Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Open-source 3D printed reactors for reproducible batch and continuous-flow photon-induced chemistry: design and characterization†‡
Tom M.
Masson
 ,
Stefan D. A.
Zondag
,
Stefan D. A.
Zondag
 ,
Jasper H. A.
Schuurmans
,
Jasper H. A.
Schuurmans
 and
Timothy
Noël
and
Timothy
Noël
 *
*
Flow Chemistry Group, Van't Hoff Institute for Molecular Sciences (HIMS), Universiteit van Amsterdam (UvA), 1098 XH Amsterdam, The Netherlands. E-mail: t.noel@uva.nl
First published on 24th May 2024
Abstract
In both batch and continuous-flow reactor technology, reproducibility can be challenging for photochemical processes due to setup variability. One major contributor to this issue is the lack of standardized reactor solutions, particularly in academic laboratories where cost is often a prohibitive factor to purchase commercially-available reactor technology. However, advancements in 3D printing technologies and the availability of high-intensity light sources present an opportunity to develop cost-effective laboratory equipment. In this work, we present a diverse set of open-source reactor designs aimed at democratizing photochemistry while reducing the barrier of expensive technology. We introduce three new reactor designs: the UFO reactor for batch reactions, the Uflow reactor for seamless transition to flow processes, and the Fidget reactor for scale-up. After detailing the design principles and rationale behind these configurations, we characterize and evaluate their performance through simulations and experiments. These designs offer a standardized and affordable point of entry for researchers interested in exploring batch and flow photochemistry.
Introduction
In recent decades, the field of photochemistry has undergone remarkable expansion, driven by the widespread adoption of visible light photocatalysis.1,2 This resurgence in interest stems from the diverse array of innovative transformations it facilitates, alongside its enhanced selectivity.3 Such methodologies have revolutionized the construction of challenging chemical bonds under remarkably mild reaction conditions, often at room temperature and through visible light activation.4 Simultaneously, advancements in technology have met chemists' demands for high-intensity, energy-efficient, and precisely controllable light sources, notably through LED technology.5–8 This transition not only streamlines routine operations but also substantially reduces the costs associated with photochemical processes.9 Moreover, the adoption of continuous-flow reactor technology, employing transparent capillaries with small diameters, has addressed scalability challenges inherent in photochemical transformations.10 The convergence of these cutting-edge technologies, alongside the growing appeal of the field, has propelled the widespread adoption of photon-driven chemistry in laboratories worldwide.The accessibility of assembling homemade photochemical setups has led to a proliferation of various designs, often lacking adequate characterization. Consequently, this diversity poses challenges to the reproducibility of photochemical transformations.11 It is important to realize that photons serve as the central reactant initiating these reactions, thereby rendering both reaction kinetics and reagent stability closely dependent on light intensity. Moreover, according to the Lambert–Beer law, light intensity diminishes rapidly as it traverses through the reaction mixture, resulting in zones of over-irradiation conducive to byproduct formation and under-irradiation where photochemistry is impeded. Thus, it becomes evident that reactor characterization and standardization are pivotal for ensuring reproducibility in photochemical reactions. While purchasing off-the-shelf reactor technology presents a potential solution, the high associated costs, particularly in academic settings, often hinder the acquisition of such expensive equipment. Additionally, the need to separately procure solutions for batch or flow technology further exacerbates the financial burden associated with initiating photochemical activities. Furthermore, most commercially available solutions are non-customizable, posing a significant challenge for tailoring them to specific applications.
However, 3D printing technologies offer a promising solution to overcome most, if not all, of these challenges.12–21 The advancement of additive manufacturing, particularly fused deposition modeling (FDM), presents a compelling option for laboratory settings where customized solutions are often required promptly.22,23 These processes cater to the global scientific community by enabling the design, modification, enhancement, production, and repair of small-scale systems.14 Notably, these technologies not only ensure precise replication of designs multiple times but also expedite the dissemination of innovations, enhancements, and reproductions among fellow scientists.
Our research group has been integrating 3D printing technology with innovative reactor design for several years.24–26 Despite having access to commercially available reactor designs, the popularity of our 3D-printed reactor designs within our group surged notably. This was primarily due to the elimination of waiting queues and the ability to have multiple setups per person, facilitating the accelerated acquisition of research data. Furthermore, these designs garnered interest from colleagues in the field, prompting us to frequently donate setups and share our blueprints. In an effort to further democratize access to our cost-effective reactor designs for photochemical applications, this paper aims to provide a comprehensive blueprint, along with characterization and experimental validation of these designs; see the Data availability section for access to the open-source files.
Our 3D printed reactor systems have been tailored around the widely-used, high-intensity LEDs developed by Kessil.27–32 These light sources offer diverse wavelength options along with tunable intensity settings, ensuring precise control over a wide range of photochemical reactions. To validate the efficacy of the proposed designs, a comprehensive assessment of the reactors' performance is conducted. This assessment encompasses standard photochemical transformations as well as thorough simulations of photon pathways within the systems to verify the irradiation efficiency. Presented herein is a versatile batch system engineered to expedite and ensure reproducible screening of photochemical reaction conditions. Additionally, we introduce two continuous-flow systems employing the same light sources. These systems facilitate more uniform irradiation of the reaction mixture, reduce reaction time, enable straightforward scale-up, and expedite the evaluation of continuous reaction variables such as reaction time and light intensity. Collectively, these systems represent invaluable tools for efficiently screening batch conditions, extrapolating to continuous-flow systems, and scaling up towards large-scale production. Finally, a brief comparison between the 3D printed reactors and state-of-the-art commercially available systems is provided for context.
3D printed batch and continuous-flow photochemical reactors: design
Our objective was to develop a versatile array of reactors capable of guiding researchers through various stages of process development. Typically, initial exploration of photochemical reactions occurs in batch mode, exploiting its potential for high-throughput experimentation to simultaneously investigate multiple discrete variables such as solvents, bases, and catalysts.33 For this purpose, we devised the UFO reactor—a batch reactor featuring multiple inserts for test tubes—customized for efficient and reproducible screening.As chemical processes progress, scaling up becomes imperative, necessitating the adoption of continuous-flow technologies for photochemical reactions.2 Thus, we introduce our second reactor design: the Uflow reactor, equipped with a single Kessil lamp, facilitating screening of continuous variables and enabling the production of a few mmol of product. However, as the goal shifts towards achieving substantial product quantities during scale-up, more photons are required to ensure high throughput capacity. This trade-off led us to develop a third design, the Fidget reactor, featuring three high-intensity Kessil lamps arranged around a central capillary reactor mounted on a fidget-resembling holder.
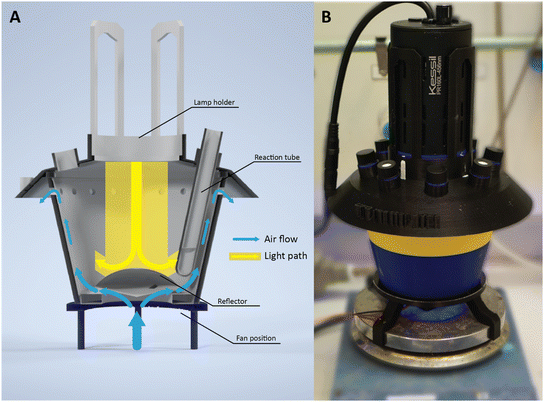
UFO batch reactor design
The UFO batch reactor§ design incorporates several reflectors, coated with reflective tape to efficiently redistribute irradiation (see Fig. 1). This intuitive redistribution strategy aims to achieve uniform irradiation across multiple reaction tubes while minimizing the formation of potential hotspots. Additionally, to maintain stable operational temperatures within the system and thereby prevent undesired thermal effects on the targeted photochemistry, an axial fan is strategically positioned on the opposing side of the lamp. This configuration facilitates the inflow of air from the reactor's base, promoting the cooling of both the reaction mixture and the reactor chamber. Typically, the latter stabilizes around 28 °C (refer to ESI‡). The cooling air is then evacuated through ventilation holes, ensuring optimal thermal conditions.The system operates with the lamp oriented vertically to maintain fixed positions of the lamp and tubes while occupying minimal space on the operator's workbench. The reaction tubes are arranged in a circular configuration surrounding the lamp. The inclined orientation of these tubes facilitates effective agitation of the reaction mixture using magnetic stir bars, as they are positioned above the magnetic stirring plate. Since mixing is a crucial factor for photochemical transformations, the position of the tube is essential to ensure uniform stirring across all tubes. Inhomogeneous stirring can result in physical limitations and reduce the performance of the reactions.34,35 Moreover, the slightly tilted positioning of the test tubes provides ample surface area for effective irradiation of the solution. Various reactor lid designs are available for inserting 4, 8, or 12 tubes. In this work, disposable reaction tubes (7.5 mL, 13 × 100 mm, Pyrex, Corning) were used; however, modifications can be made to adapt the system to any reaction tube. Opting for more available tube positions allows for increasing the number of parallel experiments using a single light source, albeit at the expense of reducing the received radiant power per tube.
Continuous-flow reactors
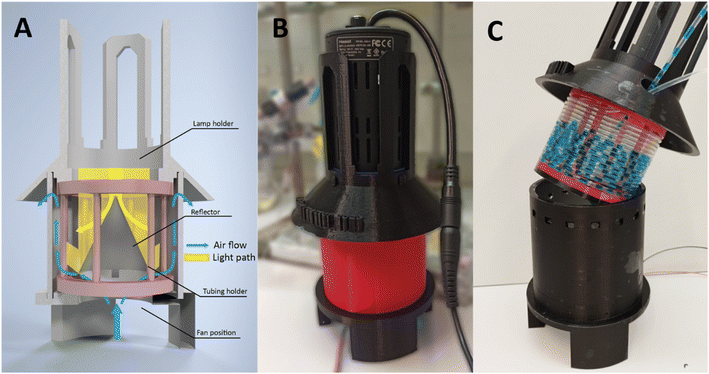
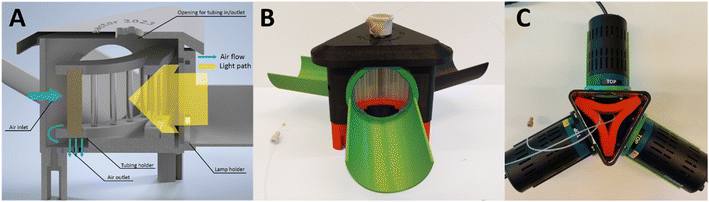
When converting a photochemical process from batch to continuous flow, maintaining consistent reaction parameters is crucial for a seamless transition.36 However, altering the light source may introduce undesired variability in light intensity and/or wavelength distribution, thereby introducing a critical parameter that varies between systems. The challenge prompted us to develop a standardized flow system that facilitates a seamless transition from our initial batch setup. Dubbed Uflow reactor, this system integrates the same light source, used in the batch configuration, directly into the flow setup without requiring additional hardware adjustments (see Fig. 2). In this setup, the vertically positioned lamp irradiates a reflective cone, redirecting the light towards the interior of the coiled transparent capillary reactor tube. Similar to the batch system, the use of reflectors assists in redistributing incident irradiation, thereby minimizing potential hotspots. Likewise, a fan is incorporated to maintain a stable internal temperature within the reactor chamber, typically stabilizing around 30 °C (see ESI‡).Due to the direct correlation between reaction kinetics and the received irradiation, photochemical systems are often constrained by the quantity of photons reaching the reaction mixture.2 To augment the photon availability in our system, we modified the existing design to accommodate three Kessil lamps for process intensification and thus increased throughput, resulting in the Fidget reactor design (see Fig. 3).
The Fidget reactor necessitated a different reactor holder design to facilitate a revised coiling of the capillary microreactor, aimed at preventing the formation of hotspots. To tackle this challenge, we conducted ray-tracing simulations utilizing a validated 3D model of the Kessil lamp37 (see ESI‡) and incorporated the optimal curvature of the reactor coil. This fidget-curvature was pivotal in achieving uniform irradiation along the illuminated surface. The tubing was intricately wound around brass and transparent PMMA pillars to achieve the desired shape while minimizing shading effects on the tubing. Additionally, the triangular configuration ensured that any light not absorbed during its initial passage through the coil had an additional opportunity to be absorbed upon striking the back of the capillary. Temperature measurements revealed that cooling with the axial fan alone was insufficient to dissipate the heat generated by the three lamps. Consequently, the reactor chamber underwent redesign to facilitate compressed air cooling, resulting in satisfactory outcomes as the temperature stabilized around 27 °C (see ESI‡).
3D printed batch and continuous-flow photochemical reactors: characterization
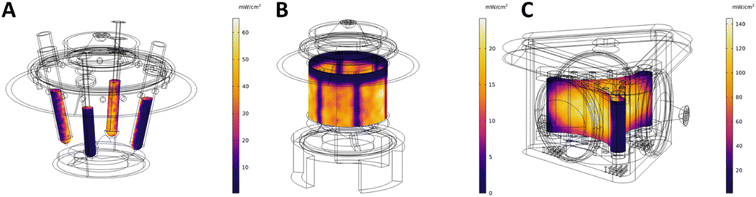
The characterization of the three reactors involved both ray-tracing simulations and experimental assessments.Central to the ray-tracing simulations of all three reactor designs is the irradiation profile from the utilized Kessil light source. The linear reflector within the Kessil lamps results in an anisotropic angular distribution of the light emission pattern, as indicated in the datasheet provided by the manufacturer.38 Consequently, the light emission profile is not circularly symmetrical, potentially leading to variations in irradiation characteristics among different reaction tube positions within the UFO reactor or different reaction zones within the coiled capillaries for the Uflow and Fidget reactors. This variation requires detailed investigation for proper comparison between different reaction mixtures. While this effect is crucial for kinetic studies of photochemical reactions, it may be negligible for systems where photons are in excess and do not limit the overall reaction rate. However, such a scenario is rarely encountered in most photochemical transformations reported in the literature, as they mostly operate in a photon-limited regime. A virtual representation of the general Kessil lamp design was previously developed by our team and validated using ray-tracing. This representation is utilized here for characterizing the irradiation profiles of the complex reactor geometries.39
The simulations for the UFO reactor using the 4-vial reactor lid are validated through chemical actinometry (see ESI‡), serving as the foundation for further simulated irradiation profiles. For the flow reactors, the wrapped coil is simply modeled as a flat surface, as this represents the generic collecting surface, making simulations more generalized and independent of the specific capillary's dimensions. The generated irradiation profiles, depicted in Fig. 4, illustrate the potential hotspots, dark areas, and homogeneously irradiated regions within the different reactor designs. Surface integration of these irradiation profiles provides the expected maximum radiant power that can be absorbed by the reaction mixture. Since all the reactors utilize Kessil lamps, the simulations of these reactors have broad applicability. Not only can the power setting be adjusted, but also the wavelength option, enabling informed decisions to set a desired radiant power experienced by the reaction mixture using our simulation data. Further details and tabulated data are available in the ESI.‡
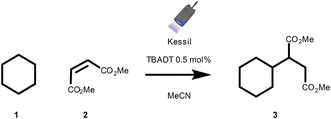
To assess the effectiveness of the presented reactor designs, we conducted a photocatalytic hydrogen atom transfer (HAT)-enabled Giese-type alkylation reaction (Scheme 1), facilitating the coupling between cyclohexane and dimethyl maleate catalyzed by tetrabutylammonium decatungstate (TBADT).40–42
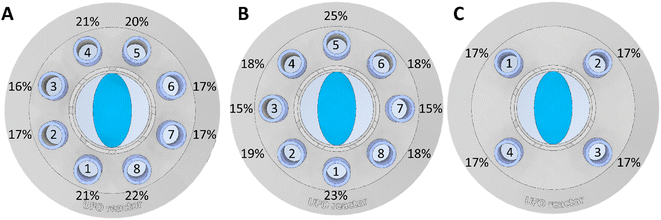
To compare photon exposure across tubes in different positions, we deliberately maintained a relatively low conversion rate by utilizing a Kessil PR160L 390 nm lamp at only 25% of its maximal power, as depicted in Fig. 5. As described in the reactor configuration, variations in yields were observed between configurations A and B due to the lamp's two planes of symmetry, with the blue ellipse representing the lamp orientation. While these differences may be insignificant during extended reaction times when photons are abundant (i.e., when the transformation reaches full conversion), they could pose challenges in precise data comparison, particularly in kinetic studies or when byproduct formation occurs at increased light intensities. To mitigate this concern, we devised a reactor insert with four equivalent positions, ensuring uniform photon exposure to all tubes, as illustrated in Fig. 5C.
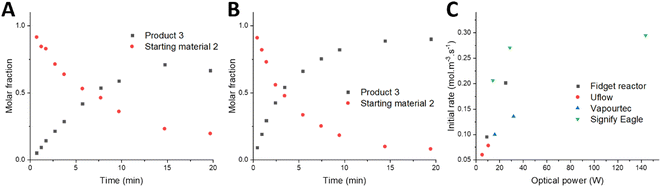
We further investigated the performances of our different flow reactor designs using the same photocatalytic benchmark transformation (Scheme 1). Our aim in this assessment was to evaluate the maximum performance of the systems under optimal reaction conditions. To achieve this, we conducted the transformation at 100% light intensity, utilizing Kessil PR160L 370 nm Gen2 lamps. This choice allowed for comparison with commercially available systems, such as the Vapourtec UV-150 photochemical reactor and the Signify Eagle reactor, which typically employ 365 nm LED light sources.41,43,44 By sampling at various time intervals (see method in the ESI‡), we generated the graphs depicted in Fig. 6A and B.
As expected, the intensified photon flux of the Fidget reactor resulted in faster photon-induced conversion compared to the Uflow reactor system, which utilizes only a single lamp and thus lower light intensity. Analyzing the reaction composition over time enabled us to determine the initial rate of the reaction in both reactors. By manipulating the power input of the lamps, we assessed the initial rate of the reaction in relation to the optical power. Comparing these output powers provided a means to evaluate and contrast the reactor designs based on the chosen reaction parameters.
To ensure fair comparisons, we screened the light intensity in both the Signify Eagle and the Vapourtec UV-150 systems. However, due to the exceptionally high-power light sources in the Signify Eagle system (144 W optical power), it was necessary to reduce the intensity setting to 10–20% for comparable temperatures and reaction rates. This adjustment aimed to provide better insight into the effect of photons on the initial rate, particularly challenging in a photon-saturated system encountered at higher light intensities.
Comparing the initial reaction rates achieved in the Fidget reactor and the Uflow system under similar optical power conditions allowed us to draw conclusions about the design efficiency of both systems. Fig. 6C illustrates that, for equivalent optical power levels, the Fidget reactor yields a higher initial reaction rate. This can be attributed to its superior design resulting in a more homogeneous irradiation pattern, thus minimizing photon losses from reflections and preventing light rays from escaping the reactor.
It is important to note that both systems hold practical value. The Uflow reactor offers a quick, easy, and cost-effective means of screening reaction conditions, while the Fidget reactor system excels in photon efficiency, scalability, and intensifying reactions. Additionally, both the Uflow and Fidget reactors demonstrate comparable performance to commercially available photochemical flow reactors. Consequently, we believe that our 3D printed designs serve as an accessible starting point for research groups entering the field of photochemistry, especially for those with limited upfront financial investment capabilities.
Conclusion
Our study introduces three novel, 3D printed reactor designs – coined the UFO batch reactor, the Uflow continuous-flow reactor, and the Fidget continuous-flow reactor – that effectively address key challenges in the field of photochemistry. The UFO reactor is meticulously engineered to facilitate energy-efficient batch photochemical processes, ensuring uniform irradiation and reproducible reactions. It enables parallel execution of multiple reactions and is particularly well-suited for screening discrete variables.The Uflow reactor system serves as a seamless transition from batch to continuous flow, offering a straightforward evolution from its batch counterpart and facilitating the evaluation of continuous variables with ease.
The Fidget reactor, optimized for photon-intensive applications, demonstrates superior performance through strategic design, ensuring uniform light irradiation of the capillary and streamlining scale-up using three Kessil light sources.
All of these 3D printed reactors, validated and standardized through simulations, actinometry, and experimental assessments, present cost-effective and versatile solutions that can rival commercial counterparts. Their demonstrated value underscores their practical utility, making them invaluable assets for researchers seeking affordable and robust photochemical setups.
We believe that this work will significantly contribute to the popularization of photochemical processes within the research community by eliminating high costs as the primary barrier to the adoption of this technology and ensuring reproducibility through its standardized design. With these innovative reactor designs, we anticipate broader accessibility and utilization of photochemistry in diverse research fields.
Data availability
Open-source 3D files are available in the online repository. See: https://github.com/Noel-Research-Group/3D-printed_UFO_Reactors.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors would like to acknowledge Dr. Zhenghui Wen and Dr. Luca Capaldo for their work on the early designs of the system and their constant support to the project. The authors would also like to thank the European Union's Horizon research and innovation program for generous funding through the projects FlowPhotoChem (T. M. M., S. D. A. Z. and T. N.; grant number 862453) and CATART (J. H. A. S. and T. N.; grant number 101046836). The materials presented and views expressed here are the responsibility of the author(s) only. The EU Commission takes no responsibility for any use made of the information set out.References
- T. Noël and E. Zysman-Colman, The Promise and Pitfalls of Photocatalysis for Organic Synthesis, Chem Catal., 2022, 2(3), 468–476, DOI:10.1016/j.checat.2021.12.015.
- L. Buglioni, F. Raymenants, A. Slattery, S. D. A. Zondag and T. Noël, Technological Innovations in Photochemistry for Organic Synthesis: Flow Chemistry, High-Throughput Experimentation, Scale-up, and Photoelectrochemistry, Chem. Rev., 2022, 122(2), 2752–2906, DOI:10.1021/acs.chemrev.1c00332.
- H. E. Bonfield, T. Knauber, F. Lévesque, E. G. Moschetta, F. Susanne and L. J. Edwards, Photons as a 21st Century Reagent, Nat. Commun., 2020, 11(1), 2–5, DOI:10.1038/s41467-019-13988-4.
- P. Melchiorre, Introduction: Photochemical Catalytic Processes, Chem. Rev., 2022, 122(2), 1483–1484, DOI:10.1021/acs.chemrev.1c00993.
- T. H. Rehm, Reactor Technology Concepts for Flow Photochemistry, ChemPhotoChem, 2020, 4(4), 235–254, DOI:10.1002/cptc.201900247.
- J. D. Williams and C. O. Kappe, Recent Advances toward Sustainable Flow Photochemistry, Curr. Opin. Green Sustainable Chem., 2020, 25, 100351, DOI:10.1016/j.cogsc.2020.05.001.
- F. Lévesque, M. J. Di Maso, K. Narsimhan, M. K. Wismer and J. R. Naber, Design of a Kilogram Scale, Plug Flow Photoreactor Enabled by High Power LEDs, Org. Process Res. Dev., 2020, 24(12), 2935–2940, DOI:10.1021/acs.oprd.0c00373.
- M. Sender and D. Ziegenbalg, Light Sources for Photochemical Processes – Estimation of Technological Potentials, Chem. Ing. Tech., 2017, 89(9), 1159–1173, DOI:10.1002/cite.201600191.
- J. R. Swierk, The Cost of Quantum Yield, Org. Process Res. Dev., 2023, 27(7), 1411–1419, DOI:10.1021/acs.oprd.3c00167.
- S. D. A. Zondag, D. Mazzarella and T. Noël, Scale-Up of Photochemical Reactions: Transitioning from Lab Scale to Industrial Production, Annu. Rev. Chem. Biomol. Eng., 2023, 14(1), 283–300, DOI:10.1146/annurev-chembioeng-101121-074313.
- A. Slattery, Z. Wen, P. Tenblad, J. Sanjosé-Orduna, D. Pintossi, T. den Hartog and T. Noël, Automated Self-Optimization, Intensification, and Scale-up of Photocatalysis in Flow, Science, 2024, 383(6681), eadj1817, DOI:10.1126/science.adj1817.
- G.-N. Ahn, M.-J. Kim, S.-J. Yim, B. M. Sharma and D.-P. Kim, Chemical-Resistant Green Luminescent Concentrator-Based Photo-Microreactor via One-Touch Assembly of 3D-Printed Modules, ACS Sustainable Chem. Eng., 2022, 10(12), 3951–3959, DOI:10.1021/acssuschemeng.1c08240.
- A. Riddell, P. Kvist and D. Bernin, A 3D Printed Photoreactor for Investigating Variable Reaction Geometry, Wavelength, and Fluid Flow, Rev. Sci. Instrum., 2022, 93(8), 084103, DOI:10.1063/5.0087107.
- E. G. Gordeev, K. S. Erokhin, A. D. Kobelev, J. V. Burykina, P. V. Novikov and V. P. Ananikov, Exploring Metallic and Plastic 3D Printed Photochemical Reactors for Customizing Chemical Synthesis, Sci. Rep., 2022, 12(1), 3780, DOI:10.1038/s41598-022-07583-9.
- F. Schiel, C. Peinsipp, S. Kornigg and D. Böse, A 3D-Printed Open Access Photoreactor Designed for Versatile Applications in Photoredox- and Photoelectrochemical Synthesis**, ChemPhotoChem, 2021, 5(5), 431–437, DOI:10.1002/cptc.202000291.
- S. Rossi, M. V. Dozzi, A. Puglisi and M. Pagani, 3D-Printed, Home-Made, UV-LED Photoreactor as a Simple and Economic Tool to Perform Photochemical Reactions in High School Laboratories, Chem. Teach. Int., 2020, 2(2), 20190010, DOI:10.1515/cti-2019-0010.
- M. R. Penny and S. T. Hilton, 3D Printed Reactors and Kessil Lamp Holders for Flow Photochemistry: Design and System Standardization, J. Flow Chem., 2023, 13(4), 435–442, DOI:10.1007/s41981-023-00278-w.
- M. Renner and A. Griesbeck, Think and Print: 3D Printing of Chemical Experiments, J. Chem. Educ., 2020, 97(10), 3683–3689, DOI:10.1021/acs.jchemed.0c00416.
- T. Aubineau, J. Laurent, L. Olanier and A. Guérinot, Design, Characterization and Evaluation of a Lab-made Photoreactor: A First Step Towards Standardized Procedures in Photocatalysis**, Chem.: Methods, 2023, 3(11), e202300002, DOI:10.1002/cmtd.202300002.
- D. Kowalczyk, P. Li, A. Abbas, J. Eichhorn, P. Buday, M. Heiland, A. Pannwitz, F. H. Schacher, W. Weigand, C. Streb and D. Ziegenbalg, Making Photocatalysis Comparable Using a Modular and Characterized Open-Source Photoreactor**, ChemPhotoChem, 2022, 6(7), e202200044, DOI:10.1002/cptc.202200044.
- G. Glotz and C. O. Kappe, Design and Construction of an Open Source-Based Photometer and Its Applications in Flow Chemistry, React. Chem. Eng., 2018, 3(4), 478–486, 10.1039/C8RE00070K.
- J. M. Aguirre-Cortés, A. I. Moral-Rodríguez, E. Bailón-García, A. Davó-Quiñonero, A. F. Pérez-Cadenas and F. Carrasco-Marín, 3D Printing in Photocatalysis: Methods and Capabilities for the Improved Performance, Appl. Mater. Today, 2023, 32, 101831, DOI:10.1016/j.apmt.2023.101831.
- C. Khositanon, S. Deepracha, S. Assabumrungrat, M. Ogawa and N. Weeranoppanant, Simple Fabrication of a Continuous-Flow Photocatalytic Reactor Using Dopamine-Assisted Immobilization onto a Fluoropolymer Tubing, Ind. Eng. Chem. Res., 2022, 61(3), 1322–1331, DOI:10.1021/acs.iecr.1c04303.
- F. Zhao, D. Cambié, J. Janse, E. W. Wieland, K. P. L. Kuijpers, V. Hessel, M. G. Debije and T. Noël, Scale-up of a Luminescent Solar Concentrator-Based Photomicroreactor via Numbering-Up, ACS Sustainable Chem. Eng., 2018, 6(1), 422–429, DOI:10.1021/acssuschemeng.7b02687.
- D. Cambié, F. Zhao, V. Hessel, M. G. Debije and T. Noël, A Leaf-Inspired Luminescent Solar Concentrator for Energy-Efficient Continuous-Flow Photochemistry, Angew. Chem., Int. Ed., 2017, 56(4), 1050–1054, DOI:10.1002/anie.201611101.
- C. Bottecchia, X.-J. Wei, K. P. L. Kuijpers, V. Hessel and T. Noël, Visible Light-Induced Trifluoromethylation and Perfluoroalkylation of Cysteine Residues in Batch and Continuous Flow, J. Org. Chem., 2016, 81(16), 7301–7307, DOI:10.1021/acs.joc.6b01031.
- F. Meinardi, A. Colombo, K. A. Velizhanin, R. Simonutti, M. Lorenzon, L. Beverina, R. Viswanatha, V. I. Klimov and S. Brovelli, Large-Area Luminescent Solar Concentrators Based on Stokes-Shift-Engineered Nanocrystals in a Mass-Polymerized PMMA Matrix, Nat. Photonics, 2014, 8(5), 392–399, DOI:10.1038/nphoton.2014.54.
- P. J. Sarver, V. Bacauanu, D. M. Schultz, D. A. DiRocco, Y. Lam, E. C. Sherer and D. W. C. MacMillan, The Merger of Decatungstate and Copper Catalysis to Enable Aliphatic C(Sp3)–H Trifluoromethylation, Nat. Chem., 2020, 12(5), 459–467, DOI:10.1038/s41557-020-0436-1.
- E. W. Webb, J. B. Park, E. L. Cole, D. J. Donnelly, S. J. Bonacorsi, W. R. Ewing and A. G. Doyle, Nucleophilic (Radio)Fluorination of Redox-Active Esters via Radical-Polar Crossover Enabled by Photoredox Catalysis, J. Am. Chem. Soc., 2020, 142(20), 9493–9500, DOI:10.1021/jacs.0c03125.
- Z. Zuo, H. Cong, W. Li, J. Choi, G. C. Fu and D. W. C. MacMillan, Enantioselective Decarboxylative Arylation of α-Amino Acids via the Merger of Photoredox and Nickel Catalysis, J. Am. Chem. Soc., 2016, 138(6), 1832–1835, DOI:10.1021/jacs.5b13211.
- M. González-Esguevillas, D. F. Fernández, J. A. Rincón, M. Barberis, O. de Frutos, C. Mateos, S. García-Cerrada, J. Agejas and D. W. C. MacMillan, Rapid Optimization of Photoredox Reactions for Continuous-Flow Systems Using Microscale Batch Technology, ACS Cent. Sci., 2021, 7(7), 1126–1134, DOI:10.1021/acscentsci.1c00303.
- T. Constantin, M. Zanini, A. Regni, N. S. Sheikh, F. Juliá and D. Leonori, Aminoalkyl Radicals as Halogen-Atom Transfer Agents for Activation of Alkyl and Aryl Halides, Science, 2020, 367(6481), 1021–1026, DOI:10.1126/science.aba2419.
- C. L. Allen, D. C. Leitch, M. S. Anson and M. A. Zajac, The Power and Accessibility of High-Throughput Methods for Catalysis Research, Nat. Catal., 2019, 2(1), 2–4, DOI:10.1038/s41929-018-0220-4.
- K. Loubière, M. Oelgemöller, T. Aillet, O. Dechy-Cabaret and L. Prat, Continuous-Flow Photochemistry: A Need for Chemical Engineering, Chem. Eng. Process.: Process Intesif., 2016, 104, 120–132, DOI:10.1016/j.cep.2016.02.008.
- I. C. Andrews, L. J. Edwards, B. S. J. McKay, B. Hudson-Curtis, G. Birkbeck, C. M. Alder and G. C. Cook, High-Throughput Exploration of a Thioxanthone-catalyzed Photoredox C−O Coupling, ChemPhotoChem, 2024, e202300272, DOI:10.1002/cptc.202300272.
- Z. Dong, Z. Wen, F. Zhao, S. Kuhn and T. Noël, Scale-up of Micro- and Milli-Reactors: An Overview of Strategies, Design Principles and Applications, Chemical Engineering Science: X, 2021, 10, 100097, DOI:10.1016/j.cesx.2021.100097.
- S. D. A. Zondag, J. H. A. Schuurmans, A. Chaudhuri, R. P. L. Visser, C. Soares, N. Padoin, K. P. L. Kuijpers, M. Dorbec, J. Van der Schaaf and T. Noël, A Facile Strategy to Determine Photon Flux and Effective Optical Path Length in Intensified Continuous-Flow Photoreactors, ChemRxiv, 2024, preprint, DOI:10.26434/chemrxiv-2024-gfk84.
- Kessil, Intensity Map for single PR160L Cross-section of Illumination Area, https://kessil.com/support/downloads.php, (accessed 2024-01-18).
- X. Liu, Y. Mou, H. Wang, R. Liang, X. Wang, Y. Peng and M. Chen, Enhanced Light Extraction of Deep Ultraviolet Light-Emitting Diodes by Using Optimized Aluminum Reflector, Appl. Opt., 2018, 57(25), 7325, DOI:10.1364/AO.57.007325.
- F. Raymenants, T. M. Masson, J. Sanjosé-Orduna and T. Noël, Efficient C(Sp 3)−H Carbonylation of Light and Heavy Hydrocarbons with Carbon Monoxide via Hydrogen Atom Transfer Photocatalysis in Flow**, Angew. Chem., Int. Ed., 2023, 62(36), e202308563, DOI:10.1002/anie.202308563.
- G. Laudadio, Y. Deng, K. Van Der Wal, D. Ravelli, M. Nunõ, M. Fagnoni, D. Guthrie, Y. Sun and T. Noël, C(Sp3)-H Functionalizations of Light Hydrocarbons Using Decatungstate Photocatalysis in Flow, Science, 2020, 369(6499), 92–96, DOI:10.1126/science.abb4688.
- Z. Wen, D. Pintossi, M. Nuño and T. Noël, Membrane-Based TBADT Recovery as a Strategy to Increase the Sustainability of Continuous-Flow Photocatalytic HAT Transformations, Nat. Commun., 2022, 13(1), 6147, DOI:10.1038/s41467-022-33821-9.
- T. Wan, Z. Wen, G. Laudadio, L. Capaldo, R. Lammers, J. A. Rincón, P. García-Losada, C. Mateos, M. O. Frederick, R. Broersma and T. Noël, Accelerated and Scalable C(Sp 3 )–H Amination via Decatungstate Photocatalysis Using a Flow Photoreactor Equipped with High-Intensity LEDs, ACS Cent. Sci., 2022, 8(1), 51–56, DOI:10.1021/acscentsci.1c01109.
- G. Laudadio, S. Govaerts, Y. Wang, D. Ravelli, H. F. Koolman, M. Fagnoni, S. W. Djuric and T. Noël, Selective C(Sp 3)−H Aerobic Oxidation Enabled by Decatungstate Photocatalysis in Flow, Angew. Chem., Int. Ed., 2018, 57(15), 4078–4082, DOI:10.1002/anie.201800818.
Footnotes |
| † This work is dedicated to Prof. Dr. Klavs F. Jensen (Dept. of Chemical Engineering, Massachusetts Institute of Technology) in honor of his 70th birthday. |
| ‡ Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4re00081a |
| § The term ‘UFO reactor’ is coined within our research group, inspired by its resemblance to science fiction UFOs. While not an acronym, this nickname has become ingrained in our discussions and usage. |
| This journal is © The Royal Society of Chemistry 2024 |