Augmentation of properties on sparingly loaded nanocomposites via functionalized single-walled carbon nanotubes using a covalent approach†
Abstract
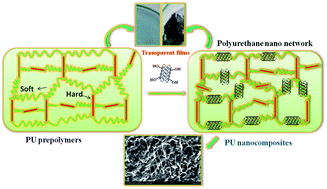
Polymer nanocomposites are developed, for the first time, as transparent films by the covalent addition of polyurethane (PU) prepolymers to trace amounts of functionalized carbon nanotubes, [OH]n–SWCNTs, via an efficient route using mild reagents. These PU nanocomposites, which were uniformly distributed with SWCNTs via covalent bonding between SWCNTs and the polyurethane network show enhanced mechanical, thermal and conductivity (10−4 S cm−1) properties.

 Please wait while we load your content...
Please wait while we load your content...