTS and 1 mM veratryl alcohol were taken for 10–40% (v/v) [EMIM][Ac] solutions (Fig. 4).In Fig. 4, increasing concentrations of the ionic liquid [EMIM][Ac] lowered the peak current. This is most likely due to the increasing viscosity of the ionic liquid. This can be explained by the substrate diffusion through the solvent towards the electrode. With higher viscosity, the solvent will provide more resistance to the substrate in solution, hindering the transport of analytes to the working electrode. Therefore, lower peak currents are measured. Although the peak current decreases with increasing ionic liquid concentration, the potential of the second oxidation peak around 900 mV remains nearly the same.
In the following experiment, cyclic voltammograms were taken at varying (0–15 mM) veratryl alcohol substrate concentrations in the presence of 1 mM ABTS mediator in 15% (v/v) [EMIM][Ac] in buffer in order to determine the relationship of peak current and substrate concentration (Fig. 5).
The experimentally obtained cyclic voltammograms in Fig. 5 show an increase in peak current with increasing veratryl alcohol substrate concentration. The peak current of the second oxidation peak of ABTS noticeably increases, and the potential only slightly increases around 900 mV. Therefore, this reaction is classified as homogeneous redox catalysis described by Bourbonnais et al.34 The ABTS dication oxidizes the veratryl alcohol to veratryl aldehyde and at the same time, regenerates to ABTS radical which can be seen in the increase in the peak current compared to the cyclovoltammograms of ABTS solution without veratryl alcohol.
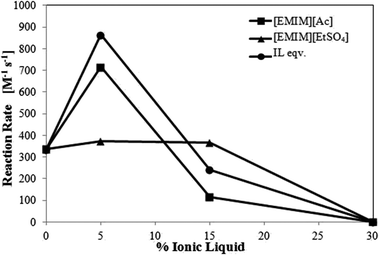
By varying both, the concentration of veratryl alcohol (0–15 mM) in the presence of 1 mM ABTS and the scan rate (10–80 mV s−1), the second order homogeneous rate constant of ABTS and veratryl alcohol reaction can be determined from peak currents obtained using cyclic voltammetry. The rate constant in 0.1 M NaAc buffer solution obtained at pH 4.5 was 335.34 M−1 s−1. Bourbonnais et al. obtained a rate constant of 170 M−1 s−1 in 0.05 M sodium citrate buffer, pH 4, and Branchi et al. obtained a rate constant of 210 M−1 s−1 in 0.1 M citrate buffer, pH 5.33,40 As the electrode reaction was shown to be independent of pH-value, it is suggested that the buffer salt or the ionic strength or the pH-value has an influence on the reaction rate between ABTS and veratryl alcohol.39 Increasing pH-values favor the oxidation of veratryl alcohol to veratryl aldehyde.41 Since at the same buffer concentration, the reaction rate obtained in our experiment at pH 4.5 in sodium acetate was higher than the one at pH 5 in sodium citrate, either reduced buffer ionic strength or specifically the acetate anion seems to increase the redox reaction rate. Fig. 6 summarizes the second order rate constants obtained at varying ionic liquid and IL-equivalent solutions.
The rate constant in buffer is lower than in 5% (v/v) ionic liquids and IL equivalent solution and even lower than in 15% (v/v) ionic liquid [EMIM][EtSO4] as shown in Fig. 6. After an initially increased reaction rate at low IL concentrations, the reaction rate decreases with further increasing ionic liquid concentration. Shipovskov et al. had also observed laccase-catalyzed oxidation in concentrations of 10–20% (v/v) for [BMIM]Br and 50–60% (v/v) for [BMIM][N(CN)2].42 This indicates that the reaction may also be dependent on ionic liquid concentration within a certain range. At concentrations outside this range, the reactions may be severely limited or undetectable. There is no detectable reaction at 30% (v/v) ionic liquid. [EMIM][EtSO4] reaction rate is relatively stable between 0–15% (v/v), but eventually decreases. Ionic liquid equivalent solutions and [EMIM][Ac] exhibit higher reaction rates at 5% (v/v), but at 15% (v/v) the reaction rate decreases significantly and below the value of [EMIM][EtSO4]. The reaction in the 15% (v/v) [EMIM][EtSO4] solution has the highest reaction rate constant of 366.57 M−1 s−1 among the 15% (v/v) ionic liquid solutions analyzed. This indicates that higher conductivities do not necessarily lead to higher reaction rates, whereas higher viscosities of the solution may significantly limit the reaction.
Influence of viscosity and conductivity on mediator–substrate reaction
To further dissect the effects of viscosity and conductivity of the ionic liquids, a set of buffer solutions adjusted to the same viscosity as 15% (v/v) [EMIM][EtSO4] and 15% (v/v) [EMIM][Ac] and another set of buffer solutions adjusted to the same conductivity as the respective 15% (v/v) ILs was analysed for the reaction rate of ABTS–veratryl alcohol redox reaction (Table 1).| Solution | k [M−1 s−1] | |
|---|---|---|
| 0.1 M sodium acetate buffer | 335.3 | |
| 15% (v/v) IL equivalent | 241.2 | |
| [EMIM][Ac] | 15% (v/v) | 115.9 |
| Viscosity equivalent | 300.3 | |
| Conductivity equivalent | 175.8 | |
| [EMIM][EtSO4] | 15% (v/v) | 366.6 |
| Viscosity equivalent | 261.4 | |
| Conductivity equivalent | 226.3 | |
Astonishingly, the rate constants of the ABTS–veratryl alcohol reaction were higher in those solutions where the viscosity of the buffer solution was adjusted to the ionic liquid solution than in those solutions that only have same conductivity. The viscosity of the buffer was increased by roughly 60% and 30% to produce viscosity equivalent solutions of 15% (v/v) [EMIM][Ac] and 15% (v/v) [EMIM][EtSO4], respectively, resulting in a 10 and 20% reduction of the reaction rate constant compared to buffer (cf. Tables 1 and A in the ESI†). However, the addition of sodium chloride to buffer, to adjust for the higher conductivity of the ionic liquids, increased the conductivity in comparison to buffer by a factor of 3 and 3.5 for 15% (v/v) [EMIM][Ac] and [EMIM][EtSO4]-equivalent, respectively, resulting in a 50 and 33% reduced reaction rate constant (cf. Tables 1 and A in the ESI†). This suggests that indeed, increased viscosity limits the reaction by retarding mass transfer, but unexpectedly, increased conductivity does not generally improve the electrochemical reaction. In support of these findings, the 15% (v/v) ionic liquid equivalent solution with average viscosity and conductivity values of the two ionic liquids shows a reaction rate constant between the two viscosity and conductivity equivalent solutions. However, the 15% (v/v) ionic liquids reaction rate constants are either well below the reaction rate constant for all viscosity and conductivity adjusted solutions as with [EMIM][Ac], or well above them as with [EMIM][EtSO4]. This suggests that the simple concept of transferring physicochemical properties of ionic liquids does not represent their actual function for improving the electrochemical reaction as with [EMIM][EtSO4], or hindering it as with [EMIM][Ac].
Conclusions
The reaction kinetics of the laccase–mediator system consisting of laccase from T. versicolor, the redox mediator ABTS and the lignin model substrate veratryl alcohol has been investigated in varying buffer and ionic liquid solutions that should represent the reaction medium after lignocellulose pretreatment. For both the laccase–mediator and the mediator–substrate reaction, reaction rates in [EMIM][Ac] and buffer solutions follow similar trends. There is a general trend of decreasing reaction kinetic parameters, namely enzyme activity Vmax, enzyme specificity Vmax/Km, enzyme deactivation rate constant kd, and the 2nd order reaction rate constant k for veratryl alcohol oxidation by ABTS with increasing viscosity. However, the same parameters tend to decrease with increasing conductivity. The quantitative analysis of the individual effects of ionic liquid viscosity and conductivity is subject to further study.Specifically, the presence of low concentrations of ionic liquids facilitates the enzymatic reaction both with respect to activity and stability, compared to IL equivalent solutions. In contrast, the reaction between the ABTS mediator and veratryl alcohol tends to be negatively influenced by ionic liquids. At the same time, there are significant deviations of the reaction behavior between IL and IL equivalent solutions, suggesting specific molecular effects of the cations and anions. The effects of the anions are interesting for both the electrochemical and the enzymatic reaction. Further studies are required for a better understanding of these effects to elucidate the contributions of particular cations and anions on the electrochemical reaction.
In summary, both the decreasing enzymatic activity, but increasing stability in the presence of ionic liquids, as well as the maximum of the electrochemical reaction rate of ABTS and veratryl alcohol indicate a process optimum, suggesting the ionic liquid type and content as an optimization parameter for future processes for the oxidative lignin degradation.
Acknowledgements
This work was performed as part of the Nordrhein–Westfalen (NRW) Research School “Brennstoffgewinnung aus nachwachsenden Rohstoffen (BrenaRo)” and the Cluster of Excellence “Tailor-Made Fuels from Biomass”, which is funded by the Excellence Initiative by the German federal and state governments to promote science and research at German universities. Additional support by the Deutsche Forschungsgemeinschaft (German Research Foundation) within the research training group 1166 “Biocatalysis using Non-Conventional Media – BioNoCo” is gratefully acknowledged.Notes and references
- P. Engel and R. Mladenov, et al., Point by point analysis: how ionic liquid affects the enzymatic hydrolysis of native and modified cellulose, Green Chem., 2010, 12(11), 1959–1966 RSC.
- T. vom Stein and P. M. Grande, et al., From biomass to feedstock: one-step fractionation of lignocellulose components by the selective organic acid-catalyzed depolymerization of hemicellulose in a biphasic system, Green Chem., 2011, 13(7), 1772–1777 RSC.
- J. Zakzeski and A. L. Jongerius, et al., Transition metal catalyzed oxidation of Alcell lignin, soda lignin, and lignin model compounds in ionic liquids, Green Chem., 2010, 12(7), 1225–1236 RSC.
- F. G. Calvo-Flores and J. A. Dobado, Lignin as renewable raw material, ChemSusChem, 2010, 3(11), 1227–1235 CrossRef CAS PubMed.
- J. Viell and W. Marquardt, Disintegration and dissolution kinetics of wood chips in ionic liquids, Holzforschung, 2011, 65(4), 519–525 CrossRef CAS.
- M. Mora-Pale and L. Meli, et al., Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass, Biotechnol. Bioeng., 2011, 108(6), 1229–1245 CrossRef CAS PubMed.
- A. P. M. Tavares and O. Rodriguez, et al., Ionic liquids as alternative co-solvents for laccase: study of enzyme activity and stability, Biotechnol. Bioeng., 2008, 101(1), 201–207 CrossRef CAS PubMed.
- O. Rodriguez and A. P. M. Tavares, et al., Ionic liquids: Alternative reactive media for oxidative enzymes, Ionic Liq.: Appl. Perspect., 2011, 499–516 CAS.
- L. E. Barrosse-Antle and A. M. Bond, et al., Voltammetry in room temperature ionic liquids: comparisons and contrasts with conventional electrochemical solvents, Chem.–Asian J., 2011, 2010(5), 202–230 Search PubMed.
- Y. Q. Pu and N. Jiang, et al., Ionic liquid as a green solvent for lignin, J. Wood Chem. Technol., 2007, 27(1), 23–33 CrossRef CAS.
- P. Hapiot and C. Lagrost, Electrochemical reactivity in room-temperature ionic liquids, Chem. Rev., 2008, 108(7), 2238–2264 CrossRef CAS PubMed.
- A. George and K. Tran, et al., The effect of ionic liquid cation and anion combinations on the macromolecular structure of lignins, Green Chem., 2011, 13(12), 3375–3385 RSC.
- X. J. Huang and E. I. Rogers, et al., The reduction of oxygen in various room temperature ionic liquids in the temperature range 293–318 K: exploring the applicability of the Stokes–Einstein relationship in room temperature ionic liquids, J. Phys. Chem. B, 2009, 113(26), 8953–8959 CrossRef CAS PubMed.
- D. S. Silvester and R. G. Compton, Electrochemistry in room temperature ionic liquids: A review and some possible applications, Z. Phys. Chem., 2006, 220(10–11), 1247–1274 CrossRef CAS.
- K. Stark and N. Taccardi, et al., Oxidative depolymerization of lignin in ionic liquids, ChemSusChem, 2010, 3(6), 719–723 CrossRef PubMed.
- M. Alcade, Laccases: biological functions, molecular structure and industrial applications, Industrial Enzymes, ed. J. Polaina and A. P. MacCabe, 2007, pp. 461–476 Search PubMed.
- H. P. Call and I. Mucke, History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems (lignozym(R)-process), J. Biotechnol., 1997, 53(2–3), 163–202 CrossRef CAS.
- O. V. Morozova and G. P. Shumakovich, et al., Laccase-mediator systems and their applications: a review, Appl. Biochem. Microbiol., 2007, 43(5), 523–535 CrossRef CAS.
- K. Piontek and M. Antorini, et al., Crystal structure of a laccase from the fungus Trametes versicolor at 1.90 angstrom resolution containing a full complement of coppers, J. Biol. Chem., 2002, 277(40), 37663–37669 CrossRef CAS PubMed.
- R. Bourbonnais and M. G. Paice, Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation, FEBS Lett., 1990, 267(1), 99–102 CrossRef CAS.
- S. Kurniawati and J. A. Nicell, Efficacy of mediators for enhancing the laccase-catalyzed oxidation of aqueous phenol, Enzyme Microb. Technol., 2007, 41(3), 353–361 CrossRef CAS PubMed.
- A. I. Cañas and S. C. Camarero, Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes, Biotechnol. Adv., 2010, 28, 694–705 CrossRef PubMed.
- I. Bento and C. S. Silva, et al., Mechanisms underlying dioxygen reduction in laccases. Structural and modelling studies focusing on proton transfer, BMC Struct. Biol., 2010, 10, 28 CrossRef PubMed.
- A. Potthast and T. Rosenau, et al., Oxidation of benzyl alcohols by the laccase-mediator system (LMS) – a comprehensive kinetic description, Holzforschung, 2001, 55(1), 47–56 CrossRef CAS.
- T. Tzanov and M. Diaz-Gonzalez, et al., Phenolic compounds as enhancers in enzymatic and electrochemical oxidation of veratryl alcohol and lignins, Appl. Microbiol. Biotechnol., 2011, 89(6), 1693–1700 CrossRef PubMed.
- R. Bourbonnais and M. Paice, Voltammetric measurement of lignin in pulp and paper samples – an electron transfer catalytic approach with mediators, J. Electrochem. Soc., 2004, 151(7), E246–E249 CrossRef CAS PubMed.
- R. Bourbonnais and D. Leech, et al., Electrochemical analysis of the interactions of laccase mediators with lignin model compounds, Biochim. Biophys. Acta, Gen. Subj., 1998, 1379(3), 381–390 CrossRef CAS.
- S. Kurniawati and J. A. Nicell, A comprehensive kinetic model of laccase-catalyzed oxidation of aqueous phenol, Biotechnol. Prog., 2009, 25(3), 763–773 CrossRef CAS PubMed.
- E. A. Macedo and R. O. Cristovao, et al., Kinetic modelling and simulation of laccase catalyzed degradation of reactive textile dyes, Bioresour. Technol., 2008, 99(11), 4768–4774 CrossRef PubMed.
- A. Dominguez and O. Rodriguez, et al., Studies of laccase from Trametes versicolor in aqueous solutions of several methylimidazolium ionic liquids, Bioresour. Technol., 2011, 102, 7494–7499 CrossRef CAS PubMed.
- M. Moniruzzaman and N. Kamiya, et al., Activation and stabilization of enzymes in ionic liquids, Org. Biomol. Chem., 2010, 8(13), 2887–2899 CAS.
- H. Liu and L. Zhu, et al., Directed laccase evolution for improved ionic liquid resistance, Green Chem., 2013, 15, 1348–1355 RSC.
- L. Rehmann and E. Ivanova, et al., Measuring the effect of ionic liquids on laccase activity using a simple, parallel method, Green Chem., 2012, 14, 725–733 RSC.
- R. Bourbonnais and D. Leech, et al., Electrochemical analysis of the interactions of laccase mediators with lignin model compounds, Biochim. Biophys. Acta, Gen. Subj., 1998, 1379(3), 381–390 CrossRef CAS.
- R. S. Nicholson and I. Shain, Theory of stationary electrode polarography – single scan + cyclic methods applied to reversible irreversible + kinetic systems, Anal. Chem., 1964, 36(4), 706–723 CrossRef CAS.
- A. E. Cass and G. Davis, et al., Ferrocene-mediated enzyme electrode for amperometric determination of glucose, Anal. Chem., 1984, 56(4), 667–671 CrossRef CAS.
- K. R. Seddon and A. Stark, et al., Influence of chloride, water, and organic solvents on the physical properties of ionic liquids, Pure Appl. Chem., 2000, 72(12), 2275–2287 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 1980, pp. 64–67 Search PubMed.
- A. P. Carneiro and O. Rodriguez, et al., Kinetic and stability study of the peroxidase inhibition in ionic liquids, Ind. Eng. Chem. Res., 2009, 48(24), 10810–10815 CrossRef CAS.
- B. Branchi and C. Galli, et al., Kinetics of oxidation of benzyl alcohols by the dication and radical cation of ABTS. Comparison with laccase–ABTS oxidations: an apparent paradox, Org. Biomol. Chem., 2005, 3(14), 2604–2614 CAS.
- A. Majcherczyk and C. Johannes, et al., Oxidation of aromatic alcohols by laccase from Trametes versicolor mediated by the 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) cation radical and dication, Appl. Microbiol. Biotechnol., 1999, 51(2), 267–276 CrossRef CAS.
- S. Shipovskov and H. Q. N. Gunaratne, et al., Catalytic activity of laccase in aqueous solutions of ionic liquid, Green Chem., 2008, 10(7), 806–810 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00733f |
| This journal is © The Royal Society of Chemistry 2014 |