Eco-friendly conversion between n- and p-type carbon nanotubes based on rationally functionalized lignin biopolymers†
Yoohyeon
Choi‡
ab,
Ngoc Tuan
Tran‡
 cd,
Doojoon
Jang
a,
Minju
Park
a,
Chun-Jae
Yoo
cd,
Jin Young
Kim
cd,
Doojoon
Jang
a,
Minju
Park
a,
Chun-Jae
Yoo
cd,
Jin Young
Kim
 b,
Hyunjoo
Lee
*cd and
Heesuk
Kim
b,
Hyunjoo
Lee
*cd and
Heesuk
Kim
 *ad
*ad
aSoft Hybrid Materials Research Center, Korea Institute of Science and Technology (KIST), Seoul 02792, Republic of Korea. E-mail: heesukkim@kist.re.kr
bDepartment of Materials Science and Engineering, Seoul National University, Seoul 08826, Republic of Korea
cClean Energy Research Center, Korea Institute of Science and Technology (KIST), Seoul 02792, Republic of Korea. E-mail: hjlee@kist.re.kr
dDivision of Energy & Environment Technology, KIST School, Korea University of Science and Technology, Seoul 02792, Republic of Korea
First published on 5th December 2023
Abstract
As global interest in carbon-neutrality policies grows, the demand for sustainable alternatives to fossil fuels intensifies. Herein, we demonstrate functionalized lignin-based n- and p-dopants for nanocarbon materials, providing promising eco-friendly alternatives to chemical dopants derived from fossil fuels. A simple microwave-assisted phenolation of organosolv lignin introduces phenol groups, offering additional reaction sites and enhancing solvent solubility for uniform, reliable and efficient carbon nanotube (CNT) doping. To create effective n- and p-dopants, the phenolated lignin is further functionalized with amine and hexafluoropropyl groups, respectively. The aminated and fluorinated lignin-doped CNTs present the Seebeck coefficients of −48.0 and 53.9 μV K−1, respectively, confirming an efficient and eco-friendly conversion between n- and p-type CNT. Notably, this n-doping performance is of particular significance since stable and reliable n-doping is challenging due to the inherent p-type semiconducting properties of CNTs. As a proof of concept, we demonstrate a flexible thermoelectric generator using 10 p–n pairs of the CNT films. The output voltage (7.86 mV) and output power (247 nW) of the flexible generator at ΔT = 15 K confirm that the aminated- and fluorinated lignin-doped CNT films exhibit n- and p-type characteristics, respectively. This study paves the way for sustainable, lignin-based doping of carbon nanomaterials, offering a green alternative to traditional fossil fuel-derived dopants and contributing to the transition towards carbon-neutral technologies.
Introduction
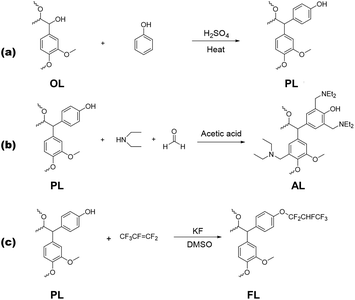
As practical interest in global carbon-neutrality (net zero) policies increases, the demand for alternative carbon sources to replace fossil fuel resources is growing. Lignin has been considered as a promising alternative carbon resource due to its second-highest abundance in nature, primarily found in wood and other plant materials.1–4 Lignin is a complex, heterogeneous polymer composed of various phenylpropane-derived monomers, such as guaiacyl, syringyl, and 4-hydroxyphenyl. However, due to its recalcitrant nature stemming from its structure as a cross-linked aromatic hetero-polymer, the utilization of lignin is highly limited. Despite the annual production of 50–70 million tons as by-products in biomass and pulp manufacturing processes, only 2% of lignin has been used for industrial purposes.1,5–7Lignin can be classified into Kraft lignin, lignosulfonates, organosolv lignin, and soda lignin depending on the separation process from biomass. However, its application remains limited due to the lack of reactive sites, its low solubility in organic solvents, and its sulfur content of 2–8 wt%, which is responsible for the characteristic odor associated with lignin. To address these issues, the acetylation or phenolation of lignin have been adopted. In acetylation, lignin is diluted in pyridine and reacted with acetic anhydride, leading to enhanced solubility in solvents. However, this acetylation method has bottlenecks, including long reaction times and the utilization of large amounts of chemicals. In contrast, lignin phenolation has proven to be an effective method for increasing reactive sites and solubility in organic solvents.8–10 By dissolving lignin in phenol in the presence of H2SO4 as a catalyst at ∼100 °C, lignin solubility is greatly enhanced, and phenol moieties are added to lignin, providing further reaction sites. This process results in lignin with superior properties compared to pristine lignin itself. With various lignin functionalization techniques, the use of lignin in biofuels, biochemicals, and composite materials is steadily growing.
Lignin serves as a versatile replacement for petrochemical-based substances across a wide range of industries, including polymers, inorganic compounds, and carbon-based materials.11 Its unique structural advantages allow lignin to effectively reduce or entirely substitute the toxic petroleum-based phenol precursor in the synthesis of phenolic resins and polyurethane foam, while maintaining their properties and minimizing the use of harmful compounds.12–15 Lignin can also replace bitumen, a component of crude oil used in asphalt for roads.16 Furthermore, the abundance of reactive functional groups in lignin enables its use in the productive synthesis of nitrogen fertilizers through graft copolymerization with cross-linking agents.17 Additionally, lignin can replace inorganic complexes like sodium chromate (Na2CrO4) and sodium nitrite (NaNO2) that are toxic synthetic corrosion inhibitors,18,19 as well as alternative photocatalysts.20 In applications such as lithium-ion batteries, lignin serves as a binder to replace carbon blacks,21,22 and it is utilized in the tire industry to enhance the properties of rubber products.23 Even though various reports highlight the potential of replacing petroleum-based materials with lignin, further intensive studies are still essential to explore how to substitute petrochemical-based chemicals with lignin while maintaining equivalent performance and achieving carbon neutrality.
Herein, we demonstrate functionalized lignin-based n- and p-dopants for carbon nanomaterials, particularly nanotubes (CNTs), as promising alternatives to chemical dopants derived from fossil fuels. CNTs have garnered extensive attention for their metallic or semiconducting applications due to their lightweight, mechanical strength and tunable electrical properties.24 Surface charge transfer doping, a method in which n- or p-type dopants physically adsorb onto the CNT surface, is considered the most feasible doping approach as it does not disrupt the CNT sp2 characteristics. Fossil-fuel based organic dopants like carbazole and 7,7,8,8-tetracyanoquinodimethane (TCNQ) as p-dopants, and 1,3-bis(diphenylphosphino)propane (dppp) and polyethyleneimine (PEI) as n-dopants have mainly been used for surface charge transfer doping of CNT.25–29 As an alternative to fossil fuel-based dopants, organosolv lignin (OL) was phenolated via a simple microwave method because the addition of phenol groups creates more reaction sites and achieves sufficiently high solubility for uniform, reliable, and efficient CNT doping. To create effective n- and p-dopants, phenolated lignin was further functionalized with diethylamine and hexafluoropropyl groups, respectively. The carrier-transport characteristics of n- and p-doped CNT samples were determined using Seebeck coefficient and electrical conductivity measurements. The aminated and fluorinated lignin-doped CNTs exhibit distinct Seebeck coefficients of −48.0 and 53.9 μV K−1, respectively, demonstrating an efficient and eco-friendly conversion between n- and p-type CNTs. These doping performances are comparable to those achieved with fossil fuel-based organic dopants. In particular, the n-doping performance using aminated lignin is noteworthy due to the inherent p-type semiconducting properties of CNTs. Finally, as a proof of concept, we fabricated a flexible thermoelectric generator using 10 pairs of the n- and p-doped CNT films, in order to highlight the potential of functionalized lignin as a promising alternative carbon resource to petrochemical-based chemicals for use in surface charge transfer doping and electronic/or energy applications.
Experimental
Materials
Organosolv lignin (OL) was purchased from SugarEn Company (South Korea). Phenol (≥99%), sulfuric acid (99.99%), acetic acid (≥99%), dioxane, and tetrahydrofuran (anhydrous, ≥99%, inhibitor-free) were obtained from Sigma-Aldrich. Methanol (99.5% HPLC grade) was supplied by Daejung Chemicals & Metals Co., Ltd. Double-walled carbon nanotube (DWCNT) was purchased from Nanoshel. Silver paste (Elcoat p-100) was purchased from CANS.Phenolation of organosolv lignin (OL)
H2SO4 (0.15 g, catalyst), phenol (2.0 g), and OL (1.0 g) were combined in a pressure glass tube reactor for the lignin phenolation, as described in a previously reported procedure.30 The reactor was heated using a microwave synthesizer (CEM Discover, power = 150 W and frequency = 2.5 GHz) for 5–20 min. After the reaction, the solution was diluted with methanol (15 mL), and the phenolated lignin was precipitated with acidic water (pH = 1), followed by washing with deionized water until the pH became neutral. The resulting precipitate was then dried in a vacuum oven at 50 °C overnight.Amination and fluoroalkylation of phenolated lignin (PL)
For the amination of phenolated lignin (PL), PL (1.0 g), dioxane (10 g), diethylamine (1.0 g), 30% formaldehyde solution (1.0 g), and acetic acid (0.2 mL, catalyst) were heated at 60 °C for 1 h.31 After the reaction, the solvent and remaining reagents were removed using a rotary evaporator. The obtained solid (aminated lignin, AL) was washed with deionized water to eliminate any remaining impurities, followed by drying in a vacuum oven at 150 °C overnight. For the fluoroalkylation of PL, PL (1.0 g), potassium fluoride (0.3 g, catalyst) and dimethyl sulfoxide (DMSO, 30 mL) were mixed in a pressure glass tube reactor (Fischer bottle). Hexafluoropropylene (HFP, 2.0 g) was then added to the mixture, followed by stirring at room temperature for 1 h.32 After the reaction, the mixture was poured into water (200 mL) and vigorously stirred to precipitate the product. The precipitate (fluoroalkylated lignin, FL) was collected using a centrifuge and washed with deionized water to remove any remaining reagents, followed by drying in a vacuum oven at 50 °C overnight.Doping of DWCNT films with functionalized lignins and thermoelectric generator (TEG) fabrication
The AL and FL solutions in tetrahydrofuran (THF) with various concentrations were prepared for DWCNT doping. The free-standing DWCNT films were cut into 2 cm × 1 cm pieces and placed onto slide glasses. The AL and FL solutions were applied to the DWCNT film surfaces using a brushing process for n- and p-doping, respectively. The brushing process was repeated three times for each film, and after each treatment, the films were dried at 40 °C for 5 min to evaporate the solvent. After the final brushing, the films were further dried at 60 °C for 5 min to ensure the removal of any remaining solvents. To characterize the doping properties of the DWCNT films, a thermoelectric device consisting of 10 pairs of n- and p-doped DWCNTs connected in series was fabricated. Copper tape and silver paste were used as electrodes.Characterization
1H, 13C, 19F and 31P NMR analyses were performed to characterize the functionalized lignin. The 1H and 31P NMR spectra were obtained using a 400 MHz NMR Bruker instrument and analyzed using MestReNova software. For the 13C NMR spectra, a 600 MHz Bruker spectrometer was used. The 1H NMR sample was prepared by mixing lignin (0.05 g) with an internal standard solution (0.10 g, 10 wt% CH2Br2 in DMSO-D6) for quantification. For 31P NMR, the sample was first dissolved in mixture of pyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CDCl3 (1
CDCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio), and then phosphorus reagent (2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane) of 50 μL was added. The mixture was allowed to react for a few minutes and then mixed with an internal standard (cyclohexanol) and a relaxation reagent (chromium(III) acetylacetonate).33 By using 1H (Fig. S1 in ESI†), 31P (Fig. S2†), and 13C NMR (Fig. S3†) experiments, the amounts of aromatic (Ar)-H and aliphatic (Al)-H, Ar-OH and Al-OH, and Ar-C and Al-C could be quantified, respectively. Fourier-transform infrared spectroscopy (FTIR) of the lignin samples was analyzed using a Nicolet™ iS™ 10 spectrometer (Thermo Scientific). Elemental analysis was employed to determine the nitrogen content (wt%) in each sample. Gel permeation chromatography (GPC, Agilent 1100 Series) was performed on lignin using a pair of Shodex LF-604 columns (8 × 300 mm) and THF as the eluent. The flow rate was 1.0 mL at 30 °C. Calibration was accomplished with a polystyrene low molecular standard (ReadyCal Set Mp ∼250–65
1 weight ratio), and then phosphorus reagent (2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane) of 50 μL was added. The mixture was allowed to react for a few minutes and then mixed with an internal standard (cyclohexanol) and a relaxation reagent (chromium(III) acetylacetonate).33 By using 1H (Fig. S1 in ESI†), 31P (Fig. S2†), and 13C NMR (Fig. S3†) experiments, the amounts of aromatic (Ar)-H and aliphatic (Al)-H, Ar-OH and Al-OH, and Ar-C and Al-C could be quantified, respectively. Fourier-transform infrared spectroscopy (FTIR) of the lignin samples was analyzed using a Nicolet™ iS™ 10 spectrometer (Thermo Scientific). Elemental analysis was employed to determine the nitrogen content (wt%) in each sample. Gel permeation chromatography (GPC, Agilent 1100 Series) was performed on lignin using a pair of Shodex LF-604 columns (8 × 300 mm) and THF as the eluent. The flow rate was 1.0 mL at 30 °C. Calibration was accomplished with a polystyrene low molecular standard (ReadyCal Set Mp ∼250–65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Sigma Aldrich) and an RI detector (G1362A, Agilent). The lignin sample was dissolved in THF at a concentration of 1 mg mL−1, sonicated and filtered using a polytetrafluoroethylene (PTFE) Whatman syringe filter with a pore size 0.45 μm. The GPC system operation and data collection were carried out using Agilent Chemstation GPC analysis software.
000, Sigma Aldrich) and an RI detector (G1362A, Agilent). The lignin sample was dissolved in THF at a concentration of 1 mg mL−1, sonicated and filtered using a polytetrafluoroethylene (PTFE) Whatman syringe filter with a pore size 0.45 μm. The GPC system operation and data collection were carried out using Agilent Chemstation GPC analysis software.
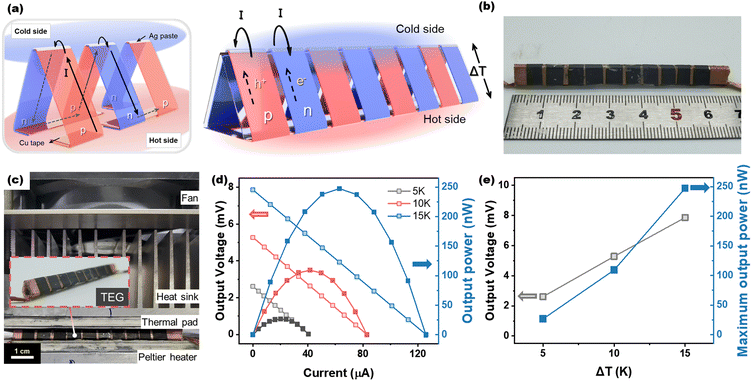
The surface morphology of the lignin-doped DWCNT films was examined using a field-emission scanning electron microscope (FE-SEM, Sigma 300, ZEISS). Energy dispersive spectrometry (EDS) was used to conduct surface elemental analysis of the doped DWCNTs. The work function of the lignin-doped DWCNTs was characterized using ultraviolet photoelectron spectroscopy (UPS, Nexsa, Thermo Fisher Scientific) with a He–I source (21.22 eV). Additionally, the electrical conductivity and Seebeck coefficient of the films with a thickness of several tens of micrometers were measured at room temperature using a four-point probe TE measurement system (TEP600, Seepel Instrument). The electrical conductivity was calculated from the equation (σ = L/RA, where L, R, and A indicate the length, the resistance and the cross-sectional area of the DWCNT films, respectively). The Seebeck coefficient was determined using the equation (α = −ΔV/ΔT, where ΔV is the potential difference across the sample at a given temperature difference). The potential differences at given temperature gradients were recorded and those with a linear correlation (R2) higher than 0.999 were selected. The output power of the assembled TEG was characterized using a homemade system (Fig. 6(c)) equipped with two Peltier modules, a heat sink, an electric fan, and a Keithley 2700 for measuring the output voltage.
Results and discussion
Eco-friendly DWCNT doping and its application to a thermoelectric generator
Fig. 1 illustrates the concept of eco-friendly DWCNT doping and its application to a thermoelectric generator. Eco-friendly organic dopants of carbon nanomaterials have been synthesized using organosolv lignin (OL) as a starting material. Organosolv lignin (OL) is separated from natural biomass by a sulfur-free process involving heat treatment in organic solvents, resulting in higher solubility in organic solvents compared to other separation methods. This enhanced solubility of OL allows its applications to biofuels, biochemical, composite materials and so on. However, OL solubility in organic solvents still presents limitations for broader application. To overcome this limitation, OL was phenolated via a simple microwave method because the addition of phenol groups creates more reaction sites and achieves sufficiently high solubility for further functionalization. Phenolated lignin (PL) was fluorinated using hexafluoropropylene with electron-withdrawing fluorine groups, serving as a p-dopant. For n-dopant, PL was functionalized with diethylamine. As depicted in Fig. 1 and Fig. S4,† the excellent solubility of aminated lignin (AL) and fluorinated lignin (FL) in THF enables a uniform, reliable and efficient CNT doping.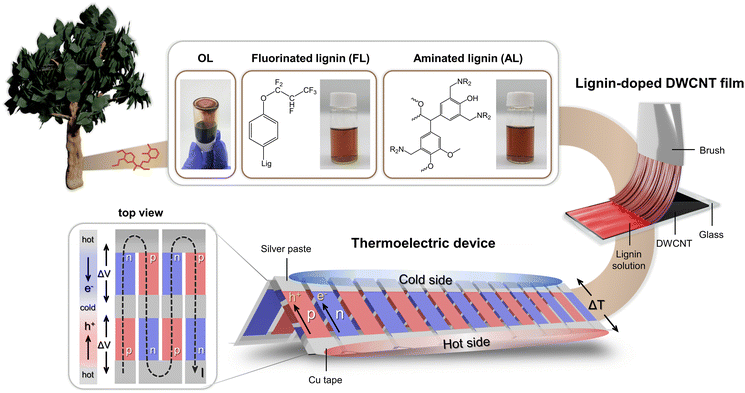 | ||
| Fig. 1 Schematic illustration of DWCNT doping by functionalized lignin and its application to a flexible thermoelectric generator. | ||
DWCNT films were n- and p-doped by AL and FL, respectively, using a brushing method. In order to determine whether the primary charge carriers of the doped DWCNT films were electrons or holes, the Seebeck coefficients of the films were characterized. The Seebeck coefficient (α), which is one of the most important factors determining the behavior of thermoelectric materials, is the magnitude of the thermoelectric voltage generated in response to a temperature difference across the material (i.e., α = −ΔV/ΔT). If the primary charge carriers of the material are holes, a positive potential is induced, while electrons as charge carriers result in a negative potential. The FL-doped DWCNT film exhibits the Seebeck coefficient of 53.9 μV K−1 and the electrical conductivity of 340 S cm−1. Following n-doping with AL, the CNT film displays the Seebeck coefficient of −48.0 μV K−1, indicating a dramatic conversion to n-type behavior. As a proof of concept, we demonstrate a flexible thermoelectric generator using 10 pairs of n- and p-type CNT films prepared by AL and FL doping, respectively. A more detailed explanation of lignin functionalization, carrier properties of lignin-doped DWCNT films, and thermoelectric performance is provided later in the manuscript.
Synthesis and characterization of PL, AL and FL
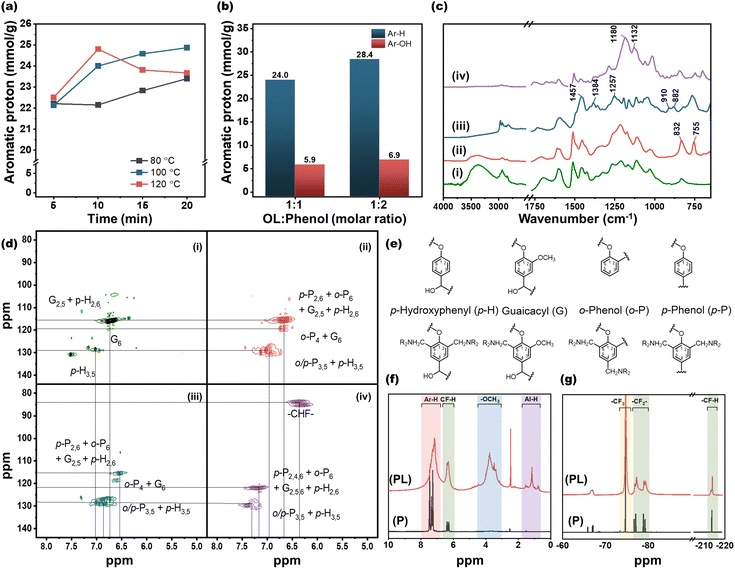
Fig. 2 illustrates the reaction schemes for lignin phenolation, amination and fluorination starting from OL. Microwave-assisted phenolation offers significant advantages in terms of reactivity, reaction time, and reaction temperature compared to conventional oil-bath heating. Table 1 presents the Ar-H and Ar-OH concentrations of PLs prepared using two different methods (i.e., oil-bath and microwave heating), which reflect the degree of phenolation. The Ar-H and Ar-OH concentrations of microwave-treated PL are 28.4 and 6.84 mmol g−1, respectively, which are 38% and 17.5% higher than those from the oil-bath method. These results indicate that the microwave-assisted phenolation provides more reaction sites in PL.| Functional group | Organosolv lignin (OL) (mmol g−1) | Phenolated lignin (PL) (mmol g−1) | |
|---|---|---|---|
| Oil-bath heating | Microwave heating | ||
| a Reaction condition: lignin 1.00 g, phenol 2.00 g and H2SO4 0.150 g at 100 °C for 10 min. | |||
| Ar-H | 13.4 | 20.5 | 28.4 |
| Ar-OH | 4.30 | 5.82 | 6.84 |
| Al-OH | 1.46 | 0.08 | 0.06 |
The microwave-assisted phenolation was optimized considering reaction temperature, time and molar ratio between OL and phenol. As shown in Fig. 3(a), regardless of the reaction temperature, the Ar-H concentration remains relatively constant at ∼22 mmol g−1 during the early reaction stages of 5 min. When the reaction time was extended to 20 min, the Ar-H concentration increases to 23.4 and 24.9 mmol g−1 at 80 and 100 °C, respectively. In contrast, at a reaction temperature of 120 °C, the Ar-H concentration reaches to 24.8 mmol g−1 after 10 min, and then decreases to 23.7 mmol g−1 with additional reaction time, possibly due to increased crosslinking or decomposition at that temperature.30Fig. 3(b) depicts the degree of phenolation as a function of the molar ratio between OL and phenol. When the phenol amount was doubled at 100 °C for 10 min, the Ar-H and Ar-OH concentrations increase to 28.4 and 6.9 mmol g−1, respectively. Based on these results, the microwave-assisted phenolation was fixed at the reaction condition of OL to phenol molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), a reaction temperature (100 °C), and a reaction time (10 min). For the synthesis of the lignin-based n-type dopant, PL was aminated using diethylamine and formaldehyde in the presence of a catalyst, acetic acid (Mannich reaction), as shown in Fig. 2(b).
2), a reaction temperature (100 °C), and a reaction time (10 min). For the synthesis of the lignin-based n-type dopant, PL was aminated using diethylamine and formaldehyde in the presence of a catalyst, acetic acid (Mannich reaction), as shown in Fig. 2(b).
As a p-type dopant, FL was synthesized from PL by reacting with hexafluoropropylene (HFP), as illustrated in Fig. 2(c). The functionalized lignins, namely OL, PL, AL, and FL were characterized by using FT-IR and NMR analyses. In FT-IR spectra (Fig. 3(c) and Fig. S5†), after phenolation, the peak at 832 cm−1 observed in OL increases with the appearance of a new peak centered at 755 cm−1. These two peaks could be attributed to the aromatic C–H bending of phenols attached to the ortho and para positions of the lignin moiety, respectively.8,30,34 The successful amination of PL can be confirmed by a C–N stretching peak of tertiary amine at 1257 cm−1, along with –CH3 symmetrical deformation at 1384 cm−1 and –CH2– deformation at 1457 cm−1 from the diethylamine group. Additionally, there are new peaks at 882 and 910 cm−1, corresponding to the aliphatic C–H bond in –N(CH2CH3)2 and the newly formed –CH2– between lignin and –N(CH2CH3)2.35 In the FL spectrum, two strong peaks appeared at 1132 and 1180 cm−1 indicate the presence of C–F bonds in FL.35,36
NMR analyses offer a more detailed insight into the functionalized lignin structures. In 13C NMR spectra (Fig. S6†), the AL exhibits two new peaks at 11.4 and 46.2 ppm, corresponding to two carbons of –N(CH2CH3)2, along with an additional peak at 53.4 ppm from the methylene group between lignin and –N(CH2CH3)2. The 1H NMR spectra (Fig. S7†) confirm these results with the ethyl group attached to the amine at 1.06 and 2.35 ppm. Further insights from 2D-heteronuclear single quantum coherence (HSQC) NMR analyses (Fig. 3(d)) reveal significant changes in the intensities of aromatic C–H peaks in the range of δH 6–8 ppm/δC 110–140 ppm in the PL spectrum (Fig. 3(d)-(ii)). After PL amination (Fig. 3(d)-(iii)), the relative intensities of peaks at δH 6.5–7.0 ppm/δC 112–120 ppm are noticeably reduced compared to the phenyl C–H peaks at δH 6.5–7.5 ppm/δC 125–130 ppm. The regions of δH 6.5–7.0 ppm/δC 112–120 ppm in PL correspond to ortho or para Ar-H positions of phenolic or guaiacyl groups, such as p-P2,6, o-P4,6, p-H2,6, and G6. 31P NMR results also indicate a substantial decrease in the concentration of guaiacyl-OH and phenolic OH with a significant increase in the 5-substituted hydroxyl group, compared to the hydroxyl groups in PL (Fig. S2 and Table S1†). Therefore, the reduction in peak intensities in that domain suggests successful attachment of the diethylamino methyl (–CH2NEt2) group via the Mannich reaction to those sites. The estimated unit structures of PL and AL are shown in Fig. 3(e).20 According to the elemental analysis in Table S2,† the N content in AL is 6.19 wt% (4.4 mmol g−1), a slightly higher value compared to the 5.44 and 4.88 wt% of ALs synthesized from thermally processed PL under similar conditions.37,38
The p-type dopant, FL, features a –PhOCF2CHFCF3 moiety, resulting from its reaction with HFP, as depicted in Fig. 2(c). Both the 13C (Fig. S4†) and 1H–13C HSQC (Fig. 3(d)-(iv)) NMR spectra confirm the presence of fluorinated carbon in FL. To clearly demonstrate the existence of the –PhOCF2CHFCF3 moiety in FL, the reaction product of phenol with HFP, PhOCF2CHFCF3, were characterized along with FL. Fig. 3(f) and (g) clearly display the similarity of 1H and 19F NMR spectra between PhOCF2CHFCF3 and FL. The 1H NMR reveals the presence of a –CHF– peak at 6.88 ppm for both products, FL and PhOCF2CHFCF3, indicating the attachment of –CF2CHFCF3 groups to the phenolic site, as expected. The 19F NMR analyses further support the results, showing three peaks at −74.7, −78.0, and −213.8 ppm, which correspond to CF3, CF2, and CHF, respectively.32,39 Although side products such as dehydrofluorinated compounds are detected at −65.9 ppm and −67.1 ppm,32,39 the purity of the desired product could be estimated to be over 90% based on the Ar-H to CHF integration ratio of 5.4 in the product of phenol and HFP. The F content in FL calculated from 19F NMR is 37.7 mmol g−1, which corresponds to ∼6.3 mmol of –C3HF6 groups per gram of the sample. Given that the total Ar-OH and Al-OH concentration of PL is 6.9 mmol g−1, it is reasonable to conclude that more than 90% of the hydroxyl groups in PL have been functionalized with HFP. 31P NMR shown in Fig. S2† also indicates the absence of hydroxyl groups in FL.
It has been reported that some side reactions such as decomposition, cross-linking, and aromatic ring exchange can occur during the phenolation of lignin, particularly at high temperatures, long reaction times, and in the presence of a catalyst such as H2SO4. To investigate structural changes in the lignin polymer during phenolation and fluoroalkylation, GPC was conducted, and the molecular weight (Mw) and polydispersity (Đ) of OL, PL, and FL were obtained. Fig. S8† shows that the Mw (1470 g mol−1) of OL slightly decreases to 1110 g mol−1 after phenolation, suggesting some fragmentation or aromatic ring exchange during phenolation. There is little change in the Đ value, which remains at 1.70 and 1.76 for OL and PL, respectively. In contrast, after fluorination, the Mw and Đ values increase to 8820 g mol−1 and 3.66, respectively. These results confirm that cross-linking reactions occur between lignin polymers during the functionalization of PL with hexafluoropropylene. However, the degree is not severe enough to reduce the solubility of the resulting polymer in organic solvents like THF and CH3CN.
n- and p-Doping of DWCNT films by functionalized lignin
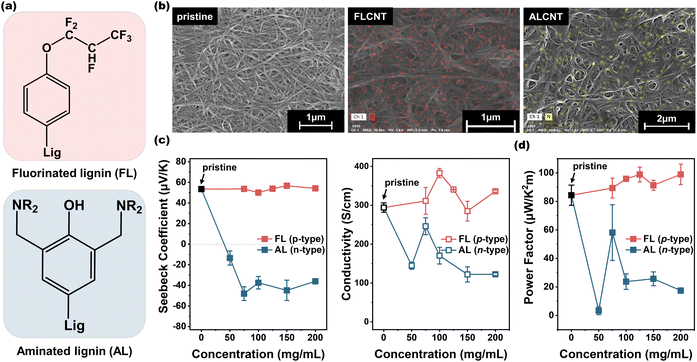
To realize and expand the applications of carbon nanomaterials, particularly in areas such as transistors, sensors and energy-storage/harvesting devices, precise control over their electrical properties, including carrier type, concentration, and mobility is essential. In this regard, the synthesized FL and AL serve as effective alternatives to fossil fuel-based dopants, efficiently facilitating p- and n-type doping of DWCNT films via surface charge transfer doping, respectively (Fig. 4). SEM and EDS characterizations in Fig. 4(b) reveal well-distributed fluorine and nitrogen elements in the FL- and AL-doped CNT films, respectively, confirming the uniform and efficient doping of DWCNTs by the functionalized lignin. Quantitative assessment of the atom quantities in the doped DWCNT films by XPS further confirms uniform doping. At three different spots within a single sample, consistent nitrogen contents of approximately 5% are observed in AL-CNT. Similarly, fluorine content ranging from 22% to 23% is observed in FL-CNT (Fig. S9†).The introduction of lignin dopants either donates or withdraws electrons from the DWCNTs. These carrier-transport characteristics of n- and p-doped CNT samples were determined using Seebeck coefficient (α) and electrical conductivity (σ) measurements, as well as power factor (α2σ) for thermoelectric applications. Fig. 4(c) and (d) show the Seebeck coefficient, electrical conductivity, and power factor of DWCNT films as a function of lignin concentration. Pristine DWCNTs exhibit inherent p-type behavior, characterized by the Seebeck coefficient of 53.5 μV K−1 and the electrical conductivity of 294 S cm−1. This p-type behavior arises from unintentional p-doping by water and oxygen molecules adsorbed on the CNT surfaces from surroundings.40–42 The FL doping leads to an increase in the electrical conductivity with the maximum of 383 S cm−1 at 100 mg mL−1 FL concentration, owing to the electron-withdrawing effect of FL from CNTs. In contrast, this effect results in a ∼10% decrease in the Seebeck coefficient as the carrier concentration is inversely proportional to the Seebeck coefficient.24 Considering this trade-off relationship, the maximum power factor of FL-doped DWCNT is determined to be 98.9 μW K−2 m−1 at 125 mg mL−1 FL concentration, which is 1.2 times higher than that of pristine DWCNT. In the case of n-doping, the AL effectively n-dopes the DWCNT films, thus converting the Seebeck coefficient from 53.5 to −48.0 μV K−1 as the AL concentration increases. However, at higher concentrations (>100 mg mL−1), the insulating properties of AL rather hinder an efficient doping. At 75 mg mL−1 AL concentration, the AL-doped DWCNT exhibits the maximum power factor of 58.2 μW K−2 m−1, along with the electrical conductivity of 246 S cm−1, indicating an effective and eco-friendly n-doping of CNTs. In particular, this n-doping performance is noteworthy due to the difficulty in stable and reliable n-doping, which is attributed to the inherent p-type semiconducting properties of CNTs. These doping performances are comparable to those of fossil fuel-based organic dopants, including carbazole and TCNQ as p-dopants, and PEI and dppp as n-dopants, as depicted in Fig. S10.†
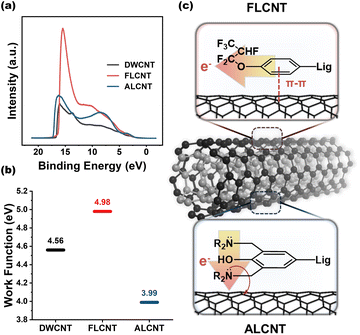
Fig. 5(a) and (b) display the UPS data and work functions of the pristine DWCNT, FL-doped DWCNT and AL-doped DWCNT films. The FL-doped DWCNT film exhibits a work function of 4.98 eV, whereas AL-doped DWCNT shows a lower work function of 3.99 eV. Although the decrease in the work function is not a direct evidence for the n-type character of AL-doped one, the combination with the Seebeck coefficient in Fig. 4(c) clearly demonstrates that AL-CNT has an n-type character. Fig. 5(c) presents a proposed mechanism of the doping process between lignin dopants and DWCNT.
Organic p-type dopants have components that effectively attract electrons from the host material to the dopant material, enabling electron movement from the host to the dopant. Similarly, the FL, which is rich in fluorine with high electronegativity, acts as an electron-withdrawing group by attracting pi electrons from lignin backbone, resulting in a weak positive charge on the aromatic rings of lignin.43–46 When the aromatic rings of lignin π-stack with those of DWCNT, the electrons could transfer from DWCNT to FL, inducing p-doping of DWCNT. Conversely, organic n-type dopants play this role effectively when they possess numerous electrons, especially lone pair electrons, readily providing electrons to the host material. Likewise, the AL contains numerous available lone-pair electrons at the nitrogen in amine, serving as an electron-donating group. The available lone-pair electrons could directly transfer from AL to DWCNT, leading to an n-doping, which is similar to the doping process by polyethyleneimine (PEI) based on saturated carbons.25,28,47
Application to a flexible thermoelectric generator (TEG)
As a proof of concept, we fabricated a flexible thermoelectric generator (f-TEG) based on the n- and p-doped DWCNT films. The thermoelectric device with 10 pairs of n- and p-doped DWCNTs connected in series using silver paste was prepared to demonstrate the doping properties of DWCNT films as shown in Fig. 6(a) and (b). Most f-TEGs based on sheet-type thermoelectric materials typically harvest heat in the in-plane direction. However, considering that heat is commonly emitted in the out-of-plane direction, we designed our f-TEG to function in the out-of-plane direction. In Fig. 6(c), the Peltier heater and cooler were placed in contact with the bottom and top surfaces of the f-TEG, respectively, to create a temperature difference. When a temperature gradient exists along the z-axis of the f-TEG, the main carriers in each semiconductor diffuse from the hot side to the cold side, resulting in the flow of electrical current. At ΔT = 5 K, the f-TEG generates the maximum output voltage of 2.61 mV and output power of 26.6 nW (Fig. 6(d) and (e)). As the temperature difference increases from 5 to 15 K, the maximum output voltage also increases from 2.61 to 7.86 mV, with the output power rising to 247 nW at ΔT = 15 K. The theoretical output voltage can be calculated by the following equation:| V = n(|Sp| + |Sn|)ΔT, |
Conclusions
We have demonstrated the use of functionalized lignin-based n- and p-dopants for DWCNTs, offering promising alternatives to chemical dopants derived from fossil fuels. Organosolv lignin (OL) was phenolated via a simple microwave method. This phenolation process introduces phenol groups, providing additional reaction sites and enhancing solvent solubility for uniform, reliable and efficient CNT doping. For effective n- and p-dopants, the phenolated lignin was further functionalized with amine and hexafluoropropyl groups, respectively. The aminated and fluorinated lignin-doped CNTs exhibit the distinguished Seebeck coefficients of −48.0 and 53.9 μV K−1, respectively, demonstrating an efficient and eco-friendly conversion between n- and p-type CNT. These doping performances are comparable to those of fossil fuel-based organic dopants including carbazole and TCNQ as p-dopants, and PEI and dppp as n-dopants. In particular, this n-doping performance is noteworthy because stable and reliable n-doping is challenging due to the inherent p-type semiconducting properties of CNTs. We have also demonstrated, as a proof of concept, the flexible TEG with 10 pairs of the AL- and FL-doped DWCNT films. The measured output voltage (7.86 mV) and output power (247 nW) of the flexible TEG at ΔT = 15 K confirm that the AL- and FL-doped DWCNT films exhibit n- and p-type characteristics, respectively. These results highlight the potential of functionalized lignin as a promising alternative carbon resource to petrochemical-based chemicals for use, particularly in the realms of surface charge transfer doping and electronic/or energy applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Korea Institute of Science and Technology (KIST) Future Resource Research Program (2E32501 & 2E32601), the National Research Foundation of Korea (NRF-2019R1A2C2091094 & 2020M3D1A2101799) and the Creative Materials Discovery Program through the NRF grant funded by Ministry of Science and ICT (2020M3D1A1110499).Notes and references
- S. Laurichesse and L. Avérous, Prog. Polym. Sci., 2014, 39, 1266–1290 CrossRef.
- M. S. Ganewatta, H. N. Lokupitiya and C. Tang, Polymers, 2019, 11, 1176 CrossRef.
- H. Liu and H. Chung, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 3515–3528 CrossRef.
- S. Bertella and J. S. Luterbacher, Trends Chem., 2020, 2, 440–453 CrossRef CAS.
- H. Ludmila, J. Michal, Š. Andrea and H. Aleš, Wood Res., 2015, 60, 973–986 Search PubMed.
- J. C. Carvajal, Á. Gómez and C. A. Cardona, Bioresour. Technol., 2016, 214, 468–476 CrossRef CAS PubMed.
- D. S. Bajwa, G. Pourhashem, A. H. Ullah and S. G. Bajwa, Ind. Crops Prod., 2019, 139, 111526 CrossRef CAS.
- F. Zhang, X. Jiang, J. Lin, G. Zhao, H. M. Chang and H. Jameel, New J. Chem., 2019, 43, 2238–2246 RSC.
- M. V. Alonso, M. Oliet, F. Rodríguez, J. García, M. A. Gilarranz and J. J. Rodríguez, Bioresour. Technol., 2005, 96, 1013–1018 CrossRef CAS PubMed.
- J. Podschun, A. Stücker, R. I. Buchholz, M. Heitmann, A. Schreiber, B. Saake and R. Lehnen, Ind. Eng. Chem. Res., 2016, 55, 5231–5237 CrossRef CAS.
- P. Jędrzejczak, M. N. Collins, T. Jesionowski and Ł. Klapiszewski, Int. J. Biol. Macromol., 2021, 187, 624–650 CrossRef PubMed.
- S. Kalami, M. Arefmanesh, E. Master and M. Nejad, J. Appl. Polym. Sci., 2017, 134, 1–9 CrossRef.
- L. Wang, L. Lagerquist, Y. Zhang, R. Koppolu, T. Tirri, I. Sulaeva, S. von Schoultz, L. Vähäsalo, A. Pranovich, T. Rosenau, P. C. Eklund, S. Willför, C. Xu and X. Wang, ACS Sustainable Chem. Eng., 2020, 8, 13517–13526 CrossRef CAS.
- R. J. Li, J. Gutierrez, Y. L. Chung, C. W. Frank, S. L. Billington and E. S. Sattely, Green Chem., 2018, 20, 1459–1466 RSC.
- Y. Zhang, H. Pang, D. Wei, J. Li, S. Li, X. Lin, F. Wang and B. Liao, Eur. Polym. J., 2019, 118, 290–305 CrossRef CAS.
- J. Wu, Q. Liu, C. Wang, W. Wu and W. Han, J. Cleaner Prod., 2021, 283, 124663 CrossRef CAS.
- J. Chen, X. Fan, L. Zhang, X. Chen, S. Sun and R. C. Sun, ChemSusChem, 2020, 13, 4356–4366 CrossRef CAS PubMed.
- E. Akbarzadeh, M. N. M. Ibrahim and A. A. Rahim, Int. J. Electrochem. Sci., 2011, 6, 5396–5416 CrossRef CAS.
- Y. Ren, Y. Luo, K. Zhang, G. Zhu and X. Tan, Corros. Sci., 2008, 50, 3147–3153 CrossRef CAS.
- A. Khan, V. Nair, J. C. Colmenares and R. Gläser, Top. Curr. Chem., 2018, 376, 1–31 CrossRef CAS.
- H. Lu, A. Cornell, F. Alvarado, M. Behm, S. Leijonmarck, J. Li, P. Tomani and G. Lindbergh, Materials, 2016, 9, 1–17 Search PubMed.
- T. Chen, Q. Zhang, J. Pan, J. Xu, Y. Liu, M. Al-Shroofy and Y. T. Cheng, ACS Appl. Mater. Interfaces, 2016, 8, 32341–32348 CrossRef CAS.
- H. Kazemi, M. Parot, T. Stevanovic, F. Mighri and D. Rodrigue, J. Appl. Polym. Sci., 2022, 139, 1–14 CrossRef.
- J. L. Blackburn, A. J. Ferguson, C. Cho and J. C. Grunlan, Adv. Mater., 2018, 30, 1870072 CrossRef.
- Y. Nonoguchi, K. Ohashi, R. Kanazawa, K. Ashiba, K. Hata, T. Nakagawa, C. Adachi, T. Tanase and T. Kawai, Sci. Rep., 2013, 3, 3344 CrossRef PubMed.
- D. Jang, K. T. Park, S.-S. Lee and H. Kim, Nano Energy, 2022, 97, 107143 CrossRef CAS.
- M. K. Ali, N. Okamoto, R. Abe, M. Pandey, A. A. Moneim and M. Nakamura, Synth. Met., 2021, 280, 116874 CrossRef.
- J. Choi, Y. Jung, C. Dun, K. T. Park, M. P. Gordon, K. Haas, P. Yuan, H. Kim, C. R. Park and J. J. Urban, ACS Appl. Energy Mater., 2020, 3, 1199–1206 CrossRef.
- K. T. Park, Y. S. Cho, I. Jeong, D. Jang, H. Cho, Y. Choi, T. Lee, Y. Ko, J. Choi, S. Y. Hong, M. Oh, S. Chung, C. R. Park and H. Kim, Adv. Energy Mater., 2022, 12, 2200256 CrossRef.
- N. T. Tran, Y. Ko, S. Kim, J. Moon, J.-W. Choi, K. H. Kim, C. S. Kim, J.-M. Ha, H. Kim, K. Jeong, H. Lee and C.-J. Yoo, Green Chem., 2022, 24, 2051–2061 RSC.
- B. Wang, T. Y. Chen, H. M. Wang, H. Y. Li, C. F. Liu and J. L. Wen, Int. J. Biol. Macromol., 2018, 107, 426–435 CrossRef.
- D. Natalia, D. Q. Nguyen, J. H. Oh, H. Kim, H. Lee and H. S. Kim, J. Fluorine Chem., 2008, 129, 474–477 CrossRef.
- A. Granata and D. S. Argyropoulos, J. Agric. Food Chem., 1995, 43, 1538–1544 CrossRef.
- Y. Ma, X. Zhao, X. Chen and Z. Wang, Colloids Surf., A, 2011, 377, 284–289 CrossRef.
- I. Fleming and D. Williams, Spectroscopic Methods in Organic Chemistry, McGraw-Hill College, Blacklick, Ohio, U.S.A., 1995 Search PubMed.
- H. T. Dang, S. Cheong, J. Kim, N. T. Tran, H. Kim and H. Lee, Catalysts, 2022, 12, 1419 CrossRef.
- G. J. Jiao, P. Peng, S. L. Sun, Z. C. Geng and D. She, Int. J. Biol. Macromol., 2019, 127, 544–554 CrossRef PubMed.
- X. Du, J. Li and M. E. Lindström, Ind. Crops Prod., 2014, 52, 729–735 CrossRef.
- H. Lee, M. H. Cho, B. S. Lee, J. Palgunadi, H. Kim and H. S. Kim, Energy Fuels, 2010, 24, 6689–6692 CrossRef.
- P. G. Collins, K. Bradley, M. Ishigami and A. Zettl, Science, 2000, 287, 1801–1804 CrossRef PubMed.
- D. Kang, N. Park, J. Ko, E. Bae and W. Park, Nanotechnology, 2005, 16, 1048–1052 CrossRef.
- G. U. Sumanasekera, C. K. W. Adu, S. Fang and P. C. Eklund, Phys. Rev. Lett., 2000, 85, 1096–1099 CrossRef PubMed.
- E. Hyun Suh, S. Beom Kim, J. Jung and J. Jang, Angew. Chem., Int. Ed., 2023, 62, e2023042 Search PubMed.
- K. Walzer, B. Maennig, M. Pfeiffer and K. Leo, Chem. Rev., 2007, 107, 1233–1271 CrossRef PubMed.
- J. Li, G. Zhang, D. M. Holm, I. E. Jacobs, B. Yin, P. Stroeve, M. Mascal and A. J. Moulé, Chem. Mater., 2015, 27, 5765–5774 CrossRef.
- C. N. R. Rao and R. Voggu, Mater. Today, 2010, 13, 34–40 CrossRef.
- M. Shim, A. Javey, N. W. S. Kam and H. Dai, J. Am. Chem. Soc., 2001, 123, 11512–11513 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Supplementary Fig. S1–S10 and Tables S1, S2. See DOI: https://doi.org/10.1039/d3gc03944g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |