Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Towards greener batteries: sustainable components and materials for next-generation batteries
Palanivel
Molaiyan†
 a,
Shubhankar
Bhattacharyya†
a,
Shubhankar
Bhattacharyya†
 b,
Glaydson Simoes
dos Reis†
*c,
Rafal
Sliz†
d,
Andrea
Paolella
e and
Ulla
Lassi
b,
Glaydson Simoes
dos Reis†
*c,
Rafal
Sliz†
d,
Andrea
Paolella
e and
Ulla
Lassi
 af
af
aResearch Unit of Sustainable Chemistry, University of Oulu, FI-90570, Oulu, Finland
bRISE Research Institutes of Sweden, Division of Bioeconomy and Health, Biorefinery and Energy, RISE Processum AB, SE-89250, Örnsköldsvik, Sweden
cDepartment of Forest Biomaterials and Technology, Swedish University of Agricultural Sciences, Biomass Technology Centre, SE-901 83 Umeå, Sweden. E-mail: glaydson.simoes.dos.reis@slu.se
dOptoelectronics and Measurement Techniques Unit, University of Oulu, 90570 Oulu, Finland
eDipartimento di Scienze Chimiche e Geologiche, Università degli Studi di Modena e Reggio Emilia, Via Campi 103, 41125 Modena, Italy
fKokkola University Consortium Chydenius, University of Jyvaskyla, FI-67100, Kokkola, Finland
First published on 3rd June 2024
Abstract
Batteries are the main component of many electrical systems, and due to the elevated consumption of electric vehicles and portable electronic devices, they are the dominant and most rapidly growing energy storage technology. Consequently, they are set to play a crucial role in meeting the goal of cutting greenhouse gas emissions to achieve more sustainable societies. In this critical report, a rational basic-to-advanced compilation study of the effectiveness, techno-feasibility, and sustainability aspects of innovative greener manufacturing technologies and processes that deliver each battery component (anodes, cathodes, electrolytes, and separators) is accomplished, aiming to improve battery safety and the circularity of end-products. Special attention is given to biomass-derived anode materials and bio-based separators utilization that indicates excellent prospects considering green chemistry, greener binders, and energy storage applications. To fully reach this potential, one of the most promising ways to achieve sustainable batteries involves biomass-based electrodes and non-flammable and non-toxic electrolytes used in lithium-ion batteries and other chemistries, where the potential of a greener approach is highly beneficial, and challenges are addressed. The crucial obstacles related to the successful fabrication of greener batteries and potential future research directions are highlighted. Bridging the gap between fundamental and experimental research will provide critical insights and explore the potential of greener batteries as one of the frontrunners in the uptake of sustainability and value-added products in the battery markets of the future.
1. Introduction
With the growth of the human population reaching 8 billion, energy demand is only expected to increase at high rates to meet society's demands for energy storage technologies, such as rechargeable batteries for electric vehicles and portable electronics.1 The battery industry is a quickly growing business area due to the increased use of portable devices and electric vehicles (EVs) in recent years. This growth is seen especially in rechargeable lithium-ion batteries (LIBs). They have a high energy density, meaning they can store a significant amount of energy in a compact and lightweight package. LIBs also have a longer cycle life compared to other rechargeable battery technologies. They can be charged and discharged hundreds to thousands of times without significant capacity fading. Furthermore, LIBs have a relatively low self-discharge rate. This enables devices to retain their charge for longer periods, making them convenient for intermittent use and reducing the need for frequent recharging. LIBs can also be charged at a faster rate compared to other battery technologies.2LIBs can be produced in various shapes and sizes, making them adaptable to a wide range of applications. They are generally considered more environmentally friendly than many other battery technologies. With the increased demand for LIBs in recent years, particularly due to their wide application in powering electric vehicles and electronic devices, there is a pressing need for the use of sustainable and environmentally friendly chemicals and the development of fabrication techniques.3,4 Some materials used in battery manufacturing related to LIBs, nickel–metal hydride, and beyond Li-ion batteries (e.g. Co, V, Li, graphite, La, Ce, Pr, and Nd) come under the category of critical raw materials (CRMs) as listed by the EU (European Commission, 2020). They do not contain e.g., heavy metals like lead or cadmium, which are harmful to the environment. However, LIBs contain several components that are of environmental concern and do not meet the standards of sustainability and green chemistry principles.
In this respect, there is a continuous search for new types of active electrode materials exhibiting high capacity and energy density. Graphite (Gr) is widely used as an anode material in commercial LIBs, because of its high coulombic efficiency and good cycle stability.5 However, owing to its low theoretical capacity of 372 mA h g−1 and poor rate capability, Gr cannot meet the urgent demand to deliver high-performance LIBs of high energy (storage) capacity and high power density.6,7 Further, Gr is also a primary raw material listed as a CRM in the European Union (EU).7
Several sustainability challenges can be addressed in LIBs and related battery value chains. The most discussed issues are extraction and mining as well as the limited availability of specific and critical raw materials, e.g., lithium, graphite, and cobalt, as well as ethical considerations related to cobalt mining. Sustainability challenges related to battery cell lines are less discussed. The environmental impact of traditional production methods, including the significant use of toxic solvents and halogen-containing binders and electrolytes,8–10 needs more attention from the scientific community. We expect that this review will bring interest from the scientific and industrial communities who seek to fabricate the next generation of sustainable batteries through greener approaches.
2. Anode challenges from the sustainability viewpoint
Anode materials are the negative electrodes in batteries. They play a crucial role in determining the performance, cost, and safety of batteries. However, there are many challenges associated with battery anode materials, such as low specific capacity, volume change, during lithiation and delithiation, and unwanted side reactions.11–13 To overcome these challenges, researchers have been developing various strategies to improve the performance and stability of anode materials, i.e. surface modification by coating, nanostructuring, and composite formation.12,14 In this review, we will focus on the sustainability challenges of anode materials. Gr is the most widely used anode material and has a layered structure that can intercalate lithium ions between its layers. It has a moderate specific capacity of 372 mA h g−1, which is much lower than the theoretical capacity of lithium metal (3860 mA h g−1).12 Gr also suffers from volume change during lithiation and delithiation,12 which can cause mechanical stress, cracking, pulverization, and loss of electrical contact. Moreover, Gr can react with the electrolyte or other components in the battery, forming a solid electrolyte interphase (SEI) layer on the surface. The SEI layer can protect the graphite from further degradation, but it also consumes lithium ions and electrolytes, reducing the available capacity and increasing the internal resistance. Furthermore, the SEI layer may not be stable and uniform, leading to uneven current distribution and dendrite formation. Dendrites are needle-like structures that grow from the graphite and can pierce through the separator, causing short circuits and safety hazards.12 To address these issues, researchers have been trying to modify the surface of graphite with various coatings or dopants that can enhance its conductivity, prevent side reactions, or accommodate volume change.15 Another example is nitrogen doping, which can increase the specific capacity and cycling stability of graphite by creating more active sites for lithium-ion storage.Another promising anode material is silicon (Si), which has a very high specific capacity of 4200 mA h g−1, which is much higher than that of graphite (372 mA h g−1). Silicon can alloy with lithium ions to form Li15Si4 at full lithiation.7,16,17 However, Si also undergoes a huge volume change of more than 300% during lithiation and delithiation, which can cause severe mechanical stress, cracking, pulverization, and loss of electrical contact. Moreover, silicon can react with the electrolyte or other components in the battery, forming a thick SEI layer on the surface.18 The SEI layer can consume a large amount of lithium ions and electrolytes, reducing the available capacity and increasing the internal resistance. Furthermore, the SEI layer may not be stable and uniform.19,20
Li metal as anode is also a promising strategy for high-performance batteries (especially solid-state batteries) due to its enormous theoretical specific capacity (∼3860 mA h g−1). However, Li metal anodes face severe issues regarding the formation of dendrites at high current densities, which leads to a high-volume expansion that leads to the partial or total destruction of the SEI, generating dead Li, which makes its practical application difficult. However, an efficient and sustainable strategy is to composite Li metal with other elements such as carbon, silicon, aluminum, and tin, such that these elements can act as a buffer to alleviate the volume expansion of the Li metal and help to avoid failure mechanisms (volume expansion, formation of dead Li and dendrite growth).21,22
2.1 Graphite (Gr) anode challenges from the sustainability viewpoint
Gr is the most common anode material for LIBs, which are the backbone of the electrified society nowadays. Although countries pledge to decarbonize energy sectors, the mining, purification, and processing of millions of metric tons of Gr (for batteries) have caused huge environmental issues to our ecosystems through pollution and CO2 emissions as well as generating socioeconomic and ethical issues/challenges within and beyond mining communities. Moreover, this urgent situation is expected to worsen due to the fast development and expansion of battery production worldwide until a simple, although not-so-easy action is taken: the complete removal of Gr. Excluding Gr from batteries would be aligned with actions taken by the European Commission (EU) that classified it as a critical raw material. In addition, the increasing demand for batteries may lead to resource depletion and geopolitical conflicts; therefore, finding sustainable materials to replace graphite is a win–win strategy. At this moment, over 90% of all graphite anodes are produced in China, making the global battery industry and EV makers dependent. Hence, the EU declared an urgent need to increase the level of self-sufficiency in this sector to tackle the monopoly.Important aspects should also be taken into consideration while graphite is being used in batteries: its purification to a battery-grade anode requires high quantities of chemicals such as sodium hydroxide (NaOH) and hydrogen fluoride (HF), which pose risks to both human health and the environment.23,24 It is worth mentioning that Gr recycling would be a sustainable approach to alleviate the issues associated with its mining. However, recycling graphite is very challenging due to the complexity and diversity of the battery chemistries, structures, and components. The recycling processes require sophisticated technologies, equipment, and infrastructure, which are often costly and energy intensive. Moreover, recycling rates and efficiencies vary depending on the type of battery and anode material.25
An interesting possibility of avoiding all the issues related to Gr mining is to produce synthetic graphite by using amorphous carbons, which can be formed into graphite by employing elevated temperatures (2300–3000 °C).26 Greener batteries can also be achieved by producing synthetic graphite from biomass-based carbons that present advantages, such as being inexpensive precursors and available abundant worldwide resources, while also having less environmental pollution concerns.26 Graphite also faces important challenges of fast Li+ intercalation due to its sluggish kinetics;27,28 when a Gr anode is charged at high rates, it suffers slow Li+ intercalation due to sluggish kinetics, lithium metal plating, and formation of severe SEI, which hinders the more widespread LIBs commercialization, especially in EV and high-capacity devices. Graphite also faces important limitations when it comes to its application in what is considered the next generation of greener batteries such as sodium-ion batteries (SIBs). Gr does not match the requirements to be employed as anodes for SIBs due to insufficient interlayer spacing for Na+ intercalation.29,30 However, a good strategy for SIBs is the employment of hard carbons as the most suitable anode materials, which use different biomass resources for their fabrication. The employment of biomass based hard carbons is a green strategy to achieve more sustainable batteries compared to the dominant battery chemistry today.
2.2 Biomass-based carbon anodes as a greener strategy for LIBs
As previously discussed, Gr is still the dominant anode material for LIBs but for the sake of the future of high-capacity energy storage systems, the use of graphite as a dominant anode material must come to an end. However, for that to take place, novel and sustainable materials must be developed for the pursuit of greener batteries with higher capacities than graphite-based ones. The development of biomass-based anodes for batteries has grown exponentially over the last decade, as evidenced by the large quantities of published articles worldwide.31–36 Considering this statement, biomass-based carbon anodes have presented themselves as suitable options for batteries due to their easier preparation processes, more sustainable approaches, low CO2 footprint, and non-toxicity.34,37–41 One advantage is that biomass can be easily turned into carbon materials via thermochemical processes, with suitable physicochemical features (required for battery anode application) under much lower energy consumption and costs, which makes their use a greener approach.42 Besides, biomass-based anode materials have been showing excellent electrochemical performances when tested in battery technologies, better than graphite-based anodes.43,44 Many studies have been devoted to the feasibility of using biomass anodes for high-capacity batteries, due to intrinsic biomass-carbon characteristics such as the combination of micro-, meso-, and macropores that is reported to have a positive impact on their electrochemical performance, such as the shortened diffusion length for Li+ ions, which improves the reversible capacity of the battery.The literature shows that amorphous carbons, including both hard and soft carbons, are also obtained from biomass resources. These biobased carbons may present different LIB performances when compared to graphite (see Fig. 1).45 With different graphite voltage profiles, both hard and soft carbons exhibit a sloping nature that will diminish the cell voltage and then limit the energy density. Hard carbons are made of small and disordered graphitic structures that originate in the presence of nanovoids within the material, which help to reduce the volume expansion. This does not happen in graphite; besides, the hard carbon structure defects may provide improved performance such as gravimetric capacities higher than that of the theoretical capacity of graphite (372 mA h g−1).
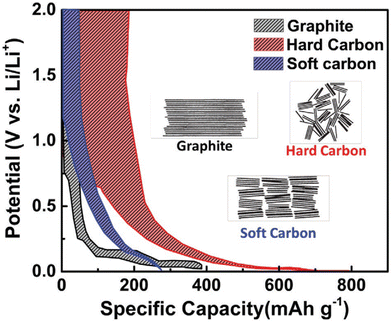 | ||
| Fig. 1 Voltage profiles comparison between Gr, hard carbon, and soft carbon. Reproduced with permission.45 Copyright 2001, Wiley-VCH GmbH. | ||
Carbon materials from biomass, which have fewer aromatic structures, can provide well-developed porous and amorphous structures (hard carbons), and hard carbons are extensively studied for application as anode materials in batteries, especially SIBs. This is an auspicious step towards greener energy storage devices. To advance into the fabrication and real application of biomass-based anodes, it is very important to understand aspects of the different carbon structure formations (hard and soft carbons and graphitic carbons). The structure of each of these carbon materials is severely dependent on the starting material and the pyrolysis temperature; these two parameters have a pivotal influence on the carbon structure formation and show the structure of graphite, hard carbon, and soft carbon, respectively. Dos Reis et al., reported the employment of an easy fabrication of biomass carbon anodes using spruce bark as the precursor.41 Two carbon anodes were prepared using different chemical activation with ZnCl2 (Biochar-1) and KOH (Biochar-2) (see Fig. 2). Biochar-1 exhibited a highly mesoporous structure while Biochar-2 displayed more micropores than Biochar-1. The anode synthesized through ZnCl2 activation exhibited a higher degree of graphitization with less disordered and defective carbon structures, while Biochar-2 displayed more defective carbon structures. When tested electrochemically in LIBs, Biochar-1 showed an excellent rate capability and the most efficient capacity retentions of 370 mA h g−1 at 100 mA g−1 at the end of 100 cycles, 332 mA h g−1 at 500 mA g−1 after 1000 cycles, and 319 mA h g−1 at 1000 mA g−1 after 5000 cycles, suggesting a more sustainable alternative to replace graphite as anode for LIBs. The same Biochar-1 had a better performance on sodium-ion batteries as well.
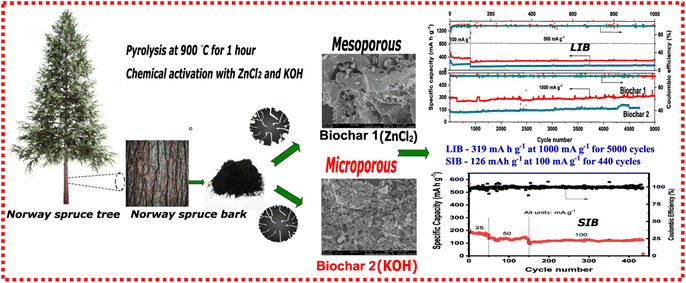 | ||
| Fig. 2 Synthesis of carbon anodes prepared using different chemical activation with ZnCl2 (Biochar-1) and KOH (Biochar-2) and tested in LIBs and SIBs. Reproduced with permission.41 Copyright 2022, American Chemical Society. | ||
Shen et al.,46 prepared an anode based on biomass garlic stem-derived porous carbon. The pyrolysis conditions were evaluated on the material's physicochemical properties and late on battery performance. It was reported that the anode prepared at 800 °C for 2 h yielded a material with a higher surface area with enough micro–meso pore structures, larger layer spacing, and rich in defect sites. Thanks to these characteristics, the biomass-carbon anode delivered a very high-capacity performance, for LIBs, of 480 mA h g−1, with no significant capacity loss at the end of 3000 cycles. The aforementioned studies of employing biomass-based materials as anodes for LIBs undoubtedly paved the path for the future development of a cleaner economy and greener ES systems. Thus, more research is needed in the direction of replacing Gr with more sustainable materials such as biomass resources.
2.3 SIBs as a greener strategy to replace Gr-based LIBs
As society needs to move towards a more sustainable future, renewable energy and energy storage technologies play an increasingly vital role. Because sodium's chemical properties are very similar to those of lithium, it too makes for good batteries.47,48 Sodium, which is extremely abundant in seawater, is thousands of times more abundant than lithium and cheaper as well.48 Moreover, harvesting it has much less environmental impact. Apart from LIBs, in recent years sodium-ion batteries (SIBs) have entered as an alternative energy storage area due to the low natural abundance of lithium. Life cycle assessment shows SIBs create lower environmental impacts compared to LIBs.49 The natural abundance of sodium is 1388 times higher (2.36%) than that of lithium (0.0017%) in the earth's crust.50 From a cost perspective, sodium is 113 times cheaper than lithium; its cost is a significant supply risk of lithium.51 Furthermore, the stability of SIBs in the fully discharged state significantly improves the safety of stored batteries, which is of particular interest for large-sized batteries. It is known that in the short run SIBs cannot overpower LIBs in terms of energy storage. However, the costs and environmental issues associated with LIB production are high, and they could be reduced by considering alternative raw materials and replacing expensive materials – or even replacing the whole chemistry – with new ones such as those in SIBs, especially in stationary storage systems because Na is much more abundant and cheaper than lithium. Several works have been dedicated to the employment of biomass-based carbon on SIBs. Lv et al., reported the use of peanut shell-derived hard carbon in SIBs.52 The hard carbons were made by following two paths; one (PSDHC-600) was prepared by pyrolysis of peanut shells under an inert atmosphere without further treatment while the second hard carbon (PSDHC-600-A) was prepared by KOH activation under the same conditions of PSDHC-600 (see Fig. 3a). Peanut shell-derived hard carbons (PSDHC-600 and PSDHC-600A) were tested in LIBs and SIBs, and their electrochemical results are seen in Fig. 3b. The authors reported large irreversible capacity losses for samples, which could be related to the thick SEI formation. After the first cycle, the irreversible capacity losses disappeared due to the stabilization of SEI, and the CEs increased fast to near 100%. Both samples PSDHC-600 and PSDHC-600A displayed excellent cycling stability, and PSDHC-600A always delivered a higher reversible capacity, indicating that the preparation method with KOH improved the hard carbon physicochemical and electrochemical properties. The capacity of PSDHC-600A (190 mA h g−1) was around 30% higher than PSDHC-600 (130 mA h g−1) at the end of 400 cycles. At a low current rate of 0.05 A g−1, the sample PSDHC-600A delivered a reversible capacity of 325 mA h g−1, which was much higher than that of graphite (35 mA h g−1).53 Similar reversible capacity performances were found by Stevens and Dahn,54 who prepared hard carbon from glucose; when tested in SIBs it delivered a reversible capacity of 325 mA h g−1. Further evaluating the stability of the peanut shell-derived hard carbon (PSDHC-600A), the long cyclability up to 3000 cycles at 1 A g−1 suggested an ultralong cycling stability at high current rate. The electrochemical impedance data indicated that the improved PSDHC-600A performance could also be due to its much lower charge transfer resistance compared to PSDHC-600. The authors reported that this behavior could be attributed to finer pore structure of the PSDHC-600A because of KOH activation that is known to increase the specific surface area of the carbons.37,41,55,56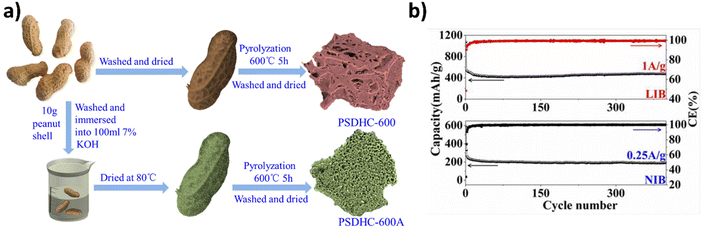 | ||
| Fig. 3 (a) Synthesis routes for the preparation of hard carbons PSDHC-600 and PSDHC-600A. (b) Cycling performances of peanut shell-derived hard carbon for LIBs and SIBs. Reproduced with permission.52 Copyright 2015, Elsevier. | ||
Although SIB is considered a realistic option for the next generation batteries, a key challenge is to fabricate cells with both high energy density and long cycling life. The successful implementation of SIBs depends on the material science involved in their fabrication. Several critical issues in the state-of-the-art of SIB chemistry remain unsolved, from the cell level to commercial products for real environment applications. The SIB electrolyte system has important challenges regarding specific formulations, an optimized solvent, sodium salts, and additives. Since SIBs must perform well under different climatic conditions the electrolytes should have a wider temperature range tolerance while enabling safe and stable cycling. The electrochemical performance of the SIB cathode material is controlled by its specific capacity and redox potential. Layered oxides exhibit high energy density characteristics, whereas the performance of polyanionic material is affected by its high molecular weight, reducing its specific capacity, and ultimately leading to lower energy density. PBAs on the other hand showcase unsatisfactory specific capacity due to the presence of inherent vacancies and coordinated water.57 It seems that coprecipitation of layered oxide materials seems to be only an industrially relevant approach, in which the radial growth of secondary particles is well controlled. This enables perfect sodiation of the whole active material particle (efficient diffusion channels for Na+ in the structure). Further, it seems that the sustainability of current SIBs is overestimated in scientific literature. Still, critical raw elements are used in SIB cathode materials, and PBAs contain cyanide-based complexes. Thus, a lot more research is needed to find and test more suitable electrolyte systems that have the potential to prolong the cycling lifetime and improve safe operation. Manufacturing and industrial aspects of SIBs should also be considered, for instance, aluminium foil (Al) can be employed as the current collector, as Na+ ions do not alloy with Al at the anode side, which implies a big reduction in the battery costs. SIBs also have lower transportation risks, as they can be transported completely discharged. All these impacts on the sustainability level of the SIBs commercialization and utilization. Although the first generation of commercial SIBs are already available in the market, they are only in the preliminary stage, and for light mobile devices. To utilize SIBs in stationary and/or grid storage applications, research efforts must shift from the academic level toward the cell/pack level, supported by industrial investments and inputs, along with policy orientations to bring down prices and popularize this battery technology worldwide.
3. Roles of carbon porosity in high-performance greener emergent batteries
Besides SIBs, other battery configurations such as lithium–sulfur (LSBs) and potassium-ion batteries (KIBs) are emerging as promising candidates for possible replacement of LIBs. LSBs own a high theoretical capacity (1675 mA h g−1) and energy density (2600 W h kg−1).58,59 The worldwide abundance reserves and low toxicity are important characteristics of sulfur, which have boosted the development of LSB technology. On the other hand, KIBs have a theoretical capacity of 591 mA h g−1, KIBs are gaining immense interest as LIBs’ alternative due to their sustainable approach because of the abundance of natural resources of potassium making it inexpensive.60,613.1 Lithium–sulfur batteries (LSBs)
LSBs can be considered a sustainable strategy for greener battery chemistry since there are large reserves of sulfur worldwide, which is also considered a low-cost resource, and are environmentally friendly compared to other elements used in batteries such as boron, phosphorus, and toxic transition metals. Besides, LSBs have a much higher specific energy density of 2600 W h kg−1 compared to LIBs.62 Despite their great suitability to meet the increasing demand for energy storage, LSBs still face several issues with their scalable fabrication, including a fading capacity due to arising from low utilization of sulfur, poor rate performance, and lifetime cycling owing to the shuttle effect of polysulfides. Under such circumstances, the design/fabrication of porous carbon–sulfur composite cathodes is regarded as an effective solution to overcome the above problems.63Porous carbon-derived materials with micro–mesoporous features have been demonstrated as excellent alternatives for improving sulfur utilization and cycle stability due to large pore volume and the reduction of Li-PS diffusion.64 Furthermore, carbon materials can easily be tailored with nanostructures, which enhance electrical conductivity with additional electron pathways and interconnected ion diffusion channels, with short ionic and electronic paths; besides it also immobilizes (by adsorption) the dissolved intermediate polysulfides, it greatly reduces the volume changes during cycling process owing to its porous structure, all these lead to a great improvement of the LSBs electrochemical performance.65,66
It is known that the pore structure of the carbon–sulfur cathode in LSBs relates to the battery electrochemical performance. For instance, an electrode rich in micropores and small mesopores has a high surface area, which allows better contact between sulfur and carbon, and provides abundant reaction/adsorption sites between electrolyte–solid interface; while large mesopores and macropores can be used as buffer vessels for volume changes and as transport channels for Li+, therefore a combination of these pore size structure is crucial for efficient LSBs. Therefore, much research is being dedicated to preparing hierarchical porous carbon, which combines the advantages of micro–mesoporosity that lead to better stability due to the rapid ion transport. Liang et al.,67 prepared a porous carbon rich in mesoporosity (pore size distribution of 7.3 nm), and then added microporosity (pore size less than 2 nm) to the existing mesopores, by further activating is with KOH and pyrolysis. Sulfur at different contents was added into the carbon structure and used as a cathode for LSBs. It was reported that when the sulfur loading was 11.7 wt%, the initial discharge capacity was 1584 mA h g−1 at a high current density of 2.5 A g−1. According to the authors, such great performance of porous sulfur–carbon cathode was attributed to the synergetic effect of the hierarchically structured meso/microporosity: the microporosity gave high surface area and the micropore volume functions as a container that retains the sulfur species at the cathode structure, while the mesoporosity served as an avenue for the mass transport of Li+ and thus confers a high ionic conductivity to the cathode.
Li et al.,68 reported a facile and scalable strategy for the in situ synthesis of sulfur nanoparticles in 3D-porous graphitic carbon and employed as a cathode for LSBs. Due to the highly porous structure, the sulfur nanoparticles were efficiently loaded within the pores of the graphitic carbon (up to 90 wt% of sulfur). Because of the high sulfur content, the nanoscale distribution of the sulfur particles, and the covalent bonding between the sulfur and the graphitic carbon, the LSBs displayed outstanding performances, with specific capacities of 1382, 1242, and 1115 mA h g−1 at 0.5, 1 and 2C, respectively. Facing only a small capacity decay (0.039% per cycle over 1000 cycles at 2C) and excellent rate capability at a high charge/discharge current. Yu et al.,69 prepared porous carbon monoliths with high surface area (1426 m2 g−1), and elevated pore volume (3.097 cm3 g−1) thanks to the presence of predominant microporosity. Because of the elevated pore volume, the sulfur particles were easily accommodated into it, yielding up to 75 wt% of sulfur into the cathode materials, which delivered a high initial discharge capacity of 1305 mA h g−1 at a current density of 0.1C.
Zhou et al.,70 prepared sustainable microporous graphitic carbon (MGC) from peanut shells via activation and graphitization promoted by K2FeO4 (Fig. 4a). Further, the prepared MGC materials were composited with sulfur (S/MGC composites) to be employed as cathodes for LSBs. The MGC materials presented more microporous features with small mesopore contribution, which were able to accommodate/confine small sulfur molecules (S2–4), a prerequisite for efficient LSBs cathodes. The microporous and mesoporous structures are interconnected so that the electrolytes and polysulfides can easily transport between the porous structures, which improves the battery's performance. Fig. 4b and c show the rate performance and discharge–charge profiles at different C-rates of 1146, 990, 886, 782, 675, 570 mA h g−1 at 0.1, 0.25, 0.5, 1, 2, and 4C, respectively, highlighting the suitability of porous carbon materials as excellent cathodes for high-performance LSBs. Further analysing the S/MGC performance on LSBs, Fig. 4d shows that the discharge capacity of 826 mA h g−1 after 1000 cycles at 1C is achieved with a CE near 100%. Even at a higher rate of 2C, 571 mA h g−1 remains after 1000 cycles. Such results evidence the potentiality of porous carbon on highly effective cathode materials for LSBs.
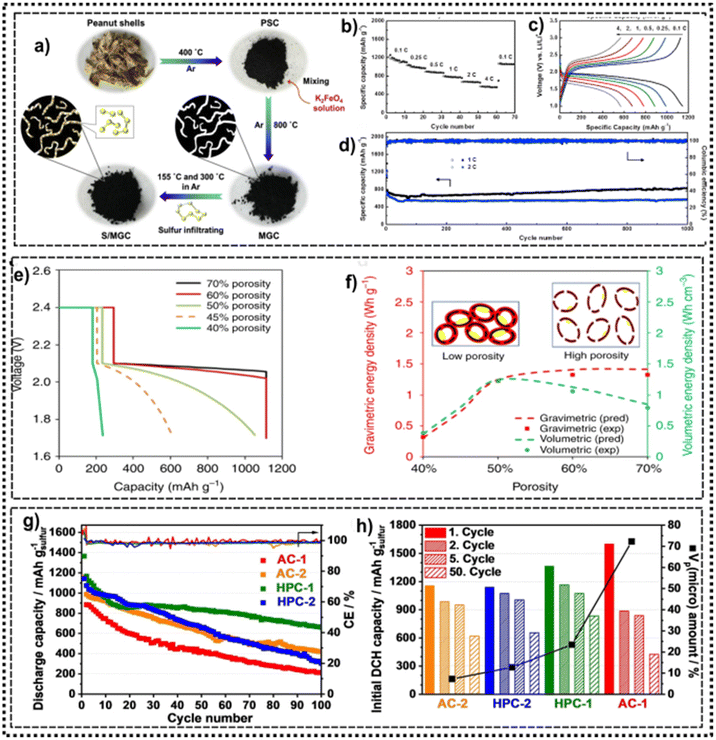 | ||
| Fig. 4 (a) The preparation process of the S/MGC composite, (b) capacity retention at different rates, (c) discharge–charge voltage profiles at different rates, (d) long-term cycling test at 1C and 2C (0.1C for the 1st cycle). Reproduced with permission.70 Copyright 2018, Elsevier. (e and f) The different range (wt%) of porosity for discharging profiles and gravimetric energy density of the cathodes for LSB. Reproduced with permission.71 Copyright 2019, Springer Nature. (g) Galvanostatic cycling performances of all samples, (h) correlation of discharge capacities and the percentage amount of the micropore volume. Reproduced with permission.72 Copyright 2021, Wiley-VCH GmbH. | ||
The effect of porosity of the cathodes on the LSBs was deeply evaluated by Kang and co-works71 and the predicted discharging curves of LSBs cells by using cathodes with different porosities are shown in Fig. 4e. The cathodes with porosities at 60–70% exhibited the highest discharging curves with negligible changes between them. Cathodes with lower porosity shortened the first plateau and depressed the second plateau, exhibiting a fast-dropping capacity, with the lowest porosity showing the lowest capacity, which was in accordance with experimental observation. The impact of porosity on the electrochemical performance can be summarized in Fig. 4f. The unutilized S, carbon matrix and deposited Li2S2/Li2S layer were represented as yellow, black and red, respectively.
Kensy et al.,72 evaluated the impact of carbon microporosity on sulfur conversion in LSBs cathodes and the electrochemical performances. Carbon materials with varying pore diameter and architecture (micropores, mesopores, and hierarchical pores) are studied as scaffold for LSBs cathodes. For carbon materials with different amounts of micropores are obtained (AC-1: 72%, AC-2: 7%, HPC-1: 24%, HPC-2: 13%). Fig. 4g shows very high initial discharge capacities of 1600 mA h gS−1, 1157 mA h gS−1, 1365 mA h gS−1, 1141 mA h gS−1 are observed for AC-1, AC-2, HPC-1 and HPC-2, respectively. Due to the reduced current at the first discharge the equilibrium time for precipitation of Li2S is enhanced and therefore, the sulfur utilization can be significantly improved.73 Further analysing the effect of microporosity on the LSBs performances, a correlation between the micropore volume and the value of the initial discharge capacity is shown in Fig. 4h. It shows that for cathode materials with higher percentage of micropores they exhibited higher initial capacities.72,74 The authors reported that the cathodes AC-2 and HPC-2 showed reduced amount of micropores, which impacted on the sulfur conversion that arose from the mesopores. The enhanced capacity degradation of AC-1 is attributed to the reaction in the first cycle, while the high micropores amount played a crucial role. Further, the porosity properties of HPC-1, (e.g., high specific surface area (SSA)), reflected in a higher number of active sites for the electron transfer from the conductive carbon to the sulfur species resulting in an improved capacity retention and cycling performance,75 suggesting that SSA played important role on the cycle stability as well as sulfur utilization. For instance, HPC-1, which exhibited the highest SSA (2717 m2 g−1), also exhibited a great sulfur utilization to maintains the highest capacity retention of 661 mA h gS−1 at 100th cycle.
3.2 Potassium ion batteries (KIBs)
In pursuit of alternatives to LIBs, another prominent battery technology is the potassium-ion battery (KIB), which is also considered a promising type of battery with a low cost and greener approach. Potassium (K+) has an estimated total concentration of 2.09 wt% in the earth's crust,76 compared with lithium (0.0017%), therefore indicating a low-cost advantage, and it can be considered an important component for shuttle-carrier in rocking-chair type barriers for stationary (off-grid) storage applications where the battery size does not matter. The large K+ radius (2.80 Å) may result in a significant volume variation of electrode materials during the K+ intercalation/deintercalation process;77 thus, the anode material for KIBs is expected to have well-developed porosity with high specific surface area, high pore size distribution, elevated pore volume, and big interlayer space within carbon lattice/framework to be able to accommodate K+ and the volume change associated to it.77 These enable achieving high performance for KIBs. The literature shows that employing porous materials with high SSA increases the contact area between electrolyte–electrode to shorten the ion diffusion distance and enhance the capacitive behaviour, which decisively boosts the rate performance and increases the cycling stability of the KIBs.78,79 Moreover, porous anodes, due to the high surface area, not only provide abundant potassium storage sites but also accommodate the volume expansion of the anodes, showing improved structural stability.Wu et al.,79 studied the effects of lignin structure (molecular weight's role) and preparation method on anode performance on storing K-ion. It was reported that K+ storage in lignin-based carbon anode depends on bulk-insertion and surface-adsorption sites, which are influenced by both characteristics of lignin precursor and preparation method. Moreover, K+ insertion effectively occurs in the interlayer spaces of Gr-like nanocrystals as well as in disordered carbon areas, which are common in porous materials. The authors reported that carbon anodes with more graphitic (less disordered carbon) structures minimize the role of K+ (Fig. 5a) due to not sufficient interlayer space between carbon graphitic sheets. In contrast, a carbon rich in disordered structures enhances the K-ion storage via the surface-adsorption mechanism (Fig. 5b and c). Carbon anode materials with a balanced combination of disordered and graphite-like structures (typically from high surface area carbons) are more suitable for better performance KIBs through the combined surface adsorption and bulk-insertion of K+ (Fig. 5b).
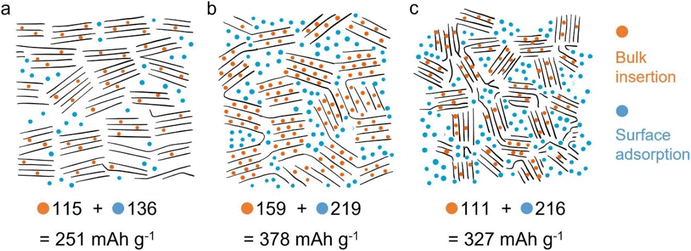 | ||
| Fig. 5 Proposed K-ion storage mechanisms in L700 (a), M700 (b), and H700 (c). Reproduced with permission.79 Copyright 2022, Elsevier. | ||
The authors highlighted that the above findings would be inspirable by biomass-derived carbon anodes for the different battery applications (KIBs, LIBs, and SIBs). The importance of the lignin molecular weight (and possibly the biomass molecular weight as well) in boosting the K-ion storage performance. Moreover, proving that lignin structures play an important role in the electrochemical performances can also expand the different biomass type precursors and their structures. The physicochemical features of the biomass-derived anodes play a crucial role in its electrochemical metrics, due to the accumulation of ions at the micropores, transport, diffusion, and storage of ions at the mesopores and macropores. The different hierarchical pores and surface properties enable the anodes to maximize their specific capacity and shed positive impacts for future developments.
Yang et al.,80 prepared a biomass-based porous anode material and when applied in KIBs it delivered a very high specific capacity of 407 mA h g−1 at 50 mA g−1, and 163.8 mA h g−1 at 200 mA g−1 after 50 and 100 cycles. They concluded such great KIB efficiency was due to the anode physicochemical properties such as its well-developed porosity, richness in structural defects/edges that helped alleviate volume change, and improved stability of the battery. Besides, the authors concluded that the hierarchical porous structures boosted the amorphous degree, which enabled the rapid K+ diffusion and improved the electrolyte wettability of the anode for better K+ insertion/extraction. These findings may show that the physicochemical features such as porosity and pore features of the new battery anodes play a crucial role in its electrochemical metrics, due to the accumulation of ions at the micropores, transport, diffusion, and storage of ions at the mesopores and macropores. The different hierarchical pores and surface properties enable the anodes to maximize their specific capacity and shed positive impacts for future developments.
4. Choice of electrolytes and challenges
A general commercial liquid electrolyte is composed of a salt (e.g., LiPF6)82 dissolved in an organic solvent like a mixture of carbonate (e.g. ethylene and diethylene carbonate83). Ethers are also used as electrolytes. A low boiling point mixture composed of dioxolane84 (boiling point 74 °C) and dimethylether85 (boiling point 85 °C) can solvate lithium ions, showing high ionic conductivities. The boiling point can be increased by using a glyme such as tetraethylene glycol dimethyl ether or tetraglyme86 (boiling point 275 °C). Ionic liquids are thermally solvents up to 300–400 °C that are ionized: they are the result of an association of an organic cation (e.g., imidazolium, phosphonium pyridinium, pyrrolidinium, alkylammonium) and an anion. Because of their properties, ionic liquids are exploited to replace liquid electrolytes in LIBs.87About salts, lithium hexafluorophosphate (LiPF6) is one of the most exploited salts in commercial Li-ion batteries88,89 because it shows high ionic conductivity values and good electrochemical stability when dissolved in dipolar aprotic organic solvents but poor thermal stability above 55 °C. LiPF6 easily decomposes forming PF5 gas that hydrolyzes forming HF and PF3O by trace amounts of water. Then, pentafluorophosphorane (PF5) reacts with the solvents to generate highly toxic chemicals and also initiates the polymerization of dioxolane acting as a nucleophilic agent.90,91 Due to the limitations of LiPF6, new salts have been explored but, unfortunately, the possible alternatives are not able to show any: LiAsF6 is poisonous92 and LiClO4 is explosive.93 The anion (BF4) in LiBF4 is not very conductive and reacts with Li metal.94 Similarly, SIBs use halogen-containing liquid electrolytes, such as NaPF6, and therefore, new types of sustainable electrolyte solutions are urgently needed.
Solid state batteries (SSBs) based on SEs are promising candidates for next-generation batteries with benefits of safety, energy density, low cost, and mechanical and thermal stabilities. Solid electrolytes can be divided into four chemistries: (a) polymers, (b) oxides, (c) sulfides, and (d) halides.95,96 Polymer electrolytes are versatile and have price competitiveness because they share properties and manufacturing processes with liquid electrolytes. Polymers of ethylene oxide (PEO)-based materials are widely used as polymer hosts in commercial solid-state electrolytes. The main limitation of PEO originates from the high crystallinity of the EO chains, which results in low ionic conductivity.97 Polyethylene carbonates can be easily converted to volatile ethylene carbonate through a water-catalyzed ring-closing reaction.98,99 Lithium metal dendrite can penetrate any electrolytes due to the existence of voids between ceramic particles.100 Oxides (e.g., garnet Li7La3Zr2O12) are thermally stable but tend to form insulator Li2CO3 without developing any hazardous gas.96,101 On the contrary, sulfides and halide electrolytes generate toxic gases (H2S and HCl, respectively)102,103 when in contact with moisture.
4.1 Nanocellulose as a battery electrolyte component
Battery components are often fossil based: thus, making batteries with biobased material is the future goal of battery researchers. Several fossil battery components have already been replaced with biobased material, although, due to their unique properties, replacing electrolytes with bio-based material is the most challenging task. In this regard, several nanocellulose-based materials have been studied in depth, both for electrolytes and as a separator application.104 Nanocellulose is a renewable, biodegradable, and nontoxic, as well as easily available biopolymer from cellulose. In plant cell walls cellulose is organized into microfibrils, which consist of the organized or crystalline form as well as the less organized or amorphous form. The amorphous parts can be removed preferentially by a chemical reaction, i.e., selective acid hydrolysis, after which only the organized or crystalline parts of the cellulose remain.105 The remainder is nanocrystalline cellulose or microcrystalline cellulose, depending on the reaction conditions and application demands. With an increasing demand for high-performance renewable materials with tailor-made mechanical and physical properties, cellulose nanocrystals or nanocrystalline cellulose (CNC/NCCs) have become the most attractive material for diverse applications. CNCs have many different, interesting properties which include a high specific Young's modulus similar to Kevlar and steel, non-toxicity, the ability to form lyotropic liquid crystals, promising reinforcing properties due to their amphiphilic nature, and a high aspect ratio.106 CNCs can also act as rheological modifiers and interface stabilizers (e.g., in emulsions, gels, and foams).107 Another interesting property of CNCs is their high colloidal stability due to the presence of highly charged functional groups that are adsorbed on the surface of CNCs during their production. Due to CNCs diverse properties and increasing demands, many producers around the globe started to produce CNCs in bulk scale (Table 1).| Producers | Process | Capacity |
|---|---|---|
| CelluForce, Canada | Sulfuric acid hydrolysis | 300 |
| American Process, USA | SO2 fractionation | 130 |
| Sweetwater Energy | Enzymatic hydrolysis | 50 |
| RISE, Sweden | Sulfuric acid hydrolysis | 3.6 |
| Alberta Innovates, Canada | Acid hydrolysis | 5 |
| U.S. Forest Products Lab, USA | Sulfuric acid hydrolysis | 3 |
| Blue Goose Biorefinery, Canada | Catalytic conversion | 2 |
| FPInnovations, Canada | Sulfuric acid hydrolysis | Pilot |
Recently, nanocellulose-based gel polymer electrolytes have gained tremendous attention due to their unique properties. However, Wang et al.,109 reported that the presence of a significant number of hydroxyl groups in a nanocellulose dense network structure can result in serious disadvantages for making gel polymer electrolytes, such as reduced electrolyte uptake ratio and electrochemical performance. Wang et al., also explained that lithium intercalation can be hindered by the presence of deposited sodium ions on nanocellulose hydroxyl groups which can result in fast fading of capacity. Possibilities of a reaction between Li+ and the hydroxyl as well as carboxyl groups can cause poor interface stability and charge–discharge efficiency. Due to the presence of hydroxyl groups, nanocellulose-based materials tend to absorb water into their crystal structure which can be detrimental to battery performance. To circumvent these issues, Wang et al., modified the nanocellulose by acetylation of hydroxyl groups. Acetylation of nanocellulose was performed by using a pyridine/acetyl chloride reagent in a dimethylacetamide (DMAc) solvent system (Fig. 6-I). All samples were evaluated by preparing nanocellulose films via the solvent casting method.109
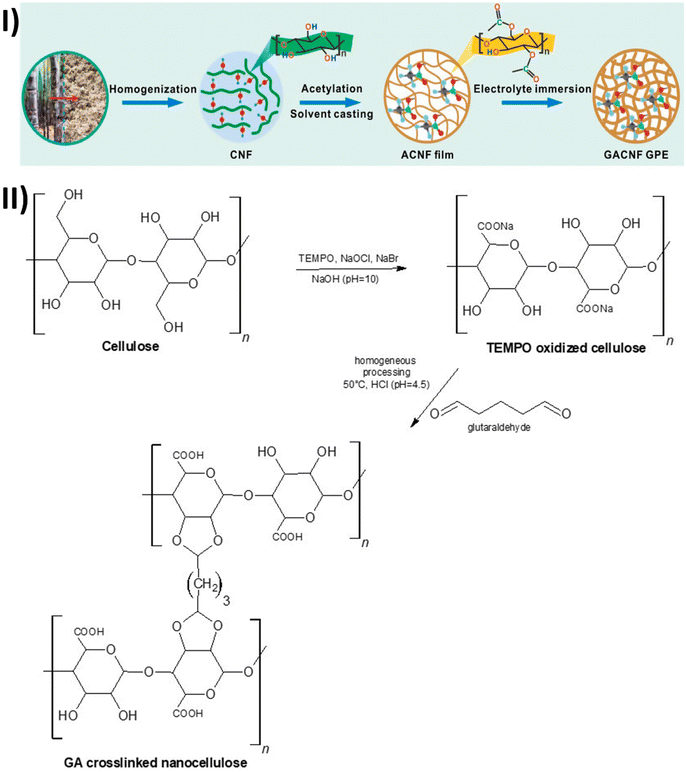 | ||
| Fig. 6 (I) A scheme of preparation of acetylated cellulose nanofibril-based gel polymer electrolyte (GPE). Reproduced with permission.109 Copyright 2022, Elsevier. (II) Scheme for preparation of glutaraldehyde crosslinked nano cellulose-based gel polymer electrolyte (GPE). | ||
From the TGAPVDF and DTG curve, Wang et al. showed that minimum thermal stability impact was observed by acetylation of nanocellulose. However, electrolyte uptake increased significantly up to 301% and ionic conductivity was found to 2.73 mS cm−1. Additionally, the lithium-ion transfer number reached up to 0.65 while the electrolyte/electrode resistance was only 152 Ω and was stable for 18 days of storage. In addition to these, 88.8% capacity retention was observed after 100 cycles at 0.2C when the battery was assembled in the Li/GPE/LiFePO4 system.
To improve the mechanical properties of nanocellulose, Gou et al.,81 performed TEMPO/NaClO/NaBr mediated oxidation of cellulose. Then the oxidized nanocellulose was cross-linked with varying weight percentages of glutaraldehyde (Fig. 6-II). The cross-linked nanocellulose membrane was prepared by freeze-drying, followed by a vacuum filtration mold. Then the cross-linked nanocellulose membrane was evaluated by preparing gel polymer electrolyte (GPE). Although modified nanocellulose did not show significant thermal stability improvement, ionic conductivity was lower than acetylated nanocellulose by 0.91 mS cm−1, which was reported by Wang et al.109 Similarly, poor performance was observed for lithium-ion transfer number 0.42 compared to 0.65, reported by Wang et al.,109 for acetylated nanocellulose. Moreover, capacity retention and coulombic efficiency were 89% and 97%, respectively, after just 50 cycles. This also signifies that this type of modification is not suitable for battery application and will result in worse quality nanocellulose material for a GPE application.
Recently, Mittal et al., reported110 that cellulose nanocrystal (CNC) alone cannot form a stable structure to use as a gel polymer electrolyte for SIBs. Due to the high crystallinity of cellulose nanocrystals, materials produced from solely CNCs are often very brittle. However, blending CNC with other cellulosic materials shows good compatibility with electrolyte counterparts through intermolecular hydrogen bonding as well as van der Waals interaction.111 Mittal et al.110 also showed that with the incorporation of flexible and long CNF fibers into CNCs (Fig. 7a), mechanical properties improved significantly, although the addition of a cross-linker, glutaraldehyde, is necessary to stabilize the gel structure via the formation of acetal linkages between diol groups of nanocelluloses.110,112 Thus, by mixing CNCs and CNFs followed by glutaraldehyde crosslinking, Mittal et al. achieved ionic conductivity values up to 2.32 mS cm−1 at room temperature with a transference number of 0.637. By assembling Na2Fe2(SO4)3/Na half cells, the capacity of the battery reaches 80.6 mA h g−1 after 25 cycles at a 1C rate which results in the gravimetric energy density of 240 W h kg−1 at a rate of 1C. Nevertheless, some commercial cellulose derivatives have also been studied significantly for gel polymer electrolyte application. Among them is hydroxyethyl cellulose (HEC), which is a nonionic water-soluble biopolymer. It is biocompatible, renewable, and low cost with excellent properties such as dispersing, emulsifying, stabilizing, binding suspending, and thickening. It has been used in numerous different applications for many decades.113 Li et al., have reported that gels114 formed from hydroxyethyl cellulose (HEC) are very dense compared to porous ones in other types of gel membranes, which can further reduce the micro-short circuit. Furthermore, the preparation of the hydroxyethyl cellulose gel membrane is simple compared to other types of cellulose membranes. Due to the water-soluble nature of hydroxyethyl cellulose, the membrane can be easily obtained by dissolving in water followed by evaporation (Fig. 7c).
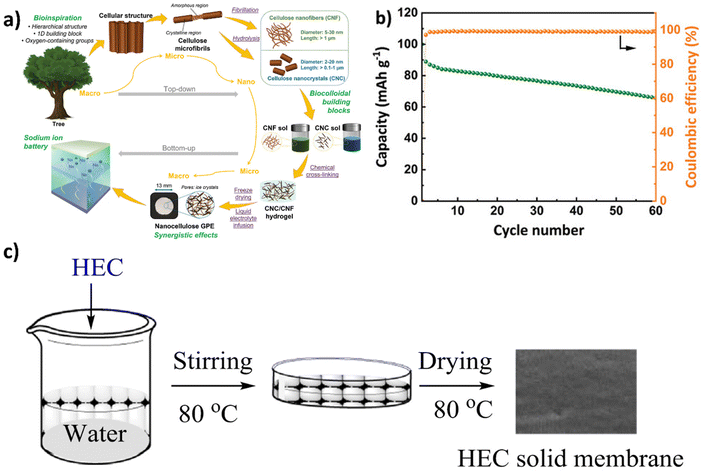 | ||
| Fig. 7 (a) The nanocellulose gel electrolyte fabrication via two different approaches (top-down and bottom-up). (b) The electrochemical testing at 1C (1C = 120 mA g−1) in Na2Fe2(SO4)3/Na half-cell configuration for the CNC/CNF 50/50 GPE discharge capacity and CE. Reproduced with permission.110 Copyright 2022, Wiley-VCH GmbH; (c) a schematic representation of simple preparation of hydroxyethyl cellulose-based membrane for gel polymer electrolyte. Reproduced with permission.114 Copyright 2015, Elsevier. | ||
The membrane made from hydroxyethyl cellulose shows very good thermal stability, up to 280 °C with organic electrolyte uptake up to 78.3%. It also enhanced the organic electrolyte retention ability; the organic electrolyte starts to evaporate after 75 °C. The lithium-ion transference number is very high for this type of gel membrane (0.48) compared to the commercial separator Celgard 2730 (0.27). When the hydroxyethyl cellulose-based gel membrane was evaluated using LiFePO4 cathode material, capacity was found to be around 110 mA h g−1 at 0.2C with 100% efficiency after 50 cycles. Cellulose-based polymers can dissociate lithium salts by absorbing organic solvents also promotes lithium-ion migration across the system.115 Zhao et al.,116 showed that by the addition of polyethylene glycol (PEG) the movement of lithium-ion was enhanced significantly by continuous dissociation–coordination interaction between lithium ions and the polyether groups. The prepared cellulose/PEG-based gel polymer electrolyte membrane showed a very good tensile strength of 33.92 MPa to 211.06 MPa with a lithium-ion conductivity from 1.49 to 3.31 mS cm−1. However, the performance depends on PEG content, and it was found that 5% PEG content was optimal with high ionic conductivity. In addition to this, the lithium-ion transference number was found to be 0.63. When the cellulose/PEG membrane was assembled in the battery, the initial discharge capacity was 159.3 mA h g−1 with a CE of 85.52%.
Some cellulose-based composite material has also been studied for use as gel polymer electrolyte (GPE). Liu et al., reported that thermoplastic polyurethane (TPU) has excellent compatibility with cellulose and can form stable composites for high-performance LIBs.117 TPU with ether bond is already a proven, promising host polymer for GPEs due to its special stability as well as structural flexibility.118,119 TPU consists of two different microstructure phases, i.e., hard as well as soft segments. Polyhydric alcohols with ether bonds are the key functionalities in soft segments that are capable of solvation of alkali metal ions and resulting in good transportation of alkali metal ions.118 Liu and his coworkers managed to prepare cellulose-based TPU composites by a solvent casting method with considerably high ionic conductivities (0.482 mS cm−1) with lithium-ion transfer number 0.68. Moreover, when constructed with LiFePO4/Li cell using a cellulose-based TPU composite, yields 91% capacity retention after 200 cycles with 2C current.
Recently Du et al., showed that it is possible to make fully biobased cellulose GPE via one-step casting and cross-linking by using a biobased cross-linker.120 Du et al., used cellulose as a main polymer matrix due to its low cost, biodegradability, and abundance, and for crosslinking, they used epichlorohydrin (ECH). Epichlorohydrin is one of the key commodity chemicals that is used in many industries including polymers, resins, plastics, etc. The global market volume of epichlorohydrin amounted to roughly 2.16 million metric tons in 2022. Currently, glycerol, which can be obtained from biofuel processing, is used as feedstock for epichlorohydrin production.121 Du et al., found that with increasing cross-linking from 5% to 9%, mechanical properties improved significantly, but the swelling ratio decreased from 542% to 297%. Thus, cross-linking with 5% ECH exhibited the most satisfactory ionic conductivity (6.34 mS cm−1) and high lithium-ion transference number (0.82). Assembled cells by using this GPE showed a discharge capacity of 145 mA h g−1 after the first cycle at a 0.2C rate with a capacity retention of 90% after 50 cycles.
Nevertheless, some commercial cellulose derivatives are also reported to be used as gel polymer electrolytes (GPEs). Zhu et al., have used carboxymethyl cellulose (CMC) for such applications. Carboxymethyl cellulose (CMC) is a linear polymeric cellulose derivative with carboxymethyl functionality (–CH2COO–). In this study, Zhu et al., used commercial CMC with a molecular weight of 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and degree of substitution (DS) = 0.9. However, the electrolyte uptake was low (75.9%) compared to cellulose derivatives reported by other authors. Zhu et al., achieved very high ionic conductivity (0.48 mS cm−1) of the CMC gel polymer membrane when soaked with electrolyte solution at room temperature. In addition to this, the lithium-ion transference number was very high (0.46) in the CMC gel membrane at room temperature compared to the commercial separator Celgard 2730 (0.27). When assembled with a LiFePO4 cathode, the reversible capacity was 140 mA h g−1 at 0.2C without any fading of capacity after 50 cycles. By contrast, commercial Celgard 2730 has a reversible capacity of 126 mA h g−1 at 0.2C, which is much lower compared to CMC-2.
000 and degree of substitution (DS) = 0.9. However, the electrolyte uptake was low (75.9%) compared to cellulose derivatives reported by other authors. Zhu et al., achieved very high ionic conductivity (0.48 mS cm−1) of the CMC gel polymer membrane when soaked with electrolyte solution at room temperature. In addition to this, the lithium-ion transference number was very high (0.46) in the CMC gel membrane at room temperature compared to the commercial separator Celgard 2730 (0.27). When assembled with a LiFePO4 cathode, the reversible capacity was 140 mA h g−1 at 0.2C without any fading of capacity after 50 cycles. By contrast, commercial Celgard 2730 has a reversible capacity of 126 mA h g−1 at 0.2C, which is much lower compared to CMC-2.
5. Biobased-based separators
A separator is an important component in the battery to prevent the short circuit of the battery by separating the anode and cathode. Although the separator acts as an inert component compatibility between the separator and electrolyte is critical for evaluating the safety as well as the performance of the battery. In addition to this, separators also allow Li+ ions to transfer between electrodes during charging and discharging cycles while acting as a physical barrier with electronic insulation properties.110Nowadays, the commercially available separators are glass fiber or polyolefin materials, which are known to be thermally unstable and lyophobic to the electrolytes, leading to short circuits and leakage of electrolyte under high temperatures and deficiency in ionic conductivity and cell performance which results in safety and quality issues with batteries. Luo et al., and others presented that the ideal separator in batteries for commercial purposes should have the following properties:122–127
(1) Physical barrier with electronic insulation between the electrodes.
(2) Correct pore size to increase ionic conductivity by transferring ions. It should be smaller than electrode materials.
(3) The thick membrane can increase the lithium-ion resistance. Thus, the membrane should be thin. For lithium ions, 25 μm thickness is preferred.
(4) Appropriate porosity (>∼40%) allows fast ion diffusion during battery operation.
(5) The separator should have uniform permeability across the whole separator membrane to uniform distribution of current density.
(6) Resistant to highly polar organic solvents with high chemical and electrochemical stability.
(7) High liquid electrolyte absorption capacity as well as wettability.
(8) Excellent tensile strength as well as mechanical properties.
(9) Excellent thermal stability without any structural deformation during battery operation.
(10) Low production cost for commercial application.
Separators can be divided into three main categories: (1) composite membranes, (2) microporous membranes, and (3) polymer blends.126,128 Various polyolefins (e.g. polyethylene or polypropylene) have been used extensively as separators. Nevertheless, polyvinylidene difluoride (PVDF) and its copolymers are widely used as separators in batteries.129 However, due to environmental concerns about these fossil-based separators, a cellulose-based separator opened a new door of separator development for battery research. The first commercial report on nanocellulose separators was published by Isao at Asahi Chemical Industry.130 Isao used nanofibrillated cellulose with sizes of 500 nm to 5000 nm to prepare a composite nanocellulose separator with a thickness of 39–85 μm and 10–200 nm average pore diameter. The maximum ionic conductivity was achieved at 1.2 mS cm−1 at 1 kHz and also exhibited high-capacity retention of 78 mA h g−1 after 41 charge/discharge cycles. Recently, Gou et al., reported a novel way of preparing nanocellulose-based highly porous separators.131 Gou and his co-workers used polystyrene spheres of 1 μm diameter as templates for controlling pore size. The polystyrene spheres were mixed with nanocellulose to form a stable dispersion. Then the gel was formed by using ethanol. At this stage, the polystyrene spheres are embedded in the nanocellulose network. Then, by immersing the gel into a toluene bath, the polystyrene will dissolve, resulting in a 1 μm porous void within the nanocellulose network. The prepared membrane showed 83% capacity retention after 100 cycles and a discharge-specific capacity of 105.6 mA h g−1 at 2C, which was an outstanding performance compared to commercial examples e.g. Celgard 2730. Apart from nonfunctionalized nanocellulose, tempo-oxidized nanocellulose has been reported as a promising candidate for battery separators. Kim et al., reported that tempo-oxidized nanocellulose-based separator membranes can be used in batteries. However, it was observed that the separator showed very poor electrochemical stability. It is due to evolution gas during battery performance which results in the deposition of sodium ions on the graphite surface. Kim et al. found out that the H2 gas evolution can be suppressed by the addition of an additive vinylene carbonate (VC). Recently, the evaporation-induced self-assembly (EISA) method has been reported by Gonçalves et al. to prepare cellulose nanocrystal (CNC) based separator membranes.132 Goncalves used tetramethyl orthosilicate (TMOS) as a template for pore size adjustment which was washed with NaOH to create pores. They obtained a high ionic conductivity of 2.7 mS cm−1 with low interfacial resistance due to loosely packed cellulose nanocrystals. When it was assembled with LiFePO4, a discharge capacity of 122 mA h g−1 and a specific capacity of 85 mA h g−1 at 0.5C and 2C rates, respectively, resulted. After 60 cycles, it shows good capacity retention, which is promising for the long-term stability of the membrane. On the other hand, Yang et al., reported TEMPO-oxidized nanocellulose is an excellent separator membrane for zinc-ion batteries,133 although, the separator membrane was doped with Zr4+ ion which coordinates with the oxygen atom of the carboxyl group. This results in an anti-swelling with ion-sieving property of the nanocellulose separator. The Zr4+ ion also works as a crosslinking agent as well as shielding the hydrogen bonding on the cellulose surface. The Zr–O layer is amorphous and thus acts as a physical barrier to reduce dendrite growth. Using the Zr–CNF separator, Yang et al. achieved a 700 h cell lifespan at a current density of 10 mA cm−2 with a capacity of 2 mA h cm−2.
In addition to this, Chun et al., reported cellulose nanofiber (CNF) based paper derived membrane which can be fabricated via an eco-friendly method by varying the isopropyl alcohol (IPA)–water composition.134 With increasing IPA content in water, the CNF membrane becomes highly interconnected with the nanoporous channel network. A separator membrane prepared from this method shows lower thermal shrinkage compared to polyolefin-based membranes. The membrane also showed very high discharge capacity retention (87%) after the 100th cycle compared to a polyolefin (PP/PE/PP) based separator (82%). The maximum ionic conductivity achieved with these types of separator membranes was 0.77 mS cm−1 with a capacity of 138 mA h g−1 at 0.2C. Due to CNFs structural uniqueness, together with high thermal stability and polarity, the CNF paper-derived membrane separator conveys significant improvement in thermal shrinkage and electrolyte wettability as well as ionic conductivity compared to the commercial polyolefin-based separator. Liu et al., first reported a polyformaldehyde (POM)–cellulose nanofibers (CNF) blend separator via thermally induced phase separation (TIPS).135 Liu and his co-workers claimed that TIPS is the most suitable method for preparing porous separators from semi-crystalline thermoplastic as well as having better control for uniform pore structure with higher mechanical strength. The POM–CNF blend separator shows very high electrolyte uptake (412%) as well as porosity (80%) compared to the PE-based commercial separator (115% electrolyte uptake and 45% porosity). The ionic conductivity of this type of blend separator is also very high (140 mS cm−1) compared to a commercial PE-based separator (0.76 mS cm−1). Due to the porous nature of the POM–CNF based separator, the lithium-ion transference number was found to be higher (tLi+ = 0.53) than with the PE-based separator (tLi+ = 0.31). Overall, the nanocellulose based separator shows a promising future to be used as the separator in lithium-ion batteries. Due to their low cost, biodegradability, hydrophilicity, thermos-chemical, and mechanical stability, they can be promising substitutes for fossil-based separators that have been used to date.
Additionally, a porous separator membrane plays a significant role in battery performance because it acts as an ion-conducting channel between the two electrodes. However, the processing cost of the separator can be up to 20% of total battery production. Nanocellulose-based separators represent a new promising door for new biobased separators. One of the challenging aspects of nanocellulose-based separators is controlling porosity and processing time and cost. Thus, the main research efforts should be towards sustainable approaches including recovery of processing solvents that being used during the separator manufacturing process. To achieve environmentally benign separators researchers and industry should focus on shifting from separator to dual functional approach e.g. Gel Polymer Electrolyte (GPE). GPE can serve as a separator between the two electrodes and reduce the electrolyte loading in battery cells via gel formation. In the future, separators as well as GPE will not be limited only to cellulose but also to other biobased materials like chitin, and alginate which can open a new paradigm of biodegradable battery components.
6. Sustainable solvents and binders used in electrode fabrication towards a greener battery
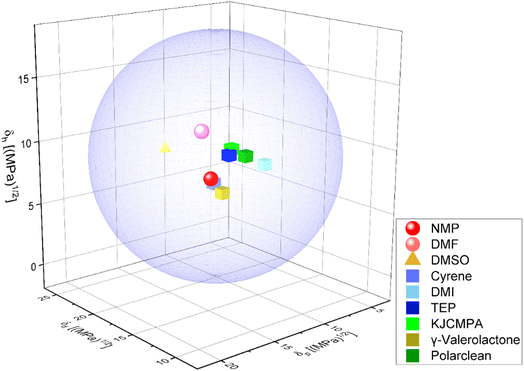
In LIB cathodes, the active material binder (typically PVDF), and carbon-based conductive additives are combined with a solvent to create a slurry. This slurry is then deposited on the surface of a current collector, usually made of aluminium. The most commonly used solvent in cathode production is N-methyl-2-pyrrolidone (NMP), a polar aprotic solvent extensively utilized in various industries, with properties detailed in Table 2. NMP is highly miscible with most organic solvents and is known for its chemical and thermal stability, along with its high boiling and flash points. In battery cell fabrication, NMP dissolves binders for cathode slurry mixing and formulation, resulting in highly adhesive and stable slurries. However, NMP is toxic, and its processing and recovery require a significant amount of energy.8 Additionally, the use of NMP is increasingly being prohibited in numerous countries. Consequently, researchers are exploring green alternatives to NMP.136–138 These alternatives should ideally possess similar properties to NMP, both in terms of processing and performance. The Hansen solubility sphere, a widely recognized system in chemistry for predicting material solubility, can be used to determine the suitability of solvents as potential replacements for NMP.138,139 This system is based on a three-parameter model: dispersion forces (δd), polar forces (δp), and hydrogen bonding forces (δh). Fig. 8 illustrates the Hansen solubility sphere for various solvents suitable for cathode slurries.140| Solvent | Surface tension [mN m−1 at 25 °C] | Viscosity [mPa s] | Boiling point [°C] | Autoignition temperature [°C] |
|---|---|---|---|---|
| NMP | 41 | 1.66 | 202 | 252 |
| DMF | 37 | 0.92 | 155 | 445 |
| DMSO | 43 | 1.99 | 189 | 215 |
| Cyrene | 72 | 14.5 | 226 | 296 |
| γ-Valerolactone | 30 | 2.18 | 207 | 402 |
| KJCMPA | 29 | 2.3 | 215 | NA |
| TEP | 30 | 1.6 | 215 | 452 |
| Polarclean | 22 | 1.04 | 280 | 390 |
| DMI | 33 | 6.8 | 94 | 285 |
From a sustainability perspective, solvents can be categorized based on criteria proposed by Gu and Jérôme,141 for evaluating solvent acceptability as a green solvent:
(i) Available on the required scale with a secure long-term source of supply.
(ii) Technical performance (including solvency) is now worse than the equivalent conventional solvent, stable during use and storage.
(iii) Low- or non-flammable and competitively priced.
(iv) Able to be recycled and purity appropriate to use.
(v) Resource and energy-efficient production (preferably life cycle assessed).
(vi) Sourced from renewable intermediates and feedstocks.
(vii) Established acceptable toxicity and ecotoxicity profiles sufficient for regulatory purposes. Meets standards and regulations for transportation.
(viii) Fully biodegradable to innocuous products.
To enhance the sustainability of battery fabrication, extensive research has focused on replacing NMP with alternative solvents such as DMSO (dimethylsulfoxide), Cyrene (dihydrolevoglucosenone), KJCMPA (3-methoxy-N,N-dimethylpropanamide), and γ-valerolactone. These solvents exhibit similar dissolving capabilities to NMP but have drawbacks, such as a higher boiling point (increasing energy requirements for cathode drying), limited sustainability, the introduction of sulfur impurities (DMSO), and poorer adhesion in cathode layers (Cyrene, γ-valerolactone), leading to reduced battery performance.142,143 Although dimethylformamide (DMF) offers more favorable properties than NMP (lower boiling point, lower viscosity, surface tension, and higher autoignition temperature) for cathode slurries, its widespread use is questionable due to toxicity concerns – it has been added to the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) list, limiting its application in consumer products.8,144 KJCMPA (3-methoxy-N,N-dimethylpropanamide) is emerging as a promising NMP alternative. This amphiphilic amide solvent has a reduced toxicity profile and can dissolve a wide range of materials. KJCMPA's properties make it particularly suitable for formulating various inks and slurries, as detailed in Table 2. Its effectiveness in fabricating NMC811 cathodes based pouch cells has been demonstrated.145
Triethyl phosphate (TEP) has been investigated as a greener alternative to NMP in Ni-rich cathode fabrication. TEP-based cells have shown similar electrochemical performance, including specific capacity and cycle stability, compared to NMP-based cells.146 PolarClean (methyl-5-(dimethylamino)-2-methyl-5-oxopentanoate) has been proposed as a potential replacement for toxic NMP in cathode fabrication, without altering the manufacturing process. Additionally, dimethyl isosorbide (DMI) has been used as an alternative to NMP in the fabrication of polyvinylidene fluoride (PVDF)-based ultrafiltration process.147 Water is another environmentally friendly solvent for formulating cathode slurries, and its use is being explored in cathode fabrication. The use of water also reduces energy consumption during drying due to its lower boiling point compared to conventional solvents.148,149 However, water does not dissolve PVDF and can negatively impact the performance of cathodes, especially those with high nickel content.150,151 Dry processing is another alternative for cathode production, where materials are mixed in dry form, without solvents, and deposited on the current collector.152,153 This promising approach addresses many issues related to solvent use and high energy consumption during drying/recovery. However, cathodes fabricated using dry processing currently do not provide satisfactory performance, especially at high C-rates.154
Binders play a pivotal role in maintaining cathodes’ structural integrity and performance and recent advancements in sustainable cathode binders signify a promising direction towards eco-friendly and high-performance batteries. Sustainable binders, derived from bio-based or green chemistry routes, offer a viable alternative to conventional petroleum-based binders, reducing the environmental footprint of battery production. These binders not only ensure the mechanical stability of the electrode but also enhance the electrochemical performance of batteries. In particular, developing water-based and bio-derived binders has been identified as a significant advancement. Bresser et al., provide a comprehensive review of green binders for batteries and supercapacitors, highlighting the transition to aqueous electrode processing and the use of bio-derived polymers.155 Authors elaborate on the benefits of using polysaccharides such as carboxymethyl cellulose and alginate, which improve cyclability and address the challenges associated with water-processable cathodes. This work also covers the emerging fields of lithium–sulphur and sodium-ion batteries, underscoring the growing recognition of the binder's role in these technologies. Dou et al., focus on the binders for sustainable high-energy-density LIBs, emphasizing the importance of binders in enhancing the electrochemical behaviour of high-capacity electroactive materials.156 They discuss the development of binders that facilitate the easy separation and recycling of electroactive materials, contributing to a more sustainable battery economy. This work also highlights the advancements in conductive binders, which lead to fewer battery chemistries and higher energy densities. At the same time, Schmidt et al., explore the use of sustainable protein-based binders for lithium–sulphur cathodes processed by a solvent-free dry-coating method.157 This work demonstrates that biodegradable sericin can replace traditional polytetrafluoroethylene (PTFE) binders without compromising the cycle stability and performance of the cathodes. This study exemplifies the potential of renewable and biodegradable materials in enhancing the sustainability of electrode processing. Work conducted by Zhang et al., compares green water-based binders with conventional polyvinylidene fluoride binders for LiFePO4/C cathodes in LIBs.158 The results highlight those green binders, with their high content of carboxyl groups, provide additional lithium-ion transfer channels, thereby improving the rate performance of the batteries.
Another avenue in improving the sustainability of binders is the introduction of fluorine-free binders. These binders are crucial in mitigating the environmental impact linked to the production and disposal of batteries. The development of fluorine-free binders, such as poly(vinylpyrrolidone) (PVP), poly(ethylene oxide) (PEO), and cellulose-based ETHOCEL™, provides viable alternatives to conventional polyvinylidene fluoride (PVDF) binders, reducing environmental hazards and improving the mechanical and electrochemical properties of electrodes. Hernández et al., discuss the environmental concerns associated with fluorinated components and highlight the potential of fluorine-free electrolytes in delivering performance comparable to traditional systems.159 Rolandi et al., propose the introduction of fluorine-free poly(ionic liquid) binders for high-voltage NMC811 cathodes, demonstrating their effectiveness in aqueous processing and contribution to sustainable battery manufacturing.160 These studies underscore the importance of developing fluorine-free materials to address battery technology safety, environmental, and performance challenges.
From the perspective of anodes, the selection of solvents strongly relies on the choice of binders, which in turn depends on the anode chemistry. A polyimine binder efficiently provides good electrochemical performance by binding graphite and silicon together, whereas styrene–butadiene rubber/carboxymethyl cellulose (SBR/CMC) and PVDF perform sufficiently with pure graphite electrodes.161 In recent years, silicon (Si) anodes and silicon–graphite composite anodes have been recognized for their high potential due to their large capacity.162 However, the implementation of Si in anodes requires countermeasures to address silicon's volumetric expansion during battery operation. This aspect must be considered when selecting an appropriate binder. While PVDF and polyimine require a similar set of solvents as used for cathodes, the use of SBR/CMC as binders enables the use of water as a solvent.163 Water is the most used solvent in anode production due to its non-toxicity, environmental friendliness, and lower energy consumption during drying, attributed to its low boiling point. From the perspective of silicon anodes, there is a range of water-soluble binders designed to accommodate the significant expansion of silicon in the anode structure. Deng et al., provide a comprehensive review of various strategies for designing water-soluble binders for silicon anodes.164 Similarly, Yim et al., have proposed a method to identify the most suitable binder for Si anodes for LIBs.165
7. Electrode fabrication process
Conventional electrode fabrication technologies involve applying layers of active material onto current collectors, such as copper for anodes and aluminium for cathodes. Common methods include slurry casting, the doctor blade technique, and extrusion coating. In slurry casting, a mixture of active material, binder, and solvent is spread evenly over the current collector. The doctor blade technique, a variation of slurry casting, employs a blade to set a uniform thickness for the electrode coating. Extrusion coating involves pushing the electrode material through a die to achieve a consistent layer. Sustainable fabrication of batteries, particularly through advanced coating and printing technologies, represents a significant advancement in the evolution of energy storage systems. This approach is crucial for addressing the environmental and economic challenges posed by traditional battery manufacturing processes.166 The primary objective is to develop batteries using methods that are not only efficient and cost-effective but also environmentally friendly. Coating and printing technologies are instrumental in achieving green battery fabrication.167 Furthermore, recent advances in the development of solid-state batteries indicate that printing techniques with controlled deposition could enable the fabrication of fully printed batteries (including the electrolyte), significantly reducing the number of fabrication steps and, consequently, the environmental impact of battery production.4In contrast, printing technologies introduce a new dimension to battery fabrication. Techniques such as inkjet, screen, spray, and 3D printing (Fig. 9) enable precise deposition of battery materials in predetermined patterns and structures.168–170 This precision not only optimizes material usage but also facilitates the design of batteries with innovative architectures that can offer improved performance and longer life cycles. For example, 3D printing can create complex, porous structures that enhance the electrode's surface area, leading to better ion transport and higher energy densities.171 However, not all additive manufacturing methods are suitable for battery fabrication; for instance, ink-jet printing might not be ideal for cathode fabrication because large (>2 μm) active material particles could cause nozzle clogging.172 Nevertheless, the inks/slurries used to print solid-electrolyte in solid-state batteries consist of materials and blends compatible with inkjet printing processes.173 Except for 3D printing, these advanced fabrication methods are fully scalable and adaptable – they can be seamlessly integrated into roll-to-roll processes, allowing for continuous, high-throughput production of batteries. This scalability is essential for meeting the increasing demand for energy storage solutions in applications ranging from portable devices and electric vehicles to grid storage. While printing technologies may not achieve fabrication speeds on par with traditional methods, they significantly improve the sustainability of battery production. Furthermore, their true potential is only fully realized when used in conjunction with eco-friendly materials and solvents, as detailed in other sections of this work.174,175
 | ||
| Fig. 9 Various printing techniques suitable to fabrication of battery layers: (a) inkjet printing, (b) 3D printing, (c) screen printing, (d) spray printing. | ||
8. Recycling strategy of greener batteries
The demand for higher energy and power densities, low-cost, safe batteries for automotive and stationary applications is propelling many research efforts toward developing advanced chemistry and battery systems. Advanced materials play an important role in new batteries to provide greater ion transport to store more energy. Under the goal of global sustainable development, the rapid growth of the EV industry has led to the proliferation of used batteries. Huge demand and consumption of LIBs in EVs and other applications would lead to the generation of mountains of spent LIB waste which if left out cause's pollution and damage to the environment and human health. Therefore, the recycling process is one of the keys to turning a raw materials shortage into a circular economy through reuse and reduction. The decarbonization of energy production requires the proliferation of efficient energy storage such as LIBs used in EVs. The projected EV market requires to estimate of the circular economy design of end-of-life products and recovery of the materials for secondary battery use.176 Spent LIBs also contain large amounts of different metals such as Li, Co, Mn, Ni, Cu, Al, and Fe in higher concentrations than in the respective ores. Hence, recycling and recovery of spent LIB is important both for critical metal supply and environmental protection.177 The choice of recycling strategy for used LIBs and materials determines the energy consumption, emissions, recovery efficiency, and profitability of battery recycling.178 Mostly the recycling of LIBs uses pyrometallurgy or hydrometallurgy methods.176 From this approach, the value of the product has a higher cost and a lower fraction of recovery. With the inevitable increase in EVs over the forthcoming years, materials should be more scalable, reusable, and suitable recycling methods are essential. The batteries could be dismantled and separated into each component like electrodes, polymer separators, and current collectors (Fig. 10a). The active cathode materials from the electrodes would be delaminated and recovered using aqueous solutions.179 A greener approach was applied for spent NMC523 cathode via water-soluble binder-based processing and reuse in LIBs (Fig. 10b).149 In this recycling process, NMP solvent was replaced by water as a solvent during the extraction of electrode fabrication and black mass removal from the current collector. Its potential approach provides a gateway towards low-cost battery materials and lowers the environmental impacts as well as the green and sustainable manufacturing of future greener batteries. | ||
| Fig. 10 (a) The spent LIBs were dismantled from the leaching experiments and recycled secondary materials via direct recycling. Reproduced with permission.179 Copyright 2015, American Chemical Society. (b) A sustainable manufacturing approach for the NMC-based cathodes from the spend LIB. Reproduced with permission.149 Copyright 2020, Elsevier. | ||
Currently, the techno-economic feasibility of recycling LIBs is strongly based on the value of elements Co and Ni (and the recovery of Cu foil). EU has released a new battery regulation. It has several improvements compared to earlier legislation. EV batteries and related battery management systems and battery passports are first time classified, and the requirement is to minimize the carbon footprint of EV batteries. From the battery recycling viewpoint, mandatory minimum levels of recycled content for Co, Li, and Ni are presented. Further, increased collection rate targets for waste portable batteries are given by 2025 and 2030. Regarding recycling efficiencies, specific material recovery targets for Co, Cu, Ni, and Li are also given. For LFP battery recycling alone, it is rather difficult to find a techno-economically feasible solution. Recycling of battery materials mitigates environmental degradation and promotes circular economy principles in energy storage.
9. Summary and perspectives
One of the major challenges in the fabrication of LIBs is the synthesis of high-capacity and long-cycling stable electrode materials, in particular anodes and cathodes eying more sustainable and greener energy storage systems.One sustainable solution to replace Gr anode would be to use biomass as a precursor material to produce carbon materials, which have already been proven to be suitable battery anode material. The utilization of graphite also faces important challenges regarding its large CO2 footprint and high costs associated with its mining. In addition, more than 65% is produced in China, and the EU has classified it as a critical raw material with a high delivery risk. Replacing graphite components in LIB electrodes with bio-based materials would not only be beneficial for the environment and security of supply but also provide opportunities for Swedish agriculture and forest industries to find applications to valorize and make a profit from waste that today costs money to dispose of. Therefore, Gr can be successfully replaced by biobased carbon materials made from biomass wastes.34
Biomass resources have intrinsic potential for the development of superior anode material for LIBs aiming to replace long-standing graphite. Biomass-based carbons have displayed intrinsic potential and superiority as high-performance anodes for greener batteries (Fig. 11). To achieve it, a rational and controllable fabrication of biomass-based anodes with different structures is the inevitable trend toward the development of a new concept of green batteries free of Gr and more toxic elements.
Cathode dependency on critical raw elements, i.e., lithium, cobalt, and phosphorus, can be minimized with new parallel technologies to LIBs. These involve the use of SIBs, LSBs, and KIBs. All these technologies enable the production of cathode active materials from abundant elements with improvements in safety and sustainability, without significant compromising on energy density.
The use of green binder materials in next-generation batteries will open advancements lowering the overall CO2 footprint for the battery manufacturing process. Recycling batteries is the key to the sustainable development of the new energy industry, which is also connected to the circular economy concept. Greening key critical raw metals is the core part of battery materials development. This will be more useful for greener batteries to a circular economy in technology metals, which could offer the decarbonization of the transportation sector.
Author contributions
Palanivel Molaiyan, Shubhankar Bhattacharyya, Glaydson Simoes dos Reis, Rafal Sliz, Andrea Paolella and Ulla Lassi: writing – original draft, visualization, investigation. Palanivel Molaiyan, Shubhankar Bhattacharyya, Glaydson Simoes dos Reis, Rafal Sliz, Andrea Paolella and Ulla Lassi: writing – review & editing, conceptualization. Ulla Lassi: supervision, resources, funding acquisition. Palanivel Molaiyan, Shubhankar Bhattacharyya, Glaydson Simoes dos Reis and Rafal Sliz: authors contributed equally to this manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors wish to thank Bio4Energy, a strategic research environment appointed by the Swedish government, as well as the Swedish University of Agricultural Sciences for supporting this work. This work was supported by the financial support of the Academy of Finland's FIRI funding (grant no. 320017), Business Finland R2B Funding (grant no. 7270/31/2022), and EU/Interreg Aurora (Project GreenBattery, grant no. 20357574). Financial support from the Kempes Foundation (Grant No. JCSMK23-0145) is gratefully acknowledged.References
- D. Wu and F. Wu, eTransportation, 2023, 16, 100224 CrossRef.
- M. S. Whittingham, Chem. Rev., 2004, 104, 4271–4301 CrossRef CAS PubMed.
- J. B. Dunn, L. Gaines, J. C. Kelly, C. James and K. G. Gallagher, Energy Environ. Sci., 2015, 8, 158–168 RSC.
- R. Sliz, P. Molaiyan, T. Fabritius and U. Lassi, Nano Express, 2022, 3, 021002 CrossRef.
- H. Zhang, Y. Yang, D. Ren, L. Wang and X. He, Energy Storage Mater., 2021, 36, 147–170 CrossRef.
- M. Ashuri, Q. He and L. L. Shaw, Nanoscale, 2016, 8, 74–103 RSC.
- M. Abdollahifar, P. Molaiyan, U. Lassi, N. L. Wu and A. Kwade, Renewable Sustainable Energy Rev., 2022, 169, 112948 CrossRef CAS.
- R. Sliz, J. Valikangas, H. Silva Santos, P. Vilmi, L. Rieppo, T. Hu, U. Lassi and T. Fabritius, ACS Appl. Energy Mater., 2022, 5, 4047–4058 CrossRef CAS PubMed.
- M.-M. Titirici, Adv. Energy Mater., 2021, 11, 2003700 CrossRef CAS.
- H. Yaghoobnejad Asl and A. Manthiram, Nat. Sustain., 2021, 4, 379–380 CrossRef.
- H. Hong, N. A. R. Che Mohamad, K. Chae, F. Marques Mota and D. H. Kim, J. Mater. Chem. A, 2021, 9, 10012–10038 RSC.
- J. Lu, Z. Chen, F. Pan, Y. Cui and K. Amine, Electrochem. Energy Rev., 2018, 1, 35–53 CrossRef CAS.
- M. Abdollahifar, P. Molaiyan, M. Perovic and A. Kwade, Energies, 2022, 15, 8791 CrossRef CAS.
- A. Skurtveit, A. Brennhagen, H. Park, C. Cavallo and A. Y. Koposov, Front. Energy Res., 2022, 10, 897755 CrossRef.
- Y. Mekonnen, A. Sundararajan and A. I. Sarwat, in SoutheastCon 2016, 2016, pp. 1–6.
- D. M. Piper, J. J. Travis, M. Young, S. B. Son, S. C. Kim, K. H. Oh, S. M. George, C. Ban and S. H. Lee, Adv. Mater., 2014, 26, 1596–1601 CrossRef CAS PubMed.
- A. Franco Gonzalez, N.-H. Yang and R.-S. Liu, J. Phys. Chem. C, 2017, 121, 27775–27787 CrossRef CAS.
- J. W. Choi and D. Aurbach, Nat. Rev. Mater., 2016, 1, 16013 CrossRef CAS.
- O. S. Taskin, D. Hubble, T. Zhu and G. Liu, Green Chem., 2021, 23, 7890–7901 RSC.
- D. Di Lecce, R. Verrelli and J. Hassoun, Green Chem., 2017, 19, 3442–3467 RSC.
- J. A. Lewis, K. A. Cavallaro, Y. Liu and M. T. McDowell, Joule, 2022, 6, 1418–1430 CrossRef CAS.
- P. Molaiyan, M. Abdollahifar, B. Boz, A. Beutl, M. Krammer, N. Zhang, A. Tron, M. Romio, M. Ricci, R. Adelung, A. Kwade, U. Lassi and A. Paolella, Adv. Funct. Mater., 2023, 2311301 Search PubMed.
- X. Zheng, Z. Zhu, X. Lin, Y. Zhang, Y. He, H. Cao and Z. Sun, Engineering, 2018, 4, 361–370 CrossRef CAS.
- D. Mustika, T. Torowati, A. Dimyati, S. Sudirman, A. Fisli, I. M. Joni and R. Langenati, Urania: J. Ilm. Daur Bahan Bakar Nukl., 2020, 26, 167–176 Search PubMed.
- J. Neumann, M. Petranikova, M. Meeus, J. D. Gamarra, R. Younesi, M. Winter and S. Nowak, Adv. Energy Mater., 2022, 12, 2102917 CrossRef CAS.
- B. P. Thapaliya, H. Luo, P. Halstenberg, H. M. Meyer, III, J. R. Dunlap and S. Dai, ACS Appl. Mater. Interfaces, 2021, 13, 4393–4401 CrossRef CAS PubMed.
- D. S. Kim, Y. E. Kim and H. Kim, J. Power Sources, 2019, 422, 18–24 CrossRef CAS.
- J. C. Garcia, I. Bloom, C. Johnson, D. Dees and H. Iddir, J. Phys. Chem. C, 2020, 124, 8162–8169 CrossRef CAS.
- W. Zhang, F. Zhang, F. Ming and H. N. Alshareef, EnergyChem, 2019, 1, 100012 CrossRef.
- W. Luo, F. Shen, C. Bommier, H. Zhu, X. Ji and L. Hu, Acc. Chem. Res., 2016, 49, 231–240 CrossRef CAS PubMed.
- G. S. dos Reis, P. Molaiyan, C. M. Subramaniyam, F. García-Alvarado, A. Paolella, H. P. de Oliveira and U. Lassi, Electrochem. Commun., 2023, 153, 107536 CrossRef CAS.
- A. Liu, T.-F. Liu, H.-D. Yuan, Y. Wang, Y.-J. Liu, J.-M. Luo, J.-W. Nai and X.-Y. Tao, New Carbon Mater., 2022, 37, 658–674 CrossRef CAS.
- B. Feng, L. Xu, Z. Yu, G. Liu, Y. Liao, S. Chang and J. Hu, Electrochem. Commun., 2023, 148, 107439 CrossRef CAS.
- P. Molaiyan, G. S. Dos Reis, D. Karuppiah, C. M. Subramaniyam, F. García-Alvarado and U. Lassi, Batteries, 2023, 9, 116 CrossRef CAS.
- S. Huang, X. Qiu, C. Wang, L. Zhong, Z. Zhang, S. Yang, S. Sun, D. Yang and W. Zhang, New Carbon Mater., 2023, 38, 40–66 CrossRef CAS.
- X. Yuan, B. Zhu, J. Feng, C. Wang, X. Cai and R. Qin, Chem. Eng. J., 2021, 405, 126897 CrossRef CAS.
- G. S. dos Reis, R. M. A. Pinheiro Lima, S. H. Larsson, C. M. Subramaniyam, V. M. Dinh, M. Thyrel and H. P. de Oliveira, J. Environ. Chem. Eng., 2021, 9, 106155 CrossRef CAS.
- D. R. Lima, E. C. Lima, P. S. Thue, S. L. P. Dias, F. M. Machado, M. K. Seliem, F. Sher, G. S. dos Reis, M. R. Saeb and J. Rinklebe, J. Environ. Chem. Eng., 2021, 9, 105865 CrossRef CAS.
- G. S. dos Reis, D. Bergna, A. Grimm, E. C. Lima, T. Hu, M. Naushad and U. Lassi, Colloids Surf., A, 2023, 669, 131493 CrossRef CAS.
- G. S. dos Reis, S. H. Larsson, M. Mathieu, M. Thyrel and T. N. Pham, Biomass Convers. Biorefin., 2023, 13, 10113–10131 CrossRef CAS.
- G. S. dos Reis, C. Mayandi Subramaniyam, A. D. Cárdenas, S. H. Larsson, M. Thyrel, U. Lassi and F. García-Alvarado, ACS Omega, 2022, 7, 42570–42581 CrossRef PubMed.
- J. Deng, M. Li and Y. Wang, Green Chem., 2016, 18, 4824–4854 RSC.
- N. A. Banek, K. R. McKenzie, D. T. Abele and M. J. Wagner, Sci. Rep., 2022, 12, 8080 CrossRef CAS PubMed.
- A. Pramanik, S. Chattopadhyay, G. De and S. Mahanty, Mater. Adv., 2021, 2, 7463–7472 RSC.
- Y. Nishi, Chem. Rec., 2001, 1, 406–413 CrossRef CAS PubMed.
- G. Shen, B. Li, Y. Xu, X. Chen, S. Katiyar, L. Zhu, L. Xie, Q. Han, X. Qiu, X. Wu and X. Cao, J. Colloid Interface Sci., 2024, 653, 1588–1599 CrossRef CAS PubMed.
- P. Yadav, A. Patrike, K. Wasnik, V. Shelke and M. Shelke, Mater. Today Sustain., 2023, 22, 100385 CrossRef.
- P. K. Nayak, L. Yang, W. Brehm and P. Adelhelm, Angew. Chem., Int. Ed., 2018, 57, 102–120 CrossRef CAS PubMed.
- J. Peters, D. Buchholz, S. Passerini and M. Weil, Energy Environ. Sci., 2016, 9, 1744–1751 RSC.
- J. Park, J. Lee, M. H. Alfaruqi, W.-J. Kwak, J. Kim and J.-Y. Hwang, J. Mater. Chem. A, 2020, 8, 16718–16737 RSC.
- S. Dühnen, J. Betz, M. Kolek, R. Schmuch, M. Winter and T. Placke, Small Methods, 2020, 4, 2000039 CrossRef.
- W. Lv, F. Wen, J. Xiang, J. Zhao, L. Li, L. Wang, Z. Liu and Y. Tian, Electrochim. Acta, 2015, 176, 533–541 CrossRef CAS.
- Y. Wen, K. He, Y. Zhu, F. Han, Y. Xu, I. Matsuda, Y. Ishii, J. Cumings and C. Wang, Nat. Commun., 2014, 5, 4033 CrossRef CAS PubMed.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2000, 147, 1271 CrossRef CAS.
- R. M. A. P. Lima, G. S. Dos Reis, M. Thyrel, J. J. Alcaraz-Espinoza, S. H. Larsson and H. P. de Oliveira, Nanomaterials, 2022, 12, 866 CrossRef CAS PubMed.
- G. S. dos Reis, D. Bergna, S. Tuomikoski, A. Grimm, E. C. Lima, M. Thyrel, N. Skoglund, U. Lassi and S. H. Larsson, ACS Omega, 2022, 7, 32620–32630 CrossRef PubMed.
- P. Gupta, S. Pushpakanth, M. A. Haider and S. Basu, ACS Omega, 2022, 7, 5605–5614 CrossRef CAS PubMed.
- Y.-X. Yin, S. Xin, Y.-G. Guo and L.-J. Wan, Angew. Chem., Int. Ed., 2013, 52, 13186–13200 CrossRef CAS PubMed.
- H.-J. Peng, J.-Q. Huang, X.-B. Cheng and Q. Zhang, Adv. Energy Mater., 2017, 7, 1700260 CrossRef.
- X. Han, X. Meng, S. Chen, J. Zhou, M. Wang, L. Sun, Y. Jia, X. Peng, H. Mai, G. Zhu, J. Li, C. W. Bielawski and J. Geng, ACS Appl. Mater. Interfaces, 2023, 15, 11713–11722 CrossRef CAS PubMed.
- X. Yang, S. Chen, W. Gong, X. Meng, J. Ma, J. Zhang, L. Zheng, H. D. Abruña and J. Geng, Small, 2020, 16, 2004950 CrossRef CAS PubMed.
- L. Sun, W. Gong, J. Zhou, J. Zhang, C. Chen, X. Meng, X. Han, H. Mai, C. W. Bielawski and J. Geng, J. Colloid Interface Sci., 2024, 653, 1694–1703 CrossRef CAS PubMed.
- M. A. Pope and I. A. Aksay, Adv. Energy Mater., 2015, 5, 1500124 CrossRef.
- J. Schuster, G. He, B. Mandlmeier, T. Yim, K. T. Lee, T. Bein and L. F. Nazar, Angew. Chem., Int. Ed., 2012, 51, 3591–3595 CrossRef CAS PubMed.
- Y. Sun, J. Ma, X. Yang, L. Wen, W. Zhou and J. Geng, J. Mater. Chem. A, 2020, 8, 62–68 RSC.
- H. Mai, Q. Wang, L. Sun, X. Meng, S. Chen, J. Zhou, Y. Jia, M. Wang, X. Han, X. Zhou, W. Gong, G. Zhu, J. Li, C. W. Bielawski and J. Geng, ACS Appl. Mater. Interfaces, 2023, 15, 41426–41437 CrossRef CAS PubMed.
- J. Liang, Z.-H. Sun, F. Li and H.-M. Cheng, Energy Storage Mater., 2016, 2, 76–106 CrossRef.
- G. Li, J. Sun, W. Hou, S. Jiang, Y. Huang and J. Geng, Nat. Commun., 2016, 7, 10601 CrossRef CAS PubMed.
- L. Yu, N. Brun, K. Sakaushi, J. Eckert and M. M. Titirici, Carbon, 2013, 61, 245–253 CrossRef CAS.
- J. Zhou, Y. Guo, C. Liang, J. Yang, J. Wang and Y. Nuli, Electrochim. Acta, 2018, 273, 127–135 CrossRef CAS.
- N. Kang, Y. Lin, L. Yang, D. Lu, J. Xiao, Y. Qi and M. Cai, Nat. Commun., 2019, 10, 4597 CrossRef PubMed.
- C. Kensy, F. Schwotzer, S. Dörfler, H. Althues and S. Kaskel, Batteries Supercaps, 2021, 4, 823–833 CrossRef CAS.
- L. Kong, J.-X. Chen, H.-J. Peng, J.-Q. Huang, W. Zhu, Q. Jin, B.-Q. Li, X.-T. Zhang and Q. Zhang, Energy Environ. Sci., 2019, 12, 2976–2982 RSC.
- Y.-J. Choi, K.-W. Kim, H.-J. Ahn and J.-H. Ahn, J. Alloys Compd., 2008, 449, 313–316 CrossRef CAS.
- S. Dörfler, P. Strubel, T. Jaumann, E. Troschke, F. Hippauf, C. Kensy, A. Schökel, H. Althues, L. Giebeler, S. Oswald and S. Kaskel, Nano Energy, 2018, 54, 116–128 CrossRef.
- I. Sultana, M. M. Rahman, Y. Chen and A. M. Glushenkov, Adv. Funct. Mater., 2018, 28, 1703857 CrossRef.
- D. Li, X. Ren, Q. Ai, Q. Sun, L. Zhu, Y. Liu, Z. Liang, R. Peng, P. Si, J. Lou, J. Feng and L. Ci, Adv. Energy Mater., 2018, 8, 1802386 CrossRef.
- G. S. Reis, S. Petnikota, C. M. Subramaniyam, H. P. de Oliveira, S. Larsson, M. Thyrel, U. Lassi and F. García Alvarado, Nanomaterials, 2023, 13, 765 CrossRef CAS PubMed.
- Z. Wu, J. Zou, Y. Zhang, X. Lin, D. Fry, L. Wang and J. Liu, Chem. Eng. J., 2022, 427, 131547 CrossRef CAS.
- M. Yang, J. Dai, M. He, T. Duan and W. Yao, J. Colloid Interface Sci., 2020, 567, 256–263 CrossRef CAS PubMed.
- J. Gou, W. Liu and A. Tang, J. Mater. Sci., 2020, 55, 10699–10711 CrossRef CAS.
- H. Yang, G. V. Zhuang and P. N. Ross, J. Power Sources, 2006, 161, 573–579 CrossRef CAS.
- Z. Zeng, W.-I. Liang, H.-G. Liao, H. L. Xin, Y.-H. Chu and H. Zheng, Nano Lett., 2014, 14, 1745–1750 CrossRef CAS PubMed.
- C. Pang, F. Ding, W. Sun, J. Liu, M. Hao, Y. Wang, X. Liu and Q. Xu, Electrochim. Acta, 2015, 174, 230–237 CrossRef CAS.
- A. Bhargav, M. Wu and Y. Fu, J. Electrochem. Soc., 2016, 163, A1543 CrossRef CAS.
- X. Liu, M. Zarrabeitia, B. Qin, G. A. Elia and S. Passerini, ACS Appl. Mater. Interfaces, 2020, 12, 54782–54790 CrossRef CAS PubMed.
- M. Galiński, A. Lewandowski and I. Stępniak, Electrochim. Acta, 2006, 51, 5567–5580 CrossRef.
- A. Mauger, C. M. Julien, A. Paolella, M. Armand and K. Zaghib, Mater. Sci. Eng., R, 2018, 134, 1–21 CrossRef.
- J.-G. Han, K. Kim, Y. Lee and N.-S. Choi, Adv. Mater., 2019, 31, 1804822 CrossRef PubMed.
- T. Kawamura, S. Okada and J. Yamaki, J. Power Sources, 2006, 156, 547–554 CrossRef CAS.
- M. Xie, Y. Wu, Y. Liu, P. P. Yu, R. Jia, W. A. Goddard and T. Cheng, Mater. Today Energy, 2021, 21, 100730 CrossRef CAS.
- A. Subramania, R. Sathiyamoorthi, T. Vasudevan and R. Gangadharan, Ionics, 2006, 12, 327–329 CrossRef CAS.
- G. H. Newman, R. W. Francis, L. H. Gaines and B. M. L. Rao, J. Electrochem. Soc., 1980, 127, 2025 CrossRef CAS.
- S. S. Zhang, K. Xu and T. R. Jow, J. Electrochem. Soc., 2002, 149, A586 CrossRef CAS.
- H. Cavers, P. Molaiyan, M. Abdollahifar, U. Lassi and A. Kwade, Adv. Energy Mater., 2022, 12, 2200147 CrossRef CAS.
- D. Campanella, W. Zhu, G. Girard, S. Savoie, S. Kaboli, Z. Feng, A. Guerfi, M. Romio, P. Molaiyan, D. Bélanger and A. Paolella, ChemSusChem, 2023, e202300399 CrossRef CAS PubMed.
- J. Qiu, L. Yang, G. Sun, X. Yu, H. Li and L. Chen, Chem. Commun., 2020, 56, 5633–5636 RSC.
- W. He, Z. Cui, X. Liu, Y. Cui, J. Chai, X. Zhou, Z. Liu and G. Cui, Electrochim. Acta, 2017, 225, 151–159 CrossRef CAS.
- B. Commarieu, A. Paolella, S. Collin-Martin, C. Gagnon, A. Vijh, A. Guerfi and K. Zaghib, J. Power Sources, 2019, 436, 226852 CrossRef CAS.
- M. Golozar, A. Paolella, H. Demers, S. Savoie, G. Girard, N. Delaporte, R. Gauvin, A. Guerfi, H. Lorrmann and K. Zaghib, Sci. Rep., 2020, 10, 18410 CrossRef CAS PubMed.
- R. H. Brugge, A. K. O. Hekselman, A. Cavallaro, F. M. Pesci, R. J. Chater, J. A. Kilner and A. Aguadero, Chem. Mater., 2018, 30, 3704–3713 CrossRef CAS.
- D. Lee, K.-H. Park, S. Y. Kim, J. Y. Jung, W. Lee, K. Kim, G. Jeong, J.-S. Yu, J. Choi, M.-S. Park and W. Cho, J. Mater. Chem. A, 2021, 9, 17311–17316 RSC.
- X. Luo, X. Hu, Y. Zhong, X. Wang and J. Tu, Small, 2023, 2306736 Search PubMed.
- D. Di Lecce, V. Marangon, H.-G. Jung, Y. Tominaga, S. Greenbaum and J. Hassoun, Green Chem., 2022, 24, 1021–1048 RSC.
- A. Dufresne, Mater. Today, 2013, 16, 220–227 CrossRef CAS.
- D. Trache, A. F. Tarchoun, M. Derradji, T. S. Hamidon, N. Masruchin, N. Brosse and M. H. Hussin, Front. Chem., 2020, 8, 392 CrossRef CAS PubMed.
- N. Grishkewich, N. Mohammed, J. Tang and K. C. Tam, Curr. Opin. Colloid Interface Sci., 2017, 29, 32–45 CrossRef CAS.
- J. Miller, Nanocellulose: producers, products and applications: a guide for end users, TAPPI Press, 2017 Search PubMed.
- W. Wang, Z. Li, H. Huang, W. Li and J. Wang, Chem. Eng. J., 2022, 445, 136568 CrossRef CAS.
- N. Mittal, S. Tien, E. Lizundia and M. Niederberger, Small, 2022, 18, 2107183 CrossRef CAS PubMed.
- A. A. Oun and J.-W. Rhim, Carbohydr. Polym., 2017, 174, 484–492 CrossRef CAS PubMed.
- J. G. Jeon, H. C. Kim, R. R. Palem, J. Kim and T. J. Kang, Mater. Lett., 2019, 250, 99–102 CrossRef CAS.
- W. E. Gloor, B. H. Mahlman and R. D. Ullrich, Ind. Eng. Chem., 1950, 42, 2150–2153 CrossRef CAS.
- M. X. Li, X. W. Wang, Y. Q. Yang, Z. Chang, Y. P. Wu and R. Holze, J. Membr. Sci., 2015, 476, 112–118 CrossRef CAS.
- J. Zhang, Z. Liu, Q. Kong, C. Zhang, S. Pang, L. Yue, X. Wang, J. Yao and G. Cui, ACS Appl. Mater. Interfaces, 2013, 5, 128–134 CrossRef CAS PubMed.
- L. Zhao, J. Fu, Z. Du, X. Jia, Y. Qu, F. Yu, J. Du and Y. Chen, J. Membr. Sci., 2020, 593, 117428 CrossRef CAS.
- K. Liu, M. Liu, J. Cheng, S. Dong, C. Wang, Q. Wang, X. Zhou, H. Sun, X. Chen and G. Cui, Electrochim. Acta, 2016, 215, 261–266 CrossRef CAS.
- N. Wu, B. Jing, Q. Cao, X. Wang, H. Kuang and Q. Wang, J. Appl. Polym. Sci., 2012, 125, 2556–2563 CrossRef CAS.
- L. Tian, W. Zhu and X. Tang, J. Appl. Polym. Sci., 2003, 90, 2310–2315 CrossRef CAS.
- Z. Du, Y. Su, Y. Qu, L. Zhao, X. Jia, Y. Mo, F. Yu, J. Du and Y. Chen, Electrochim. Acta, 2019, 299, 19–26 CrossRef CAS.
- B. M. Bell, J. R. Briggs, R. M. Campbell, S. M. Chambers, P. D. Gaarenstroom, J. G. Hippler, B. D. Hook, K. Kearns, J. M. Kenney, W. J. Kruper, D. J. Schreck, C. N. Theriault and C. P. Wolfe, Clean: Soil, Air, Water, 2008, 36, 657–661 CAS.
- W. Luo, S. Cheng, M. Wu, X. Zhang, D. Yang and X. Rui, J. Power Sources, 2021, 509, 230372 CrossRef CAS.
- Y. Wang, S. Zhu, D. Sun and Y. Jin, RSC Adv., 2016, 6, 105461–105468 RSC.
- W. Lu, Z. Yuan, Y. Zhao, H. Zhang, H. Zhang and X. Li, Chem. Soc. Rev., 2017, 46, 2199–2236 RSC.
- J. Nunes-Pereira, C. M. Costa and S. Lanceros-Méndez, J. Power Sources, 2015, 281, 378–398 CrossRef CAS.
- C. M. Costa, Y. H. Lee, J. H. Kim, S. Y. Lee and S. Lanceros-Méndez, Energy Storage Mater., 2019, 22, 346–375 CrossRef.
- E. Lizundia, C. M. Costa, R. Alves and S. Lanceros-Méndez, Carbohydr. Polym. Technol. Appl., 2020, 1, 100001 Search PubMed.
- W. Pantoja, J. A. Perez-Taborda and A. Avila, Batteries, 2022, 8, 105 CrossRef CAS.
- J. C. Barbosa, J. P. Dias, S. Lanceros-Méndez and C. M. Costa, Membranes, 2018, 8, 45 CrossRef PubMed.
- I. Kuribayashi, J. Power Sources, 1996, 63, 87–91 CrossRef CAS.
- J. Gou, W. Liu and A. Tang, J. Membr. Sci., 2021, 639, 119750 CrossRef CAS.
- R. Gonçalves, E. Lizundia, M. M. Silva, C. M. Costa and S. Lanceros-Méndez, ACS Appl. Energy Mater., 2019, 2, 3749–3761 CrossRef.
- S. Yang, Y. Zhang, Y. Zhang, J. Deng, N. Chen, S. Xie, Y. Ma and Z. Wang, Adv. Funct. Mater., 2023, 33, 2304280 CrossRef CAS.
- S.-J. Chun, E.-S. Choi, E.-H. Lee, J. H. Kim, S.-Y. Lee and S.-Y. Lee, J. Mater. Chem., 2012, 22, 16618–16626 RSC.
- J. Liu, K. Yang, Y. Mo, S. Wang, D. Han, M. Xiao and Y. Meng, J. Power Sources, 2018, 400, 502–510 CrossRef CAS.
- F. Gao, R. Bai, F. Ferlin, L. Vaccaro, M. Li and Y. Gu, Green Chem., 2020, 22, 6240–6257 RSC.
- R. Sliz, J. Valikangas, P. Vilmi, T. Hu, U. Lassi and T. Fabritius, in 2021 IEEE 16th Nanotechnology Materials and Devices Conference (NMDC), 2021, pp. 1–5.
- R. Sliz, I. S. Roy, P. Molaiyan, J. Välikängas, T. Jakkila, M. H. Christophliemk, T. Hu, H. H. Nguyen, E. Hannila, S. Illikainen, U. Lassi and T. Fabritius, Batteries Supercaps, 2024, e202300527 CrossRef CAS.
- S. Bapat, S. O. Kilian, H. Wiggers and D. Segets, Nanoscale Adv., 2021, 3, 4400–4410 RSC.
- F. Russo, F. Galiano, F. Pedace, F. Aricò and A. Figoli, ACS Sustainable Chem. Eng., 2020, 8, 659–668 CrossRef CAS.
- Y. Gu and F. Jérôme, Chem. Soc. Rev., 2013, 42, 9550–9570 RSC.
- A. Jordan, C. G. J. Hall, L. R. Thorp and H. F. Sneddon, Chem. Rev., 2022, 122, 6749–6794 CrossRef CAS PubMed.
- V. R. Ravikumar, A. Schröder, S. Köhler, F. A. Çetinel, M. Schmitt, A. Kondrakov, F. Eberle, J.-O. Eichler-Haeske, D. Klein and B. Schmidt-Hansberg, ACS Appl. Energy Mater., 2021, 4, 696–703 CrossRef CAS.
- J. Välikangas, P. Laine, T. Hu, P. Tynjälä, M. Selent, P. Molaiyan, K. Jürgen and U. Lassi, Small, 2023, 2305349 Search PubMed.
- G. Yang, M. Zhang, I. Majeed, W. Fan, J. Zhao and Z. Zeng, ACS Sustainable Chem. Eng., 2023, 11, 14582–14590 CrossRef CAS.
- C. Chen, V. Reddy Tatagari, H. Lin and L. Shaw, J. Energy Chem., 2023, 78, 240–245 CrossRef CAS.
- A. Sarkar, R. May, Z. Valmonte and L. E. Marbella, Energy Adv., 2022, 1, 671–676 RSC.
- M. Wood, J. Li, R. E. Ruther, Z. Du, E. C. Self, H. M. Meyer, C. Daniel, I. Belharouak and D. L. Wood, Energy Storage Mater., 2020, 24, 188–197 CrossRef.
- J. Li, Y. Lu, T. Yang, D. Ge, D. L. Wood and Z. Li, iScience, 2020, 23, 101081 CrossRef CAS PubMed.
- M. Bichon, D. Sotta, N. Dupré, E. De Vito, A. Boulineau, W. Porcher and B. Lestriez, ACS Appl. Mater. Interfaces, 2019, 11, 18331–18341 CrossRef CAS PubMed.
- W. B. Hawley, A. Parejiya, Y. Bai, H. M. Meyer, D. L. Wood and J. Li, J. Power Sources, 2020, 466, 228315 CrossRef CAS.
- G. Park, H.-S. Kim and K. J. Lee, Nanomaterials, 2023, 13, 324 CrossRef CAS PubMed.
- N. Phattharasupakun, J. Wutthiprom, S. Duangdangchote, S. Sarawutanukul, C. Tomon, F. Duriyasart, S. Tubtimkuna, C. Aphirakaramwong and M. Sawangphruk, Energy Storage Mater., 2021, 36, 485–495 CrossRef.
- M. Ryu, Y.-K. Hong, S.-Y. Lee and J. H. Park, Nat. Commun., 2023, 14, 1316 CrossRef CAS PubMed.
- D. Bresser, D. Buchholz, A. Moretti, A. Varzi and S. Passerini, Energy Environ. Sci., 2018, 11, 3096–3127 RSC.
- W. Dou, M. Zheng, W. Zhang, T. Liu, F. Wang, G. Wan, Y. Liu and X. Tao, Adv. Funct. Mater., 2023, 33, 2305161 CrossRef CAS.
- F. Schmidt, S. Kirchhoff, K. Jägle, A. De, S. Ehrling, P. Härtel, S. Dörfler, T. Abendroth, B. Schumm, H. Althues and S. Kaskel, ChemSusChem, 2022, 15, e202201320 CrossRef CAS PubMed.
- X. Zhang, X. Ge, Z. Shen, H. Ma, J. Wang, S. Wang, L. Liu, B. Liu, L. Liu and Y. Zhao, New J. Chem., 2021, 45, 9846–9855 RSC.
- G. Hernández, R. Mogensen, R. Younesi and J. Mindemark, Batteries Supercaps, 2022, 5, e202100373 CrossRef.
- A. C. Rolandi, C. Pozo-Gonzalo, I. de Meatza, N. Casado, D. Mecerreyes and M. Forsyth, Adv. Energy Sustainability Res., 2023, 4, 2300149 CrossRef CAS.
- M. Hong, S. Lee, S. Choi and J. Mun, J. Electroanal. Chem., 2020, 871, 114317 CrossRef CAS.
- X. Zuo, J. Zhu, P. Müller-Buschbaum and Y.-J. Cheng, Nano Energy, 2017, 31, 113–143 CrossRef CAS.
- S. Gao, F. Sun, A. Brady, Y. Pan, A. Erwin, D. Yang, V. Tsukruk, A. G. Stack, T. Saito, H. Yang and P.-F. Cao, Nano Energy, 2020, 73, 104804 CrossRef CAS.
- L. Deng, Y. Zheng, X. Zheng, T. Or, Q. Ma, L. Qian, Y. Deng, A. Yu, J. Li and Z. Chen, Adv. Energy Mater., 2022, 12, 2200850 CrossRef CAS.
- T. Yim, S. J. Choi, Y. N. Jo, T.-H. Kim, K. J. Kim, G. Jeong and Y.-J. Kim, Electrochim. Acta, 2014, 136, 112–120 CrossRef CAS.
- V. Singh, S. Kuthe and N. V. Skorodumova, Batteries, 2023, 9, 184 CrossRef CAS.
- X. Jia, Y. Ge, L. Shao, C. Wang and G. G. Wallace, ACS Sustainable Chem. Eng., 2019, 7, 14321–14340 CrossRef CAS.
- H. Ragones, S. Menkin, Y. Kamir, A. Gladkikh, T. Mukra, G. Kosa and D. Golodnitsky, Sustainable Energy Fuels, 2018, 2, 1542–1549 RSC.
- R. Sliz, E. Hannila, I. S. Roy, J. Valikangas, P. Molaiyan, U. Lassi and T. Fabritius, in 2023 IEEE 23rd International Conference on Nanotechnology (NANO), 2023, pp. 363–366.
- R. Gonçalves, P. Dias, L. Hilliou, P. Costa, M. M. Silva, C. M. Costa, S. Corona-Galván and S. Lanceros-Méndez, Energy Technol., 2021, 9, 2000805 CrossRef.
- M. Zhang, H. Mei, P. Chang and L. Cheng, J. Mater. Chem. A, 2020, 8, 10670–10694 RSC.
- J. Choi, T. Ingsel and R. K. Gupta, Solid State Batteries Volume 2: Materials and Advanced Devices, American Chemical Society, 2022, vol. 1414, pp. 14–311 Search PubMed.
- S. Zhou, M. Li, P. Wang, L. Cheng, L. Chen, Y. Huang, S. Yu, F. Mo and J. Wei, Electrochem. Energy Rev., 2023, 6, 34 CrossRef.
- B. Clement, M. Lyu, E. Sandeep Kulkarni, T. Lin, Y. Hu, V. Lockett, C. Greig and L. Wang, Engineering, 2022, 13, 238–261 CrossRef.
- C. M. Costa, R. Gonçalves and S. Lanceros-Méndez, Energy Storage Mater., 2020, 28, 216–234 CrossRef.
- M. He, X. Jin, X. Zhang, X. Duan, P. Zhang, L. Teng, Q. Liu and W. Liu, Green Chem., 2023, 25, 6561–6580 RSC.
- D. L. Thompson, J. M. Hartley, S. M. Lambert, M. Shiref, G. D. J. Harper, E. Kendrick, P. Anderson, K. S. Ryder, L. Gaines and A. P. Abbott, Green Chem., 2020, 22, 7585–7603 RSC.
- J. J. Roy, S. Rarotra, V. Krikstolaityte, K. W. Zhuoran, Y. D.-I. Cindy, X. Y. Tan, M. Carboni, D. Meyer, Q. Yan and M. Srinivasan, Adv. Mater., 2022, 34, 2103346 CrossRef CAS PubMed.
- X. Chen, C. Luo, J. Zhang, J. Kong and T. Zhou, ACS Sustainable Chem. Eng., 2015, 3, 3104–3113 CrossRef CAS.
Footnote |
| † The authors equally contributed to the manuscript. |
| This journal is © The Royal Society of Chemistry 2024 |