Large-scale colloidal films with robust structural colors†
Jing
Zhang
 a,
Zhijie
Zhu
a,
Ziyi
Yu
a,
Zhijie
Zhu
a,
Ziyi
Yu
 ab,
Luting
Ling
a,
Cai-Feng
Wang
ab,
Luting
Ling
a,
Cai-Feng
Wang
 *a and
Su
Chen
*a and
Su
Chen
 *a
*a
aState Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemical Engineering, Jiangsu Key Laboratory of Fine Chemicals and Functional Polymer Materials, Nanjing Tech University, Nanjing 210009, P. R. China. E-mail: caifengwang@njtech.edu.cn; chensu@njtech.edu.cn; Fax: +86-25-83172258; Tel: +86-25-83172258
bDepartment of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, UK
First published on 15th October 2018
Abstract
Inspired by the commonly seen “milk skin” phenomenon, a general scheme, namely, a “colloid skin”-regulated assembly route, is demonstrated for colloidal film deposition, which easily suppresses the ubiquitous coffee ring effect accompanying the asymmetrical evaporation processes, implying an alternative colloidal film-forming strategy.
Conceptual insightsWaterborne paints with low toxicity and flammability represent a major direction of coating research and development. However, it is challenging to achieve fine control over asymmetrical drying processes, thus producing defects on the resulting film, such as sagging, orange skin, casing, cratering, etc. In this work, inspired by the commonly seen “milk skin” phenomenon of hot milk-containing liquids, a general scheme, namely, a polymer colloid ensemble (named “colloid skin”)-regulated assembly route, is demonstrated, presenting alternative insights to regulate the microdroplet drying processes to suppress the coffee-ring effect and facilitate the formation of colloidal deposits with defined morphologies. Evidenced by its exemplary application in the construction of fine colloidal photonic crystal (CPC) prints, this strategy exhibits high compatibility when combined with inkjet-printing, spray painting or bar coating techniques. Also, the regulating processes occur irrespective of the substrate properties, and are thus free from the limited choice of printing substrates that most previously reported work encountered. Accordingly, we believe that this strategy represents significant progress in the field of colloidal assembly, especially CPC patterning areas, and may provide valuable insights into the elimination of film defects associated with the asymmetrical drying processes. |
Due to the increased environmental awareness and tighter related regulations, waterborne resins with advantages of low volatile organic compounds (VOCs) and toxicity are becoming an increasingly popular alternative to conventional solvent-based ones, playing a leading role in chemicals, e.g. cosmetics, dyeing, printing and coatings. Their market will go up to $7.824 billion according to the Zion Market Research by 2021. Although waterborne resins have predominated in coating fields, the film forming strategy of these resins still highly relies on conventional volatilization coalescence or chemical cross-linking principles. Additionally, due to the asymmetrical drying processes, film defects, such as sagging, orange skin, casing and cratering could easily be caused, thus limiting their widespread use. For example, colloidal photonic crystals (CPCs) with periodic micro/nanostructures could produce iridescent colors,1–3 but they suffer from the ubiquitous “coffee ring” phenomenon, resulting in limited pattern resolution and low colour saturation. Hence, it is still challenging to construct large-scale, uniform CPC prints towards applications such as in advanced inks, printing, flexible display, packaging, textile printing and dyeing and chemical/biological sensors.4–12 In this respect, Song et al. proposed the use of low surface energy substrates to generate closely packed deposits through creating a sliding three-phase contact line.13,14 Hu and co-workers unveiled that the enhanced Marangoni flow could be adopted to neutralize the outward capillary flows, promoting the formation of uniform deposits.15–17 More recently, researchers demonstrated that irregular morphologies could be helpful in deforming the air–water interface and thus suppressing the coffee ring effect.18 Despite these achievements, the development of a new broad-spectrum kind of film forming strategy is still an urgent issue, especially in frontiers of nanoimprinting, nano-ink, etc.19
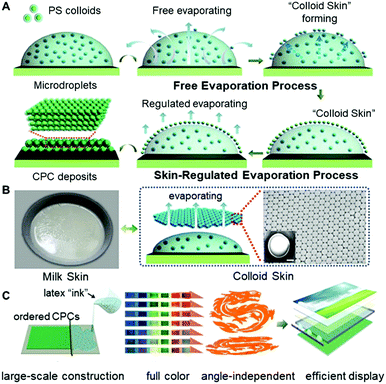
In this work, we provided an alternative strategy to regulate the microdroplet drying processes, facilitating the formation of colloidal deposits with selective morphologies. The method is mainly based on the fact that, just as the commonly seen “milk skin” phenomenon of hot milk-containing liquids, a regular layer of colloid ensembles, “colloid skin”, would form on the top surface of the drying microdroplets (Fig. 1A). This “skin” would seal the drying liquid surfaces, break the outward capillary flow, and greatly vary the evaporative flux across the air–liquid surface. Hence, the coffee ring effect, the major obstacle for uniform colloid deposition, could be suppressed, enabling the preparation of closely packed polymer colloids on diverse substrates with different wettability (Fig. 1B). Also, this strategy exhibits high compatibility when it is combined with either the spray painting or bar coating techniques, allowing facile construction of large-scale CPC prints (about 90 × 70 cm) with uniform morphologies, enriched colors and angle-independent spectrum characteristics (Fig. 1C). These findings undoubtedly provide valuable insights into the elimination of film defects associated with the asymmetrical drying processes. Additionally, evidenced by the colloidal Bragg reflector uses of the resulting CPCs, this strategy would greatly broaden the potential uses of colloidal assemblies in areas of advanced materials.
Specifically, 15 wt% of polystyrene (PS) colloids (Fig. S1 and S2, ESI†) dispersed in a mixture of ethylene glycol (EG) and deionized water (mass ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) were used as the printing “ink”, and a commercial printer (Jetlab 4, MicroFab Corp.) was used to generate tiny ink droplets with sizes ranging from 100 to 300 μm (Fig. 2A, B and Fig. S3, ESI†). As shown in Fig. 2C, after the ejected droplets were deposited onto the glass substrates, the PS colloids at the droplet edge pinned the contact line during drying, and meanwhile, a layer of colloidal assemblies was gradually formed atop the droplets, generating the so-called “colloid skin”. The possible mechanism could be attributed to the fact that, similar to the commonly seen “milk skin” phenomenon of hot milk-containing liquids,20 here, although hard polystyrene particles cannot coalesce together, they can still form very stable colloidal aggregates (i.e. “colloidal skin”) atop the droplet surface. This is explained as follows: due to the high evaporation flux, the PS colloids protrude easily from the droplet surface, and further assemble to ordered regions due to the strong attractive capillary force; these regions grow while the colloids continuously transport to the ordered region, forming the colloidal skin; once the colloidal skin is formed, its effective density is lower than the density of the solvents, and hence they float stably across the droplet surface.21 More attractively, this preformed “skin” would result in different late-stage colloidal assembly behavior, facilitating the formation of CPC deposits with diverse morphologies.
9) were used as the printing “ink”, and a commercial printer (Jetlab 4, MicroFab Corp.) was used to generate tiny ink droplets with sizes ranging from 100 to 300 μm (Fig. 2A, B and Fig. S3, ESI†). As shown in Fig. 2C, after the ejected droplets were deposited onto the glass substrates, the PS colloids at the droplet edge pinned the contact line during drying, and meanwhile, a layer of colloidal assemblies was gradually formed atop the droplets, generating the so-called “colloid skin”. The possible mechanism could be attributed to the fact that, similar to the commonly seen “milk skin” phenomenon of hot milk-containing liquids,20 here, although hard polystyrene particles cannot coalesce together, they can still form very stable colloidal aggregates (i.e. “colloidal skin”) atop the droplet surface. This is explained as follows: due to the high evaporation flux, the PS colloids protrude easily from the droplet surface, and further assemble to ordered regions due to the strong attractive capillary force; these regions grow while the colloids continuously transport to the ordered region, forming the colloidal skin; once the colloidal skin is formed, its effective density is lower than the density of the solvents, and hence they float stably across the droplet surface.21 More attractively, this preformed “skin” would result in different late-stage colloidal assembly behavior, facilitating the formation of CPC deposits with diverse morphologies.
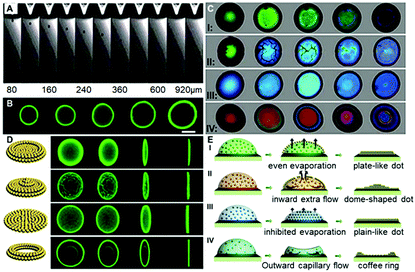
To carefully investigate the “colloid skin” formation and its impact on the late-stage colloidal assembly behavior, the ink droplet drying processes were recorded under different drying conditions. As shown in Case I of Fig. 2C, when the droplets were drying at a temperature of 30 °C and a relative humidity of 40%, the droplet surface exhibited a bright green structural color, implying the formation of the densely-packed “colloid skin”. Herein, the accumulation of colloids at the droplet surface and subsequent formation of the “colloid skin” could be explained using the interfacial growth model. The diffusion constant D calculated from the Stokes–Einstein equation D = (kBT)/6πηr is about 1.7 μm2 s−1. In this equation, kB is Boltzmann's constant, T is the temperature (303 K in Case II), η is higher than 0.97 mPa s for the solution (90 wt% water with 10 wt% ethylene glycol), r is 195 nm, the hydrodynamic radius of the PS colloids. The droplet with a thickness of ca. 20 μm on the substrate dries within 30 s, and on this timescale, the PS microparticles can diffuse a vertical distance less than (2Dt)1/2, 10 μm, which is smaller than the quick evaporative shrink caused by the high evaporation flux J in this case. Therefore, the interface velocity (ν) is faster than diffusion, and PS colloids would accumulate at the descending interface and crystallize to form colloid skins. These “skins” floated across the entire droplet surface, and according to the evaporative lithography model established by Jennifer A. Lewis,22 this would generate free-evaporation areas (leading to the maximum evaporative flux) and hindered evaporation regions (leading to the minimum evaporative flux). In Case I, as the skins were moving all the time, the free-evaporation and hindered evaporation regions kept changing, and hence promoted the uniform distribution of colloids, producing plate-like deposits (Case I of Fig. 2D and E). It should be noted that herein the colloid skin formation could also be explained by the Peclet number, which reflects the ratio of the time for the colloids to diffuse a given distance (normally refers to the initial film thickness) to the droplet drying time.21a However, as it is also calculated from the Stokes–Einstein equation, which is essentially the same as the interfacial growth model, to avoid duplication, the following discussions are mainly based on the interfacial growth model.
When the drying temperature was increased to 40 °C and the relative humidity was increased to 50%, the ink droplet dried within 10 s; however, according to the above-mentioned interfacial growth model, the colloid diffusion distance did not improve much on this timescale. Hence, PS colloids would accumulate more quickly at the descending interface and form a more dense and thicker “colloid skin”. As shown in Case II of Fig. 2C, the crystallized colloids were quickly transformed into some micron-sized “island”-like colloidal skins floating across the droplet surface. Due to the gravity effect, these “islands” slid along the microdroplet surface and finally settled down at its periphery (Movie S1, ESI†). According to Jennifer's model, this would generate the free-evaporation areas with no coverage and the hindered evaporation regions with aggregated colloid coverage around the droplet center and periphery, respectively. The fluid would flow toward the open regions to compensate for the fluid loss (causing an extra “inward flow”) and the entrained particles accumulate beneath the droplet center, giving rise to dome-shaped colloid deposits (Case II in Fig. 2D and E).
When the drying temperature was decreased to 20 °C and the relative humidity was increased to 80%, no colloid skins appeared at the early stage of drying, while the color of droplets varied gradually from pale color to green and finally blue,23 finally giving rise to flat plane-like deposits (Case III in Fig. 2C and D). The phenomena could also be explained using the interfacial growth model. The low temperature and high moisture level led to a significantly prolonged drying time (over 6 min). On this timescale, the colloid diffusion distance far exceeded the interface descending distance, and hence the colloids could not accumulate near the drying droplet interface. Colloid crystallization could not take place, and no skins formed. However, though no “skins” formed in this case, the high humidity significantly decreased the evaporation flux differences between the droplet center and its periphery, and thus the outward flow was suppressed.24 The solvents in drying droplets evaporated evenly and a uniform colloid distribution was finally achieved (Case III in Fig. 2E).
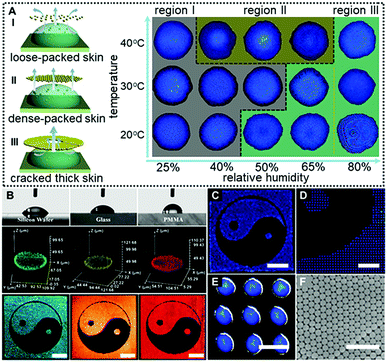
In a control experiment, due to the absence of EG (Case IV in Fig. 2C and 2D), similarly, no colloidal patches were found near the droplet surfaces throughout the drying processes, and ring-like deposits were produced. This could be attributed to the fact that the outward capillary flow caused by the huge evaporation difference between the center of the drying microdroplets and their edges rapidly carried most of the PS colloids to the periphery of the microdroplets. It left no chance for colloids to move towards the microdroplet surfaces, and hence no “colloid skin” could be observed (Movie S2, ESI†). It should be noted that the non-uniform evaporation would also cause extra temperature gradients due to the “evaporative cooling” process25 which resulted in an inward Marangoni flow to compensate for the outward capillary flow. However, as shown by the control experiment, the Marangoni flow was obviously negligible in our cases. The fact that much less EG was used and diverse-shaped deposits were finally obtained points to a substantially different mechanism from those previously reported.16,26 To sum up, Fig. 3A shows the microscopy images of deposits that could be attained under different drying temperature and relative humidity conditions. Region III corresponds to the quick evaporation area. As revealed, a bump is noticed at the deposit centre, suggesting the formation of the dome-shaped deposits. As demonstrated above, this is because the high evaporative flux leads to the formation of a thick “colloid skin” settling around the droplet edge, hence, an inward extra flow would dominate the drying processes. Region II corresponds to medium evaporation areas, and plate-like deposits are formed (Fig. S4, ESI†). This is attributed to the fact that “colloidal skins” with appropriate sizes would dynamically float across the entire droplet surfaces, facilitating even evaporation. Region I corresponds to the slow evaporation areas. As shown, a dark area appears in the middle of the deposits, because its top surface is lower than the focal plane. This characteristic indicates that a concave mirror-like deposit is formed. This may be attributed to the fact that the limited scope and size of the colloid skins under a relatively low evaporative flux could not sufficiently compensate for the outward capillary flow. Consequently, simply by adjusting the drying temperature and humidity, we could facilely achieve fine control over the size and scope of the colloidal skin, and hence spontaneously guide the subsequent colloidal deposition behavior to attain desired CPC deposits.
More attractively, this “colloid skin”-regulated self-assembly strategy is applicable to inkjet printing of fine CPC patterns on diverse substrates irrespective of their wettability or surface roughness. Fig. 3B shows the printed CPC patterns on hydrophilic substrates with contact angles ranging from 52° (silicon wafer), 60° (glass) to 87° (PMMA). Fine Tai Chai prints were finally attained on all these substrates under the same conditions as in Case I of Fig. 2C. The uniform fluorescence intensities for individual printing dots indicate the homogenously distributed PS colloids. Also, as shown in Fig. 3C–E, these printing microdots exhibit nearly uniform sizes, plate-shaped morphologies and brilliant structural colors due to the ordered arrangement of colloidal building blocks (Fig. 3F). In the case of low-adhesion hydrophobic surfaces, the contact line of the drying droplets moves inwards whilst evaporating. These phenomena together with the “colloid skin” promote the formation of uniform colloid deposits (Movie S3, ESI†). Hence, we for the first time realized uniform deposition of CPCs on diverse substrates irrespective of the substrate surface properties. To enhance the mechanical strength of these CPC patterns, polyurethane (PU) and polyvinyl alcohol (PVA) could be added into the inks. A minor addition of these polymers into the printing ink imposes a negligible impact on colloidal assembly processes but greatly enhances interactions between PS colloids and substates, producing robust CPC optical films (Fig. S5–S8, ESI†). However, it should be noted that highly concentrated polymers should be avoided beacuse they would cause different assembly behaviors due to the significant diffusiophoresis phenomenon.27
The “colloid skin”-regulated self-assembly strategy could also be applied to produce large-area colloidal crystal films through the spray-painting technology. Specifically, a gravity feed-type airbrush connected with an air compressor was used, and the airbrush was moved in a line-by-line fashion at a speed of ca. 1 cm s−1, with each stroke overlapping with the previously sprayed area. The interval time between each stroke was set at 5 min to ensure sufficient time for droplet drying. As shown in Fig. S9 (ESI†), the printing inks were intermittently air-sprayed towards a glass substrate, and discrete ink droplets were evenly covered on the glass surface. Under conditions depicted in Case II of Fig. 2C, dome-shaped colloidal crystal deposits were finally attained. Accordingly, a well-executed palette of structural colors was prepared by alternately spraying the inks containing PS colloids of 195, 215 and 272 nm onto the glass substrate (Fig. 4A). The optical characteristics of multiple photonic bandgaps indicate that their colors originate from the overlay of different colloidal crystal deposits (Fig. 4B and Fig. S10, ESI†). More attractively, the intermittently air-sprayed film displays identical colors rather than angle-dependent colors shown in conventional colloidal crystal films (Fig. 4C). The goniometry measurement of the typical sprayed photonic crystal film composed of 195 nm PS colloids (Fig. S11, ESI†) confirms that the position of the spectral peaks remains almost constant at various viewing angles. This is because, due to the hemispherical symmetry of the dome-shaped deposits, the viewing and light incidence angles would remain at the same values of 90° whilst merely rotating the CPC film, and hence the color observed would not change. Similarly, when the film was fixed, if the viewing and light incidence angles changed synchronously with the same amplitude (that is the real case of using a digital camera to capture the CPC films using the flashing mode), the color observed still would not change for the same reason.28 Accordingly, an orange-colored “Dragon” pattern was facilely prepared on a flexible polyethylene glycol terephthalate (PET) film (Fig. 4D). Compared to the colloidal crystals prepared with a single bandgap located at the orange wavelength, the orange color herein was produced by alternately spraying the inks containing PS colloids of 215 and 272 nm. This feature frees us from the burden of heavy synthesis, thus providing a simple but effective route to prepare full-spectrum structural colors with the synergy effects of colloid species (Fig. S12, ESI†). It should be noted that when the nozzle and the glass substrate are too close or using the continuous spraying process, a thin liquid layer appears, forming a continuous colloidal crystal film with angle-dependent optical properties.
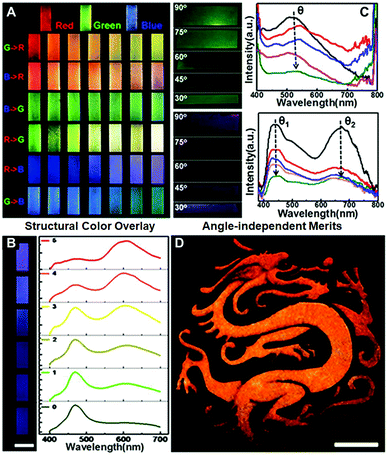 | ||
| Fig. 4 Spray painting of colloidal photonic crystals. (A) A well-executed palette of structural colours attained by alternately spraying the inks containing PS colloids of 195 nm (“blue” ink), 215 nm (“green” ink) and 272 nm (“red” ink). R, G, and B represent red, green and blue inks, respectively. As for the “G → R” row, 180 strokes of the red ink were first applied to the glass substrate, and column N represents the case where an extra (N − 1) × 30 strokes of the green ink were also applied to the substrate. (B) Corresponding reflectance spectra of the prints shown in the R → B row in Fig. 4A. (C) Optical images and reflectance spectra of the films captured with the viewing and light incidence angles changed synchronously with the same amplitude. The arrows show the CPCs with decreasing viewing and light incidence angles. (D) Angle-independent dragon patterns on PET films. Scale bar = 5 cm. To construct the yellow-coloured pattern, 30 strokes of the green ink together with 30 strokes of the red ink were applied. The interval time between each stroke is set at 5 min. | ||
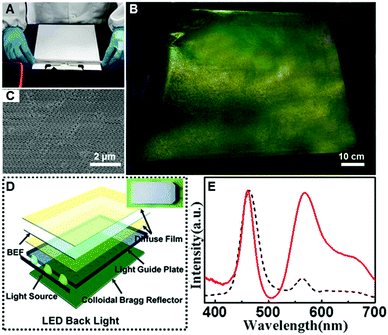
Finally, we applied the “colloid skin”-regulated drying process to the preparation of compact CPC films in 2-D directions. Up to now, it has been a great challenge to achieve large-area high-quality colloidal crystal films. By the assistance of “colloid skin”-regulated drying processes, large-scale colloidal crystal films without cracks could be achieved easily. Typically, the colloid suspension was cast onto a PET film, and spread with a 9 μm-depth spiral bar as illustrated on an A4 glossy paper in Fig. 5A and Movie S4 (ESI†). After water evaporation, a large-scale CPC film with bright structural color (Fig. 5B) and highly ordered internal structure was achieved (Fig. 5C). We successfully fabricated fine CPC films with a size of over 90 × 70 cm. The uniform structure and high optical performance of the as-prepared CPC film should be ascribed to the uniform evaporation of the solvents caused by the “colloidal skin” during the drying process. The whole process takes less than 35 seconds, thus providing an ultra-fast strategy for high-quality colloidal crystal preparation.
After doping with the dye molecule, rhodamine 6G (R6G), the resulting closely packed colloidal crystal film can potentially be used as an efficient luminous film for white-lighting LED backlights (Fig. 5D). As shown in the inset of Fig. 5D, combining with the yellow fluorescence of the composite luminous film, the blue lighting LED devices can thus generate bright white light. A control sample was prepared from the same R6G-doped structures by melting over its glass transition temperature (Tg = 110 °C), to ensure that the thickness and the dye concentration of the film were the same. Impressively, the emission intensity of the luminous film with the CPC-structure is dramatically enhanced, about 3 times more than that of the control sample (Fig. 5E), presenting great potential in the design of efficient lighting devices.
Conclusions
In this work, we use a “colloid skin” in analogy to the “milk skin” phenomenon to describe the crystallization of colloids atop drying droplets and unveil that the scope and size of this preformed “skin”, determined by the drying temperature and humidity, are highly correlated with the subsequent colloidal assembly behavior. Furthermore, evidenced by its exemplary application to CPC prints, this strategy is used irrespective of the substrate properties, and is thus free from the limited choice of printing substrates; also, it exhibits high compatibility when combined with inkjet-printing, spray painting or bar coating techniques, facilitating its practical use in the construction of large-scale colloidal films. These findings would undoubtedly advance the understanding of colloidal film forming processes and provide valuable insights into the elimination of film defects associated with the asymmetrical drying processes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21474052, 21736006, 21706122), the National Key Research and Development Program of China (2016YFB0401700), the Natural Science Foundation of Jiangsu Province (BK20171013), the Fund of State Key Laboratory of Materials-Oriented Chemical Engineering (ZK201704), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).References
- (a) J. W. Dong, X. D. Chen, H. Zhu, Y. Wang and X. Zhang, Nat. Mater., 2017, 16, 298 CrossRef CAS PubMed; (b) Y. Wang, D. Aurelio, W. Li, P. Tseng, Z. Zheng, M. Li, D. L. Kaplan, M. Liscidini and F. G. Omenetto, Adv. Mater., 2017, 29, 1702769 CrossRef PubMed; (c) G. Freymann, V. Kitaev, B. V. Lotsch and G. A. Ozin, Chem. Soc. Rev., 2013, 42, 2528 RSC.
- (a) B. J. de Gans, P. C. Duineveld and U. S. Schubert, Adv. Mater., 2004, 16, 203 CrossRef CAS; (b) J. Zhou, P. Han, M. Liu, H. Zhou, Y. Zhang, J. Jiang, P. Liu, Y. Wei, Y. Song and X. Yao, Angew. Chem., Int. Ed., 2017, 56, 10462 CrossRef CAS PubMed; (c) A. C. Arsenault, D. P. Puzzo, I. Manners and G. A. Ozin, Nat. Photonics, 2007, 1, 468 CrossRef CAS.
- (a) F. Fu, Z. Chen, Z. Zhao, H. Wang, L. Shang, Z. Gu and Y. Zhao, PNAS, 2017, 114, 5900 CrossRef CAS PubMed; (b) H. Shen, Z. Wang, Y. Wu and B. Yang, RSC Adv., 2016, 6, 4505 RSC; (c) B. Hatton, L. Mishchenko, S. Davis, K. H. Sandhage and J. Aizenberg, PNAS, 2010, 107, 10354 CrossRef CAS PubMed.
- (a) J. Li, W. Gao, R. Dong, A. Pei, S. Sattayasamitsathit and J. Wang, Nat. Commun., 2014, 5, 5026 CrossRef CAS PubMed; (b) J. Li, G. Q. Liang, X. Zhu and S. Yang, Adv. Funct. Mater., 2012, 22, 2980 CrossRef CAS.
- (a) S. S. Lee, H. J. Seo, Y. H. Kim and S. H. Kim, Adv. Mater., 2017, 29, 1606894 CrossRef PubMed; (b) C. Xiong, J. Zhao, L. Wang, H. Geng, H. Xu and Y. Li, Mater. Horiz., 2017, 4, 862 RSC; (c) K. G. Noh and S. Y. Park, Mater. Horiz., 2017, 4, 633 RSC.
- (a) J. V. Barth, G. Costantini and K. Kern, Nature, 2005, 437, 671 CrossRef CAS PubMed; (b) X. Li, L. Peng, J. Cui, W. Li, C. Lin, D. Xu, T. Tian, G. Zhang, D. Zhang and G. T. Li, Small, 2012, 8, 612 CrossRef CAS PubMed; (c) X. Fei, T. Lu, J. Ma, S. Zhu and D. Zhang, Nanoscale, 2017, 9, 12969 RSC.
- (a) A. G. Mark, J. G. Gibbs, T. C. Lee and P. Fischer, Nat. Mater., 2013, 12, 802 CrossRef CAS PubMed; (b) Z. Y. Yu, C. F. Wang, L. Ling, L. Chen and S. Chen, Angew. Chem., Int. Ed., 2012, 51, 2375 CrossRef CAS PubMed; (c) W. Zhang, N. Gao, J. Cui, C. Wang, S. Wang, G. Zhang, X. Dong, D. Zhang and G. Li, Chem. Sci., 2017, 8, 6281 RSC.
- (a) M. Kuang, L. Wang and Y. Song, Adv. Mater., 2014, 26, 6950 CrossRef CAS PubMed; (b) S. H. Kim, J. G. Park, T. M. Choi, V. N. Manoharan and D. A. Weitz, Nat. Commun., 2014, 5, 3068 CrossRef PubMed; (c) Y. Wang, Z. Zheng, H. K. Bisoyi, K. G. Gutierrez-Cuevas, L. Wang, R. S. Zola and Q. Li, Mater. Horiz., 2016, 3, 442 RSC.
- (a) H. Kim, J. Ge, J. Kim, S. E. Choi, H. Lee, H. Lee, W. Park, Y. Yin and S. Kwon, Nat. Photonics, 2009, 3, 534 CrossRef CAS; (b) S. N. Yin, S. Yang, C. F. Wang and S. Chen, J. Am. Chem. Soc., 2016, 138, 566 CrossRef CAS PubMed; (c) Y. Li, Q. Yang, M. Li and Y. Song, Sci. Rep., 2016, 6, 24628 CrossRef CAS PubMed.
- (a) D. P. Yang, S. Y. Ye and J. P. Ge, Adv. Funct. Mater., 2014, 24, 3197–3205 CrossRef CAS; (b) J. Zhang, L. Ling, C. F. Wang, S. Chen, L. Chen and D. Y. Son, J. Mater. Chem. C, 2014, 2, 3610 RSC; (c) J. Zhang, Y. Tian, W. Q. Ji, Z. Zhu, C. F. Wang and S. Chen, J. Mater. Chem. C, 2016, 4, 6750 RSC.
- (a) W. Wang, J. Feng, Y. Ye, F. Lyu, Y.-S. Liu, J. Guo and Y. Yin, Nano Lett., 2017, 17, 755 CrossRef CAS PubMed; (b) J. Zhang, S. Yang, Y. Tian, C. F. Wang and S. Chen, Chem. Commun., 2015, 51, 10528 RSC; (c) J. P. Ge and Y. D. Yin, Angew. Chem., Int. Ed., 2011, 50, 1492 CrossRef CAS PubMed; (d) M. Kuang, J. Wang and L. Jiang, Chem. Soc. Rev., 2016, 45, 6833 RSC.
- (a) M. Xiao, Z. Hu, Z. Wang, Y. Li, A. D. Tormo, N. L. Thomas, B. Wang, N. C. Gianneschi, M. D. Shawkey and A. Dhinojwala, Sci. Adv., 2017, 3, e1701151 CrossRef PubMed; (b) J. Zhang, L. Wang, J. Luo, A. Tikhonov, N. Kornienko and S. A. Asher, J. Am. Chem. Soc., 2011, 133, 9152 CrossRef CAS PubMed.
- J. Zhou, J. Yang, Z. Gu, G. Zhang, Y. Wei, X. Yao, Y. Song and L. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 22644 CrossRef CAS PubMed.
- L. Wu, Z. Dong, M. Kuang, Y. Li, F. Li, L. Jiang and Y. Song, Adv. Funct. Mater., 2015, 25, 2237 CrossRef CAS.
- H. Hu and R. G. Larson, J. Phys. Chem. B, 2006, 110, 7090 CrossRef CAS PubMed.
- H. Hu and R. G. Larson, Langmuir, 2005, 21, 3972 CrossRef CAS PubMed.
- H. Hu and R. G. Larson, Langmuir, 2005, 21, 3963 CrossRef CAS PubMed.
- P. J. Yunker, T. Still, M. A. Lohr and A. G. Yodh, Nature, 2011, 476, 308 CrossRef CAS PubMed.
- (a) H. S. Kang, J. Lee, S. M. Cho, T. H. Park, M. J. Kim, C. Park, S. W. Lee, K. L. Kim, D. Y. Ryu, J. Huh, E. L. Thomas and C. Park, Adv. Mater., 2017, 29, 1700084 CrossRef PubMed; (b) X. Su, Y. Jiang, X. Sun, S. Wu, B. Tang, W. Niu and S. Zhang, Nanoscale, 2017, 9, 17877 RSC.
- T. Okuzono, K. Ozawa and M. Doi, Phys. Rev. Lett., 2006, 97, 136103 CrossRef PubMed.
- (a) A. F. Routh, Rep. Prog. Phys., 2013, 76, 046603 CrossRef PubMed; (b) S. Erkselius, L. Wadsö and O. J. Karlsson, J. Colloid Interface Sci., 2007, 317, 83 CrossRef PubMed; (c) S. H. Im, Y. T. Lim, D. J. Suh and O. O. Park, Adv. Mater., 2002, 14, 1367 CrossRef CAS.
- D. J. Harris, H. Hu, J. C. Conrad and J. A. Lewis, Phys. Rev. Lett., 2007, 98, 148301 CrossRef PubMed.
- G. Pan, R. Kesavamoorthy and S. A. Asher, J. Am. Chem. Soc., 1998, 120, 6525 CrossRef CAS.
- R. D. Deegan, O. Bakajin, T. F. Dupont, G. Huber, S. R. Nagel and T. A. Witten, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 62, 756 CrossRef CAS.
- A. M. Cazabat and G. Guéna, Soft Matter, 2010, 6, 2591 RSC.
- H. Hu and R. G. Larson, J. Phys. Chem. B, 2002, 106, 1334 CrossRef CAS.
- R. P. Sear and P. B. Warren, Phys. Rev. E, 2017, 96, 062602 CrossRef PubMed.
- Y. Zhao, L. Shang, Y. Cheng and Z. Gu, Acc. Chem. Res., 2014, 47, 3632 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8mh00248g |
| This journal is © The Royal Society of Chemistry 2019 |