Facile fabrication of nanocomposite microcapsules by combining layer-by-layer self-assembly and Pickering emulsion templating†
Hao Liu,
Xiaoyu Gu,
Meng Hu,
Yang Hu and
Chaoyang Wang*
Research Institute of Materials Science, South China University of Technology, Guangzhou 510640, China. E-mail: zhywang@scut.edu.cn; Fax: +86-20-2223 6269; Tel: +86-20-2223 6269
First published on 14th February 2014
Abstract
Nanocomposite polysaccharide microcapsules composed of biocompatible polyelectrolyte complexes are prepared via electrostatic layer-by-layer (LbL) self-assembly based on a Pickering emulsion template method. Polyethyleneimine (PEI)–Laponite based Pickering emulsions are obtained regardless of the polarity and viscosity of the oils, at a PEI/Laponite mass ratio of 0.50 and a Laponite concentration of 0.25 wt%, and these emulsions show good long-term stability for more than two months. Four-bilayer sodium alginate–chitosan microcapsules, with dimensions of about 43.9 μm and wall thickness of 55 nm, are prepared by alternate adsorption of negatively charged sodium alginate and positively charged chitosan on Pickering emulsions. Hollow microcapsules are obtained after core removal using a mild method of washing with excess 2-propanol. Ibuprofen (IBU), as a model drug, is loaded into the hollow microcapsules, and the release rate of IBU from the microcapsules at pH 7.4 is obviously faster than the release rate at pH 2.0. The greater the number of polyelectrolyte layers of the IBU-loaded microcapsules, the more difficult the IBU release. Consequently, nanocomposite microcapsules composed of natural polysaccharides fabricated by a Pickering emulsion templated LbL assembly method offer great potential applications in the food and medical industries.
Introduction
Over the past decades, nano- or microcapsules with unique and tailored properties, that are biocompatible and biodegradable, or environmentally responsive, have been extensively studied as an important class of materials because of their potential applications in self-healing materials,1 catalysts,2 environment protection,3 drug delivery or controlled release systems,4 and so on. A number of approaches have been proposed to prepare these capsules: interfacial condensation polymerization,5 in situ radical polymerization,6 internal phase separation,7 coacervation,8 and layer-by-layer (LbL) assembly.4,9–11Among these various approaches, LbL assembly is one of the most prominent methods. Hollow microcapsules can be produced by the LbL assembly method on sacrificial templates with charged polyelectrolytes and/or charged inorganic nanoparticles, followed by subsequent decomposition of cores. The properties of the microcapsules, such as the thickness, particle size, composition, surface features, wall permeability, can be tailored on a nanoscale range.12–14 These sacrificial templates can be solid particles3,4,10,11,15 or soft bodies such as emulsion droplets.9,16,17 In particular, the development of emulsion-based microcapsules has gathered increasing interest in the pharmaceutical fields. LbL-based microcapsules are emerging as a novel potential therapeutic tool because of the associated mild template removal conditions, high loading capability of guest materials, good biocompatibility, high stability to environmental stresses and stimulus-responsive behaviour.12–14 In previous literature, oil-in-water (O/W) emulsion-based microcapsules have been prepared via the LbL technique using small molecule surfactants,9,18 proteins,16,19 and phospholipids17 as emulsifiers.
Recently, colloidal particles acting as particulate emulsifiers to stabilize emulsion droplets have aroused great interest. These are known as Pickering emulsions.20 Once the colloidal particles have been adsorbed at oil–water interfaces, the particles need high energy to desorb from the interfaces, in contrast to surfactant molecules.21,22 Thus, compared to conventional emulsions, Pickering emulsions have a unique advantage in stabilization, hence they are useful as templates for preparing functional materials or structures.23–27
However, to the best of our knowledge, only Stöver and Li28 have used Pickering emulsions as templates to make poly(diallyldimethylammonium chloride) (PDADMAC)–poly(sodium styrenesulfonate) (PSS) and PSS–PDADMAC–LUDOX HS–PDADMAC microcapsules via LbL assembly.
Usually, the polyelectrolyte materials for the LbL assembly of microcapsules are mainly focus on non-degradable synthetic polyelectrolytes, such as PDADMAC,18,28 PSS,18,28,29 poly(allylamine hydrochloride) (PAH),29,30 polyethyleneimine (PEI),31 and poly(acrylic acid) (PAA).28 However for practical and biomedical applications, the use of natural polyelectrolytes seems an attractive alternative because of their biocompatibility and biodegradability. Sodium alginate (ALG) is a natural biopolymer extracted from brown algae. It is composed of linear chains of α-L-guluronic acid (G) and β-D-mannuronic acid (M). Sodium alginate is a weak acid, a polyanion with a pKa between 3–4.15 Chitosan (CS) is a basic linear polysaccharide containing β [1 → 4]-linked 2-acetamido-2-deoxy-D-glucopyranose and 2-amino-2-deoxy-D-glucopyranose units. CS is a weak base, a positively charged polyelectrolyte in acidic medium with a pKa of 6.5.23 These are economical and nontoxic biomaterials, and have received considerable research attention in recent years.
Herein, the LbL deposition of natural polysaccharides of ALG and CS on Pickering emulsion droplets, which were stabilized by poly(ethyleneimine) (PEI) surface-modified Laponite particles, was performed for the first time. A mild method was used to obtain hollow microcapsules after core removal by washing with excess 2-propanol. A schematic drawing of the production of these hollow microcapsules is presented in Fig. 1. Ibuprofen (IBU) was used as the model drug to study the release behaviour of the hollow microcapsules. The advantages of this microcapsule fabrication method are: (i) the high stability of Pickering emulsions can suppress deformation and flocculation of emulsion droplets during the LbL assembly process, (ii) the encapsulation of molecules both in the polyelectrolyte shell (IBU) and the oil core simultaneously, and (iii) the scalability of this method to obtain other microcapsules.
 | ||
| Fig. 1 Formation of multilayer microcapsules by the Pickering emulsion templated LbL assembly method. | ||
Experimental
Materials
Laponite RD was kindly provided by another laboratory. Poly(ethyleneimine) (branched, MW = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 by GPC; MW = 25
000 by GPC; MW = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 by light scattering), chitosan (CS, degree of deacetylation ≥ 90%, viscosity-average molecular weight Mv = 60
000 by light scattering), chitosan (CS, degree of deacetylation ≥ 90%, viscosity-average molecular weight Mv = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), sodium alginate (ALG, MW = 120
000), sodium alginate (ALG, MW = 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), ibuprofen (IBU), and fluorescein isothiocyanate (FITC) were purchased from Sigma-Aldrich, and used without further purification. Xylene, chloroform, sunflower oil, dodecane, liquid paraffin, 2-propanol, glacial acetic acid, tetrahydrofuran (THF), and dimethyl sulfoxide (Guangzhou Chemical Reagent Factory, China) were of analytical grade. Water used in all experiments was purified by deionization and filtration with a Millipore purification apparatus to a resistivity higher than 18.0 MΩ cm.
000), ibuprofen (IBU), and fluorescein isothiocyanate (FITC) were purchased from Sigma-Aldrich, and used without further purification. Xylene, chloroform, sunflower oil, dodecane, liquid paraffin, 2-propanol, glacial acetic acid, tetrahydrofuran (THF), and dimethyl sulfoxide (Guangzhou Chemical Reagent Factory, China) were of analytical grade. Water used in all experiments was purified by deionization and filtration with a Millipore purification apparatus to a resistivity higher than 18.0 MΩ cm.
Preparation of Pickering emulsions
PEI–Laponite complexes were prepared according to ref. 32. First, stock aqueous solutions of PEI and Laponite were prepared. Then, these two solutions were mixed to fabricate an aqueous solution of 0.25 wt% Laponite, and 0–1.0 wt% PEI. The obtained PEI-coated Laponite aqueous suspension was added into a 14 mL glass vial, followed by an addition of 2 mL oil (xylene, chloroform, sunflower oil, dodecane, liquid paraffin). The mixture was homogenized at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min by an IKA Ultra Turrax T25 homogenizer equipped with a 10 mm dispersing tool in an ice bath.
000 rpm for 2 min by an IKA Ultra Turrax T25 homogenizer equipped with a 10 mm dispersing tool in an ice bath.
The primary Pickering emulsion was purified as follows. The resulting emulsion was diluted with deionized water. The excess water was removed from the bottom using a syringe after a few minutes of quiescent storage. This dilution–quiescence cycle was repeated three times. The volume of clean Pickering emulsion was kept at 6 mL.
Microcapsule fabrication via LbL assembly
Stock 1 mg mL−1 ALG and CS solutions were prepared by dispersing ALG and CS in an aqueous solution. The NaCl concentration in these two solutions was 0.5 M. The pH of the CS solution was adjusted to 4 with glacial acetic acid. The resulting CS solution was stirred overnight to ensure complete dispersion, and then filtered in order to remove the impurities before use.The clean Pickering emulsions stabilized by PEI–Laponite (0.25 wt% Laponite, PEI/Laponite (mass ratio) = 0.5) were added into a vial. Then, 5 mL ALG solution was added, and the mixture was oscillated at 300 rpm for 20 min to ensure ALG adsorption. This was followed by 3 washing cycles that involved creaming of the secondary emulsions after a few minutes of quiescent storage, removing the excess liquid from the bottom with a syringe, and replenishing with a 0.5 M NaCl solution to keep a constant volume. The adsorption of CS onto the resulting cleaned secondary Pickering emulsion template was carried out in exactly the same manner as for the ALG coating, including three washing steps. This coating sequence was repeated until the desired double layers of ALG–CS had been deposited.
Tetrahydrofuran challenge and hollow microcapsule preparation
The obtained (ALG–CS)4 (6 mL) at optimized conditions was transferred to a 14 mL vial, followed by an addition of excess THF (5 mL) and vigorous shaking. The same THF challenge was used to assess the corresponding Pickering emulsions stabilized by PEI–Laponite (0.25 wt% Laponite, PEI/Laponite = 0.5).Hollow microcapsules was prepared by dissolving the oil core with an excess of 2-propanol for 20 min and centrifuging at 5000 rpm for 5 min. This dissolving process was repeated two times to ensure the complete removal of the oil. Then, the microcapsules were washed two times with water, centrifuged to remove the 2-propanol, and finally redispersed in water.
Loading and release experiments
0.01 g hollow (ALG–CS)3 microcapsules were dispersed in 20 mL phosphate buffered saline (PBS, pH = 7.4) containing 0.5 mg mL−1 IBU. After being incubated for two hours at 37 °C, the IBU-loaded microcapsules were centrifuged to remove the excess IBU, and washed twice with water.The resulting IBU-loaded microcapsules were dispersed into 20 mL PBS (pH = 7.4 or 2.0), and then transferred into a dialysis bag (Mw cut-off of 3500). Next, the dialysis bag was immersed into 180 mL of the PBS at 37 °C under magnetic stirring. After the desired time intervals, 2.0 mL sample solution was taken out to analyse the IBU concentration. This 2.0 mL solution was then poured back into the PBS. This process proceeded until the concentration of IBU in the PBS remained unchanged. The quantification of IBU can be analysed with a UV-vis spectrophotometer. The relationship between the fluorescence intensity and concentration of IBU was linear in our calibration curve, which was established from standard solutions of IBU at pH = 7.4 or 2.0.
Further adsorption of the ALG and CS layers onto the IBU-loaded microcapsules was performed using the procedures described above, except that the IBU-loaded microcapsules acted as the self-assembly templates. All the supernatants produced during the washing process were collected. Loss of IBU during this process was calculated by measuring the total amount of IBU in the supernatants.
Characterization
Pickering emulsion droplets were observed with an optical microscope (Carl Zeiss, Germany) and the average diameter was measured by a laser scattering particle size distribution analyzer (LA950, Horiba). The zeta potential of the Pickering emulsion was determined with a Malvern Zetasizer Nano ZS90 and the ζ-potential value was the average of three measurements. The confocal micrographs were taken with a Leica TCS-SP2 confocal laser scanning microscope (CLSM) with a 10× objective. CS was visualized by a FITC-labelled polymer at an excitation wavelength of 488 nm. FTIR spectra were recorded using a Bruker Vector-33 FTIR spectrometer under ambient conditions. The spectra were taken from 400 to 2000 cm−1. Scanning electron microscopy (SEM) was carried out with a Zeiss EVO 18 electron microscope equipped with a field emission electron gun. The samples were sputter-coated with gold prior to measurement. An Auto Probe CP Research atomic force microscope (AFM, XE-100, Park systems) with a silicon nitride (Si3N4) cantilever of a force constant of 0.58 N m−1 was used for observing the morphology and topology of the microcapsules in tapping mode at room temperature. The sample was prepared by drying a drop of the microcapsule suspension on a freshly cleaved mica substrate. The changes in frequency (Δf/3) and dissipation (ΔD) of an Au sensor crystal during the LbL assembly were examined by a quartz crystal microbalance equipped with dissipation monitoring (QCM-D) (Q-Sense E1). Prior to the ALG and CS deposition, PEI–Laponite particles were firstly coated onto the Au surface to ensure a positively charged coating. Then, five-bilayer films of alginate–chitosan were built by alternating ALG and CS depositions onto the quartz crystal. During the coating, fresh polyelectrolyte solutions were injected into the measurement chamber for 20 min at a flow rate of 150 μL min−1. A rinsing step of 10 min with a 0.5 M NaCl solution was included after the adsorption of each polyelectrolyte. For all measurements, the temperature was set at 25 °C. The IBU concentrations were evaluated by a Hitachi U-3010 UV-vis spectrophotometer at 223 nm.Results and discussion
Particle surface modification and Pickering emulsion preparation
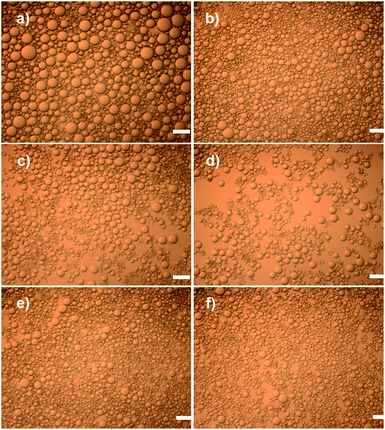
It is well known that Laponite alone is too hydrophilic to act as a particulate emulsifier.33,34 In order to produce effective emulsifiers with Laponite particles, many studies have reported increasing the hydrophobicity of Laponite by adding various small molecule additives or polymeric modifiers, such as salt,33,35 amines,34 and other polymers.36 In this study, the properties of the Laponite particles were tailored by positively charged PEI,32 which could bind irreversibly with negatively charged Laponite particle surfaces to produce an effective Pickering emulsifier. Moreover, PEI could give enough positive charge to absorb the first layer of ALG. The resulting polyelectrolyte-modified particles should form stable Pickering emulsions that could serve as robust templates for the LbL assembly of ALG and CS, as shown in Fig. 1.The morphologies of the Pickering emulsions were investigated at different PEI/Laponite mass ratios and a constant Laponite concentration of 0.25 wt%, as shown in Fig. 2. A drop test23 confirmed that we had prepared a xylene-in-water type emulsion. It is noted that emulsion droplets were discrete and spherical at various mass ratios. Furthermore, the mean droplet diameter of the emulsions gradually decreased with increasing mass ratio until a plateau was reached (Fig. 3).
 | ||
| Fig. 3 Mean droplet diameters of xylene-in-water Pickering emulsions as a function of the PEI/Laponite mass ratio. | ||
The ζ-potential value of colloid particles represents the hydrophilicity, which is crucial for the particle to form stable Pickering emulsions. According to Armes et al.,32 with increasing PEI content, the ζ-potential values of the Laponite particles gradually changed from a highly negative charge to a positive charge, remaining at a plateau at about 15 mV. At the first stage, Laponite particles modified by few PEI particles were relatively hydrophilic, so emulsions prepared under these conditions exhibited relatively poor stability toward coalescence, resulting in big droplets. With an increasing PEI/Laponite mass ratio, suitable hydrophilicity of PEI–Laponite particles ensured stable Pickering emulsions and small droplets. Further increasing the PEI content played no obvious role in reducing the size of the Pickering emulsions.
A minimum droplet diameter of around 59 μm was achieved at a mass ratio of 0.5 in ref. 32. However, in this study, the droplet diameter of the xylene-in-water emulsion at a mass ratio of 0.5 was about 43.9 μm, which was not the minimum value. Presumably, this difference was attributed to a different oil and oil to water ratio. Significantly, the xylene-in-water emulsions at a mass ratio of 0.5 displayed good stability against coalescence for several months.
Then, four other oils (chloroform, sunflower oil, dodecane, and liquid paraffin) were chosen to prepare Pickering emulsions for comparison with xylene. The parameters of these oils are listed in Table 1. The polarity can be characterized by the dielectric constant, ε. Xylene and chloroform are polar oils. Sunflower oil, dodecane and liquid paraffin are weakly polar oils. Liquid paraffin has a high viscosity of approximately 40 cP, but the viscosities of the other oils are very low. In all cases, these emulsions showed good long-term stability for more than two months regardless of the polarity and viscosity of the oils at a PEI/Laponite mass ratio of 0.50 and a Laponite concentration of 0.25 wt%. The morphologies of the corresponding emulsions are shown in Fig. S1 (ESI†), and the droplet diameters of the emulsions are listed in Table 1. In this system, xylene was selected as the model oil for the Pickering emulsions because of its low toxicity and high volatility. Thus, it was convenient to make hollow microcapsules. For practical requirements, the oil could be easily replaced by other oils.
The use of Pickering emulsions as templates for LbL assemblies of polyelectrolytes requires proper charged emulsion droplet surfaces, and sufficient stability to survive the removal of excess polyelectrolyte with water. We performed all LbL assembly studies at a PEI/Laponite mass ratio of 0.50, Laponite concentration of 0.25 wt%, and a oil to water volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. These conditions ensured that there was no PEI or Laponite remaining in the aqueous continuous phase after creaming of the emulsion droplets, which could reduce the aggregates of particulate emulsifiers after adding the first polyelectrolyte layer. In addition, the adsorption of PEI onto Laponite was close to monolayer coverage32 in these conditions, which promoted the electrostatic deposition of ALG and formed well ordered and compact microcapsules.
2. These conditions ensured that there was no PEI or Laponite remaining in the aqueous continuous phase after creaming of the emulsion droplets, which could reduce the aggregates of particulate emulsifiers after adding the first polyelectrolyte layer. In addition, the adsorption of PEI onto Laponite was close to monolayer coverage32 in these conditions, which promoted the electrostatic deposition of ALG and formed well ordered and compact microcapsules.
Polyelectrolyte complex shell formation by LbL assembly
During the LbL assembly process, the main problem with using the LbL technique to prepare multilayer emulsions is the tendency for droplet aggregation.19,38 To avoid droplet aggregation during preparation, different volumes of polyelectrolyte solutions were added to the clean emulsions to form a series of emulsions with different polyelectrolyte concentrations.In the experiments, when the ALG concentration was 0–0.04 wt% or above 0.06 wt%, obvious droplet aggregation was observed at the top of the aqueous solution due to bridging flocculation or depletion flocculation.9,37 Therefore, 0.045 wt% ALG and CS solutions (made by adding 5 mL fresh ALG and CS solutions of 1 mg mL−1 to the 6 mL emulsions) were chosen to prepare the microcapsules in the following experiments.
The surface charge is important for the LbL process, especially since the initial Pickering emulsion template has to be sufficiently charged. If not, the adsorbed layers may be partially removed upon adsorption of the next layer.9 The ζ-potential value of the clean primary emulsion stabilized by PEI–Laponite particles is +44.3 mV. This charge value is sufficiently applied to absorb the next polyelectrolyte layer. Fig. 4a shows a typical charge inversion plot for the investigated LbL systems. The surface charge changed from +44.3 mV to about −30 mV, indicating successful adsorption with the oppositely charged polyelectrolytes of ALG. The following deposition of the cationic CS led to the achievement of positive ζ-potential values (+34.8 mV). Overall, an obvious alternate ζ-potential value with the sequential deposition of polysaccharides was observed. This suggested the successful alternate adsorption of ALG and CS on the Pickering emulsions.
The deposition of ALG and CS on planar surfaces by means of a QCM-D technique was applied to simulate the assembly behaviour on the emulsion templates (Fig. 4b). The decrease of Δf/n and increase of D after each polyelectrolyte adsorption step demonstrated that a mass was being deposited on the PEI–Laponite hybrid surface. The first layer of ALG only showed a decrease in frequency of 40 Hz, while for the other polyelectrolyte layers, a decrease in frequency of about 100 Hz was observed. Presumably, the inhomogeneous and incomplete coating layer of PEI–Laponite on the Au surface, whereby there was not enough positive charge, resulted in the difficult adsorption of the first ALG layer. Actually, PEI was usually chosen to complete the first coating on the Au surface, ensuring a uniform and positive enough charge to support LbL assembly.38 Further adsorption of polyelectrolytes generated a stable frequency decrease due to the uniformly charged coating after ALG and CS deposition. With the increment of the adsorption numbers of the polyelectrolytes, only a small change of D was attributed to a transformation from a dissipative viscoelastic film to a compact and rigid structure, given that the change of each frequency remained unchanged.39,40
CS was labelled by FITC, and the CLSM image is shown in Fig. 5a. The microcapsules exhibited a highly localized green fluorescence in their shells. Furthermore, fluorescence intensity profiles along its diameter demonstrated that green fluorescence existed in the microcapsule’s shell, with just a small trace of green fluorescence in the core of the microcapsule (Fig. 5b). This result, with the experimental data of the ζ-potentials and the QCM-D experiments, allowed us to confirm that the ALG and CS were successfully assembled on the surface of the Pickering emulsion droplets.
 | ||
| Fig. 5 (a) Confocal microscopy of (ALG–CS)4 microcapsules with oil cores. (b) Corresponding fluorescence intensity profile along the line indicated in (a). | ||
Furthermore, LbL assembly on Pickering emulsions was assessed by a THF challenge. Intact microcapsules were observed by optical microscopy after inserting a small sample of the microcapsule suspension into excess THF (Fig. S2b†). In contrast, only a small number of droplets were observed when the original Pickering emulsions were subjected to THF challenge (Fig. S2a†). Most of the emulsion droplets broke up immediately after addition of THF. This also proved that the microcapsules coated by ALG and CS were successfully prepared and showed that the microcapsules had a high stability against to the outside environment, which is important in further potential applications.9,17
Characterization of hollow microcapsules
FTIR spectra of the hollow microcapsules are shown in Fig. S3.† The characteristic peaks of the above mentioned materials, such as 1417 cm−1 (ALG–COO+), 1380 cm−1 (–CH2 bending, CS), 1355 cm−1 (C–N stretching, PEI), 1006 cm−1 (Si–O–Si stretching frequencies, Laponite), were presented in the FTIR spectra of the microcapsules (Fig. S3c†), and indicated that we had successfully fabricated composite microcapsules.Optical images of the four-bilayer sodium alginate–chitosan ((ALG–CS)4) microcapsules before and after the complete removal of xylene by washing with excess 2-propanol are presented in Fig. S4.† The morphologies and droplet diameters before core removal (Fig. S4a†) were identical to the clean emulsion in Fig. 2c and the microcapsules in Fig. 5a. After core removal, hollow microcapsules (Fig. S4b†) were obtained with a similar size. Because the shells of the microcapsules consisting of natural polyelectrolytes are very thin, the microcapsules are difficult to find after core removal using an optical microscope.
The surface morphology of the hollow microcapsules was also investigated by means of SEM. Fig. 6 shows SEM images of the hollow (ALG–CS)4 microcapsules. All microcapsules preserved intact and well dispersed spherical structures, which were similar to the morphologies shown in Fig. S4b.† This intact structure may be attributed to low osmotic pressure induced by oil dissolved in 2-propanol. As expected, the ζ-potential value of the hollow microcapsules showed a positive charge, given that the outer layers were coated by cationic CS, which accounted for the well dispersed microcapsules in the SEM images. There were many creases and folds in the microcapsules because of a collapse during the drying process caused by the evaporation of the aqueous media. It was noteworthy that the textures of the microcapsules had a “grainy” appearance (more noticeable in Fig. 4b), probably reflecting the presence of Laponite particles inside the microcapsules after the core dissolution process, or local aggregation of the polyelectrolyte components.41,42
 | ||
| Fig. 6 (a) SEM images of (ALG–CS)4 microcapsules with water cores after drying. (b) A high magnification image. | ||
The structural study of the hollow microcapsules was further complemented with AFM observations (Fig. 7). A single hollow (ALG–CS)4 microcapsule also exhibited many folds and creases, which was in accordance with the SEM images. AFM measurements showed that the thickness of the (ALG–CS)4 microcapsules reached about 110 nm. Given that the measured height is twice the thickness of a single microcapsule wall, the height was estimated to be about 55 nm (Fig. 7b). This height was much larger than previously fabricated (ALG–CS)n microcapsules43 made by LbL assembly, which was attributed to the existence of Laponite, or local polyelectrolyte aggregation, as described above.
 | ||
| Fig. 7 (a) AFM micrograph of a hollow (alginate–chitosan)4 microcapsule. (b) Corresponding height distribution profile along the line indicated in (a). | ||
Controlled release from (ALG–CS)n microcapsules
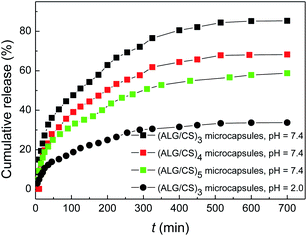
Nanocomposite microcapsules composed of natural polyelectrolytes of ALG and CS have been widely studied in the controlled release field.4,43 IBU is widely used as a clinical drug, and is a good candidate for the development of oral controlled release formulations. The research on IBU delivery and release has attracted much interest. In this report, IBU was loaded into (ALG–CS)n microcapsules as a model drug. The loading time was fixed at two hours, which was long enough for IBU to be loaded onto the (ALG–CS)n microcapsules. The loading capacity of IBU in the microcapsules was determined by the difference in drug concentrations in PBS before and after loading, and the capacity value was 150 mg g−1, which is comparable to other CS microcapsules.44The IBU release from the (ALG–CS)3 microcapsules as a function of time was investigated at pH 7.4 and 2.0 at 37 °C (Fig. 8). It was observed that IBU was gradually released at pH 2.0, where only about 33.7% of the loaded IBU was released after 11 hours. Compared to the release at 2.0, the release of IBU at pH 7.4 increased sharply with a prolonged release time (within 100 min), and then the release rate decreased until an equilibrium was reached after 500 min, with a cumulative release amount of 85.3%. This release is a typical release pattern of IBU from ALG–CS microcapsules.4 This significant difference of IBU release at different pH conditions is derived from the different solubility of IBU at pH 7.4 and 2.0.4,44 IBU exhibits better solubility at pH 7.4 than at pH 2.0 because it is an acidic molecule. The initial rapid release of IBU could mostly be attributed to the release of IBU on the surface and near the exterior surface.
Next, (ALG–CS)3, (ALG–CS)4 and (ALG–CS)5 microcapsules were prepared to investigate the effect of different adsorption double layers on IBU release. IBU-loaded (ALG–CS)4 microcapsules were obtained by additional polyelectrolyte layers coated on IBU-loaded (ALG–CS)3 microcapsules, and IBU-loaded (ALG–CS)5 microcapsules were obtained by additional polyelectrolyte double layers coated on IBU-loaded (ALG–CS)4 microcapsules. During self-assembly based on the previously described microcapsules and washing process, the loss dosage of IBU was 25–35 wt%. The IBU release from these three microcapsules at pH 7.4 is also shown in Fig. 8. As expected, with increasing polyelectrolyte double layers, the release rate was obviously reduced and the remaining IBU in the microcapsules increased correspondingly, indicating that additional deposited layers on former microcapsules can result in a greater obstacle for the release channel of IBU.44 Thus, the permeability of hollow microcapsules can be controlled by tuning the adsorption layers coated on the Pickering emulsion template.
Conclusion
In summary, ALG–CS multilayer microcapsules were firstly fabricated through LbL self-assembly on Pickering emulsion templates. Regardless of the polarity and viscosity of oils, PEI–Laponite based Pickering emulsions exhibited good long-term stability for more than 2 months at a PEI/Laponite mass ratio of 0.50 and a Laponite concentration of 0.25 wt%. The Pickering emulsion not only provided a highly stable LbL assembly template that could efficiently suppress deformation and flocculation of emulsion droplets during the LbL assembly process, but also ensured the control of the size distribution of the resulting microcapsules.Hollow microcapsules were obtained following core removal after washing with excess 2-propanol. The mild washing process could preserve the integrality and mechanism strength of the hollow microcapsules. The experimental data confirmed the successful formation of an ALG and CS multilayer on the Pickering emulsion template, and the formation of intact and well dispersed hollow (ALG–CS)n microcapsules. The size of the microcapsules can be tuned from several to tens of micrometers by varying the size of the Pickering emulsion templates, which was easily changed by altering either the PEI/Laponite mass ratio or the Laponite concentration.
IBU-loaded microcapsules were prepared by incubating these microcapsules into IBU solutions of PBS, and the release rate of IBU from (ALG–CS)n microcapsules was obviously faster at pH 7.4 than at pH 2.0. The permeability of the microcapsules can be controlled by tuning the adsorption layers coated on the Pickering emulsion templates according to the particular final purpose. The greater the number of polyelectrolyte layers on the IBU-loaded microcapsules, the more difficult the IBU release.
Herein, the proposed approach to prepare biocompatible polyelectrolyte microcapsules via Pickering emulsion templates can be considered as a general method that can be easily extended to other emulsion systems. Besides, nanocomposite microcapsules composed of natural polysaccharides prepared by a Pickering emulsion templated LbL assembly method offer great potential applications in the food and pharmaceutical industries.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, 2012CB821500), the National Natural Science Foundation of China (21274046) and the Natural Science Foundation of Guangdong Province (S20120011057).Notes and references
- S. R. White, N. R. Sottos, P. H. Geubelle, J. Moore, M. R. Kessler, S. R. Sriram, E. N. Brown and S. Viswanathan, Nature, 2001, 409, 794–797 CrossRef CAS PubMed.
- G. E. Lawson, Y. Lee, F. M. Raushel and A. Singh, Adv. Funct. Mater., 2005, 15, 267–272 CrossRef CAS PubMed.
- Y. Ding, Y. Zhao, X. Tao, Y. Z. Zheng and J. F. Chen, Polymer, 2009, 50, 2841–2846 CrossRef CAS PubMed.
- S. Q. Ye, C. Y. Wang, X. X. Liu, Z. Tong, B. Y. Ren and F. Zeng, J. Controlled Release, 2006, 112, 79–87 CrossRef CAS PubMed.
- P. Chaiyasat, M. Z. Islama and A. Chaiyasat, RSC Adv., 2013, 3, 10202–10207 RSC.
- R. Balasubramanian, S. Han and C. Chamberlayne, RSC Adv., 2013, 3, 11525–11528 RSC.
- R. Atkin, P. Davies, J. Hardy and B. Vincent, Macromolecules, 2004, 37, 7979–7985 CrossRef CAS.
- C. Carrick, M. Rud, B. Pettersson, P. T. Larsson and L. Wågberg, RSC Adv., 2013, 3, 2462–2469 RSC.
- D. Guzey and D. J. McClements, Adv. Colloid Interface Sci., 2006, 128–130, 227–248 CrossRef CAS PubMed.
- W. Qi, L. Duan and J. B. Li, Soft Matter, 2011, 7, 1571–1576 RSC.
- H. H. Dam and F. Caruso, Langmuir, 2013, 29, 7203–7208 CrossRef CAS PubMed.
- A. A. Antipov, G. B. Sukhorukov, E. Donath and H. Möhwald, J. Phys. Chem. B, 2001, 105, 2281–2284 CrossRef CAS.
- K. Breitenkamp and T. Emrick, J. Am. Chem. Soc., 2003, 125, 12070–12071 CrossRef CAS PubMed.
- R. Tangirala, Y. Hu, M. Joralemon, Q. Zhang, J. He, T. P. Russell and T. Emrick, Soft Matter, 2009, 5, 1048–1054 RSC.
- F. Cuomo, F. Lopez, M. G. Miguel and B. Lindman, Langmuir, 2010, 26, 10555–10560 CrossRef CAS PubMed.
- F. J. Rossier-Miranda, K. Schroen and R. Boom, Food Hydrocolloids, 2012, 27, 119–125 CrossRef CAS PubMed.
- S. Ogawa, E. A. Decker and D. J. McClements, J. Agric. Food Chem., 2003, 51, 5522–5527 CrossRef CAS PubMed.
- D. O. Grigoriev, T. Bukreeva, H. Möhwald and D. G. Shchukin, Langmuir, 2008, 24, 999–1004 CrossRef CAS PubMed.
- Y. S. Han, D. Radziuk, D. Shchukin and H. Möhwald, Macromol. Rapid Commun., 2008, 29, 1203–1207 CrossRef CAS PubMed.
- S. U. Pickering, J. Chem. Soc. Trans., 1907, 91, 2001–2021 RSC.
- B. P. Binks, Curr. Opin. Colloid Interface Sci., 2002, 7, 21–41 CrossRef CAS.
- R. Aveyard, B. P. Binks and J. H. Clint, Adv. Colloid Interface Sci., 2003, 100–102, 503–546 CrossRef CAS.
- H. Liu, C. Y. Wang, S. W. Zou, Z. J. Wei and Z. Tong, Langmuir, 2012, 28, 11017–11024 CrossRef CAS PubMed.
- Y. Yang, C. Y. Wang and Z. Tong, RSC Adv., 2013, 3, 4514–4517 RSC.
- Y. Ning, C. Y. Wang, T. Ngai, Y. Yang and Z. Tong, RSC Adv., 2012, 2, 5510–5512 RSC.
- G. N. Yin, Z. Zheng, H. T. Wang, Q. G. Du and H. D. Zhang, J. Colloid Interface Sci., 2013, 394, 192–198 CrossRef CAS PubMed.
- H. Liu and C. Y. Wang, RSC Adv., 2014, 4, 3864–3872 RSC.
- J. Li and H. D. H. Stöver, Langmuir, 2010, 26, 15554–15560 CrossRef CAS PubMed.
- A. A. Antipov, G. B. Sukhorukov, E. Donath and H. Möhwald, J. Phys. Chem. B, 2001, 105, 2281–2284 CrossRef CAS.
- Y. J. Wang and F. Caruso, Chem. Mater., 2006, 18, 4089–4100 CrossRef CAS.
- S. F. Yan, J. Zhu, Z. C. Wang, J. B. Yin, Y. Z. Zheng and X. S. Chen, Eur. J. Pharm. Biopharm., 2011, 78, 336–345 CrossRef CAS PubMed.
- M. Williams, S. P. Armes and D. W. York, Langmuir, 2012, 28, 1142–1148 CrossRef CAS PubMed.
- N. P. Ashby and B. P. Binks, Phys. Chem. Chem. Phys., 2000, 2, 5640–5646 RSC.
- W. Li, L. J. Yu, G. P. Liu, J. J. Tan, S. Y. Liu and D. J. Sun, Colloids Surf., A, 2012, 400, 44–51 CrossRef CAS PubMed.
- J. Faucheu, C. Gauthier, L. Chazeau, J. Y. Cavaille, V. Mellon, F. Pardal and E. B. Lami, Polymer, 2010, 51, 4462–4471 CrossRef CAS PubMed.
- E. Bourgeat-Lami, T. R. Guimarães, A. M. C. Pereira, G. M. Alves, J. C. Moreira, J. L. Putaux and A. M. d. Santos, Macromol. Rapid Commun., 2010, 31, 1874–1880 CrossRef CAS PubMed.
- S. Mun, E. A. Decker and D. J. McClements, J. Colloid Interface Sci., 2006, 296, 581–590 CrossRef CAS PubMed.
- G. M. Liu, Y. Hou, X. Xiao and G. Z. Zhang, J. Phys. Chem. B, 2010, 114, 9987–9993 CrossRef CAS PubMed.
- M. V. Voinova, M. Rodahl, M. Jonson and B. Kasemo, Phys. Scr., 1999, 59, 391–399 CrossRef CAS.
- A. A. Feiler, A. Sahlholm, T. Sandberg and K. D. Caldwell, J. Colloid Interface Sci., 2007, 315, 475–481 CrossRef CAS PubMed.
- S. Markutsya, C. Jiang, Y. Pikus and V. V. Tsukruk, Adv. Funct. Mater., 2005, 15, 771–780 CrossRef CAS PubMed.
- V. Kozlovskaya, E. Kharlampieva, I. Drachuk, D. Cheng and V. V. Tsukruk, Soft Matter, 2010, 6, 3596–3608 RSC.
- S. Q. Ye, C. Y. Wang, X. X. Liu and Z. Tong, J. Biomater. Sci., Polym. Ed., 2005, 16, 909–923 CrossRef CAS PubMed.
- X. B. Zhao, P. C. Du and P. Liu, Mol. Pharmaceutics, 2012, 9, 3330–3339 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Optical micrographs, THF challenge experiments, FTIR spectra. See DOI: 10.1039/c4ra00089g |
| This journal is © The Royal Society of Chemistry 2014 |