In vitro digestibility and bioaccessibility of lipid-based delivery systems obtained via enzymatic glycerolysis: a case study of rosemary extract bioaccessibility
Marta
Corzo-Martínez
a,
Celia
Bañares
a,
Alejandro
Díaz
a,
Luis
Vázquez
a,
Guillermo
Reglero
ab and
Carlos F.
Torres
 *a
*a
aDept. Production and Characterization of Novel Foods, Institute of Food Science Research (CIAL, CSIC-UAM), Madrid, Spain. E-mail: carlos.torres@uam.es; Fax: +34 910017905; Tel: +34 910017912
bDepartment of Production and Development of Foods for Health, IMDEA-Food Institute, CEI (UAM-CSIC), C/Faraday 7, 28049 Madrid, Spain
First published on 6th January 2020
Abstract
This work studies the effect of enzymatic glycerolysis on digestibility and bioaccessibility of ratfish liver oil (RLO) rich in alkylglycerols (AKGs), as well as the capability of the glycerolysis product (GP) to act as lipid-based delivery system (LBDS) for a supercritical rosemary extract. For comparison purposes, digestibility and bioaccessibility of two additional lipid systems i.e. original RLO and RLO with addition of GRAS monoolein (MO) as emulsifier agent (RLO + MO), have been evaluated. We have studied the efficiency of the GP and RLO + MO lipid systems as LBDS by combining them with a supercritical rosemary extract (RE), i.e. RE lipid-based formulations. In vitro digestibility and bioaccessibility of un-loaded lipid systems, RE lipid-based formulations and un-carried RE have been determined. The results show a higher digestibility and bioaccessibility of the GP as compared to those of original RLO and RLO + MO. Likewise, a substantial improvement of RE bioaccessibility has been observed when GP is used as lipid carrier of RE. The present work demonstrates that enzymatic glycerolysis is an efficient strategy to obtain highly bioaccessible and potentially bioactive alkylglycerol-based delivery systems, which can be used to increase the bioaccessibility of low water-soluble bioactive compounds.
1. Introduction
Over the last decade, many public and private resources in developed countries have been devoted to the development of nutritional supplements or nutraceuticals from natural sources, with beneficial effects on human health. Despite their promising effects, clinical use of most of these products is limited. That is because the in vivo efficiency of the bioactive ingredients used in supplement or nutraceutical manufacture is not as high as expected based on in vitro results. Low in vivo bioactivity of these compounds is mainly due to their low water solubility or lipophilic character. Low water solubility limits the luminal solubilization and dissolution after intake,1,2 leading to a low stability during gastric digestion that may promote their precipitation and degradation. All this together results in a low bioaccessibility (amount of product that is soluble in the intestinal tract and, hence, available for absorption) and bioavailability (amount of product that is absorbed and transported across the intestinal epithelium toward the blood circulation system for cellular utilization).3 This highlights the importance of developing new strategies addressed to the design of nutritional supplements or nutraceuticals of high bioaccessibility and bioavailability and, hence, highly bioefficient. In this sense, over last years, special attention has been paid to the association to lipids, usually referred as lipid-based delivery systems (LDS). Concretely, particular emphasis has been paid on self-emulsifying delivery systems (SEDS).4–9The potential mechanism of self-emulsifying delivery systems by which they increase bioaccessibility of bioactive compounds and ultimately its bioactivity is as follows. After oral intake of drug or bioactive compound and once in the stomach, lipid system is quickly dispersed in oil drops under gastric lipase and peristalsis effect. Oil dispersion increases drug solubility and dissolution in the aqueous medium, avoiding its precipitation and degradation. Once in the small intestine, pancreatic lipase hydrolyzes triacylglycerol (TAG) into free fatty acids (FFA), di- and monoacylglycerol (DAG and MAG) which along with bile salts and PL from gallbladder form vesicles and micellar structures. These stabilize the bioactive compounds, avoiding their precipitation and interaction with absorption inhibitors and favoring the bioactive transportation until the absorption area.
Oils commonly used to manufacture this kind of formulations are edible plant oils due to their high content in medium chain fatty acids with high dissolution capacity and stability against oxidation.10 Fish oils are not often used in the formulation of lipid carriers due to their high content in PUFAs and, hence, to their lower oxidative stability. However, an interesting and novel alternative to plant oils is the Ratfish Liver Oil (RLO), which offers several advantages with respect to the rest fish oils. On the one hand, due to its higher content in medium chain fatty acids and lower PUFAs content is more stable against oxidation than the rest of fish oils. And, on the other hand, it is exceptionally rich in alkyglycerols (AKG), the ether analogous of acylglycerols.11 Previous studies, some of them carried out in our laboratory, have demonstrated their security and special health-promoting effects in humans, including anticarcinogenic and immunomodulatory activity, hematopoiesis stimulation, increase of sperm motility, and antiviral activity.12–20 Therefore, besides increasing the bioaccessibility of the carried bioactive compound, the use of RLO, rich in AKG, to prepare SEDS has an extra advantage, as it might provide an additional or even synergistic bioactive effect to that of the carried compound, giving rise to highly bioefficient formulations.
AKGs, previously purified from shark liver oil, have been used in previous works as delivery systems for bioactive substances such as butyric acid21 and hydroxytyrosol esters.22 Likewise, Patent ES2294956A123 refers to the procedure for obtaining modified alkoxyglycerols from shark liver oil by fractionation, to obtain a fraction rich in non-esterified AKGs, and subsequent re-esterification with long chain fatty acids, which can be used as lipid carrier of bioactive compounds. However, to the best of our knowledge, studies on the use of self-emulsifying lipid-based delivery systems, rich in AKG, in combination with bioactive compounds for the preparation of highly bioaccessible and bioefficient formulations do not exist.
The use of RLO as lipid carrier presents, however, a limitation. O-Alkyl residue of AKG at sn-1 position of the glycerol backbone affects significantly oil digestibility, which limits its capacity to act as efficient lipid carrier.8,24 This highlights the need of strategies that improves the digestibility, miscibility and emulsifying capacity of this oil. In this respect, enzymatic glycerolysis results of great interest, as it leads to mixtures of minor glycerides and alkylglycerls with better dissolution and self-dispersion capacity than the original oil. Moreover, these mixtures are similar to the natural end products of lipid intestinal digestion and, hence, more biocompatible. In a work previously performed by our group,25 enzymatic glycerolysis, under optimized conditions, shown to be an efficient, cost-effective, environmentally friendly and easily scalable method for the design of potential self-emulsifying systems from ratfish liver oil, with a high content of bioactive alkylglycerols.
In the present work, the in vitro digestibility and bioaccessibility of a lipid system obtained by enzymatic glycerolysis from RLO under optimized conditions have been studied with the aim of evaluating the potential of this glycerolysis product rich in bioactive AKGs to act as efficient lipid-based delivery system of bioactive compounds for obtaining nutritional supplements and functional foods of high bioavailability and bioefficacy.
2. Materials and methods
2.1. Reagents and materials
Ratfish Liver Oil (RLO) was kindly provided by Rosita RatfishOil® (Helgeland, Norway).Food grade monoolein (MO) (99% purity) and cyclopentanone (CPN) used in glycerolysis process, considered as food grade flavoring agent, were obtained from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Glycerol was purchased from ICN Biomedicals (Aurora, OH) and the biocatalyst Novozym 435 (Nov435) (immobilized on acrylic resin, ≥5000 U g−1 according to manufacturer) (Candida Antarctica) was kindly supplied by Novozymes A/S (Bagsvaerd, Denmark).
Reagents used for in vitro digestion, including trizma, maleic acid, pancreatin from porcine pancreas, bile salts and cholesterol were purchased from Sigma-Aldrich. Phosphatidyl choline from egg yolk (PC) and Phospholipon 90H (PL) were supplied by Lipoid (Ludwigshafen, Germany). Hydrochloric acid, sodium sulfate anhydrous, sodium chloride, and calcium chloride were from Panreac (Barcelona, Spain).
Regarding reagents used for chromatographic analysis, pure standards of oleic acid (99% purity) and batyl alcohol (99% purity) as well as the menhaden fish oil were obtained from Sigma-Aldrich. Isooctane was obtained from Carlo Erba Reagents (Val de Reuil, France). Hexane, metyl-tertbutyl ether (MTBE) and chloroform were obtained from Lab-Scan (Gliwice, Poland) and formic acid (98% purity) from Panreac (Barcelona, Spain). All these solvents were of HPLC grade.
Rosemary extract (RE): Stabiloton OS was acquired to RAPS GmbH & Co KG (Kulmbach, Germany) with a phenolic diterpenes content of 30%.
2.2. Production of lipid systems and formulations of rosemary extract
- RLO glycerolysis product (GP) with potential self-emulsifying properties. RLO-derived product obtained by an enzymatic glycerolysis process at pilot plant scale, which was previously optimized by our group to result in the maximum diacylglycerol ether (DAGE) and triacylglycerol (TAG) conversion and the monoacylglycerol (MAG) formation in sufficient amount to provide good emulsifying properties to the product mixture (32%, w/w).25 Optimal glycerolysis conditions at pilot plant scale were 40 °C, 48 h, RLO to glycerol molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, use of immobilized commercial lipase Nov435 as biocatalyst, enzyme to substrate molar ratio of 1
1, use of immobilized commercial lipase Nov435 as biocatalyst, enzyme to substrate molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w
10 (w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w), and 67% CPN (GRAS solvent).
w), and 67% CPN (GRAS solvent).
- Original RLO, which is constituted only by DAGE and TAG.
- RLO with addition of food grade monoolein (MO) as emulsifier agent in a molar ratio RLO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MO of 1
MO of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which contains DAGE, TAG and MO. This will be called from now on RLO + MO.
1, which contains DAGE, TAG and MO. This will be called from now on RLO + MO.
2.3. In vitro gastrointestinal digestion
With the aim of studying the effect of glycerolysis on RLO digestibility, an in vitro gastrointestinal digestion model consisting of a first stage of gastric digestion and a second stage of intestinal digestion was used.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate ratio of 1
substrate ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (w
12 (w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w). Reaction was continued for 60 min at 37 °C.
w). Reaction was continued for 60 min at 37 °C.
On the other hand, a solution trying to simulate biliary secretion was prepared by mixing 200 mg of PC, 250 mg of bile salts, 40 mg of cholesterol (Sigma-Aldrich Chemie GmbH, Steinheim, Germany), 1 mL of a 325 mM CaCl2 solution, 3 mL of a 3.25 mM NaCl solution (Panreac Química S.A.U, Barcelona, Spain), and 20 mL of Trizma-maleate buffer 0.1 M pH 7.5. This mixture was homogenized for 2 min at 3500 rpm.
Then, the pre-emulsified sample and the simulated biliary secretion were mixed and homogenized together for 2 more min at 3500 rpm. The whole media was placed in a thermostatically controlled vessel at 37 °C and continuously stirred at 1000 rpm. Simulated intestinal digestion was started by the addition of fresh porcine pancreatin extract, which was prepared as follows: 1.167 g of pancreatin in 7 mL of Trizma-maleate buffer, stirred for 10 min and centrifuged at 1600g at 5 °C for 15 min. 6 mL of aqueous supernatant were added to the reaction medium together with 10 mg of a food grade PLA2 from Streptomyces violaceoruber (103 U mg−1) (Nagase Chemtex Corporation, Fukuchiyama Factory, Kyoto, Japan). After addition of enzymes, reaction was continued for 60 min at 37 °C, taking off aliquots at 0, 5, 10, 30, and 60 min in order to study the evolution of lipid digestion.
In vitro digestion of each sample was performed at least in duplicate.
2.4. Lipid extraction
The total lipids from digestion aliquots were extracted in three sequential steps by using solvent mixtures with increasing polarity in a solvent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sample ratio of 3
sample ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) and centrifuging for 10 min at 14
1 (v/v) and centrifuging for 10 min at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm each time. Used solvents were: (1) n-hexano
500 rpm each time. Used solvents were: (1) n-hexano![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methyl-tert-butyl-ether (MTBE) (50
methyl-tert-butyl-ether (MTBE) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v
50, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v); (2) MTBE
v); (2) MTBE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether (PE) (50
petroleum ether (PE) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v
50, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v); and (3) PE
v); and (3) PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1
ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6, v
0.6, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v).
v).
Organic phases containing separated lipids were collected after each extraction, put together into a vial with anhydrous sodium sulfate for humidity removal and evaporated under a nitrogen stream by using a Stuart Block Heater SBH200D/3 (Staffordshire, U.K.) until constant weight residue. Finally, samples were diluted with chloroform to a final concentration of 20 mg mL−1 before injection in the LC system.
2.5. Lipid identification and quantification by LC-ELSD
LC analyses were carried out using an Agilent Technologies 1200 Series HPLC system (Santa Clara, CA, USA) containing a thermostated column compartment, a quaternary pump, an autosampler, a vacuum degasser, and evaporative light scattering detector (ELSD) (Agilent 1260 Infinity). Conditions of the ELSD were 2 × 105 Pa, 50 °C, and gain 4, which was adjusted to accurately quantify minor compounds. Chromatographic separations were carried out on an Agilent Poroshell column (Sil 2.7 μm, 4.6 × 100 mm) at 35 °C, a flow rate of 2 mL min−1 and mixing eluents A (100% isooctane), B (0.02% (v/v) formic acid in isooctane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTBE (50
MTBE (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v)) and C (MTBE
50, v/v)) and C (MTBE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) propan-2-ol (50
propan-2-ol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v)) to form a ternary gradient system as previously described by Torres et al.26 The injection volume was 1 μL (∼20 μg of total lipids). Data acquisition and processing were performed using Agilent ChemStation software (Agilent Technologies, Boeblingen, Germany).
50, v/v)) to form a ternary gradient system as previously described by Torres et al.26 The injection volume was 1 μL (∼20 μg of total lipids). Data acquisition and processing were performed using Agilent ChemStation software (Agilent Technologies, Boeblingen, Germany).
Lipids in the reaction mixtures were identified by comparing their retention times (tR) with those of different standard lipids. Commercial oleic acid and batyl alcohol were used for identification of free fatty acid (FFA) and AKG, respectively. DAGE and TAG were identified by using commercial RLO and menhaden fish oil.27 And finally, products derived from glycerolysis of menhaden fish oil and purified by semi-preparative HPLC were used for identification of MAGE, DAG and MAG. Quantitative analysis was performed by the external standard method, using calibration curves of each standard in the range 0.4–25 mg mL−1. Relative standard deviation (RSD) values were below 10% in all cases.
Quantitative data were expressed as both, conversion of DAGE and TAG during the in vitro digestion (E1), and percentage of each compound of the digestion mixture in weight respect to the total weight of the digestion medium (E2).
| 100 − [(%final/%initial) × 100] | (E1) |
| [Wcomp/Wd.m] × 100 | (E2) |
2.6. Separation of the bioaccessible lipid fraction from undigested lipids
Digestion products were centrifuged at 4000 rpm for 40 min at 20 °C (5810R Eppendorf Iberica, Madrid, Spain) according to the method previously described by Soler-Rivas et al.28 After centrifugations, the digested samples were separated into three well-separated phases: an upper phase, called oil phase (OP), which comprises the undigested lipid fraction and part of the RE; an intermediate aqueous phase, called micellar phase (MP), which is a turbid liquid emulsion containing the digested lipid fraction and rosemary extract included in micellar and vesicular structures; and a minor and insoluble lower phase, called precipitated pellet phase (PP). These will be more extensively described in the section of Results and discussion.2.7. Characterization of the bioaccessible fraction
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000).
5000).
All measurements were performed by triplicate, data provided being the mean particle size ± SD.
3. Results and discussion
3.1. Characterization of the studied lipid systems
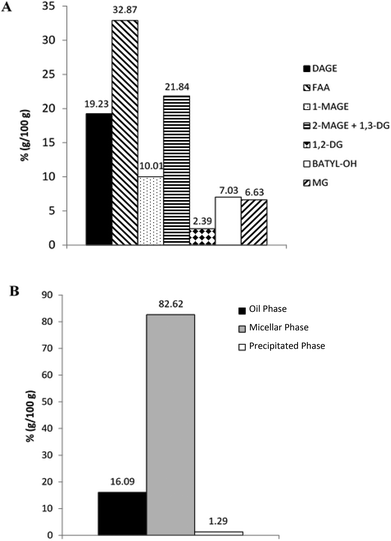
Characterization of the studied lipid systems was performed in our laboratory in a previous work.25 In the present work, we will show only the results than can help to explain the behavior of studied lipid systems during in vitro GTI digestion.Fig. 1 shows LC-ELSD profiles of RLO original, RLO + MO and GP. Original RLO was mainly comprised of two compounds, diacylglycerol ether (DAGE, peak 1, tR = 6.7 min) and triacylglycerol (TAG, peak 2, tR = 7.7 min), the content in DAGE (80%, w/w) of RLO being much higher than TAG content (20%, w/w). LC-ELSD chromatogram of RLO + MO showed one peak corresponding to MO (peak 5, tR = 18.2 min, ∼28%, w/w) in addition to peaks of DAGE (∼57%, w/w) and TAG (∼15%, w/w). Finally, the glycerolysis product showed a composition more complex than that one of RLO and RLO + MO. The final reaction mixture obtained after optimal glycerolysis conditions (previously indicated in section 2.2.1) was constituted by the reactants DAGE (∼25%, w/w) and TAG (∼1%, w/w), but also by the different products of glycerolysis reaction, namely 2-monoacylglycerol ether (2-MAGE), which coeluted with 1,3-DAG (peak 3, tR = 12.9 min, ∼42%, w/w), 1,2-DAG (peak 4, tR = 14.2 min, ∼2%, w/w) and MAG (peak 5, tR = 18.2 min, ∼32%, w/w). MAGE, DAG and, particularly, MAG have higher polarity and, hence, higher emulsifying capacity than starting DAGE and TAG.
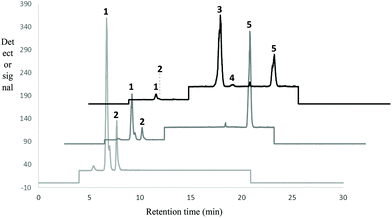 | ||
| Fig. 1 Chromatographic profile obtained by LC-ELSD of original RLO, RLO + MO and glycerolysis product (GP). 1 = DAGE; 2 = TAG; 3 = 2-MAGE + 1,3-DAG; 4 = 1,2-DAG; 5 = MO/MAG. | ||
3.2. In vitro digestibility of lipid systems and RE formulations
The behavior of studied lipid systems during in vitro GTI digestion will determine, on the one hand, the RLO bioacessibility, and, on the other hand, the capacity of studied lipid systems to act as efficient carrier of bioactive RE by increasing its bioaccessibility. Improved bioaccessibility might result, ultimately, in an increased bioactivity or bioefficiency of RE when it is ingested orally in this kind of lipid-based formulations.To evaluate the bioaccessibility of studied lipid systems and RE lipid-based formulations, these were subjected to an in vitro gastrointestinal digestion model as explained in the Method section.
As expected, during the gastric phase the lipid profile was not modified with respect to the initial one due to the absence of lipase in the digestion medium (data not shown). However, during the intestinal phase, lipids were hydrolyzed mainly by the action of the pancreatic lipase, so that the digests present a lipid composition different from the initial one.
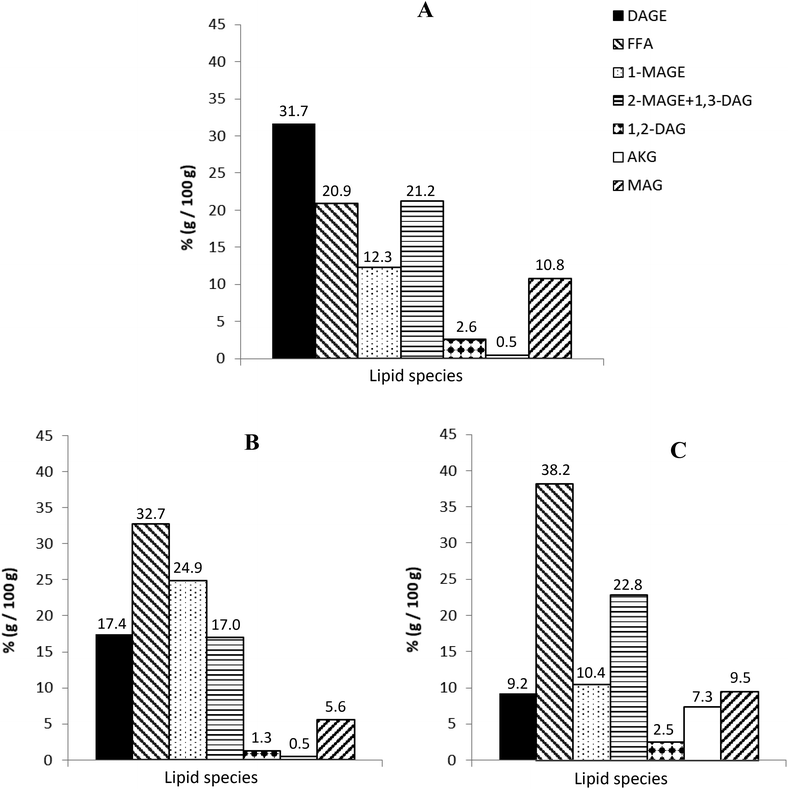
Fig. 2 depicts the composition of the final digestion products of original RLO (Fig. 2A), RLO + MO (Fig. 2B) and GP (Fig. 2C). For all of them, TAGs were completely hydrolyzed, whereas non-hydrolyzed DAGEs were still detected at the end of the digestion process. This agrees with previous studies (Martin et al., 2011),24 in which a DAGE digestibility lower than that of TAG was observed, suggesting that digestibility of studied lipid systems is determined by their initial content in DAGEs. Thus, as observed in Fig. 2, the highest hydrolysis degree of DAGEs and formation of FFA, DAG, MAGE and MAG were observed for GP, followed by the system RLO + MO. Original RLO, with the highest initial content in DAGEs, was the least digestible of all the studied lipid systems.
Moreover, these results indicate that lipid digestion is favored in the presence of an emulsifier in the medium. Lipids with emulsifying character, as MAG/MO, increase lipid dispersion at the beginning of digestion, which may enhance both the TAG and DAGE hydrolysis and the formation of micellar structures, inside which digestion products can be more easily included.30 Likewise, it is noteworthy the higher content of free AKG (non-esterified) in the final digestion product of GP as compared to that in original RLO and RLO + MO digests. This could be attributed to the higher content in MAGE of GP, which is preferably hydrolyzed before than DAGE to give free AKG.
Regarding GTI digestion of RE formulations, the presence of 4% (w/w) and 9% (w/w) of RE did not alter the digestion of the lipid carrier (GP or RLO + MO), as no notable differences with respect un-loaded lipid systems were observed. However, digestion product of formulation with 16% of RE presented a substantially higher content in DAGE and a lower percentage of FFA, MAGE, DAG and MAG, indicating a lower digestibility of lipid fraction. According to previous works,31–33 this could be due to the inhibition of pancreatic lipase in the presence of a high RE content.
3.3. Determination of in vitro bioaccessibility of lipid systems and RE formulations
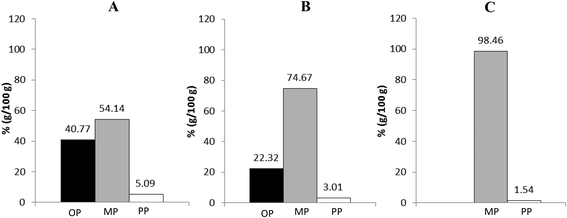
As explained in section 2.6 of Materials and methods, after GTI digestion, final digestion products were centrifuged to obtain three well-separated phases: a poorly emulsified oil phase (OP) that typically contains dispersed oil including part of the rosemary extract. This fraction should mimic the one that in vivo is wasted in faeces or transformed by colonic microbiota; a precipitated pellet phase (PP), which contains insoluble (calcium) soaps of fatty acids liberated during the pancreatic digestion; and a highly emulsified aqueous phase, called micellar phase (MP). This comprises the digested lipid fraction, which is emulsified by the action of bile salts and phospholipids, forming micellar structures and vesicles. In the case of RE formulations, these micellar structures incorporate RE inside. MP corresponds to the “bioaccessible” fraction, i.e. that one that is available to be absorbed by the cells of intestinal epithelium.28Bioaccessibility of studied lipid systems (RLO, RLO + MO and GP), RE lipid-based formulations and non-carried RE was evaluated by LC-ELSD through the characterization of their MP or bioaccessible fraction. As observed in Fig. 4, MP, mainly constituted by the digestion products (FFA, MAGE, DAG and MAG), is the predominant one for all studied systems. However, according to the results derived from digestibility assays, MP is especially abundant in the digestion product of GP, as ca. 98.5% (w/w) of the lipid fraction is in form of micelles, mixed micelles or vesicles. This indicates that almost all the products released during GTI digestion of GP are bioaccessible. The system RLO + MO and, particularly, the original RLO were substantially less bioaccessible than GP, as showed a lower percentage of lipid fraction in the micellar phase (ca. 75% and 45% (w/w), respectively) but higher in the oil phase (ca. 22% and 41% (w/w), respectively), mainly constituted by non-digested DAGE and MAGE (Fig. 5A and B).
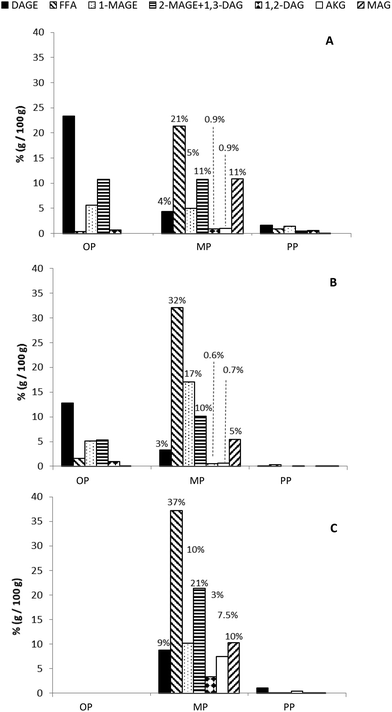 | ||
| Fig. 5 Lipid composition of the different phases (OP, MP and PP) obtained after centrifugation of the final digestion products of original RLO (A), RLO + MO (B) and GP (C). | ||
It is particularly of interest the high content in total AKG and non-esterified AKG in the MP of the GP. As shown in Fig. 5C, most of MAGE was in micellar form and, hence, bioaccessible. Likewise, MP of the GP also comprised a content of free AKG (ca. 7.5%, w/w) higher than that of systems RLO + MO (ca. 1%, w/w) and original RLO (ca. 0.7%, w/w). Both MAGE and, particularly, free AKG possess a bioactive potential higher than DAGE in agreement with several studies previously carried out with colon cancer cells.34
Based on digestibility and bioaccessibility results, it was decided to discard the original RLO as a potentially effective RE lipid carrier. That is why in the present work RE was formulated only with the lipid systems GP and RLO + MO. The incorporation of a 4% (w/w) and 9% (w/w) of RE did not affect the bioaccessibility of the lipid fraction. No remarkable differences in the distribution and composition of OP, MP and PP with respect un-loaded lipid systems were observed (data not shown). However, the presence of a 16% (w/w) RE clearly decreased the bioacessibility of the lipid fraction as compared to that of un-loaded lipid systems, as indicated by the more abundant OP and the important loss of MP (Fig. 3B). These results are in concordance with the decrease in the digestibility previously observed in these formulations due to the RE-induced inhibition of pancreatic lipase when RE is at high concentration.
RE bioaccessibility of lipid-based formulations and non-carried RE was evaluated by means of the determination of total polyphenols in the micellar phase. Non-carried RE (4% and 9%, w/w) showed a relatively low intestinal bioaccessibility, as only a ca. 39% of the initial RE content (before digestion) was detected in MP. As commented above, this could be attributed to the degradation or precipitation of RE during the gastric phase.28 However, when 4% and 9% (w/w) of RE was formulated with both GP and RLO + MO, its intestinal bioaccessibility improved notably with respect to that of non-carried RE. Such bioaccessibility improvement was particularly remarkable with GP, formulations GP + 4%/9% RE showing a ca. 91% of the initial RE content in the MP (vs. ca. 84% in the MP of formulation RLO + MO + 4%/9% RE).
3.4. Further characterization of the bioaccessible fraction of lipid systems and RE formulations
With the aim of gaining more insight that helps to confirm and explain previous results of digestibility and bioaccessibility, additional analysis of micelle quantification, size, stability and morphology were performed.Fig. 6 shows several pictures taken during quantification of micelles in the MP of studied lipid systems and RE formulations with the optical microscope. Under the optical microscope, micelles presented spherical and bright shape. At naked view, it can be observed clearly a high concentration of micellar structures in MP of GP and GP + 9% RE (Fig. 6E and F), followed (in decreasing order) by RLO + MO + 9% RE, RLO + MO, original RLO and non-carried RE (Fig. 6A–D). As observed in Table 1, the subsequent count of the micelles confirmed these observations, which correlates with the order from higher to lower digestibility and bioaccessibility of these systems (sections 3.2 and 3.3). Based on these results, we can infer that a direct relationship between the digestibility and bioaccessibility of a system and the micelle concentration in the MP exists.
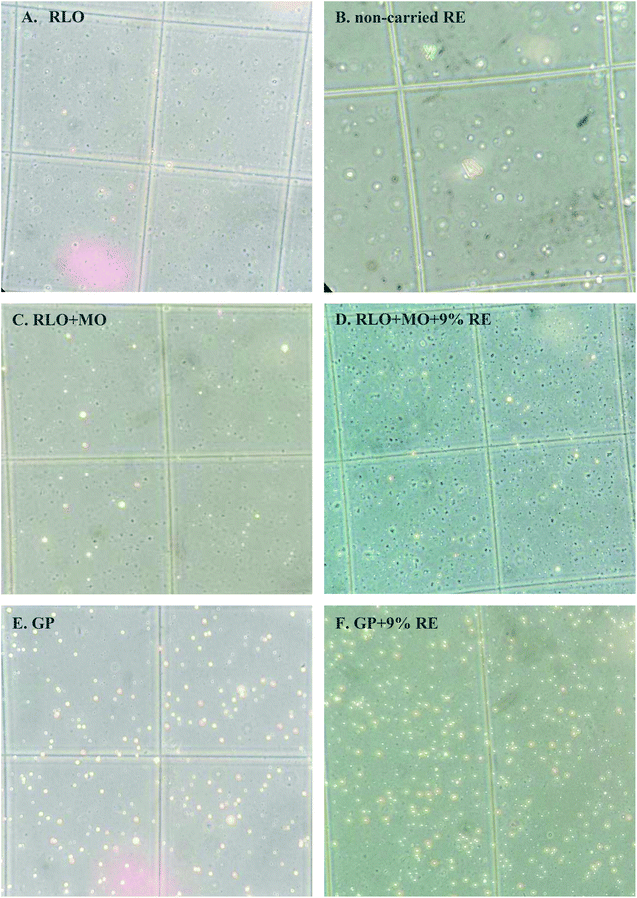 | ||
| Fig. 6 Pictures of the MP of all studied systems taken during the count of micelles with the optical microscope. | ||
| Sample | No. of micelles per mL MP |
|---|---|
| RLO | 61 ± 11 |
| RLO + MO | 113 ± 17 |
| RLO + MO + 9% RE | 147 ± 10 |
| GP | 324 ± 24 |
| GP + 9% RE | 376.5 ± 75 |
| Non-carried RE | 11 ± 3 |
NTA results also provided data of micelle concentration in the MP of lipid systems and RE formulations, as well as of size micelle distribution. In agreement with the count of micelles with the optical microscope, systems with the highest number of particles per mL of MP were GP and GP + 9% RE with 1.38 × 109 and 1.16 × 109 particles per mL, respectively, vs. 1.12 × 109, 1.08 × 109 and 1.04 × 109 particles per mL of RLO + MO + 9% RE, RLO + MO and original RLO, respectively.
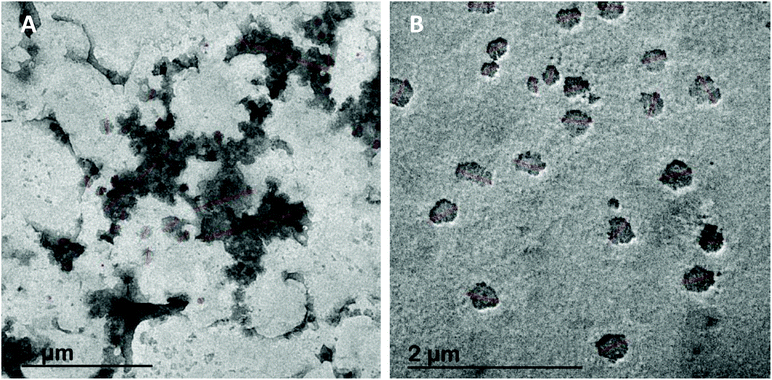
The average size of micelles in all systems was similar, around 200–300 nm. However, as shown in Fig. 7, notable differences in the micelle size distribution of different systems can be observed. Micellas phases of RLO + MO + 9% RE, RLO + MO and original RLO presented a similar size distribution (Fig. 7A), in which it can be distinguished a population of minor particles (ca. 100–250 nm) and two populations of higher size (ca. 250–500 and 500–700 nm), probably due to the micellar aggregation. Such micellar aggregates could be clearly observed in micrographs obtained by HR-TEM (Fig. 8A). Micellar phase of GP and GP + 9% RE (Fig. 7B) showed a size distribution more homogeneous, with a major population of particles of ca. 100–200 nm and a lower concentration of aggregates. In concordance, micrographs obtained by HR-TEM (Fig. 8B) showed individual micelles with spherical shape. The higher concentration of micellar aggregates (>900 nm) in the MP of RLO + MO + 9% RE, RLO + MO and original RLO suggests a lower stability as compared to MP of GP and GP + 9% RE, in which it seems that a more stable emulsion forms after intestinal digestion. However, further studies are needed to confirm this and conclude that GP acts as an efficient self-emulsifying system.
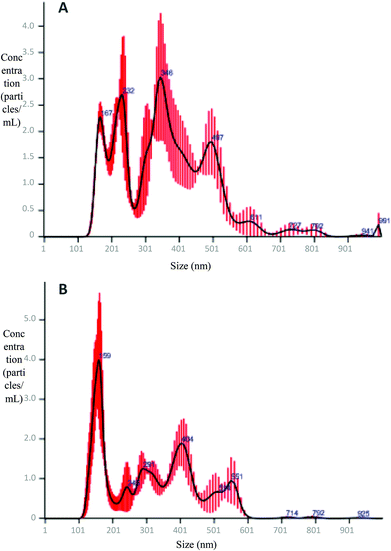 | ||
| Fig. 7 Particle size distribution obtained by NTA of the MP of RLO, RLO + MO, and RLO + MO + 9% RE (A); and RLO, GP, and GP + 9% RE (B). | ||
4. Conclusions
The glycerolysis product presented a higher percentage of total lipids in micellar phase, i.e. in bioaccessible form, as compared to original RLO and RLO + MO. This indicates that enzymatic glycerolysis process is an efficient lipid formulation to improve in vitro RLO digestibility and, consequently, its bioaccessibility.The use of the GP as lipid carrier of rosemary extract efficiently increased stability during GTI digestion, leading to an increase of its intestinal bioaccessibility.
Therefore, the present work has demonstrated that enzymatic glycerolysis is an efficient formulation strategy to obtain highly bioaccessible and potentially bioactive alkylglycerol-based delivery systems, which can be used to increase the bioaccessibility of low water-soluble bioactive compounds. Further studies are needed to evaluate the bioactivity of these new LBDS and resulting formulations when they are combined with bioactive compounds.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study has been funded by Comunidad Autónoma de Madrid (ALIBIRD, S2018/BAA-4343) and by Ministerio de Economia y Competitividad (project number AGL2016-76736-C3-1-R). Celia Bañares also thanks Ministerio de Economia y Competitividad and the European Social Fund for a pre-doctoral FPI grant (BES-2017-080853). Marta Corzo-Martínez also thanks Ministerio de Economia y Competitividad and the European Social Fund: BES-2014-070395 for a Juan de la Cierva contract.References
- R. Chillemi, S. Sciuto, C. Spatafora and C. Tringali, Hydroxytyrosol Lipophilic Analogues: Synthesis, Radical Scavenging Activity and Human Cell Oxidative Damage Protection, in Olives and Olive Oil in Health and Disease Prevention, ed. R. P. Victor and W. Ronald Ross, Academic Press, San Diego, 2010, vol. 135, pp. 1233 Search PubMed.
- K. Kohli, S. Chopra, D. Dhar, S. Arora and R. K. Khar, Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability, Drug Discovery Today, 2010, 15, 958 CrossRef CAS PubMed.
- F. Aqil, R. Munagala, J. Jeyabalan and M. V. Vadhanam, Bioavailability of phytochemicals and its enhancement by drug delivery systems, Cancer Lett., 2013, 334, 133 CrossRef CAS PubMed.
- C. W. Pouton, Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems, Eur. J. Pharm. Sci., 2000, 11, S93 CrossRef CAS PubMed.
- C. W. Pouton, Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system, Eur. J. Pharm. Sci., 2006, 29, 278 CrossRef CAS PubMed.
- V. Jannin, J. Musakhanian and D. Marchaud, Approaches for the development of solid and semi-solid lipid-based formulations, Adv. Drug Delivery Rev., 2008, 60, 734 CrossRef CAS PubMed.
- B. Chengaiah, M. Alagusundaram, S. Ramkanth and C. C. Madhusudhana, Self-emulsifying drug delivery system: a novel approach for drug delivery, Res. J. Pharm. Technol., 2011, 4, 175 Search PubMed.
- S. Kalepu, M. Manthina and V. Padavala, Oral lipid-based drug delivery systems – an overview, Acta Pharm. Sin. B, 2013, 3, 361 CrossRef.
- S. Gupta, R. Kesarla and A. Omri, Formulation strategies to improve the bioavailability of poorly absorbed drugs with spedal emphasis on self-emulsifying systems, ISRN Pharm., 2013, 1, 848043 Search PubMed.
- A. B. Bilia, B. Isacchi, C. Righeschi, C. Guccione and M. C. Bergonzi, Flavonoids loaded in nanocarriers: an opportunity to increase oral bioavailability and bioefficacy, Food Nutr. Sci., 2014, 5, 1212 CAS.
- A. A. Guney, Synthesis of controlled-release products in supercritical medium, AIChE J., 2002, 48, 856 CrossRef.
- A. Brohult, J. Brohult, S. Brohult and I. Joelsson, Effect of alkoxyglicerols on the frecuency of fistulas following radiation-therapy for carcinoma of the uterine cervix, Acta Obstet. Gynecol. Scand., 1979, 58, 203 CrossRef CAS PubMed.
- R. Andreesen, Ether lipids in the therapy of cancer, Prog. Biochem. Pharmacol., 1988, 22, 118 CAS.
- J. Palmblad, J. Samuelsson and J. Brohult, Interactions between Alkylglycerols and human neutrophil Granulocytes, Scand. J. Clin. Lab. Invest., 1990, 50, 363 CrossRef CAS PubMed.
- R. N. Firshein, Method of treating cancer using alkylglycerols in conjunction with chemotherapy, US Patent, 6121245A, 1997 Search PubMed.
- L. Diomede, F. Colotta, B. Piovani, F. Re, E. J. Modest and M. Salmona, Induction of apoptosis in human leukemic cells by the ether lipid 1-octadecyl-2-methyl-RAC-glycero-3-phosphocholine. A possible basis for its selective action, Int. J. Cancer, 1993, 53, 124 CrossRef CAS PubMed.
- J. L. Hammond, D. L. Koontz, H. Bazmi, J. Beadle, S. Hostetler, G. Kini, K. A. Aldern, D. D. Richman, K. Y. Hostetler and J. W. Mellors, Alkylglycerol prodrugs of phosphonoformate are potent in vitro inhibitors of nucleoside-resistant human immunodeficiency virus type 1 and select for resistance mutations that suppress zidovudine resistance, Antimicrob. Agents Chemother., 2001, 45, 1621 CrossRef CAS PubMed.
- R. Mitre, C. Cheminade, P. Allaume, P. Legrand and A. B. Legrand, Oral intake of shark liver oil modifies lipid composition and improves motility and velocity of boar sperm, Theriogenology, 2004, 62, 1557 CrossRef CAS PubMed.
- F. Pédrono, B. Martin, C. Leduc, J. Le Lan, B. Saïag and P. Legrand, Natural Alkylglycerols Restrain Growth and Metastasis of Grafted Tumors in Mice, Nutr. Cancer, 2004, 48, 64 CrossRef PubMed.
- A. Anadón, M. A. Martínez, I. Ares, E. Ramos, F. J. Señoráns, G. Reglero and C. Torres, Acute and repeated dose (28 days) oral safety studies of an Alkosyglycerol extract from shark liver oil in rats, J. Agric. Food Chem., 2010, 58, 2040 CrossRef PubMed.
- C. F. Torres, L. Vázquez, F. J. Señoráns and G. Reglero, Enzymatic synthesis of short-chain diacylatedalkylglycerols: A kinetic study, Process Biochem., 2009, 44, 1025 CrossRef CAS.
- A. Madrona, G. Pereira-Caro, R. Mateos, G. Rodríguez, M. Trujillo, J. Fernández-Bolaño and J. L. Espartero, Synthesis of hydroxytyrosyl alkyl ethers from olive oil waste waters, Molecules, 2009, 14, 1762 CrossRef CAS PubMed.
- G. Reglero, L. Vázquez, C. F. Torres, T. Fornari, F. J. Señorans and F. Moreno, PatentES2294956A1, Modified Alkoxyglycerols, 2006 Search PubMed.
- D. Martín, M. Morán-Valero, F. Señoráns, G. Reglero and C. Torres, In vitro intestinal bioaccessibility of alkylglycerols versus triacylglycerols as vehicles of butyric acid, Lipids, 2011, 46, 277 CrossRef PubMed.
- M. Corzo-Martínez, L. Vázquez, P. Arranz-Martínez, N. Menéndez, G. Reglero and C. F. Torres, Production of a bioactive lipid-based delivery system from ratfish liver oil by enzymatic glycerolysis, Food Bioprod. Process., 2016, 100, 311 CrossRef.
- C. F. Torres, L. Vázquez, F. J. Señoráns and G. Reglero, Study of the analysis of alkoxyglycerols and other non-polar lipids by liquid chromatography coupled with evaporative light scattering detector, J. Chromatogr. A, 2005, 28, 1078 Search PubMed.
- J. C. Mbanya, J. K. Mfopou, E. Sobngwi, M. D. Mbanya and J. Y. Ngogang, Metabolic and hormonal effects of five common African diets eaten as mixed meals: the Cameroon Study, Eur. J. Clin. Nutr., 2003, 57, 580 CrossRef CAS PubMed.
- C. Soler-Rivas, F. R. Marín, S. Santoyo, M. R. García-Risco, F. J. Señoráns and G. Reglero, Testing and enhancing the in vitro bioaccessibility and bioavailability of Rosmarinus officinalis extracts with a high level of antioxidant abietanes, J. Agric. Food Chem., 2010, 58, 1144 CrossRef CAS PubMed.
- M. Bocevska and H. Sovová, Supercritical CO2 extraction of essential oil from yarrow, J. Supercrit. Fluids, 2007, 40, 360 CrossRef CAS.
- C. Porter and W. Charman, In vitro assessment of oral lipid based formulations, Adv. Drug Delivery Rev., 2001, 50, S127 CrossRef CAS PubMed.
- K. Ninomiya, H. Matsuda, H. Shimoda, N. Nishida, N. Kasajima, T. Yoshino, T. Morikawa and M. Yoshikawa, Carnosic acid, a new class of lipid absorption inhibitor from sage, Bioorg. Med. Chem. Lett., 2004, 14, 1943 CrossRef CAS PubMed.
- T. Harach, O. Aprikian, I. Monnard, J. Moulin, M. Membrez, J. C. Béolor, T. Raab, K. Macé and C. Darimont, Rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and liver steatosis in mice fed a high-fat diet, Planta Med., 2010, 76, 566 CrossRef CAS PubMed.
- A. Ibarra, J. Cases, M. Roller, A. Chiralt-Boix, A. Coussaert and C. Ripoll, Carnosic acid-rich rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and improves cholesterol levels and glycaemia in mice on a high-fat diet, Br. J. Nutr., 2011, 106, 1182 CrossRef CAS PubMed.
- S. Molina, M. I. Morán-Valero, D. Martin, L. Vázquez, T. Vargas, C. F. Torres, A. Ramírez de Molina and G. Reglero, Antiproliferative effect of alkylglycerols as vehicles of butyric acid on colon cancer cells, Chem. Phys. Lipids, 2013, 50, 175–176 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |