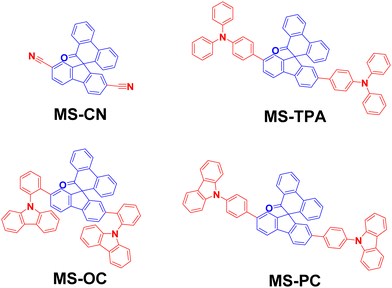
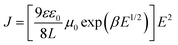Highly efficient (EQE > 27%) Yellow OLEDs using spiro[fluorene-9,9′-phenanthren-10′-one]-carbazole-based donor–acceptor–donor host materials†
Yu Hsuan
Lin
a,
Wei-Han
Lin‡
b,
Yi-Sheng
Huang‡
c,
Chen-Hsun
Wu
b,
Premkumar
Gnanasekaran
a,
Yi-Min
Chang
a,
Sheng-Wei
Teng
c,
Chin-Wei
Lu
 *c,
Chih-Hao
Chang
*c,
Chih-Hao
Chang
 *b and
Yuan Jay
Chang
*b and
Yuan Jay
Chang
 *a
*a
aDepartment of Chemistry, Tunghai University, No. 1727, Sec. 4, Taiwan Boulevard, Xitun District, Taichung 40704, Taiwan. E-mail: jaychang@thu.edu.tw; Tel: +886-4-23590121-32224
bDepartment of Electrical Engineering, Yuan Ze University, Chungli 32003, Taiwan. E-mail: chc@saturn.yzu.edu.tw; Tel: +886-3-4638800-7517
cDepartment of Applied Chemistry, Providence University, Taichung 43301, Taiwan. E-mail: cwlu@pu.edu.tw; Tel: +886-4-26328001-15213
First published on 15th February 2023
Abstract
Four bipolar host materials, with spiro[fluorene-9,9′-phenanthrene-10′-one] acceptors with nitrile fragments and ortho, para-phenyl carbazole, and triphenylamine as donor fragments, namely MS-CN, MS-OC, MS-PC, and MS-TPA, respectively, were designed, synthesized, and their photophysical and electrochemical studies were investigated. All MS-host materials exhibited adequate energy gaps, making them suitable candidates for use as host materials for green, yellow, and red phosphorescent organic light-emitting diodes (OLEDs). We have also designed a series of OLED devices with three different emitting layered architectures to explore their potential in electroluminescence applications. Among the devices using MS-OC, the CN-T2T-blended cohost achieved the highest efficiency among the tested yellow-emitting OLEDs, having a luminance efficiency of 80.0 cd A−1, power efficiency of 113.0 lm W−1, low turn-on voltage of 2.1 V, high maximum luminance of 142464 cd m−2, and peak EQE of 27.1%. The maximum efficiency of the device is one of the highest values reported in the literature for PO-01-based devices, demonstrating the high potential of our molecular design for host materials.
1. Introduction
Organic light-emitting diodes (OLEDs) from transition-metal-based phosphors with efficient triplet exciton harvesting can achieve 100% internal quantum efficiency (IQE) through heavy-atom-enhanced strong spin–orbit coupling, thus facilitating extensive development in the fields of displays and solid-state lighting devices.1–3 Regardless of the ongoing research and achievements in high-performance phosphorescent OLEDs (PhOLEDs), much room remains for the improvement of their efficiency and brightness.4–13 Typically, the emitting layer (EML) of PhOLEDs is configured with an emitter as a guest homogeneously dispersed in a suitable organic host material to reduce the chances of triplet–triplet annihilation to enhance the efficiency of the device.14–16 For maximum exciton harvesting as phosphorescence emission, the host materials should possess (i) large ΔEST, (ii) higher lying singlet (S1) and triplet levels (T1) than guest materials to have better Förster resonance energy transfer (FRET) and Dexter energy transfer (DET),8 (iii) better energy level matching for efficient charge injection into the guest molecules,17 and (iv) a high glass transition temperature (Tg) and thermal decomposition temperature (Td) to ensure thermal stability.18,19 There is a high demand for excellent host materials for efficient PHOLEDs with the above-mentioned thermal stability and photophysical and electrochemical properties, which continues to be a challenging task.20,21In 2021, we reported MS-host materials (MSs) with a spiro-[fluorene-9,9′-phenanthren-10′-one] moiety for high-efficiency perovskite solar cells (PSCs) with a device architecture, ITO/NiOx/MSs/perovskite/PC61BM/BCP/Ag. MSs were utilized as the interfacial layer, and the surface appearance of NiOx was improved. The characteristics of NiOx/MSs on the perovskite layer, quantified in terms of the photoluminescence (PL), were better than those of bare-NiOx in terms of the hole-transport ability and power conversion efficiency. The power efficiency reached 20.34%, comparable to polycrystalline silicon solar cells (16%). The NiOx/MS-OC PSCs had good thermal stability and retained the original power conversion efficiency of 93.16% even after 370 days under argon (25 °C). Furthermore, MS host exhibited suitable highest occupied molecular orbital (HOMO) (ranging from −5.18 to −5.27 eV) and lowest unoccupied molecular orbital (LUMO) (ranging from −1.94 to −2.98 eV) energy levels, as well as high triplet energy levels with large ΔEST, indicating their potential as host materials.
In this study, we report four host materials, namely MSs, based on spiro-[fluorene-9,9′-phenanthren-10′-one] moiety with ortho-, para-phenyl carbazole, and triphenylamine as a donor fragment (control nitrile fragment) satisfying the following requirements: (i) suitable HOMO and LUMO energy levels as well as triplet energies; (ii) adequate charge mobilities; (iii) low cost (18–22 USD g−1) and simple synthesis (three to five steps for acquiring MS-host materials). The last point was the most critical point we focused on for developing this series because the cost determines the market acceptance of materials. MS-host materials were constructed using spiro[fluorene-9,9′-phenanthrene-10′-one] core configurations decorated with different donor moieties as follows: para-connected cyano groups for MS-CN, para-connected triarylamine moieties for MS-TPA, para-connected phenyl-carbazole moieties for MS-PC, and ortho-connected phenyl-carbazole moieties for MS-OC. The HOMO and LUMO energy levels of this series of target molecules are in the ranges of −5.1 to −5.3 eV and −1.8 to −2.4 eV, respectively. The adequate triplet energies (2.47–2.66 eV) of these MSs allowed them to serve as ideal host materials for phosphorescent emitters of green Ir(ppy)3, yellow PO-01, and red Ir(piq)2(acac). Furthermore, we fabricated three different device architectures using different EML structures with MSs materials designed to seek balanced electron–hole recombination. We used MS-host materials in three combinations with phosphorescent dopants as EML layers in the OLED devices as follows: a single EML based on a single host material (i.e., MSs only) for the A series devices, a single EML with a blended host (i.e., MSs mixed with 3′,3′′′,3′′′′′-(1,3,5-triazine-2,4,6-triyl)tris(([1,10-biphenyl]-3-carbonitrile)) (CN-T2T) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) for the B series devices, and double EMLs with a single host (i.e., TCTA) and a blended host (i.e., MSs mixed with CN-T2T (1
1)) for the B series devices, and double EMLs with a single host (i.e., TCTA) and a blended host (i.e., MSs mixed with CN-T2T (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) for the C series devices. Overall, the external quantum efficiency (EQE) of the B and C series devices using cohost EML structures exceeded the A series device with the EML using a single host material, which was attributed to the excellent carrier balance in the B and C series devices. Significantly, the spiro[fluorene-9,9-phenanthren-10-one] core was decorated with phenyl-carbazole donor moieties in the ortho-orientation (i.e., MS-OC), resulting in a densely packed structure according to the single-crystal experiments. As indicated, organic light-emitting diodes (OLEDs) adopting MS-OC as the host material exhibited the best efficiency among the three device types, regardless of the emitter employed. In the A series device with a single host material structure, the maximum efficiency of the MS-OC-based device with the yellow-emitting PO-01 achieved 22.5% (67.1 cd A−1 and 81.1 lm W−1). Moreover, the respective peak efficiencies of 27.1% (80.0 cd A−1 and 113.0 lm W−1) and 22.3% (78.2 cd A−1 and 111.1 lm W−1) were obtained for the yellow- and green-emitting MS-OC devices in the B series. It is worth mentioning that the yellow-emitting device's performance in the B series made an excellent breakthrough as compared with the peak efficiencies of 25.3% (77.2 cd A−1 and 59.8 lm W−1) for a typical PO-01-based device.22 These outcomes show that MSs could effectively regulate the carrier balance via mixing with appropriate electron-transport materials to realize highly efficient PhOLEDs, further underscoring the great potential of this molecular design.
1)) for the C series devices. Overall, the external quantum efficiency (EQE) of the B and C series devices using cohost EML structures exceeded the A series device with the EML using a single host material, which was attributed to the excellent carrier balance in the B and C series devices. Significantly, the spiro[fluorene-9,9-phenanthren-10-one] core was decorated with phenyl-carbazole donor moieties in the ortho-orientation (i.e., MS-OC), resulting in a densely packed structure according to the single-crystal experiments. As indicated, organic light-emitting diodes (OLEDs) adopting MS-OC as the host material exhibited the best efficiency among the three device types, regardless of the emitter employed. In the A series device with a single host material structure, the maximum efficiency of the MS-OC-based device with the yellow-emitting PO-01 achieved 22.5% (67.1 cd A−1 and 81.1 lm W−1). Moreover, the respective peak efficiencies of 27.1% (80.0 cd A−1 and 113.0 lm W−1) and 22.3% (78.2 cd A−1 and 111.1 lm W−1) were obtained for the yellow- and green-emitting MS-OC devices in the B series. It is worth mentioning that the yellow-emitting device's performance in the B series made an excellent breakthrough as compared with the peak efficiencies of 25.3% (77.2 cd A−1 and 59.8 lm W−1) for a typical PO-01-based device.22 These outcomes show that MSs could effectively regulate the carrier balance via mixing with appropriate electron-transport materials to realize highly efficient PhOLEDs, further underscoring the great potential of this molecular design.
2. Results and discussion
2.1 Synthesis of MSs
The structures of MS-host materials are shown schematically in Fig. 1 and their synthesis procedures are presented in Scheme S1 (ESI†). The MSs contain two side substituents (cyano, arylamine, and carbazole) at the core moiety of spiro[fluorene-9,9′-phenanthren-10′-one]. For each of the MSs, the synthesis was initiated using 2,7-dibromo-10′H-spiro[fluorene-9,9′-phenanthren]-10′-one (1), yielding MS-CN through the cyanation reduction with CuCN.23 The final products of MS-TPA, MS-OC, and MS-PC were obtained via the Suzuki–Miyaura coupling reactions with high yields (total yield 63–90%), and were easily purifiable.24 Detailed descriptions of the synthesis procedures and nuclear magnetic resonance (NMR) data (Fig. S1–S3) are presented in the ESI.†2.2 Photophysical properties
The ultraviolet-visible absorption, fluorescence, and phosphorescence emission spectra of all MSs were obtained in a toluene solution, and the corresponding spectral profiles and numerical data are shown in Fig. 2(a) and Table 1. MS-CN and MS-OC exhibited higher energy absorption bands in the 280–340 nm range, which were attributed to the high π–π* transitions of the core moiety of spiro[fluorene-9,9′-phenanthren-10′-one] and the weak red-shift absorption for MS-OC at 340 nm, owing to the addition of ortho-phenyl-carbazole at the core moiety of spiro[fluorene-9,9′-phenanthren-10′-one]. In contrast, MS-TPA and MS-PC showed major red-shifted broad absorption peaks at 372 and 343 nm, respectively, owing to the addition of the electron-donating triphenylamine and phenyl carbazole at the para-position of the core moiety, which increased the coplanarity and conjugation of the molecular backbone. MS-TPA exhibited a more red-shifted absorption peak, which was mainly attributed to the strong electron-donating nature of triphenylamine, which destabilized the HOMO energy level as compared with phenyl carbazole.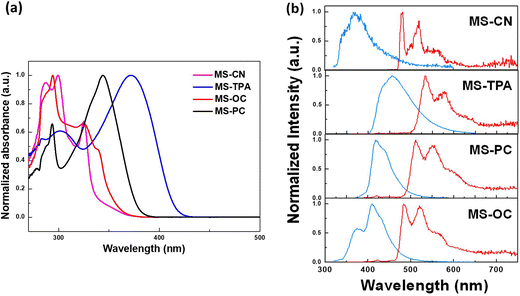 | ||
| Fig. 2 (a) Absorption spectra. (b) Fluorescence and phosphorescence spectra of MS-CN, MS-TPA, MS-PC, and MS-OC obtained in a toluene solution. | ||
| Material | λ abs (nm) | HOMOa (eV) | LUMOb (eV) | Flemc (nm) | S1b (eV) | Phc (nm) | T1c (eV) | QYd (%) |
|---|---|---|---|---|---|---|---|---|
| a Estimated from the CV curve. b Estimated from LUMO calculated by HOMO + E0–0 (E0–0 was determined from the onset of the UV-Visible spectra in toluene). c Estimated from the onset of the fluorescence and phosphorescence measured in toluene. d Photoluminescence quantum yield of 5 weight percent PO-01 doped in MS-CN, MS-TPA, MS-PC, and MS-OC films. | ||||||||
| MS-CN | 286, 298, 324 | −5.12 | −1.81 | 345, 370, 383 (sh) | 3.55 | 481, 520, 553 (sh) | 2.63 | 43 |
| MS-TPA | 282, 300, 372 | −5.30 | −2.40 | 432 (sh), 455 | 3.07 | 533, 578 | 2.47 | 83 |
| MS-OC | 294, 324, 338 (sh) | −5.20 | −1.81 | 376, 410, 430 (sh) | 3.31 | 484, 520, 562 (sh) | 2.66 | 94 |
| MS-PC | 293, 334 (sh), 343 | −5.19 | −2.08 | 418, 436 (sh) | 3.14 | 511, 550, 595 (sh) | 2.55 | 90 |
We also studied the PL properties of the prepared MSs in a toluene solution, and the corresponding emission profiles and spectral data are shown in Fig. 2(b) and Table 1. All of the studied MSs showed broad and structured violet to deep-blue fluorescence (S1) emission at ambient temperature. Among all the studied host materials, MS-CN exhibited violet emission at 370 nm because of the strong electron-withdrawing dicyano group substituted at the core moiety of spiro[fluorene-9,9′-phenanthren-10′-one], while MS-OC exhibited a structured emission maximum at 410 nm with a shoulder peak at 376 nm. On the other hand, MS-PC and MS-TPA showed broad and red-shifted emission maxima at 455 and 418 nm, respectively, owing to their strong electron-donating characteristics, and the free rotation through the molecular backbone of triphenylamine showed broad and red-shifted emission as compared with phenyl carbazole. Moreover, all of the studied host materials showed highly structured phosphorescence (T1) emission profiles in toluene solution at −77 K. The energy levels were calculated based on the fluorescence (S1) and phosphorescence (T1) emission onset data, and the resulting values for the corresponding energy gaps are listed in Table 1. The triplet energy gaps of these compounds imply that the synthesized MSs are promising as host materials for phosphorescent OLEDs. On the other hand, thin-film samples of 5-weight percent PO-01 doped in MSs materials were prepared to determine the PL quantum yields (PLQYs). The PLQY values of the doped film samples were evaluated as 0.43 (MS-CN), 0.83 (MS-TPA), 0.94 (MS-PC), and 0.90 (MS-OC). The high PLQY values obtained in the last two samples revealed their potential for electroluminescence (EL) applications.
2.3 Thermal stability and electrochemical properties
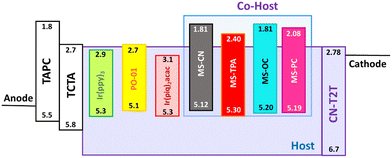
The thermal properties of the host materials were determined using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) at a heating rate of 10 °C min−1; the spectra are shown in Fig. S4 and S5 (ESI†). MS-CN, MS-TPA, and MS-PC all exhibited a flat line in their DSC spectra for the glass transition temperature (Tg), which was attributed to the poor crystallinity and poor stacking interactions; this is supported by the conclusions of our previously reported X-ray studies, except for MS-OC (Tg = 179 °C).24 The thermal decomposition temperatures (Td) of MS-CN, MS-PC, MS-OC, and MS-TPA were 352, 396, 426, and 475 °C, respectively, as shown in Fig. S4 (ESI†). This suggests that the studied host materials exhibit excellent thermal stability, which is advantageous for OLED fabrication by thermal vacuum deposition.Cyclic voltammetry studies of all MS-host materials revealed reversible oxidation curves, as shown in Fig. S6 (ESI†) and the data summarized in Table 1. The onset of ICT transition bands determined the zero–zero energy, and was used to further calculate the LUMO energy level in Fig. 2(a). MS-TPA has the smallest energy gap (2.9 eV), which is attributed to the strong electron-donating moiety; this result is also consistent with the UV-vis and PL data. MSs have low HOMO and high LUMO levels, and the energy gap is between 2.9 and 3.39 eV in Fig. 3. This indicated that all MSs are suitable as host materials for the yellow emitter PO-01 and the red emitter Ir(piq)2acac.
2.4 Computational analysis
To gain further insight into the properties of the optimized structures, the electrostatic potential (ESP) map and the frontier energy level information of MSs were obtained using time-dependent density functional theory (TD-DFT) calculations, using the B3LYP/6-31G(d) basis set in Gaussian 16 W; the results are shown in Fig. S7, S8 (ESI†) and the numerical values are listed in Table S1 (ESI†). As shown in Fig. S8 (ESI†), the optimized structure of MSs exhibited structural conformations, with a dihedral angle between the acceptor core and phenyl spacer and donor fragment. MS-CN is a simple spiro-core moiety with an electron-withdrawing nitrile group substituted on a fluorene fragment and without a donor. The electrostatic potential map shows that the positive ESP was localized on the phenyl rings while negative ESP was localized on the electron-withdrawing carbonyl and nitrile fragments. The calculated frontier molecular orbitals suggested the inadequate separation of MS-CN with the HOMO (−6.83 eV) and LUMO (−2.70 eV) energy levels. For MS-TPA, the predicted dihedral angle between the central core of spiro[fluorene-9,9′-phenanthren-10′-one] and donor triphenylamine was 38.0°, and the electrostatic potential (ESP) map revealed that the highly negative ESP was concentrated on the carbonyl group, while positive ESP values were confined to the fluorene-triphenyl amine donor backbone. The HOMO (−5.07 eV) energy level was localized over the fluorene-triphenyl amine donor backbone while the LUMO (−2.08 eV) energy level was localized on the phenanthrene-10′-one fragment. Similarly, for MS-PC, the predicted dihedral angle between the central core of spiro[fluorine-9,9′-phenanthren-10′-one] and the phenyl-carbazolyl fragment was 39.5°. The HOMO (−5.34 eV) energy level was localized on the fluorene-phenyl-carbazolyl fragment while the LUMO (−2.06 eV) energy level was localized on the phenanthrene-10′-one fragment. The ESP map showed that the positive ESP on the carbonyl group and positive ESP values were localized on the fluorene-phenyl-carbazolyl donor backbone. In contrast, MS-OC exhibited a highly twisted molecular structure with large dihedral angles for the central core of spiro[fluorene-9,9′-phenanthren-10′-one] and a phenyl-carbazolyl fragment, at 51.3°. The ESP map and frontier molecular orbitals (HOMO = −5.14 eV and LUMO = −2.11 eV) exhibited similar trends to MS-TPA and MS-PC.2.5 Exciplex experiments
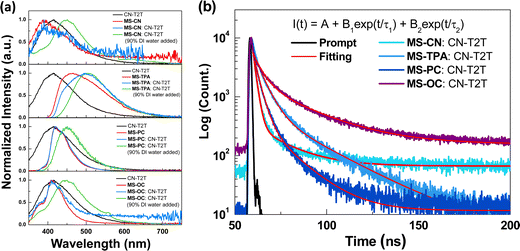
The four synthesized host materials were then used to fabricate OLEDs to examine their EL applications. Because all the studied compounds consisted of a single electron-deficient core decorated with two electron-donating moieties, it was expected that these compounds would possess a relatively weak electron-transporting ability. Therefore, subsequent device tests investigated the EML structures that were constructed using pure synthesized compounds or a blended host consisting of the synthesized compounds with an electron transport material. Before the EL tests, we obtained the PL spectra of the blended hosts to determine whether the mixtures exhibited exciplex formation. Herein, we chose CN-T2T as the electron transport part for the mixtures, owing to its adequate LUMO level, wide triplet energy bandgap, and good electron transport mobility.25 Since CN-T2T with a Y-shaped molecular structure would increase the probability of producing physical intermolecular interactions for the successful formation of an exciplex, the fast-screening method was introduced to investigate the exciplex formation by obtaining the PL spectra of the donor:acceptor (D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A) blends in a relatively high-polarity solvent.26 Four D
A) blends in a relatively high-polarity solvent.26 Four D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A (1
A (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed samples, MS-CN
1) mixed samples, MS-CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN-T2T, MS-TPA
CN-T2T, MS-TPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN-T2T, MS-PC
CN-T2T, MS-PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN-T2T, and MS-OC
CN-T2T, and MS-OC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN-T2T, were prepared for the fast-screening method. The mixed samples were initially dissolved in tetrahydrofuran (THF), and then different quantities of deionized (DI) water were added to the solution to obtain the colloidal suspension.27Fig. 4(a) shows the PL spectra of CN-T2T, the synthesized MSs, and the mixtures, in pure THF and with 90% water added. Highly polar solutions can force the D/A molecules to aggregate, thereby increasing the likelihood of intermolecular interactions that stimulate exciplex formation. On comparing the PL spectra of the mixtures with those of the four synthesized molecules and CN-T2T dissolved in pure THF, the spectral profiles of the mixtures were similar to those of the synthesized molecules. When 90% DI water was added to THF, the spectra of the four corresponding blends were red-shifted as compared with those in pure THF. However, the red shift was insignificant in all cases, indicating that an exciplex could have been generated but was very weak.26 Furthermore, the transient PL decays monitored at the emission peak were measured to study the relaxation behavior of the mixtures in THF with 90% DI water added. Fig. 4(b) shows the excited-state decay characteristics of the four blends measured at room temperature. The formulas for the curve fitting and simulation results are presented in Table S1 (ESI†). Although these samples exhibited two-component decay profiles, both components were on the nanosecond timescale, suggesting that the excited state was dominated by fluorescence. We also made blended samples by mixing CN-T2T and the synthesized MSs. Samples A (MS-CN
CN-T2T, were prepared for the fast-screening method. The mixed samples were initially dissolved in tetrahydrofuran (THF), and then different quantities of deionized (DI) water were added to the solution to obtain the colloidal suspension.27Fig. 4(a) shows the PL spectra of CN-T2T, the synthesized MSs, and the mixtures, in pure THF and with 90% water added. Highly polar solutions can force the D/A molecules to aggregate, thereby increasing the likelihood of intermolecular interactions that stimulate exciplex formation. On comparing the PL spectra of the mixtures with those of the four synthesized molecules and CN-T2T dissolved in pure THF, the spectral profiles of the mixtures were similar to those of the synthesized molecules. When 90% DI water was added to THF, the spectra of the four corresponding blends were red-shifted as compared with those in pure THF. However, the red shift was insignificant in all cases, indicating that an exciplex could have been generated but was very weak.26 Furthermore, the transient PL decays monitored at the emission peak were measured to study the relaxation behavior of the mixtures in THF with 90% DI water added. Fig. 4(b) shows the excited-state decay characteristics of the four blends measured at room temperature. The formulas for the curve fitting and simulation results are presented in Table S1 (ESI†). Although these samples exhibited two-component decay profiles, both components were on the nanosecond timescale, suggesting that the excited state was dominated by fluorescence. We also made blended samples by mixing CN-T2T and the synthesized MSs. Samples A (MS-CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN-T2T, 1
CN-T2T, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), B (MS-TPA
1), B (MS-TPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN-T2T, 1
CN-T2T, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), C (MS-PC
1), C (MS-PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN-T2T, 1
CN-T2T, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and D (MS-OC
1), and D (MS-OC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN-T2T, 1
CN-T2T, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were thin film samples composed of donor
1) were thin film samples composed of donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor (D
acceptor (D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A) blends. Fig. S9 (ESI†) shows the PL spectra of the thin film samples. Again, the results indicated no exciplex emission in the four samples. The spectra of the four corresponding blends were slightly red-shifted as compared to their original PL spectra measured in toluene. In addition, the phosphorescence spectra of the samples measured at 77 K were significantly red-shifted as compared to those measured at room temperature, indicating the absence of exciplex emission. Thus, we concluded that the blended samples used as the host system in the devices should be categorized as mixed hosts rather than an exciplex hosts.
A) blends. Fig. S9 (ESI†) shows the PL spectra of the thin film samples. Again, the results indicated no exciplex emission in the four samples. The spectra of the four corresponding blends were slightly red-shifted as compared to their original PL spectra measured in toluene. In addition, the phosphorescence spectra of the samples measured at 77 K were significantly red-shifted as compared to those measured at room temperature, indicating the absence of exciplex emission. Thus, we concluded that the blended samples used as the host system in the devices should be categorized as mixed hosts rather than an exciplex hosts.
2.6 Electroluminescence properties
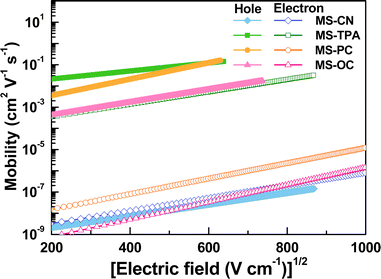
Because the charge mobilities of host materials adopted in OLEDs influence the performance and should be concerned before device designs, single carrier devices were fabricated to implement the space-charge-limited current (SCLC) measurements. The hole-only devices adopted the following architecture: ITO/MoO3 (5 nm)/MSs (250 nm)/MoO3 (10 nm)/Al (120 nm), for which MoO3 was used as the hole injection layer and the electron blocking layer. The architecture of the electron-only devices was as follows: ITO/CN-T2T (10 nm)/MSs (150 nm)/CN-T2T:Li2CO3 10 wt% (5 nm)/Al (120 nm), where CN-T2T and CN-T2T:Li2CO3 were used as the hole blocking layer and the electron injection layer, respectively.As shown in Fig. S10 (ESI†), the J–V curves of the hole-only and electron-only devices revealed the detected current densities for MSs. Since the target compounds possess similar HOMO/LUMO levels, this result was mainly related to the charge transport conditions. The hole mobilities were estimated according to the SCLC theory (J = 9εε0μE2/8L), assuming that the evaporated organic solid films have inherently disordered morphologies. This theory states that the charge mobility dependence on the electric field is ascribed to organic semiconductors (μ = μ0 exp(βE1/2)), resulting in the following expression:
According to the mobility results of these series, most of the compounds presented higher hole transport capabilities as compared to their electron counterparts. The construction of MSs-based EMLs with additional electron-transporting materials was considered to boost electron transport. Therefore, to investigate this series as the hosts for constructing EMLs in OLEDs, we designed three device architectures to examine the carrier balance conditions. In addition, based on the energy bandgaps of the tested host materials, phosphorescent emitters with different energy bandgaps were chosen for fabricating red, yellow, and green OLEDs. When designing an EML, the triplet energy bandgap of the host should be higher than that of the selected emitter. Therefore, green-emitting Ir(ppy)3 was used as the emitter for the host materials MS-CN and MS-OC, while yellow-emitting PO-01 and red-emitting Ir(piq)2acac were doped into all synthesized host materials to construct the EML.28–30 Two commonly used hole-transporting materials, namely, bis[(di-4-tolylamino)phenyl]cyclohexane (TAPC) and 4,4′,4′′-tris(N-carbazolyl)triphenylamine (TCTA), were chosen to form step-wise hole-transport layers (HTLs), aiming to promote smooth hole injection from the HTL into the EML.31 Moreover, as mentioned above, CN-T2T with appropriate electron transport properties was used as the electron transport layer (ETL). The three device architectures were nearly identical, except for the EML structures. The EML structures corresponding to the three devices were as follows: (1) a single EML based on a single host material for the A series devices, (2) a single EML with a blended host for the B series devices, and (3) double EMLs with a single host and a blended host for the C series devices. Consequently, the structures of the A series devices used a configuration of indium tin oxide (ITO) (90 nm)/TAPC (30 nm)/TCTA (10 nm)/MSs doped with an emitter (25 nm)/CN-T2T (50 nm)/LiF (1.5 nm)/Al (150 nm), where ITO and aluminum were used as the anode and cathode, respectively. The B series devices with a blended host were fabricated with the following generalized architecture: ITO (90 nm)/TAPC (30 nm)/TCTA (10 nm)/MSs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN-T2T (1
CN-T2T (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) doped with an emitter (25 nm)/CN-T2T (50 nm)/LiF (1.5 nm)/Al (150 nm). In addition, the C series devices used a double EML configuration including a mixed EML and an auxiliary EML using a hole-transport material as the host: ITO (90 nm)/TAPC (30 nm)/TCTA (5 nm)/TCTA doped with an emitter (5 nm)/MSs
1) doped with an emitter (25 nm)/CN-T2T (50 nm)/LiF (1.5 nm)/Al (150 nm). In addition, the C series devices used a double EML configuration including a mixed EML and an auxiliary EML using a hole-transport material as the host: ITO (90 nm)/TAPC (30 nm)/TCTA (5 nm)/TCTA doped with an emitter (5 nm)/MSs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN-T2T (1
CN-T2T (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) doped with an emitter (25 nm)/CN-T2T (50 nm)/LiF (1.5 nm)/Al (150 nm). The doping concentrations for all emitters were 8 wt.% for green-emitting Ir(ppy)3, 5 wt.% for yellow-emitting PO-01, and 8 wt.% for red-emitting Ir(piq)2acac. To facilitate the identification of device architectures, R, Y, and G were added to the names of different series devices to represent different colors. In addition, the device names were also followed by adding 1, 2, 3, and 4 to represent the devices using MS-CN, MS-TPA, MS-PC, and MS-OC as hosts. For instance, the yellow-emitting device with MS-PC host in the A series devices was denoted as device AY3, and so on. The materials used and the schematic architecture of the fabricated OLEDs are shown in Fig. 6.
1) doped with an emitter (25 nm)/CN-T2T (50 nm)/LiF (1.5 nm)/Al (150 nm). The doping concentrations for all emitters were 8 wt.% for green-emitting Ir(ppy)3, 5 wt.% for yellow-emitting PO-01, and 8 wt.% for red-emitting Ir(piq)2acac. To facilitate the identification of device architectures, R, Y, and G were added to the names of different series devices to represent different colors. In addition, the device names were also followed by adding 1, 2, 3, and 4 to represent the devices using MS-CN, MS-TPA, MS-PC, and MS-OC as hosts. For instance, the yellow-emitting device with MS-PC host in the A series devices was denoted as device AY3, and so on. The materials used and the schematic architecture of the fabricated OLEDs are shown in Fig. 6.
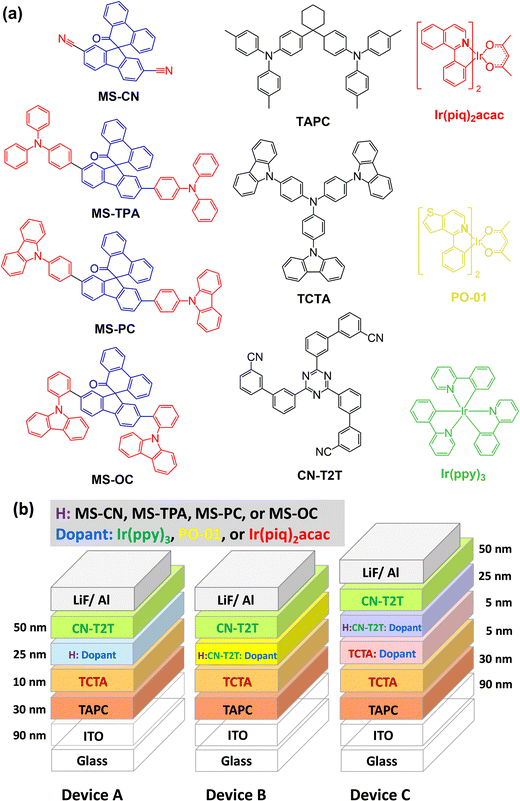 | ||
| Fig. 6 (a) Schematics of the materials used in the OLEDs; (b) schematics of the fabricated OLEDs with different emitters. | ||
We designed three series of devices with different EML structures to investigate the carrier transport and energy transfer capabilities of the synthesized compounds from the host to the guest. Owing to the great number of devices tested, we used a histogram to present the peak EQE values of the various devices using red (R), yellow (Y), and green (G) emitters. Fig. 7 summarizes the peak EQE values of the A, B, and C series devices. The B and C series devices exhibited quite similar performances regardless of the emitter used. Still, upon closer inspection of their EL characteristics, the B series device with a single mixed host had the highest efficiency, while the C series device with an additional auxiliary EML exhibited the highest luminance. In contrast, the A series device with a single EML consisting of synthesized MSs as the host slightly underperformed as compared with the other series, indicating that these materials have slightly imbalanced carrier transport properties. Although the A series devices exhibited relatively low efficiency, the peak efficiencies of yellow-emitting devices AY3 (MS-PC) and AY4 (MS-OC) were 22.8% (66.2 cd A−1 and 86.0 lm W−1) and 22.5% (67.1 cd A−1 and 81.1 lm W−1), respectively, which could still be considered excellent performances for yellow emitters. Considering the host compounds with more extraordinary hole transport ability, the carrier recombination zone located at the EML/ETL interface could be expected in the device A series. Therefore, the carrier balance of the devices was determined by the hole transport of the host and the electron transport of the ETL in a low operation voltage range. Although MS-TPA possesses bipolar carrier transport ability, the lower efficiency might result from the mismatch between MS-TPA and CN-T2T. As for the B and C series, the hole–electron mismatch was significantly improved by blending the electron transport material CN-T2T.
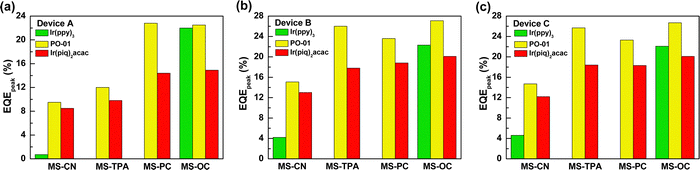 | ||
| Fig. 7 Peak EQEs of the tested devices for different hosts and emitters: (a) A series; (b) B series; (c) C series. | ||
Because the highest-efficiency performance was obtained for the B series devices, we collected the numerical data for the B series devices, including green, yellow, and red emitters from the EL characteristics, and summarized them in Table 2. For comparison, the remaining EL characteristic data for the A and C series devices are provided in the ESI† (Tables S3 and S4). Moreover, on comparing all tested emission colors for the B series devices, device BY using the yellow-emitting PO-01 showed outstanding performance; thus, it was selected as the representative case, as shown in Fig. 8. The EL characteristics of the BR and BG series devices are shown in Fig. S11 and S12 (ESI†).
| Device | BG | BY | BR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | 1 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Host | MS-CN | MS-OC | MS-CN | MS-TPA | MS-PC | MS-OC | MS-CN | MS-TPA | MS-PC | MS-OC |
| Emitter | Ir(ppy)3 | PO-01 | Ir(piq)2acac | |||||||
| a Maximum efficiency. b Measured at 102 cd m−2. c Turn-on voltage measured at 1 cd m−2. d Current density at half of the peak external quantum efficiency. | ||||||||||
| External quantum efficiencyab [%] | 4.2 | 22.3 | 15.1 | 26.0 | 23.6 | 27.1 | 13.0 | 17.8 | 18.8 | 20.1 |
| 4.1 | 21.9 | 11.1 | 26.0 | 23.5 | 27.1 | 7.7 | 14.4 | 18.1 | 18.9 | |
| Luminance efficiencyab [cd A−1] | 12.6 | 78.2 | 43.5 | 77.2 | 68.4 | 80.0 | 7.9 | 10.7 | 10.7 | 12.0 |
| 12.4 | 76.6 | 31.8 | 77.2 | 68.0 | 80.0 | 4.7 | 6.0 | 10.3 | 11.3 | |
| Power efficiencyab [lm W−1] | 11.3 | 111.1 | 46.7 | 109.0 | 92.8 | 113.0 | 9.5 | 12.3 | 15.3 | 16.6 |
| 10.2 | 94.8 | 21.4 | 92.3 | 87.4 | 104.4 | 3.3 | 8.7 | 12.2 | 12.7 | |
| V on [V] | 2.5 | 2.1 | 2.9 | 2.0 | 2.1 | 2.1 | 2.9 | 2.4 | 2.2 | 2.2 |
| J 1/2 [mA cm−2] | 381.9 | 72.3 | 4.5 | 52.4 | 56.7 | 94.4 | 6.3 | 40.7 | 121.3 | 77.6 |
| Max luminance [cd m−2] [V] | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 152 [10.8] 152 [10.8] |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6900 [9.6] 6900 [9.6] |
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 168 [13.0] 168 [13.0] |
127![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 451 [11.6] 451 [11.6] |
122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 753 [12.0] 753 [12.0] |
142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 464 [10.4] 464 [10.4] |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 857 [11.4] 857 [11.4] |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 423 [12.0] 423 [12.0] |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 959 [11.2] 959 [11.2] |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 719 [10.4] 719 [10.4] |
| CIE1931 coordinatesb | (0.42, 0.55) | (0.34, 0.61) | (0.51, 0.48) | (0.51, 0.49) | (0.51, 0.49) | (0.51, 0.49) | (0.68, 0.31) | (0.69, 0.31) | (0.69, 0.31) | (0.69, 0.31) |
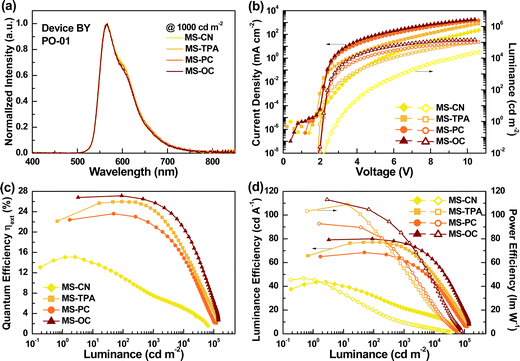
Fig. 8 shows the EL characteristics of the BY series device with yellow-emitter PO-01, featuring a co-host-based EML consisting of MSs and CN-T2T. As shown in Fig. 8(a), all of the tested devices with the different host materials exhibited nearly identical EL spectra originating from PO-01, indicating valid energy transfer from the blended host to the guest.32 Furthermore, the emission of pure PO-01 confirmed that exciton diffusion and migration to adjacent layers were avoided.33 This phenomenon was observed not only for the B series devices with different emitters but also for the A and C series devices. Accordingly, we concluded that the designed EML structures for the A, B, and C series devices were practical and reasonable. Fig. 8(b) shows the current density–voltage–luminance (J–V–L) curves for device BY, for different host materials. As indicated, the recorded current density curves followed the sequence MS-CN < MS-TPA < MS-PC < MS-OC, where the last two devices exhibited relatively close current densities. Similar results were obtained for the B series device with red and green emitters, or even for the A and C series devices. The low current density observed in devices with MS-CN hosts is reasonable, mainly due to the poor carrier transport capability of MS-CN. However, according to the results of SCLC experiments, MS-TPA has bipolar and excellent transport capability. Thus, the lower current density obtained in MS-TPA-based devices may be due to dopant-induced carrier imbalance or suboptimal energy transfer from host to guest. The luminance curves of the tested devices exhibited the same trends. The observed maximum luminance values for devices BY1, BY2, BY3, and BY4 were 64168, 127451, 122753, and 142464 cd m−2, respectively. Table S5 (ESI†) summarizes the EL characteristics of the selected PO-01-based devices reported in the literature. It is worth noting that device BY4 with the MS-OC/CN-T2T mixed host exhibited a high maximum luminance, one of the highest values reported in the literature for PO-01-based devices.34–37 On the other hand, in contrast to device BY1, which exhibited a high turn-on voltage of 2.9 V (defined at the luminance of 1 cd m−2), devices BY2, BY3, and BY4 exhibited extremely low turn-on voltages of approximately 2.0–2.1 V. On comparing device BY4 with the previously reported PO-01-based devices (Table S5, ESI†) in terms of the device performance and luminance, the MS-OC/CN-T2T mixed host presented an outstanding balance in terms of the carrier mobility, yielding excellent performance, with high power efficiency, high maximum luminance, and low Von.
Fig. 8(c) and (d) show the EQEs, luminance efficiency, and power efficiency curves, respectively, against luminance, for the BY series devices. The peak efficiencies for devices BY1, BY2, BY3, and BY4 were respectively 15.1% (43.5 cd A−1 and 46.7 lm W−1), 26.0% (77.2 cd A−1 and 109.0 lm W−1), 23.6% (68.4 cd A−1 and 92.8 lm W−1), and 27.1% (80.0 cd A−1 and 113.0 lm W−1). Given the PLQY of the blended films mentioned above, devices BY2, BY3, and BY4 demonstrated that carrier balance can be approximately achieved in these mixed EML structures. Notably, satisfactory efficiency together with ultra-high luminance values could be harvested in the PO-01-based device BY4 via a co-host of MS-OC and CN-T2T (MS-OC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN-T2T = 1
CN-T2T = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); this was attributed to the combined effect of the specific molecular and device architecture designs. Furthermore, these results indicated that the mixture of MS-OC and CN-T2T was even and sustained both compounds’ inherent carrier transport abilities. In addition, devices BY1, BY2, BY3, and BY4 exhibited efficiency drops of 26.5, 0, 0.5, and 0%, respectively, from the peak efficiency to those recorded at a practical luminance of 100 cd m−2. The lower efficiency roll-off of devices BY2–BY4 suggested mitigating TTA and stable carrier balance in the EML, mainly attributed to the mixed host enlarging the exciton formation zone.38
1); this was attributed to the combined effect of the specific molecular and device architecture designs. Furthermore, these results indicated that the mixture of MS-OC and CN-T2T was even and sustained both compounds’ inherent carrier transport abilities. In addition, devices BY1, BY2, BY3, and BY4 exhibited efficiency drops of 26.5, 0, 0.5, and 0%, respectively, from the peak efficiency to those recorded at a practical luminance of 100 cd m−2. The lower efficiency roll-off of devices BY2–BY4 suggested mitigating TTA and stable carrier balance in the EML, mainly attributed to the mixed host enlarging the exciton formation zone.38
As for the B series device with red-emitting Ir(piq)2acac, device BR4, which also used MS-OC to blend with CN-T2T for the EML, demonstrated the highest performance, as shown in Fig. S11 (ESI†). Akin to the BY series device, the efficiencies of the tested red-emitting devices were relative to the host materials; the lowest one was for the MS-CN-based device, whereas the efficiencies of devices BR2–BR4 gradually increased. The pure red-emitting device BR4 exhibited a high maximum luminance of 18719 cd m−2 at 10.4 V and a low turn-on voltage of 2.2 V. The peak efficiency of device BR4 reached 20.1% (12.0 cd A−1 and 16.6 lm W−1), while a slightly lower efficiency of 18.9% (11.3 cd A−1 and 12.7 lm W−1) was recorded at a practical luminance of 100 cd m−2. However, we only investigated MS-CN and MS-OC as host materials in the green-emitting B series device because their triplet energy bandgaps exceeded that of Ir(ppy)3. The corresponding EL characteristics of the BG series devices are shown in Fig. S12 (ESI†). As indicated, the green-emitting devices BG1 and BG4 offered very different performances, and their respective peak efficiencies reached 4.2% (12.6 cd A−1 and 11.3 lm W−1) and 22.3% (78.2 cd A−1 and 111.1 lm W−1). Again, BG4 demonstrated the best performance among the tested devices, indicating that mixing MS-OC with CN-T2T as the host enables devices with various emitters to achieve significant carrier balance.
Regarding the performance of all the B series devices with mixed EMLs, most of the tested MS-OC-based devices with different emitters demonstrated the best performances, including high efficiency, high luminance value, low turn-on voltage, as well as mitigated efficiency roll-off. Only a few MS-PC-based devices exhibited the highest efficiency and luminance. A similar trend was observed for the AR, AY, AG, CR, CY, and CG series. From the molecular design and the single crystal data, the para-oriented MS-PC stacking is a phenanthrenone–phenanthrenone configuration at a distance of 4.606 Å through intermolecular π–π interactions. Additionally, the ortho-oriented MS-OC stacking is a fluorene-to-fluorene configuration with a spacing of 3.202 Å, which not only leads to the densest stacking but also demonstrates its high potential as a host material for OLED applications among four-targeted MSs.24 These results demonstrate that the spiro[fluorene-9,9′-phenanthren-10′-one] core decorated with the 9-phenyl carbazole moiety in the ortho- or para-orientation could be considered an appropriate design.39 Furthermore, this phenomenon illustrates that mixing EMLs with different synthesized compounds helps to regulate the carrier balance and increase luminance.
3. Conclusions
We have demonstrated four MSs based on the spiro[fluorene-9,9′-phenanthrene-10′-one] moiety as host materials, that is, MS-CN, MS-TPA, MS-PC, and MS-OC. The MSs have a wide energy gap, suitable energy levels and good energy level matching with Ir(ppy)3, PO-01, and Ir(piq)2acac emitters. Three device architectures were considered. MS-OC showed good performance in the device A series with a single EML and single host; in particular, the yellow emitter of PO-01 can be obtained with an EQE of 22.5% (67.1 cd A−1 and 81.1 lm W−1). Device B series was fabricated with a co-host with the following generalized architecture: ITO (90 nm)/TAPC (30 nm)/TCTA (10 nm)/host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CN-T2T (1
CN-T2T (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) doped with an emitter (25 nm)/CN-T2T (50 nm)/LiF (1.5 nm)/Al (150 nm). MS-OC exhibited good dense packing at the molecular level, which enhanced its charge transport; consequently, it was used as a host material in the A–C series devices, which were superior to other MS series devices. In particular, MS-OC and CN-T2T as co-hosts for the yellow emitter PO-01 in device BY (i.e., BY4) demonstrated the best performance with a luminance efficiency of 80.0 cd A−1, power efficiency of 113.0 lm W−1, and a low turn-on voltage of 2.1 V. Notably, device BY4 achieved a high maximum luminance of 142464 cd m−2 and an EQE of 27.1%, among the highest values reported in the literature for PO-01-based devices. These results suggest that this series of bipolar molecules are promising multifunctional materials for OLED applications.
1) doped with an emitter (25 nm)/CN-T2T (50 nm)/LiF (1.5 nm)/Al (150 nm). MS-OC exhibited good dense packing at the molecular level, which enhanced its charge transport; consequently, it was used as a host material in the A–C series devices, which were superior to other MS series devices. In particular, MS-OC and CN-T2T as co-hosts for the yellow emitter PO-01 in device BY (i.e., BY4) demonstrated the best performance with a luminance efficiency of 80.0 cd A−1, power efficiency of 113.0 lm W−1, and a low turn-on voltage of 2.1 V. Notably, device BY4 achieved a high maximum luminance of 142464 cd m−2 and an EQE of 27.1%, among the highest values reported in the literature for PO-01-based devices. These results suggest that this series of bipolar molecules are promising multifunctional materials for OLED applications.
4. Experimental section
All detailed synthesis experiment processes, OLED device fabrication, and characterizations can be found in the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Financial support from the Ministry of Science and Technology of Taiwan (MOST 110-2221-E-155-036, 110-2221-E-155-033-MY2, 111-2221-E-155-012-MY2, 111-2113-M-126-001, and 109-2113-M-029-009), Yuan Ze University, Providence University, and Tunghai University are gratefully acknowledged.References
- C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1987, 51, 913–915 CrossRef CAS.
- M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 4–6 CrossRef CAS.
- M. Hack, M. S. Weaver and J. J. Brown, SID Symp. Dig. Tech. Pap., 2017, 48, 187–190 CrossRef CAS.
- M. A. Baldo, D. F. O’Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151–154 CrossRef CAS.
- J. Lee, J.-I. Lee, J. Y. Lee and H. Y. Chu, Appl. Phys. Lett., 2009, 94, 193305 CrossRef.
- C.-H. Chang, C.-L. Ho, Y.-S. Chang, I. C. Lien, C.-H. Lin, Y.-W. Yang, J.-L. Liao and Y. Chi, J. Mater. Chem. C, 2013, 1, 2639–2647 RSC.
- Y. Seino, H. Sasabe, Y.-J. Pu and J. Kido, Adv. Mater., 2014, 26, 1612–1616 CrossRef CAS PubMed.
- C. Han, L. Zhu, J. Li, F. Zhao, Z. Zhang, H. Xu, Z. Deng, D. Ma and P. Yan, Adv. Mater., 2014, 26, 7070–7077 CrossRef CAS PubMed.
- M. Hu, Q. Xu, Y. Jiang, H. Mu, L. Gao, P. Hu, J. Huang and J. Su, Dyes Pigm., 2018, 150, 185–192 CrossRef CAS.
- H. J. Park, E. A. Chae, H. W. Seo, J.-H. Jang, W. J. Chung, J. Y. Lee, D.-H. Hwang and U. C. Yoon, J. Mater. Chem. C, 2020, 8, 13843–13851 RSC.
- C. Murawski, K. Leo and M. C. Gather, Adv. Mater., 2013, 25, 6801–6827 CrossRef CAS PubMed.
- S. Xia, R. C. Kwong, V. Adamovich, M. S. Weaver and J. J. Brown, 2007 IEEE International Reliability Physics Symposium Proceedings. 45th Annual, 2007, pp. 253–257.
- F. So and D. Kondakov, Adv. Mater., 2010, 22, 3762–3777 CrossRef CAS PubMed.
- Y. Tao, C. Yang and J. Qin, Chem. Soc. Rev., 2011, 40, 2943–2970 RSC.
- A. Chaskar, H.-F. Chen and K.-T. Wong, Adv. Mater., 2011, 23, 3876–3895 CrossRef CAS PubMed.
- T. Zhu and T. Van Voorhis, J. Phys. Chem. C, 2019, 123, 10311–10318 CrossRef CAS.
- T. Ito, H. Sasabe, Y. Nagai, Y. Watanabe, N. Onuma and J. Kido, Chem. – Eur. J., 2019, 25, 7308–7314 CrossRef CAS PubMed.
- M.-H. Tsai, Y.-H. Hong, C.-H. Chang, H.-C. Su, C.-C. Wu, A. Matoliukstyte, J. Simokaitiene, S. Grigalevicius, J. V. Grazulevicius and C.-P. Hsu, Adv. Mater., 2007, 19, 862–866 CrossRef CAS.
- J. Jayakumar, W.-L. Wu, C.-L. Chang, T.-Y. Han, L.-Y. Ting, C.-M. Yeh, H.-W. Hung and H.-H. Chou, Org. Electron., 2021, 88, 106013 CrossRef CAS.
- Y. Zheng, A. S. Batsanov, V. Jankus, F. B. Dias, M. R. Bryce and A. P. Monkman, J. Org. Chem., 2011, 76, 8300–8310 CrossRef CAS PubMed.
- Z. Li, Z. Cheng, J. Lin, N. Xie, C. Li, G. Yang and Y. Wang, J. Mater. Chem. C, 2019, 7, 13486–13492 RSC.
- N. H. Cho, J. Y. Lee, O. Y. Kim and S.-H. Hwang, New J. Chem., 2020, 44, 3868–3873 RSC.
- Y. C. Chen, S. K. Huang, S. S. Li, Y. Y. Tsai, C. P. Chen, C. W. Chen and Y. J. Chang, ChemSusChem, 2018, 11, 3225–3233 CrossRef CAS PubMed.
- Y.-M. Chang, C.-W. Li, Y.-L. Lu, M.-S. Wu, H. Li, Y.-S. Lin, C.-W. Lu, C.-P. Chen and Y. J. Chang, ACS Appl. Mater. Interfaces, 2021, 13, 6450–6460 CrossRef CAS PubMed.
- W.-Y. Hung, P.-Y. Chiang, S.-W. Lin, W.-C. Tang, Y.-T. Chen, S.-H. Liu, P.-T. Chou, Y.-T. Hung and K.-T. Wong, ACS Appl. Mater. Interfaces, 2016, 8, 4811–4818 CrossRef CAS PubMed.
- D. Luo, C.-W. Liao, C.-H. Chang, C.-C. Tsai, C.-W. Lu, T. C. Chuang and H.-H. Chang, J. Phys. Chem. C, 2020, 124, 10175–10184 CrossRef CAS.
- Y.-T. Hung, D. Luo, L.-M. Chen, D.-C. Huang, J.-Z. Wu, Y.-S. Chen, C.-H. Chang and K.-T. Wong, J. Mater. Chem. C, 2022, 10, 4748–4756 RSC.
- C. Adachi, R. C. Kwong, P. Djurovich, V. Adamovich, M. A. Baldo, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 2001, 79, 2082–2084 CrossRef CAS.
- H.-L. Huang, K.-H. Shen, M.-C. Jhu, M.-R. Tseng and J.-M. Liu, Mater. Res. Soc. Symp. Proc., 2020, 846, 95 CrossRef.
- W.-L. Chen, S.-Y. Chen, D.-C. Huang, D. Luo, H.-W. Chen, C.-Y. Wang and C.-H. Chang, Materials, 2021, 14, 5723 CrossRef CAS PubMed.
- R.-H. Yi, Y.-C. Lei, Y.-H. Tseng, Y.-F. Lin, Y.-C. Cheng, Y.-C. Fang, C.-Y. Ho, W.-W. Tsai, C.-H. Chang and C.-W. Lu, Chem. – Eur. J., 2022, 28, e202102966 CrossRef CAS PubMed.
- C.-W. Lu, C.-C. Tsai, Y.-S. Huang, H.-Y. Chih, W.-C. Li, T.-Y. Chiu and C.-H. Chang, Dyes Pigm., 2019, 163, 145–152 CrossRef CAS.
- B. Patil, J. Lade, S.-S. Chiou, Y.-C. Cheng, Y.-F. Lin, Y. Jadhav, P. Chetti, C.-H. Chang and A. Chaskar, Org. Electron., 2021, 92, 106090 CrossRef CAS.
- M. Kim and J. Y. Lee, Synth. Met., 2015, 199, 105–109 CrossRef CAS.
- K. Gao, K. Liu, X.-L. Li, X. Cai, D. Chen, Z. Xu, Z. He, B. Li, Z. Qiao, D. Chen, Y. Cao and S.-J. Su, J. Mater. Chem. C, 2017, 5, 10406–10416 RSC.
- C. Cao, W.-C. Chen, J.-X. Chen, L. Yang, X.-Z. Wang, H. Yang, B. Huang, Z.-L. Zhu, Q.-X. Tong and C.-S. Lee, ACS Appl. Mater. Interfaces, 2019, 11, 11691–11698 CrossRef CAS PubMed.
- B. Sun, K.-N. Tong, X. Chen, J.-L. He, H. Liu, M.-K. Fung and J. Fan, J. Mater. Chem. C, 2021, 9, 7706–7712 RSC.
- Y. Chen, J. Chen, Y. Zhao and D. Ma, Appl. Phys. Lett., 2012, 100, 213301 CrossRef.
- R.-H. Yi, C.-M. Shao, C.-H. Lin, Y.-C. Fang, H.-L. Shen, C.-W. Lu, K.-Y. Wang, C.-H. Chang, L.-Y. Chen and Y.-H. Chang, J. Phys. Chem. C, 2020, 124, 20410–20423 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: The 1H and 13C NMR spectra of all compounds, mass spectra, TDDFT calculated orbitals, thermogravimetric analysis (TGA) of HTMs, differential scanning calorimetry (DSC), OLED device A and device C performances. See DOI: https://doi.org/10.1039/d2tc05296b |
| ‡ Equal contribution first author. |
| This journal is © The Royal Society of Chemistry 2023 |