Analytical-scale purification of trichostatin A from bacterial culture in a single step and with high selectivity using immobilised metal affinity chromatography
Najwa
Ejje
a,
Ernest
Lacey
b and
Rachel
Codd
*a
aSchool of Medical Sciences (Pharmacology) and Bosch Institute, The University of Sydney, New South Wales 2006, Australia. E-mail: rachel.codd@sydney.edu.au; Fax: 61 2 9351 4717; Tel: 61 2 9351 6738
bMicrobial Screening Technologies, Building A, 28-54 Percival Road, Smithfield, New South Wales 2164, Australia. Fax: 61 2 9757 2586; Tel: 61 2 9757 4515
First published on 8th November 2011
Abstract
The potent histone deacetylase inhibitor trichostatin A (TSA) has been captured in a single step with high selectivity from the culture supernatant of the native TSA-producing strain Streptomyces hygroscopicusMST-AS5346 using analytical-scale Ni(II)-based immobilised metal affinity chromatography.
Introduction
R-(+)-Trichostatin A (TSA, 1, Fig. 1) is a natural hydroxamic acid-based antibiotic first discovered from cultures of Streptomyces hygroscopicusY-50.1 Other Streptomyces species, including S. platensis and S. sioyaensis, also produce TSA.2 As characteristic of hydroxamic acids, TSA has an affinity towards Fe(III) and other transition metal ions.3 The red-purple complex Fe(TSA(1–))3 (named trichostatin B) was formed from trace amounts of Fe(III) present in the S. hygroscopicusY-50 culture medium and isolated viachloroform extraction.1 Analogues of TSA that feature β-glucosyl (trichostatin C, 2) or α-glucosyl (trichostatin D, 3) units substituted at the NH–O motif of the parent scaffold have been isolated.4,5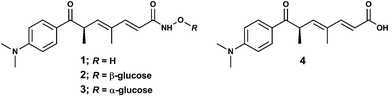 | ||
| Fig. 1 R-(+)-Trichostatin A (1), trichostatin C (2), trichostatin D (3) and trichostatic acid (4). | ||
The ability to coordinate metal ions confers therapeutic value upon hydroxamic acids as inhibitors of metalloenzymes and as modulators of conditions involving metal ion dyshomeostasis.3,6 The mesylate salt of the trihydroxamic acid desferrioxamine B, an Fe(III)-sequestering compound (siderophore) native to Streptomyces pilosus, is used for the treatment of iron overload therapy, which occurs as a secondary complication of transfusion-dependent haemoglobin disorders.7,8 The most recent use of a hydroxamic acid in the clinic is suberoylanilide hydroxamic acid (SAHA, Vorinostat) for cutaneous T-cell lymphoma. SAHA and TSA are potent inhibitors of the histone deacetylases (HDACs), a class of Zn(II)-containing enzymes under scrutiny as oncology drug targets.9–13 The activity of HDACs and the histone acetyltransferases (HATs) controls the acetylation status of lysine residues in nucleosomal histones in chromatin. Increased HDAC activity causes an excess of unmasked lysine residues on the histone protein, resulting in higher concentrations of condensed chromatin and transcriptional repression and gene silencing.
The total synthesis of R-(+)-TSA has been reported in 99% e.e.14 Other methods have described the synthesis of rac-(±)-TSA.15–18 The number of steps in early synthetic work ranged from nine to 18 and provided modest yields of product (6%, 17%).15,16 Synthetic efficiency towards rac-(±)-TSA17,18 and trichostatic acid as a precursor19 has been improved in more recent methods. As one of the most potent in vitro HDAC inhibitors ever documented (IC50 ∼5 nM against the HDAC1 isoform),20 ready access to TSA is important for oncology drug development programs and for continued studies of its potential in other disease states, such as has been shown recently in Niemann-Pick disease type C,21 cardiac hypertrophy,22 cystic fibrosis23 and polyglutamine toxicity.24 Methods able to resolve mixtures of trichostatin derivatives (Fig. 1) are also sought, since TSC, TSD and trichostatic acid have each been shown to induce differentiation of Friend leukaemia cells and could have potential as anti-cancer agents.5,25,26 Improved access to TSA and congeners from bacterial culture requires methodological advances that are selective, require few steps and minimise the use of organic solvents.
Here, we describe the analytical-scale capture of TSA in a single step and with high selectivity from the crude bacterial culture of S. hygroscopicusMST-AS5346 on a Ni(II)-loaded immobilised metal affinity chromatography (IMAC) resin. We demonstrate the resolution of TSA from TSC and trichostatic acid from the culture supernatant, which contains at least 150–200 components. The results underscore the potential for IMAC as a green chemistry platform for the streamlined capture and resolution of selected high-value bacterial secondary metabolites as potential new anti-cancer and anti-infective agents.
Results and discussion
The binding capacity of a Ni(II)-loaded IMAC resin towards pure TSA (Sigma, >98%, from Streptomyces sp.) was first investigated. A 1-mL aliquot of TSA (500 nmol) in basified water (1 μM NaOH, pH 8) was sorbed onto a column (i.d. 0.7 cm × 2.5 cm) containing 1 mL of Ni(II)-loaded IMAC resin that had been preconditioned.27 After TSA loading, the column was washed with 10 column volumes (CV) of basified water (1 μM NaOH, pH 8) and 6 CV of low-pH elution buffer (20 mM Na2HPO4/NaH2PO4, 0.5 M NaCl, pH 6.5) and 1-mL fractions were collected. An aliquot of Fe(ClO4)3 in 0.2 M HClO4 (100 μL, 10 mM) was added to a 200-μL subsample of each fraction and after 10 min, the absorbance value of the solution was measured at λ = 450 nm using an Amersham Biosciences Biotrak™ II Visible Plate Reader. Standard solutions of Fe(III) and TSA (5 μM–350 μM) were prepared and analysed at λ = 450 nm.The binding capacity of Ni(II)-loaded IMAC resin towards a pure sample of TSA was >0.5 μmol mL−1, with the recovery ≥95% (Fig. 2a). The binding capacity of this resin with respect to desferrioxamine B has been shown to be 3 μmol mL−1.28 As typical for hydroxamic acids, TSA is a weak acid (N–OH, pKa ∼9.0)3,29 and will act as a monoanionic hydroxamate ligand under the conditions of the Ni(II)-based IMAC procedure (pH 8). Elution of neutral TSA from the column was achieved by decreasing the pH value of the elution buffer to pH 6.5.
![Processing pure TSA (a); or S. hygroscopicusMST-AS5346 culture supernatant (b), using Ni(ii)-based IMAC. Iron(iii) responsive components were detected and the values normalised to standard solutions of Fe(iii) and TSA. Step elution conditions (dotted line) involved reducing the pH value (a) or increasing [imidazole] (b).](/image/article/2012/RA/c1ra00864a/c1ra00864a-f2.gif) | ||
| Fig. 2 Processing pure TSA (a); or S. hygroscopicusMST-AS5346 culture supernatant (b), using Ni(II)-based IMAC. Iron(III) responsive components were detected and the values normalised to standard solutions of Fe(III) and TSA. Step elution conditions (dotted line) involved reducing the pH value (a) or increasing [imidazole] (b). | ||
The promise of the high-yielding capture of pure TSA prompted us to examine the use of Ni(II)-based IMAC to capture native TSA from the culture supernatant of S. hygroscopicusMST-AS5346. This strain is used for the commercial supply of TSA and TSC (BioAustralis). The 1.5-mL aliquot of bacterial culture supernatant that was processed contained about 52 nmol of TSA.30 In the bacterial system, components bound to the Ni(II)-based IMAC resin were eluted in 0.5-mL fractions using an imidazole buffer, rather than using the low-pH regimen, since it was reasoned that the acid conditions might affect the integrity of selected bacterial metabolites. In this experiment, the intensity of the unbound peak was significantly greater than the peak representing the bound components (Fig. 2b) and indicated that about 20% of TSA was retained on the resin. The secondary metabolites and components of the bacteriological medium in the S. hygroscopicusMST-AS5346 culture appeared to reduce the TSA-binding capacity of the Ni(II)-based IMAC resin via competition and other mechanisms.
The identities of TSA and other components in the culture extracts were confirmed using LC-PDA-MS measurements (positive-ion mode ESI-MS) from standard solutions.31 The chromatogram from a solution of pure TSA (0.85 nmol, 2 μL injection volume) showed a single, well-resolved peak at tR 23.5 min (Fig. 3a).
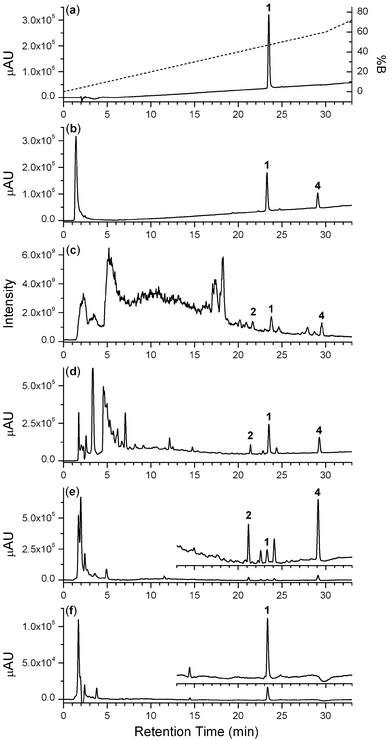 | ||
| Fig. 3 Chromatograms from solutions of pure TSA (a); Fe(III) and TSA (b); crude bacterial culture supernatant ((c), (d)); unbound fraction (fraction 7) from Ni(II)-based IMAC (e); or bound fraction (fraction 42) from Ni(II)-based IMAC (f), displayed in total scan (200–320 nm) photodiode array ((a)–(b), (d)–(f)) or total ion current (positive-ion ESI-MS) (c) detection mode. The gradient in (a) was used in (b)–(f), but has been omitted for clarity. | ||
The trace from a solution of Fe(III) and TSA (Fig. 3b) showed three major peaks, which corresponded with free Fe(III) (tR 1.42 min), which was present in excess, TSA (tR 23.3 min, 1), and trichostatic acid (tR 29.1 min, 4). The acidic Fe(ClO4)3 solution promoted the formation of trichostatic acid (4, Fig. 1) from TSA in a fashion similar to the acid-catalysed formation of acetic acid from acetohydroxamic acid.32,33 The peaks at tR 23.5 min or tR 29.1 min gave m/zobs 303.04 or m/zobs 288.03, respectively, which corresponded with the m/zcalc values for TSA (M + H+, m/zcalc 303.37) or trichostatic acid (M + H+, m/zcalc 288.35) (Fig. 4a and 4b, respectively). No peaks in the solution of Fe(III) and TSA were ascribable to Fe(TSA(1–))3 (trichostatin B) or to Fe(III)-TSA complexes of different stoichiometries. The acidic pH appeared to mitigate against the formation of Fe(III)-TSA complexes.
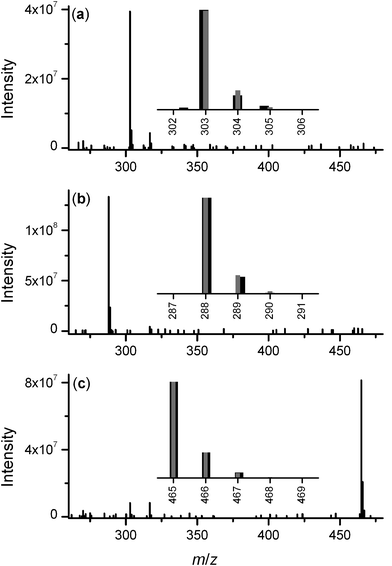 | ||
| Fig. 4 Mass spectra (positive-ion mode) from LC-MS measurements of TSA (a); trichostatic acid (b) and TSC (c) resolved from S. hygroscopicusMST-AS5346 culture supernatant using Ni(II)-based IMAC. Experimental (black) and calculated (grey) isotope patterns are shown as insets. | ||
The complexity of the S. hygroscopicusMST-AS5346 culture supernatant was evident from the LC-MS total ion current (TIC) trace (Fig. 3c). A conservative estimate would have at least 150–200 MS-active components present. The peaks at tR 23.8 min and tR 29.5 min gave m/zobs values that were consistent with TSA and trichostatic acid, respectively. Trichostatic acid is both a precursor for the biosynthesis of TSA and a hydrolysis product,4,14–17,19 and its presence in the culture supernatant was not unexpected. The peak at tR 21.6 min gave m/zobs 465.01, which corresponded with the presence of trichostatin C (2, Fig. 1) (M + H+, m/zcalc 465.51) (Table 1, Fig. 4c). No other signal with m/z 465 was observed, which indicated that there was no TSD present. Several strains of Streptomyces, including S. hygroscopicusMST-AS5346 and S. platensis 145 are known to co-produce TSA and TSC.9
| [M + H+]+, m/z | ||||
|---|---|---|---|---|
| t r (min) | Calculated | Observed | clogP | |
| 1 | 23.3–23.8 | 303.37 | 303.04 | 2.05 |
| 2 | 21.2–21.6 | 465.51 | 465.01 | 0.91 |
| 4 | 29.1–29.5 | 288.35 | 288.03 | 3.26 |
As would be expected, the chromatogram from the S. hygroscopicusMST-AS5346 culture supernatant using the PDA detection mode (Fig. 3d) showed fewer peaks than the TIC mode. There were at least 50 UV-active species present in the mixture. Peaks at tR 21.4 min, tR 23.5 min and tR 29.3 min gave m/zobs values consistent with TSC, TSA and trichostatic acid, respectively, and the UV traces were consistent with spectra from available standards (TSC, BioAustralis). The order of elution of the trichostatin derivatives from a C18 column operated under reverse-phase conditions at acid pH values where TSA, TSC and trichostatic acid would be charge neutral correlated with the clogP values of the components (Table 1).
One fraction (fraction 7) from the culture that was not retained on the Ni(II)-based IMAC resin (Fig. 3e) and one fraction (fraction 42) that was retained and subsequently eluted from the resin (Fig. 3f) was analysed by LC-PDA-MS. The presence of TSC, TSA and trichostatic acid in the unbound fraction was evident from the peaks at tR 21.2 min, tR 23.3 min and tR 29.1 min, respectively; each peak gave m/z values consistent with the assignment. Compared to the relative concentrations of the trichostatin derivatives in the crude supernatant ([TSA] > [trichostatic acid] > [TSC]) (Fig. 3d), the unbound fraction was enriched with trichostatic acid and TSC and depleted of TSA ([trichostatic acid] > [TSC] > [TSA]) (Fig. 3e). Consistent with this observation, was the presence of TSA in the fraction eluted from the Ni(II)-based IMAC column (Fig. 3f). In this fraction, there were no detectable levels of TSC or trichostatic acid, which demonstrated the high selectivity of Ni(II)-based IMAC towards TSA to >95% purity and its effectiveness in resolving TSA from TSC and trichostatic acid. A minor species at tR 14.5 min (m/zobs 432.89) was present in this fraction.
The estimated retention of 20% of native TSA from the bacterial culture on the resin as determined by the Fe(III) addition assay was conservative, since components other than TSA in the unbound fraction could form complexes with Fe(III) and contribute to the intensity of this peak. The isoflavone genistein could be a candidate, since this endogenous S. hygroscopicusMST-AS5346 metabolite has been shown to coordinate Fe(III).34Genistein and analogues would have a low affinity towards Ni(II) and would be expected to appear in the unbound fraction from an Ni(II)-based IMAC procedure. A more accurate estimate of the TSA retained on the resin was obtained from the areas of the peaks from the LC-MS data, which indicated that about 45% of TSA (24 nmol from the 52 nmol that was processed) was retained on the resin.35 The complete resolution of TSA from TSC and trichostatic acid could be achieved by using a larger volume of resin or a second cycle of the IMAC procedure.
The resolution behaviour of the trichostatin derivatives correlated with the availability of the hydroxamic acid motif to bind to the vacant coordination sites of the immobilised iminodiacetic acid–Ni(II) complex. This is akin to the more traditional use of IMAC resin for the purification of histidine-tagged recombinant proteins. TSA was retained on the Ni(II)-based IMAC resin via coordination with the single-deprotonated hydroxamate group at pH 8. In contrast, the capped NH–O–β–glucosyl unit of TSC prevented coordination. The carboxylic acid-based trichostatic acid was unable to coordinate to the resin and together with TSC, passed through as unbound species (Fig. 5).
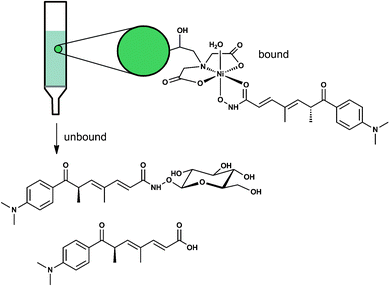 | ||
| Fig. 5 Trichostatin A was retained on a Ni(II)-based IMAC column (pH 8). Trichostatin C and trichostatic acid were not bound. | ||
Early reports of the isolation of trichostatin derivatives from bacterial culture described multi-step procedures involving ethyl acetate extraction of the culture, in vacuoevaporation, silica chromatography with chloroform–methanol, in vacuoevaporation, and HPLC.25 Other steps, including acetone extraction, 1-butanol-water partitioning, XAD-7 and LH-20 chromatography, were used in different purification protocols.1,4,5,36 The two key advantages of Ni(II)-based IMAC for the resolution of trichostatin derivatives described here is that the method shows high selectivity towards TSA in only a single step; and the process is aqueous-based and does not require the use of organic solvents during isolation.
Conclusions
The potent histone deacetylase inhibitor trichostatin A (TSA) has been captured in a single step from the culture supernatant of the native TSA-producing strain Streptomyces hygroscopicusMST-AS5346 using analytical-scale Ni(II)-based immobilised metal affinity chromatography (IMAC). The method shows high selectivity towards TSA from a complex bacteriological mixture of at least 150–200 components. The NH–O–glycosylated derivative trichostatin C and trichostatic acid were not retained on the resin and were identified in the unbound fraction from LC-PDA-MS measurements. The resolution of TSA and congeners using analytical-scale IMAC may have potential for the industrial-scale capture of these bioactives direct from culture.The ability to directly process aqueous bacterial culture supernatants viaIMAC without pre-treatment or extraction with organic solvents marks an advance in green chemistry techniques for accessing high-value bacterial metabolites. This technique has significant potential to value-add to metabolomics programs and for expediting drug discovery from Nature.
Acknowledgements
Funding from The University of Sydney (Bridging Support 2009 and Faculty of Medicine Strategic Research Fund 2009 to R.C.; University Postgraduate Award co-funded to N.E.) is gratefully acknowledged. Ms Vivian Liao is acknowledged for useful discussions.References
- N. Tsuji, M. Kobayashi, K. Nagashima, Y. Wakisaka and K. Koizumi, J. Antibiot., 1976, 29, 1–6 CAS.
- R. Codd, N. Braich, J. Liu, C. Z. Soe and A. A. H. Pakchung, Int. J. Biochem. Cell Biol., 2009, 41, 736–739 CrossRef CAS.
- R. Codd, Coord. Chem. Rev., 2008, 252, 1387–1408 CrossRef.
- N. Tsuji and M. Kobayashi, J. Antibiot., 1978, 31, 939–944 CAS.
- Y. Hayakawa, M. Nakai, K. Furihata, K. Shin-Ya and H. Seto, J. Antibiot., 2000, 53, 179–183 CAS.
- C. J. Marmion, D. Griffith and K. B. Nolan, Eur. J. Inorg. Chem., 2004, 3003–3016 CrossRef CAS.
- D. J. Weatherall, Blood, 2010, 115, 4331–4336 CrossRef CAS.
- J. Liu, D. Obando, L. G. Schipanski, L. K. Groebler, P. K. Witting, D. S. Kalinowski, D. R. Richardson and R. Codd, J. Med. Chem., 2010, 53, 1370–1382 CrossRef CAS.
- M. Yoshida, S. Nomura and T. Beppu, Cancer Res., 1987, 47, 3688–3691 CAS.
- M. Yoshida, M. Kijima, M. Akita and T. Beppu, J. Biol. Chem., 1990, 265, 17174–17179 CAS.
- C. Monneret, Eur. J. Med. Chem., 2005, 40, 1–13 CrossRef CAS.
- S. Minucci and P. G. Pelicci, Nat. Rev. Cancer, 2006, 6, 38–51 CrossRef CAS.
- T. Liu, S. Kuljaca, A. Tee and G. M. Marshall, Cancer Treat. Rev., 2006, 32, 157–165 CrossRef CAS.
- S. Zhang, W. Duan and W. Wang, Adv. Synth. Catal., 2006, 348, 1228–1234 CrossRef CAS.
- I. Fleming and J. Iqbal, Tetrahedron, 1983, 39, 841–846 CrossRef CAS.
- K. Mori and K. Koseki, Tetrahedron, 1988, 44, 6013–6020 CrossRef CAS.
- A. Chatterjee, J. Richer, T. Hulett, V. B. R. Iska, O. Wiest and P. Helquist, Org. Lett., 2010, 12, 832–834 CrossRef CAS.
- C. C. Cosner and P. Helquist, Org. Lett., 2011, 13, 3564–3567 CAS.
- J. T. Markiewicz, D. J. Schauer, J. Löfstedt, S. J. Corden, O. Wiest and P. Helquist, J. Org. Chem., 2010, 75, 2061–2064 CrossRef CAS.
- S. H. Woo, S. Frechette, E. A. Khalil, G. Bouchain, A. Vaisburg, N. Bernstein, O. Moradei, S. Leit, M. Allan, M. Fournel, M.-C. Trachy-Bourget, Z. Li, J. M. Besterman and D. Delorme, J. Med. Chem., 2002, 45, 2877–2885 CrossRef CAS.
- N. H. Pipalia, C. C. Cosner, A. Huang, A. Chatterjee, P. Bourbon, N. Farley, P. Helquist, O. Wiest and F. R. Maxfield, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 5620–5625 CrossRef CAS.
- D. J. Cao, Z. V. Wang, P. K. Battiprolu, N. Jiang, C. R. Morales, Y. Kong, B. A. Rothermel, T. G. Gillette and J. A. Hill, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 4123–4128 CrossRef CAS.
- D. M. Hutt, D. Herman, A. P. C. Rodrigues, S. Noel, J. M. Pilewski, J. Matteson, B. Hoch, W. Kellner, J. W. Kelly, A. W. Schmidt, P. J. Thomas, Y. Matsumura, W. R. Skack, M. Gentzsch, J. R. Riordan, E. J. Sorscher, T. Okiyoneda, J. R. I. Yates, G. L. Lukacs, R. A. Frizzell, G. Manning, J. M. Gottesfeld and W. E. Balch, Nat. Chem. Biol., 2010, 6, 25–33 CrossRef CAS.
- A. McCampbell, A. A. Taye, L. Whitty, E. Penney, J. S. Steffan and K. H. Fischbeck, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 15179–15184 CrossRef CAS.
- M. Yoshida, Y. Iwamoto, T. Uozumi and T. Beppu, Agric. Biol. Chem., 1985, 49, 563–565 CrossRef CAS.
- H. Morioka, M. Ishihara, M. Takezawa, K. Hirayama, E. Suzuki, Y. Komoda and H. Shibai, Agric. Biol. Chem., 1985, 49, 1365–1370 CAS.
- Preconditioning: Five column volumes (CV) of imidazole buffer (0.5 M imidazole, 20 mM Na2HPO4/NaH2PO4, 0.5 M NaCl, pH 8), 25 CV of phosphate buffer (20 mM Na2HPO4/NaH2PO4, 0.5 M NaCl, pH 7) and 25 CV of basified water (1 μM NaOH, pH 8).
- N. Braich and R. Codd, Analyst, 2008, 133, 877–880 RSC.
- A. E. Fazary, J. Chem. Eng. Data, 2005, 50, 888–895 CrossRef CAS.
- A 50-mL volume of S. hygroscopicusMST-AS5346 culture supernatant grown at 28 °C for 4 d in ISP2 medium was lyophilised and resuspended in 7.5 mL of an aqueous solution of NaCl (0.5 M, pH 9). An aliquot of this solution (1.5 mL) was subject to processing using a Ni(II)-based IMAC resin, as described for the pure TSA system, with altered elution conditions (0.5-mL fractions, imidazole buffer: 0.5 M imidazole, 20 mM Na2HPO4/NaH2PO4, 0.5 M NaCl, pH 8).
-
LC-MS measurements: Thermo Separation System, P400 pump, UV6000LP photodiode array detector coupled to a Thermoquest Finnigan LCQ Deca ion trap MS (ESI) (m/z range, 150-2000). A Phenomenex Luna C18 column was used under the following conditions. A, H2O
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formic acid 100
formic acid 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1; B, acetonitrile
0.1; B, acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formic acid 100
formic acid 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1; 0% B to 60% B over 30 min, PDA 200-320 nm, injection volume, 2 μL, flow rate, 0.2 mL min−1.
0.1; 0% B to 60% B over 30 min, PDA 200-320 nm, injection volume, 2 μL, flow rate, 0.2 mL min−1. - K. K. Ghosh, Ind. J. Chem., Sect B., 1997, 36, 1989–1102.
- K. K. Ghosh, S. K. Patle, P. Sharma and S. K. Rajput, Bull. Chem. Soc. Jpn., 2003, 76, 283–290 CrossRef CAS.
- S. Dowling, F. Regan and H. Hughes, J. Inorg. Biochem., 2010, 104, 1091–1098 CrossRef CAS.
- The areas of the TSA peaks in the chromatograms from the unbound (Fig. 3e, 1620 μAU) or bound (Fig. 3f, 3430 μAU) fractions were referenced to the single data point of the corresponding fraction analysed by the Fe(III) addition assay (Fig. 2). The total TSA in the unbound fractions (2–5 mL) and the bound fractions (20–21 mL) was summed using these reference points to obtain the final estimated binding ratio. Based on the chromatogram from the solution of the TSA standard (Fig. 3a), about 23.6 nmol of TSA was retained on the resin, from a total of 51.5 nmol TSA in the 1.5-mL volume of culture that was processed.
- H. Morioka, M. Ishihara, M. Takezawa, H. Shibai and Y. Komoda, Agric. Biol. Chem., 1988, 52, 251–253 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
