Relationship between basicity, reducibility and partial oxidation properties of chromium containing MCM-41
Maciej Trejda*,
Małgorzata Bryś and
Maria Ziolek
Adam Mickiewicz University in Poznań, Faculty of Chemistry, Umultowska 89b, 61-614 Poznań, Poland. E-mail: tmaciej@amu.edu.pl; Tel: +48 618291305
First published on 14th November 2014
Abstract
MCM-41 mesoporous solids were modified with chromium species using Cr(NO3)3 and CrO3 as precursors. The impact of metal amount and metal sources on the structural and textural parameters of the materials obtained was investigated. Their surface properties were analyzed using XRD, UV-Vis, H2-TPR (the state of Cr species) as well as test reactions (2-propanol decomposition and cyclization and dehydration of 2,5-hexsanedione). The catalytic activity of Cr/MCM-41 materials was tested in methanol partial oxidation. The relationships of reducibility, kind of chromium species, basicity of material surface and the catalysts performance in partial oxidation of methanol were examined and discussed. The impact of basicity on methanol oxidation was clearly documented. Di- and polychromate species were found to be responsible for high yields of formaldehyde.
Introduction
The design of catalysts continues to be an attractive and important issue even in view of the large number of catalytic processes already applied in industry. Development of new catalytic systems or optimization of those used already is essential to decrease the cost of production and limit the number and amount of side-products, which is also in line with the principles and rules of so-called “green chemistry”.1 Therefore, development of new methods supporting catalyst design is important. Huge progress in this regard has been made thanks to the application of new methods of physicochemical characterization of materials providing information on the catalyst structure and single processes that occur on the surface of solid materials.2 However, these techniques are applied usually under conditions very different to those of the actual catalyst application, i.e. the conditions of the catalytic process. A new perspective has been offered by Operando techniques.3 Nevertheless, the application and development of test reactions that examine the surface properties at “working” conditions are still crucial. For example, the test processes involving the acid–base or redox properties of the solids are well known and described in literature.4–7 Methanol oxidation belongs to the well-defined test reactions but it is also used in industry for the production of fine chemicals.Partial oxidation of methanol is an important industrial process since it provides valuable products such as formaldehyde, dimethoxymethane (methylal) or methyl formate.8 The production of the first product mentioned above at industrial level has been known for more than 100 years and involves Fe–Mo or Ag containing catalysts. The mechanism of this reaction, depending on the kind of catalyst used, has been described in literature, e.g. ref. 8–18 but some of its aspects are still debatable. Partial oxidation of methanol has been also proposed as a test reaction for different kinds of catalysts basing on mechanisms proposed, e.g. ref. 19 and 20. In our previous work we have shown that partial oxidation of methanol can be combined with other transformation of CH3OH, namely the sulphurisation process.20 As a consequence, these two complementary reactions give more detail information concerning the properties of catalyst surface.
The application of different test reactions or characterization techniques is essential especially when different kinds of species can be formed in course of catalyst preparation. This is often in the case of transition metals, e.g. iron21,22 or chromium,23,24 applied in the catalysts preparation. The latter element was incorporated into different kinds of mesoporous materials (MCM-41, SBA-15 and SBA-3) using CrO3 as a metal precursor.24 We have shown that a kind of support strongly influences chromium species obtained after impregnation. In this paper we focus on MCM-41 support that is modified with different amounts of chromium using CrO3 and Cr(NO3)3 as metal precursors. By changing the amount and sources of chromium we expected to obtain different chromium species on mesoporous support having different properties.
The aim of this study was to examine basicity and redox properties of model catalysts of MCM-41 type containing chromium by the use of selected test reaction (2-propanol decomposition, cyclization and dehydration of 2,5-hexsanedione and methanol oxidation). In particular, we would like to present the correlation of basic properties of Cr/MCM-41 materials (measured by test reactions) and reducibility of chromium species (testified by H2-TPR) with redox properties estimated from methanol oxidation to formaldehyde.
Experimental
Preparation of MCM-41 materials
MCM-41 was prepared by the classical hydrothermal synthesis in the polypropylene (PP) bottles.25 The reactant mixture consisted of sodium silicate (27% SiO2 in 14% NaOH – Aldrich), cetyltrimethylammonium chloride (CTACl) (Aldrich) and water. The gel formed from these components was stirred for 0.5 h. Next, pH was adjusted to 11 by H2SO4, and distilled water was added. The gel, with the molar ratio: 1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.78CTA+
1.78CTA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.09H2SO4
0.09H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 201H2O, was moved into a PP bottle and heated without stirring at 373 K for 24 h. The reactants were cooled down to room temperature (RT) in order to adjust pH again to 11 with H2SO4. After this, the mixture was heated once more to 373 K and left for another 24 h. The product was filtered and washed with distilled water. After drying at 373 K the template was removed by calcination at 823 K for 2 h in helium flow and then 14 h in air in static conditions (heating rate 5 K min−1).
201H2O, was moved into a PP bottle and heated without stirring at 373 K for 24 h. The reactants were cooled down to room temperature (RT) in order to adjust pH again to 11 with H2SO4. After this, the mixture was heated once more to 373 K and left for another 24 h. The product was filtered and washed with distilled water. After drying at 373 K the template was removed by calcination at 823 K for 2 h in helium flow and then 14 h in air in static conditions (heating rate 5 K min−1).
Incorporation of chromium species
Chromium was introduced via incipient wetness impregnation. Prior to the modification, the mesoporous silica was outgassed in evaporator flask for 1 h at 353 K. Then the material was filled with such an amount of the aqueous solution of chromium(VI) oxide (Aldrich) or chromium(III) nitrate nonahydrate (Aldrich) which filled only the pores of the support. The amount of chromium in the solution was sufficient to obtain 1 wt%, 3 wt% or 5 wt% of this metal in the final product. The mixture was located in an evaporator flask, rotated and heated at 353 K for 1 h. The impregnated powder was dried at 393 K for 12 h and calcined at 823 K for 5 h in air in static conditions (heating rate 5 K min−1).Characterization methods
The materials prepared were characterized using different techniques like: XRD, N2 adsorption/desorption, UV-Vis, H2-TPR.XRD patterns were recorded at room temperature on a Bruker AXS D8 Advance apparatus using CuKα radiation (λ = 0.154 nm), with a step of 0.02° and 0.05° in the small-angle and wide-angle range, respectively.
N2 adsorption/desorption isotherms were obtained on a Micromeritics ASAP equipment, model 2010. The samples (200 mg) were pre-treated in situ under vacuum at 573 K for 3 h. The surface area was calculated using the BET method. The pore diameter and the mesopore volumes were determined from the adsorption branch of the isotherms.
UV-Vis spectra were recorded using Varian-Cary 300 Scan UV-Visible Spectrophotometer. Powdered samples were placed into the cell equipped with a quartz window. The spectra were recorded in the range from 800 to 190 nm. Spectralon was used as a reference material.
The temperature-programmed reduction of the samples was carried out using H2/Ar (10 vol% in Ar) as a reducing agent (flow rate = 32 cm3 min−1). The sample (0.025 g) was introduced to a quartz tube, treated in a flow of helium at 723 K for 2 h (flow rate = 40 cm3 min−1) and cooled to room temperature. Then it was heated at the rate of 10 K min−1 to 1273 K under the flow of the reducing mixture. Hydrogen consumption was measured by a thermal conductivity detector.
2-Propanol decomposition
The 2-propanol dehydration and dehydrogenation was performed using a microcatalytic pulse reactor (Ø 6 mm; length 80 mm) inserted between the sample inlet and the column of a CHROM-5 chromatograph. A portion of 0.02 g (3 mm height in the reactor) of granulated catalyst was activated at 673 K (heating rate 10 K min−1) for 2 h under helium flow (40 cm3 min−1). The 2-propanol (Chempur Poland) conversion was studied at 573 K using 3 μl pulses of alcohol under helium flow (40 cm3 min−1). The substrate was vaporized before being passed through the catalyst bed with the flow of helium carrier gas. The products such as propene, 2-propanone (acetone) and diisopropyl ether were identified by CHROM-5 gas chromatograph online with microreactor. The reaction mixture was separated on a 2 m column filled with Carbowax 400 loaded on Chromosorb W (80–100 mesh) at 338 K in helium flow (40 cm3 min−1) and detected by TCD.Cyclization and dehydration of 2,5-hexanedione (2,5-DHN)
The catalysts were tested in 2,5-hexanedione (2,5-DHN) dehydration and cyclization as the probe reaction. A tubular, down-flow reactor (Ø 8 mm; length 80 mm) was used for (2,5-DHN) cyclization reaction that was carried out at atmospheric pressure, using nitrogen as the carrier gas. The catalyst bed (0.05 g, 2 mm height in the reactor) was first activated for 2 h at 673 K (heating rate 15 K min−1) under nitrogen flow (40 cm3 min−1). Subsequently, a 0.5 cm3 of 2,5-hexanedione (Fluka, GC grade) was passed continuously into the catalyst at 623 K. The substrate was delivered with a pump system (KD Scientific) and vaporized before being passed through the catalyst with the flow of nitrogen carrier gas (40 cm3 min−1). The reaction products were collected for 30 min downstream of the reactor in a cold trap (liquid nitrogen + 2-propanol) and analyzed by gas chromatography (SRI 310 C, MXT-1 column 30 m, temperature of column 373 K) with TCD detector. Helium was applied as a carrier gas.Methanol oxidation
The methanol oxidation reaction was performed in a fixed-bed flow reactor (Ø 5 mm; length 70 mm). A portion of 0.04 g of the pure (not diluted) catalyst of the size fraction of 0.5 < Ø < 1 mm was placed in the reactor (4 mm height in the reactor). The samples were activated in argon flow (40 cm3 min−1) at 673 K for 2 h (the rate of heating was 15 K min−1). Then, the temperature was decreased to that of the reaction. The reactant mixture of Ar/O2/MeOH (88/8/4 mol%) was supplied at the rate of 40 cm3 min−1. Methanol (Chempur Poland) was introduced to the flow reactor by bubbling argon gas through a glass saturator filled with methanol. The reactions were conducted at GHSV = 1220 h−1. The reactor effluent was analyzed using an online two gas chromatographs. One chromatograph GC 8000 Top equipped with a capillary column of DB-1 operated at 313 K – FID detector was applied for analyses of organic compounds and the second GC containing Porapak Q and 5A molecular sieves columns for analyses of O2, CO2, CO, H2O and CH3OH – TCD detector. The columns in the second chromatograph with TCD were heated according to the following programme: 5 min at 358 K, increase of the temperature to 408 K (heating rate 5 K min−1), 4 min at 408 K, cooling down to 358 K (for the automatic injection on the column with 5 A), 10 min at 358 K, increase of the temperature to 408 K (heating rate 10 K min−1), 11 min at 408 K. Argon was applied as a carrier gas. The outlet stream line from the reactor to the gas chromatograph was heated at about 373 K to avoid condensation of reaction products.Results and discussion
Characterization
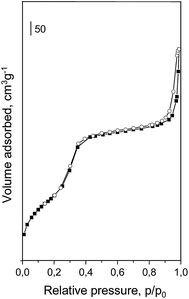
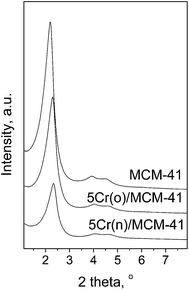
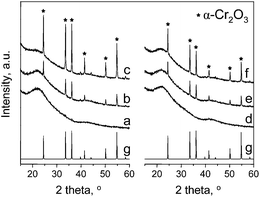
The support for chromium species was prepared by typical hydrothermal synthesis. The list of materials obtained, including their colors, is presented in Table 1. MCM-41 obtained shows characteristic properties of ordered mesoporous solid with relatively large surface area (950 m2 g−1), pore volume of 1.11 m2 g−1 and average pore diameter of 4.1 nm (Table 2). The adsorption/desorption isotherm of the support is presented in Fig. 1. It shows a typical shape of type IV isotherm according to IUPAC classification.26 Two regions of high increase in N2 volume can be observed. The first, in the range of relative pressure p/p0 = 0.2–0.4 is caused by capillary condensation inside mesopores. The second, at p/p0 ca. 0.9 testifies to the presence of macroporosity (interparticle macroporosity) or secondary mesopore system in the space between mesoporous channels.27 The incorporation of chromium does not significantly change the textural parameters of final materials as indicated in Table 2, especially in the case of samples prepared from CrO3. The latter show a small decrease of surface area up to 3 wt% of chromium loading, whereas for samples prepared from Cr(NO3)3 the drop of surface area is remarkable. This difference is not much seen for 5 wt% of Cr loading. Other textural parameters, i.e. pore volume and pore diameter decrease as well, however the latter is similar for all chromium containing samples. Mesoporous channels of MCM-41 are well ordered even after Cr impregnation as it is determined by XRD measurements for the highest chromium loading (Fig. 2). The appearance of a peak at 2 theta ca. 2.3° related to (100) is caused by the presence of channels that are regular in shape. The position of this peak is associated with the distance between material walls. The next two peaks at 2 theta ca. 4° and 4.6° appear due to the regular arrangement of channels inside MCM-41 and can be used as an indicator of pore ordering. The intensity of the main peak depends on the kind of chromium precursor that was applied for catalyst preparation, i.e. CrO3 (labeled as (o) in the catalyst symbol) or Cr(NO3)3 (labeled as (n) in the catalyst symbol). The latter gives lower intensity of the (100) peak, which can be explained by the chromium species located to a greater extend inside the hexagonal pores of the support. The incorporation of chromium in MCM-41 leads to the formation of some crystal phase on the material surface (both, inside and outside the pores) which is testified by the presence of new XRD peaks at 2 theta between 20–55° (Fig. 3). They are not observed when 1 wt% of Cr was used for the impregnation. For higher metal loading, the crystal phase that can be assigned to α-Cr2O3 (calculated pattern in Fig. 3g) becomes visible and the highest intensity of XRD peaks is observed for 5 wt% of chromium. Moreover, if CrO3 is used as the metal precursor, the intensity of the peaks related to α-Cr2O3 is higher than if Cr(NO3)3 is used indicating relatively high concentration of chromium(III) oxide phase in sample 5Cr(o)/MCM-41.| Catalysta | Chromium source | Chromium loading, wt% | Colour of sample |
|---|---|---|---|
| a (o) – obtained from CrO3; (n) – obtained from Cr(NO3)3. | |||
| 1Cr(o)MCM-41 | CrO3 | 1 | Pale yellow |
| 3Cr(o)MCM-41 | CrO3 | 3 | Yellow-green |
| 5Cr(o)MCM-41 | CrO3 | 5 | Dark green |
| 1Cr(n)MCM-41 | Cr(NO3)2 | 1 | Pale yellow |
| 3Cr(n)MCM-41 | Cr(NO3)2 | 3 | Yellow-green |
| 5Cr(n)MCM-41 | Cr(NO3)2 | 5 | Green |
Some conclusions concerning the nature of chromium species can be deduced from UV-Vis measurements. The spectra of samples prepared are shown in Fig. 4. The introduction of chromium on the surface of MCM-41 results in the appearance of some characteristic bands coming from different coordinations of Cr species. In particular the bands at ca. 260 and 350 nm are assigned to the charge transfer from O2− → Cr6+ in monochromate species.28–30 These bands prevail for the samples with the lowest amount of chromium (1 wt%) thus one can postulate that monochromate species are dominant for lower Cr loading on material surface. This conclusion is also supported by the pale yellow (characteristic of Cr6+) color of the samples having the lowest chromium concentration. The introduction of higher than 1 wt% amount of chromium results in the appearance of a band at ca. 465 nm (this band shows a very low intensity for 1 wt% of Cr) that is assigned to di- and poly-chromate species.28 For samples containing 3 and 5 wt% of chromium also a band at ca. 600 nm is formed. Moreover, the intensity of the band at 600 nm is higher when CrO3 is applied as the source of metal for the impregnation. The band mentioned is usually assigned in literature to Cr3+ in α-Cr2O3 particles.28,30 The presence of this species can be additionally testified by the color of the samples (yellow-green for 3 wt% of Cr and green or dark green for 5 wt% of Cr). For the lower concentration of chromium (1 wt%), the XRD peaks do not show the presence of α-Cr2O3, which is in line with UV-Vis results.
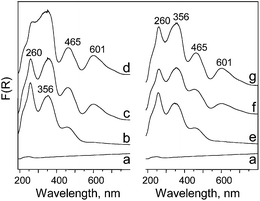 | ||
| Fig. 4 UV-Vis spectra of: (a) MCM-41; (b) 1Cr(o)/MCM-41; (c) 3Cr(o)/MCM-41; (d) 5Cr(o)/MCM-41; (e) 1Cr(n)/MCM-41; (f) 3Cr(n)/MCM-41; (g) 5Cr(n)/MCM-41. | ||
Fig. 5 presents the H2-TPR profiles of MCM-41 samples containing chromium. For all catalysts one main maximum on H2-TPR profile is observed and according to literature this peak can be assigned to the reduction of Cr6+ species.31 The temperature of Cr6+ reduction depends on metal loading. For both chromium sources applied, the increase of metal loading from 1 wt% to 3 wt% leads to an increase in Cr6+ reducibility. This is in agreement with the formation of lager aggregates, i.e. polychromate species that show lower temperature of reduction.30 However, for samples with 5 wt% of chromium loading the temperature of Cr6+ reduction is higher than for those with 3 wt% of chromium. This fact could be correlated with a decrease in polychromate species in the sample. The latter species decomposes forming α-Cr2O3 observed in XRD patterns (Fig. 3). For the samples that show the presence of α-Cr2O3 (3 wt% and 5 wt% of chromium) a small reduction peak is also visible at ca. 550 K. This peak is the most intense for the highest Cr loading. According to literature, it can be assigned to the reduction of Cr6+ species dispersed on α-Cr2O3.31 Moreover, the amount of hydrogen consumption differs depending on the metal loading and this dissimilarity is not proportional to the chromium content. The highest H2 consumption is observed for 3 wt% of Cr loading independently of chromium source applied for the MCM-41 impregnation. This observation is consistent with XRD and UV-Vis measurements that show the formation of α-Cr2O3 phase especially in samples 5Cr(o)/MCM-41 and 5Cr(n)/MCM. Thus the increase in chromium concentration from 1 wt% to 3 wt% is accompanied by increasing concentration of Cr6+ in the samples, whereas a further increase in the metal content leads mainly to the formation of α-Cr2O3 and limits the amount of different kinds of chromates species (Cr6+) inside or outside the pores of material.
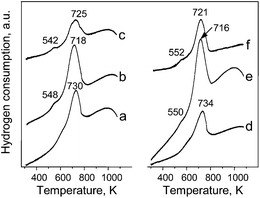 | ||
| Fig. 5 H2-TPR profiles of: (a) 1Cr(o)/MCM-41; (b) 3Cr(o)/MCM-41; (c) 5Cr(o)/MCM-41; (d) 1Cr(n)/MCM-41; (e) 3Cr(n)/MCM-41; (f) 5Cr(n)/MCM-41. | ||
Catalytic test reactions
As demonstrated above, the highest amount of Cr6+ species is present on the samples containing 3 wt% of chromium. However, for methanol oxidation process not only the amount of active species is important but also their kind should be taken into account. In this context, the acid–base properties of the catalysts are very important because both kinds of centers take part in the formation of products in partial oxidation of methanol. Two different test reactions were applied for the characterization of acidic and basic centers, i.e. cyclization and dehydration of 2,5-hexanedione (2,5-DHN) and 2-propanol decomposition.All catalysts prepared were tested in cyclization and dehydration of 2,5-hexanedione (2,5-DHN) after activation of the samples at 623 K. The cyclization of 2,5-DHN is a well-known reaction for determination of basicity/acidity of materials and was proposed by Dessau4 and Alcaraz et al.5 as Brønsted acid–base test. The formation of 2,5-dimethylfuran (DMF) occurs on acidic centers, whereas basic centers take part in the production of 3-methyl-2-cyclopentenone (MCP). The ratio of MCF to DMF can be taken as an indicator of acid (<1), basic (>1) or acid-basic properties (∼1).
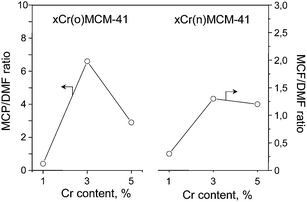
The results obtained in cyclization and dehydration of 2,5-DHN are presented in Fig. 6 as the MCP/DMF ratio vs. chromium loading in the samples. The samples containing the lowest concentration of Cr species (evidenced on material surface mainly as monochromate ones) show acidic character (MCP/DMF < 1). The impregnation of MCM-41 using higher amount that 1 wt% of Cr species results in an increase in the MCP/DMF ratio. For 3 wt% of metal loading (most pronounced for 3Cr(o)/MCM-41) the MCP/DMF value is the highest, however the introduction of 5 wt% of chromium decreases the basicity of samples. On the basis of these results one can propose to assign the origin of moderate basicity in the materials prepared to the presence of di- and polychromate species, which dominate for the samples with 3 wt% of chromium loading.
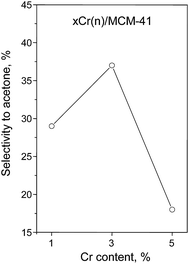
The acid–basic properties of materials prepared were also examined in 2-propanol decomposition. In general, propene and diisopropyl ether are formed on acid sites (or on the acid–base pairs), whereas the formation of acetone requires the presence of basic (or redox) centers.6,7 The materials obtained using CrO3 as a metal precursor (xCr(o)/MCM-41) showed negligible activity in the test reaction carried out at 573 K, which was not the case for the catalyst prepared using the other Cr precursor. Therefore in Fig. 7 the selectivity to acetone vs. chromium concentration is presented only for xCr(n)/MCM-41 samples. It can be observed that the profile presented in Fig. 7 shows a volcanic shape (with the maximum for 3 wt% of Cr) similar as for cyclization and dehydration of 2,5-DHN (Fig. 6). These results confirm the moderate basic or redox character of di- and polychromate species on MCM-41 surface.
Methanol oxidation
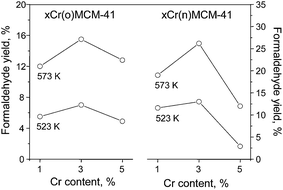
As reported in literature, e.g. ref. 9 and 10 acidic and basic centers play a key role in the oxidation of methanol by solid catalysts. The first step in methanol oxidation is suggested to be the abstraction of hydrogen from methanol leading to methoxy groups.9 The further transformation of adsorbed species depends on the kind and strength of active centers, as reported earlier.10 In the presence of acidic centers only methoxy groups interact with a second CH3OH molecule to form dimethyl ether. On the other hand, the presence of strong basic centers leads to the total oxidation. The partial oxidation products are determined by the presence of moderate acidic and basic centers.Table 3 shows the results of methanol oxidation carried out at 523 and 573 K. Unsupported MCM-41 was not active in this reaction. The main product obtained on chromium containing samples is formaldehyde (FA), whose formation demands the presence of moderate acidic and basic centers (the side product detected are methyl formate and CO2). The selectivity to FA does not change systematically with the chromium loading and seems to depend in some extent on a source of chromium. For materials modified with CrO3 the highest loading leads to the lowest selectivity to formaldehyde. This can be correlate with Cr2O3 formation leading to total methanol oxidation. Indeed, 5Cr(o)/MCM-41 sample shows the highest selectivity to CO2 among all catalysts at both reaction temperatures. In case of Cr(NO3)3 applied for MCM-41 modification the selectivity to FA first decrease a little and then does not change significantly. However, the higher amount of polychromates species deduced for samples containing 3 wt% of chromium (UV-Vis, H2-TPR) reflects in the increase of methanol conversion. This in consequence influences the amount of FA formed. The yield of FA depends on the chromium loading as demonstrated in Fig. 8. However, one should take into account that the acid–base properties of catalysts also depend on chromium loading. Irrespectively of the chromium source used for MCM-41 impregnation, as well as the reaction temperature applied, the volcanic shapes of the profiles in Fig. 8 are the same with the maximum for 3 wt% of chromium. The formation of FA requires rather weak acidity (necessary for methanol adsorption and formation of surface methoxy species) and moderate basicity (abstraction of hydrogen from methoxy species). Moreover, the week acidity enhances desorption of formaldehyde from materials surface (a similar effect is observed with increasing reaction temperature). Therefore, the results presented in Fig. 8 suggest the important role of di- and polychromate species (characterized as moderate basic) that participate in partial methanol oxidation process. The nucleophilic character of oxygen linking chromium atoms (Cr–O–Cr) could explain the highest activity of samples containing 3 wt% of chromium. These species are probably responsible for hydrogen abstraction from methoxy species adsorbed on Cr6+ (Lewis acid centers). Therefore, the amount and relative concentration of monochromate, di- and polychromate as well as α-Cr2O3 species is crucial for the results obtained in the methanol partial oxidation process. The relative content of each above-mentioned species can be measured and therefore controlled by the test reactions for acid–base properties and physico-chemical analyses. In consequence the knowledge of the amount, types and properties of active species permits anticipation and design of new catalytic systems.
| Catalyst | Methanol conversion (%) | Selectivity (%) | ||||
|---|---|---|---|---|---|---|
| (CH3)2O | HCHO | (CH3O)2CH2 | HCOOCH3 | CO2 | ||
| Reaction temperature: 523 K | ||||||
| 1Cr(o)/MCM-41 | 7 | Traces | 79 | Traces | 12 | 9 |
| 3Cr(o)/MCM-41 | 9 | Traces | 78 | Traces | 12 | 10 |
| 5Cr(o)/MCM-41 | 7 | Traces | 70 | Traces | 11 | 19 |
| 1Cr(n)/MCM-41 | 14 | Traces | 83 | Traces | 6 | 11 |
| 3Cr(n)/MCM-41 | 18 | Traces | 72 | Traces | 15 | 13 |
| 5Cr(n)/MCM-41 | 4 | Traces | 72 | Traces | 14 | 13 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Reaction temperature: 573 K | ||||||
| 1Cr(o)/MCM-41 | 18 | Traces | 67 | Traces | 7 | 25 |
| 3Cr(o)/MCM-41 | 21 | Traces | 74 | Traces | 8 | 18 |
| 5Cr(o)/MCM-41 | 22 | Traces | 58 | Traces | 8 | 34 |
| 1Cr(n)/MCM-41 | 26 | Traces | 73 | Traces | 8 | 19 |
| 3Cr(n)/MCM-41 | 38 | Traces | 69 | Traces | 6 | 25 |
| 5Cr(n)/MCM-41 | 18 | Traces | 67 | Traces | 11 | 21 |
Conclusions
• CrO3 is the primary chromium species formed on MCM-41 impregnated with CrO3 or Cr(NO3)2.• The increase in chromium loading on MCM-41 results in an increase in di- and polychromate species and later α-Cr2O3 irrespective of chromium source used for modification.
• Higher number of polychromate species are obtained using Cr(NO3)2 than CrO3 as the precursor, however these species show similar reducibility for the same Cr loading.
• Reducibility of chromium species is influenced by Cr loading – the highest reducibility is observed for the catalysts with 3 wt% of chromium.
• Polychromate species are responsible for basicity of the catalyst tested in 2-propanol decomposition and 2,5-hexanedione transformation. The basic character of the solid is the highest for materials with 3 wt% of chromium loading.
• There is a clear relationship between the basicity of the catalyst surface, reducibility of chromium species and redox properties manifested by the formaldehyde yield in methanol oxidation.
Acknowledgements
National Science Centre in Poland is acknowledged for partial support (project no. 2011/01/B/ST5/00847).Notes and references
- P. T. Anastas and J. C. Warner, in Green Chemistry: Theory and Practice, Oxford University Pres, New York, 1998, p. 30 Search PubMed.
- A. J. Foster and R. F. Lobo, Chem. Soc. Rev., 2010, 39, 4783 RSC.
- S. Rousseau, O. Marie, P. Bazin, M. Daturi, S. Verdier and V. Harle, J. Am. Chem. Soc., 2010, 132, 10832 CrossRef CAS PubMed.
- R. M. Dessau, Zeolites, 1990, 10, 205 CrossRef CAS.
- J. J. Alcaraz, B. J. Arena, R. D. Gillespie and J. S. Holmgren, Catal. Today, 1998, 43, 89 CrossRef CAS.
- A. Gervasini, J. Fenyvesi and A. Auroux, Catal. Lett., 1997, 43, 219 CrossRef CAS.
- C. Lahousse, J. Bachelier, J. C. Lavalley, H. Lauron-Pernot and A. M. Le Govic, J. Mol. Catal., 1994, 87, 329 CrossRef CAS.
- C. H. Bartholomew and R. J. Farrauto, in Fundamentals of Industrial Catalytic Processes, John Wiley & Sons. Inc., 2nd edn, 2005, ch. 8, pp. 584–597 Search PubMed.
- J. M. Tatibouet, Appl. Catal., A, 1997, 148, 213 CrossRef.
- G. Busca, A. S. Elmi and P. Forzatti, J. Phys. Chem., 1987, 91, 5263 CrossRef CAS.
- G. Busca, J. Lamotte, J. C. Lavalley and V. Lorenzelli, J. Am. Chem. Soc., 1987, 109, 5197 CrossRef CAS.
- L. J. Burcham and I. E. Wachs, Catal. Today, 1999, 49, 467 CrossRef CAS.
- X. Gao, I. E. Wachs, M. S. Wong and J. Y. Ying, J. Catal., 2001, 203, 18 CrossRef CAS.
- T. Kim and I. E. Wachs, J. Catal., 2008, 255, 197 CrossRef CAS PubMed.
- M. Muhler, Handbook of Heterogeneous Catalysis, ed. G. Ertl, H. Knozinger and J. Weitkamp, VCH, 1997 Search PubMed.
- G. Centi and S. Perathoner, in Selective oxidation – Industrial, in Encyclopedia of Catalysis, ed. I. Horvath, Wiley – Interscience: Hoboken, New York, 2003 Search PubMed.
- M. Ziolek, P. Decyk, I. Sobczak, M. Trejda, J. Florek, H. Golinska, W. Klimas and A. Wojtaszek, Appl. Catal., A, 2011, 391, 194 CrossRef CAS PubMed.
- M. Trejda, M. Ziolek, Y. Millot, K. Chalupka, M. Che and S. Dzwigaj, J. Catal., 2011, 281, 169 CrossRef CAS PubMed.
- M. Badlani and I. E. Wachs, Catal. Lett., 2001, 75, 137 CrossRef CAS.
- M. Trejda, J. Kujawa and M. Ziolek, Catal. Lett., 2006, 108, 141 CrossRef CAS PubMed.
- P. Decyk, M. Trejda, M. Ziolek and A. Lewandowska, Stud. Surf. Sci. Catal., 2002, 142, 1785 CrossRef.
- P. Decyk, M. Trejda and M. Ziolek, C. R. Chim., 2005, 8, 635 CrossRef CAS PubMed.
- S. C. Laha and R. Glaser, Microporous Mesoporous Mater., 2007, 99, 159 CrossRef CAS PubMed.
- M. Trejda, A. Wojtaszek, M. Ziolek and J. Kujawa, Appl. Catal., A, 2009, 365, 135 CrossRef CAS PubMed.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS.
- K. S. W. Sing, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- M. Kruk and M. Jaroniec, Chem. Mater., 1999, 11, 492 CrossRef CAS.
- B. M. Weckhuysen, A. A. Verberckmoes, A. R. De Baets and R. A. Schoonheydt, J. Catal., 1997, 166, 160 CrossRef CAS.
- H. Yamashita, K. Yoshizawa, M. Ariyuki, S. Higashimoto, M. Che and M. Anpo, Chem. Commun., 2001, 435 RSC.
- V. Elias, E. Sabre, K. Sapag, S. Casuscelli and G. Eimer, Appl. Catal., A, 2012, 413–414, 280 CrossRef CAS PubMed.
- A. Hakuli, M. E. Harlin, L. B. Backman and A. O. I. Krause, J. Catal., 1999, 184, 349 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |