Enhancement of afterglow in SrAl2O4:Eu2+ long-lasting phosphor with swift heavy ion irradiation
Tianzhuo
Zhan
a,
Chao-Nan
Xu
*abc,
Hiroshi
Yamada
ab,
Yujin
Terasawa
a,
Lin
Zhang
b,
Hiroshi
Iwase
d and
Masayoshi
Kawai
d
aInterdisciplinary Graduate School of Engineering Sciences, Kyushu University, 6-1, Kasugakoen, Kasuga, Fukuoka, 816-8580, Japan. E-mail: cn-xu@aist.go.jp
bNational Institute of Advanced Industrial Science and Technology (AIST), Kyushu, 807-1, Shuku, Tosu, Saga, 841-0052, Japan
cCREST, Japan Science and Technology Agency (JST), 4 Honcho, Kawaguchi, Saitama, 332-0012, Japan
dHigh Energy Accelerator Research Organization (KEK), 1-1 Oho, Tsukuba, Ibaraki, 305-0801, Japan
First published on 4th November 2011
Abstract
We present a novel approach for improving the afterglow of long-lasting phosphors with swift heavy ion irradiation. The afterglow of famous long-lasting phosphor SrAl2O4:Eu2+ was dramatically enhanced with swift heavy ion irradiation. Furthermore, it was found that the enhancement was dependent on the electronic stopping power as well as the irradiation fluence. Thermoluminescence analyses revealed that the SHI irradiation had no significant influence on the trap depth, but increased the trap density in the phosphor. In addition, the mechanism underlying afterglow enhancement phenomenon was discussed.
Introduction
Long-lasting phosphors (LLPs) emit bright light for a long time after exposure to daylight; this luminescence decay phenomenon is referred to as phosphorescence or afterglow.1 Afterglow has been used for decades in applications such as decorative items, luminous watches, emergency passageway illumination, exit signs, and traffic signs.1 Moreover, afterglow also shows great potential for novel applications such as fiber-optic thermometers,2 radiation detection,3 and in energy saving devices. The formerly most widely used LLP, ZnS:Cu, was very sensitive to moisture and the duration of its afterglow was quite short. Therefore, rare earth ion-doped alkali earth aluminate LLPs such as SrAl2O4:Eu2+ and its derivatives have been developed.4 Nevertheless, the afterglow intensity of the alkali earth aluminate LLPs still needs further improvement.The mechanism of afterglow is still not fully understood, however the afterglow process for LLPs is generally attributed to the thermal release of electrons and holes trapped in lattice defects (traps) and their recombination at the emission centers.5,6 The initial intensity and the decay time of the afterglow depend on the trap density and the trap depth, respectively. Thus, the formation of lattice defects suitable for afterglow is an effective strategy for improving the afterglow properties of LLPs. In phosphors, the formation of lattice defects is typically realized by chemical methods such as nonstoichiometry and the incorporation of auxiliary activators, which are typically rare earth ions.7–11 However, progress in improving the afterglow further by conventional chemical methods has been slow over the past decade. Therefore, there is a great need for new approaches to improve the afterglow of LLPs.
Swift heavy ion (SHI) irradiation has a wide variety of applications in many fields such as biology, medicine, physics, and material science.12–14 The intense energy deposition from SHI irradiation in the target material is several orders of magnitude higher than that of conventional ionizing radiation processes such as γ-rays and electrons. The resultant dense electronic excitation leads to defect formation along the trajectory of the incident ions. The formation of defects has been observed in many materials, including semiconductors, insulators and various metals.15–18 Therefore, SHI irradiation is a strong candidate for improving the afterglow of LLPs through the formation of suitable lattice defects.
We report the dramatic enhancement of the afterglow in the SrAl2O4:Eu2+ (SAOE) LLP with SHI irradiation. The effects of the electronic stopping power and the irradiation fluence on the afterglow properties are examined, and possible mechanisms underlying the phenomenon are discussed.
Experimental
Sample preparation
The SAOE phosphor powders were synthesized by a solid-state reaction.19 High purity SrCO3 (99.9%), Al2O3 (99.9%), Eu2O3 (99.9%) and a small amount of Ho2O3 (99.9%) (Kojundo Chemical Laboratory Co., Ltd.) were thoroughly mixed with ethanol and ground for 1 h. The mixture was calcined at 900 °C for 1 h in air. Then, the mixture was sintered at 1300 °C for 4 h in a reducing atmosphere (H2 + Ar). Pellet and film samples were fabricated by mixing the SAOE powders with optical epoxy resin in a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.20
1.20
SHI irradiation
The SHI irradiation was performed at room temperature at HIMAC (Chiba, Japan). The irradiation fluence was measured with an ionization chamber. The effects of the electronic stopping power on the afterglow property were investigated by exposing the SAOE pellet samples to the following different types of SHI irradiation:Fe (500 MeV/u), Si (100 MeV/u), C (430 MeV/u), N (430 MeV/u), Ne (600 MeV/u) and He (230 MeV/u). The irradiation fluence was 1.4 × 1012 ions/cm2. To evaluate the effects of the irradiation fluence on the afterglow property, the SAOE film samples were irradiated with Fe ions (500 MeV/u) with irradiation fluence of 4.4 × 1011, 9.2 × 1011, 4.4 × 1012, and 1.7 × 1013 ions/cm2, respectively. The powder samples were irradiated with Fe ions (500 MeV/u) at an irradiation fluence of 1.4 × 1012 ions/cm2 for the X-ray diffraction (XRD), photoluminescence (PL) and thermoluminescence (THL) measurements.Measurement
The afterglow of the SAOE samples was measured with a lab-made charge-coupled device (CCD) camera.21 The afterglow intensity decay curves of the samples were constructed using the grayscale value of the afterglow pictures and normalized. The afterglow pictures of the samples were recorded with the CCD camera after UV excitation was stopped. The crystal structure characterization of the Fe-irradiated and the non-irradiated samples were analyzed by XRD (Rint-2000; Rigaku Co.). A spectrofluorometer (FP6600, JASCO Co., Japan) equipped with a 150 W Xe lamp was used for the PL measurement. The measurements for the THL glow curves were obtained using a system consisting of a fluorescent spectrometer (FP6600, JASCO Co., Japan) and a lab-made temperature controlling unit. The THL glow curves were measured between 83 K and 500 K with a linear heating rate of 1 K s−1. The THL measurement was recorded after UV excitation under 83 K for 5 min to fill the traps with excited electrons.Results and discussion
Afterglow enhancement with irradiation of different ions
The effects of the electronic stopping power on the afterglow properties of the SAOE LLP were investigated using pellet samples. Fig. 1 shows the afterglow of the SAOE pellet samples (captured 30 s after UV excitation was stopped) and the afterglow decay curves of the non-irradiated and the SHI-irradiated areas on the pellet samples. The six different areas irradiated by Fe, Si, C, N, Ne, and He ions were brighter than the non-irradiated area, indicating that the afterglow was enhanced by the SHI irradiation. The shape of the afterglow decay curves were similar, but the afterglow intensity of the SHI-irradiated areas was stronger than that of the non-irradiated area; the afterglow intensity of the non-irradiated area 200 s after the UV (365 nm) excitation was stopped was ∼2, whereas that of the Si-irradiated area was ∼3. The decay time to a normalized intensity of 2 was increased from 200 s to 300 s after irradiation by Si ions. In addition, the afterglow enhancement was different for different ions; the heavier ions such as Si and Fe showed the greater enhancement, whereas the lighter ions such as He and C had a weaker effect.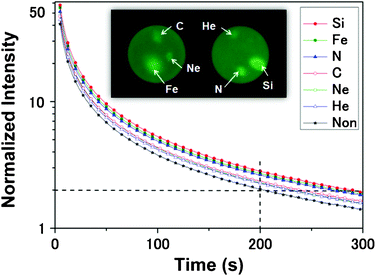 | ||
| Fig. 1 Afterglow of the SAOE pellet samples and the afterglow decay curves of the non-irradiated and the SHI-irradiated areas on the pellet samples. | ||
Afterglow enhancement as a function of the electronic stopping power
The electronic stopping power is determined by the type and the energy of the ion as well as the properties of the material it strikes. In order to investigate the effects of the electronic stopping power on the afterglow enhancement, the electronic stopping power of the SAOE sample was calculated for all the ions using the SRIM, the Stopping and Range of Ions in Matter22 (Table 1). Fig. 2 shows the afterglow enhancement as a function of the electronic stopping power for all the ions. The afterglow intensity of the non-irradiated area was normalized to 100 to allow easy comparison of values. The dotted line shows that the afterglow enhancement varied with the electronic stopping power exponentially. The afterglow increased in an almost linear manner in the low electronic stopping power regime, then steadily increased and reached saturation. These results demonstrated that the afterglow of the SAOE LLP could be significantly improved by exposure to SHI irradiation with a higher electronic stopping power above 0.2 KeV nm−1. | ||
| Fig. 2 Afterglow enhancement as a function of the electronic stopping power for all the ions (data collected 10 s after UV excitation was stopped). | ||
| SHI (MeV/u) | Fe at 500 | Si at 100 | Ne at 600 | N at 430 | C at 430 | He at 230 |
| Electronic Stopping Power (KeV/nm) | 0.492 | 0.384 | 0.068 | 0.038 | 0.028 | 0.004 |
Afterglow enhancement as a function of the irradiation fluence
Fe ions were selected to investigate the effects of the SHI irradiation fluence on the afterglow of SAOE, because the heavier ions enhanced the afterglow intensity of the SAOE phosphor the most, due to their high electronic stopping power. The afterglow intensity of the Fe-irradiated and non-irradiated SAOE samples was measured with different irradiation fluence. Fig. 3 shows the afterglow intensities measured 30 min after UV excitation was stopped as a function of the irradiation fluence, where the afterglow intensity of the non-irradiated sample was normalized to 100. It was found that the afterglow intensity increased almost linearly with the irradiation fluence; the afterglow intensity of the sample exposed to an irradiation fluence of 1.7 × 1013 ions/cm2 was stronger by nearly one order of magnitude than that of the non-irradiated sample. Therefore, the afterglow of the SAOE phosphor was dramatically improved using a high fluence of SHI irradiation. | ||
| Fig. 3 Afterglow enhancement as a function of the irradiation fluence. | ||
XRD and PL spectra
XRD was used to analyze the Fe-irradiated and non-irradiated SAOE samples in order to investigate the mechanisms underlying the afterglow enhancement phenomena;23 these results showed that the SHI irradiation did not change the crystal structure of the sample. Fig. 4 (a) shows the PL spectra of the Fe-irradiated and the non-irradiated samples. The spectra were similar in shape, consisting of a broad band emission peaking at 510 nm, caused by the transition from the excited state 4f65d1 to the ground state 4f7 (8S7/2) of Eu2+. However, the PL intensity was weakened by the SHI irradiation. | ||
| Fig. 4 (a) PL spectra of the Fe-irradiated and non-irradiated samples. (b) The THL glow curves of the Fe-irradiated and non-irradiated samples. | ||
Evaluation of trap properties by THL
THL is used to evaluate the properties of carrier trapped centers, including the trap density and trap depth.24,25 The THL glow curves of the Fe-irradiated and the non-irradiated samples were measured at a heating rate of 1 K s−1 (Fig. 4 (b)). Three bands were observed in the THL glow curves for both samples, peaking at 200 K, 300 K, and 380 K, indicating that there are at least three types of traps in the SAOE LLP. The THL glow curves for the both samples had a similar shape, although the THL intensity increased after exposure to SHI irradiation. The THL intensity and THL peak position are related to the trap density and the trap depth respectively. Thus the THL results indicate that the SHI irradiation increased the trap density, but had no significant effect on the trap depth. This can be confirmed by comparing the afterglow decay curves in Fig. 1; if the trap depth is affected by SHI irradiation, the shape of the afterglow decay curves should change because the afterglow decay speed would be altered. However, the afterglow decay curves showed that only the intensity increased, and the shape of the curves was not significantly affected. The afterglow decay curves of the SHI-irradiated and the non-irradiated samples were further examined using a tri-exponential equation. | (1) |
| Sample | I 1 | I 2 | I 3 | τ 1 | τ 2 | τ 3 |
|---|---|---|---|---|---|---|
| Non | 55670 | 17823 | 4784 | 4.91 | 26.9 | 178.1 |
| Fe | 77650 | 23879 | 6438 | 4.81 | 26.4 | 176.7 |
Discussion of mechanisms
The defect creation mechanism during SHI irradiation has been extensively investigated by many researchers, and three models are proposed.26–29 Fleischer et al. put forward the Coulomb explosion model, where the coulomb repulsion between the atoms ionized by SHI results in damage along the incident ion tracks.26,27 The thermal spike model assumes that the energy of SHI is transferred to the target electrons and then to the lattice vibrations via the electron–phonon interaction. The lattice temperature along the SHI path can exceed the melting point of the material; the rapid quenching causes the damage.28 However, Itoh et al. proposed the self-trapped exciton model, where excitons are generated during the SHI irradiation process.15 Previous works have shown that ions with much lower incident energy could modify the band gap of materials due to ion embedment.30,31 However, in this study it should be noted that all the incident ions passed through our samples and no incident ions embedded in them. On the basis of the three models, we consider that the process of the afterglow enhancement by SHI irradiation consists of the following three steps:(1) When SHIs pass through the SAOE samples, energy deposition by SHIs induces electronic excitations from the valence band and core levels; the excited electrons cause further excitations of the same type and also lattice vibrations via the electron–phonon interaction. Following SHI irradiation, dense electron–hole pairs are generated which are rapidly converted to excitons.15
(2) In ideal crystals, excitons are delocalized and propagate freely. However, self-trapping of excitons occurs when the exciton–lattice coupling overcomes the freedom of the translational motion of an exciton.29 This phenomenon has previously been detected in alkali halides.32,33 In SAOE we propose that the free excitons were trapped in their own lattice distortion field, which formed self-trapped excitons.
(3) The subsequent relaxation of the self-trapped excitons induces strong lattice distortion and collective atom displacement. Moreover, the excitons may also interact with impurity ions to produce defects.34,35Fig. 5 shows a diagram of the defect creation process; new defects are created in the lattice along the SHI tracks. The trap density was therefore increased following exposure to SHI irradiation, which caused the afterglow enhancement. Furthermore, the similar shape of the non-irradiated and Fe-irradiated THL glow curves indicates that defects similar to the original ones in non-irradiated SAOE were created after SHI irradiation.
 | ||
| Fig. 5 Diagram of the defect creation process. | ||
Conclusions
Enhancement of the afterglow of famous LLP SrAl2O4:Eu2+ has been realized with SHI irradiation, which opened up a novel strategy for improving the afterglow of LLPs. The afterglow enhancement was found to be dependent on the electronic stopping power and irradiation fluence. Moreover, THL analyses showed that the SHI irradiation had no significant influence on the trap depth, but increased the trap density in the phosphor. The results provided new information for understanding the interaction of SHIs and materials as well as the mechanism of afterglow process. The discussion of the mechanisms underlying afterglow enhancement phenomenon is of crucial importance for future development of new LLPs.Acknowledgements
This work was partially supported by the JST CREST program. We gratefully acknowledge the researchers and technical staffs at AIST, particulary Dr Xiaoyan Fu, Dr Chenshu Li, Ms. Etsuko Kawasaki and Mr. Masayoshi Kubo, for assisting with this work.References
- S. Shionoya and W. M. Yen, Phosphor Handbook, CRC Press, LLC. 1999 Search PubMed.
- H. Aizawa, T. Katsumata, J. Takahashi, K. Matsunaga, S. Komuro, T. Morikawa and E. Toba, Rev. Sci. Instrum., 2002, 74, 1344 CrossRef.
- M. Kowatari, D. Koyama, Y. Satoh, K. Iimura and S. Uchida, Nucl. Instrum. Methods Phys. Res., Sect. A, 2002, 480, 431 CrossRef CAS.
- T. Matsuzawa, Y. Aoki, N. Takeuchi and Y. Murayama, J. Electrochem. Soc., 1996, 143, 2670 CrossRef CAS.
- F. Clabau, X. Rocquefelte, S. Jobic, P. Deniard, M.-H. Whangbo, A. Garcia and T. Le Mercier, Chem. Mater., 2005, 17, 3904 CrossRef CAS.
- F. Clabau, X. Rocquefelte, T. Le Mercier, P. Deniard, S. Jobic and M.-H. Whangbo, Chem. Mater., 2006, 18, 3212 CrossRef CAS.
- C. N. Xu, T. Watanabe, M. Akiyama and X. G. Zheng, Appl. Phys. Lett., 1999, 74, 2414 CrossRef CAS.
- C. N. Xu, H. Yamada, X. S. Wang and X. G. Zheng, Appl. Phys. Lett., 2004, 84, 3040 CrossRef CAS.
- X. S. Wang, C. N. Xu, H. Yamada, K. Nishikubo and X. G. Zheng, Adv. Mater., 2005, 17, 1254 CrossRef CAS.
- P. C. Ricci, C. M. Carbonaro, A. Casu, C. Cannas, R. Corpino, L. Stagi and A. Anedda, J. Mater. Chem., 2011, 21, 7771 RSC.
- G. P. Dong, X. D. Xiao, L. L. Zhang, Z. J. Ma, X. Bao, M. Y. Peng, Q. Y. Zhang and J. R. Qiu, J. Mater. Chem., 2011, 21, 2194 RSC.
- N. Itoh and A. M. Stoneham, Materials Modification by Electronic Excitation, Cambridge University press, 2001 Search PubMed.
- N. Kishimoto, N. Umeda, Y. Takeda, C. G. Lee and V. T. Gritsyna, Nucl. Instrum. Methods Phys. Res., Sect. B, 1999, 148, 1017 CrossRef CAS.
- N. Kishimoto, Y. Takeda, N. Umeda, V. T. Gritsyna, C. G. Lee and T. Saito, Nucl. Instrum. Methods Phys. Res., Sect. B, 2000, 166–167, 840 CrossRef CAS.
- M. Toulemonde, S. Bouffard and F. Studer, Nucl. Instrum. Methods Phys. Res., Sect. B, 1994, 91, 108 CrossRef CAS.
- C. Trautmann, K. Schwartz, J. M. Costantini, T. Steckenreiter and M. Toulemonde, Nucl. Instrum. Methods Phys. Res., Sect. B, 1998, 146, 367 CrossRef CAS.
- E. H. Lee, Nucl. Instrum. Methods Phys. Res., Sect. B, 1999, 151, 29 CrossRef CAS.
- K. Nordlund, J. Keinonen, M. Ghaly and R. S. Averback, Nature, 1999, 398, 49 CrossRef CAS.
- T. Z. Zhan, C. N. Xu, O. Fukuda, H. Yamada and C. S. Li, Ultrason. Sonochem., 2011, 18, 436 CrossRef CAS.
- C. S. Li, C. N. Xu, L. Zhang, H. Yamada and Y. Imai, J. Visualization, 2008, 11, 329 CrossRef.
- D. Ono, C. Z. Li, N. Bu and C. N. Xu, J. JSEM, 2010, 10, 152 Search PubMed.
- J. F. Ziegler, J. P. Biersack and M. D. Ziegler, the Stopping and Range of Ions in Matter, SRIM Co., 2008 Search PubMed.
- H. Yamada, H. Kusaba and C. N. Xu, Appl. Phys. Lett., 2008, 92, 101909 CrossRef.
- H. Yamada, X. Y. Fu and C. N. Xu, J. Electrochem. Soc., 2007, 154, 348 CrossRef.
- H. Zhang, H. Yamada, N. Terasaki and C. N. Xu, Appl. Phys. Lett., 2007, 91, 081905 CrossRef.
- R. L. Fleischer, P. B. Price and R. M. Walker, Nuclear Tracks in Solids, Univ. California Press, Berkeley, CA, 1975 Search PubMed.
- E. M. Bringa and R. E. Johnson, Phys. Rev. Lett., 2002, 88, 165501 CrossRef CAS.
- M. Toulemonde, J. M. Costantini, C. Dufour, A. Meftah, E. Paumier and F. Studer, Nucl. Instrum. Methods Phys. Res., Sect. B, 1996, 116, 37 CrossRef CAS.
- N. Itoh, Nucl. Instrum. Methods Phys. Res., Sect. B, 1996, 116, 33 CrossRef CAS.
- M. Anpo, M. Takeuchi, K. Ikeue and S. Dohshi, Curr. Opin. Solid State Mater. Sci., 2002, 6, 381 CrossRef CAS.
- H. Yamashita, M. Harada, J. Misaka, M. Takeuchi, B. Neppolian and M. Anpo, Catal. Today, 2003, 84, 191 CrossRef CAS.
- K. Schwartz, R. A. U. Scientific Reports & Solid State Electronics and technologies, 1996, 1, 3 Search PubMed.
- Ch. Lushchik, Physics of Radiation Effects in Crystals, Elsevier Science Publ., Amsterdam, 1986, 473 Search PubMed.
- K. Schwartz, A. E. Volkov, M. V. Sorokin, C. Trautmann, K.-O. Voss, R. Neumann and M. Lang, Phys. Rev. B, 2008, 78, 024120 CrossRef.
- R. T. Williams and K. S. Song, J. Phys. Chem. Solids, 1990, 51, 679 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
