Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stabilizing the electrode–electrolyte interface for high-voltage Li‖LiCoO2 cells using dual electrolyte additives†
Jiwei
Ding
a,
Chao
Yang
a,
Wenxi
Hu
a,
Xiaowei
Liu
a,
Anran
Zhang
b,
Deda
Peng
a,
Jin
Han
*b and
Ya
You
 *abc
*abc
aState Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, P. R. China. E-mail: youya@whut.edu.cn
bInternational School of Materials Science and Engineering, School of Materials Science and Microelectronics, Wuhan University of Technology, Wuhan 430070, P. R. China. E-mail: jinhan@whut.edu.cn
cHubei Longzhong Laboratory, Wuhan University of Technology, Xiangyang Demonstration Zone, Xiangyang 441000, China
First published on 19th June 2025
Abstract
Rechargeable Li‖LiCoO2 batteries are attractive due to their high energy density. However, the growth of lithium dendrites and the degradation of cathode at high potential hinder their practical application. Herein, we propose an advanced fluorinated carbonate-based electrolyte consisting of trans-4,5-difluoro-1,3-dioxolan-2-one (DFEC) and tri-(trimethylsilyl) phosphite (TMSPi) as dual additives to construct a stable interface on both the anode and cathode. The results show that DFEC can promote the formation of a stable solid-electrolyte-interface (SEI) layer on the lithium anode to inhibit the growth of dendrites. Additionally, TMSPi is conducive to the production of an inorganic-rich cathode–electrolyte interface (CEI) layer on the LiCoO2 cathode to inhibit the dissolution of cobalt. Finally, the Li‖LiCoO2 cells with this electrolyte could obtain an initial capacity of 211.6 mAh g−1 (846.4 Wh kg−1, active substances based on cathodes) at 1C (1C = 274 mA g−1) with a high-capacity retention of 81.6% after 200 cycles at a high upper cut-off voltage of 4.6 V. This work provides valuable insights into the development of electrolytes for high-voltage Li‖LiCoO2 cells.
Introduction
Rechargeable Li‖LiCoO2 cells have broad application prospects because of their high energy density. Unfortunately, LiCoO2 cathodes can only deliver a relatively low reversible capacity (∼140 mAh g−1) at the upper cut-off voltage of 4.20 V, despite their high theoretical capacity (274 mAh g−1).1–3 Raising the upper cut-off voltage of LiCoO2 has been proven to be an effective way to increase the practical capacity. However, once the upper cut-off voltage approaches 4.55 V, the electrolyte can suffer from severe decomposition in the absence of a stable interface on the cathode, leading to continuous capacity degradation of LiCoO2.4–6 Additionally, lithium metal anodes are attractive due to their outstanding theoretical capacity (3860 mAh g−1) and low redox potential (−3.04 V vs. SHE).7–11 However, lithium-dendrite growth and the accumulation of dead lithium lead to a short cycle life and low Coulomb efficiency, hindering the practical application of lithium metal anodes. To address the above-mentioned issues with both the cathode and anode of Li‖LiCoO2 cells, electrode/electrolyte interface engineering seems to be an effective strategy.12–17Electrode/electrolyte interface engineering can be mainly classified into artificial interface and electrolyte engineering.18–21 However, artificial interfaces often require a relatively complex preparation process, which is not conducive to large-scale production. In contrast, electrolyte engineering through the use of additives is a simple and feasible approach. For instance, 2,3-dimethylmaleic anhydride was used as a film-forming additive for constructing a dense and uniform CEI film on an LiCoO2 cathode, thereby enhancing the interface stability of the LiCoO2 cathode.22 2-Fluoro-5-iodopyridine has been proposed as a film-forming additive for lithium metal anodes, and addressed the issue of dendrite growth on lithium metal anodes.23 However, these electrolyte formulations can only solve the problem with the LiCoO2 cathode or the lithium metal anode individually.24–26 Therefore, there is an urgent need to explore novel electrolyte formulations capable of simultaneously stabilizing both the electrodes.
In this work, we adopted a dual-additive strategy and selected DFEC with a low lowest unoccupied molecular orbital (LUMO) energy level and TMSPi with a high highest occupied molecular orbital (HOMO) energy level as the film-forming additives for the lithium metal anode and the LiCoO2 cathode, respectively. The results show that DFEC can contribute to the generation of a stable SEI layer on the lithium metal anode to inhibit the lithium-dendrite growth. TMSPi can induce the formation of an inorganic-rich CEI layer on the LiCoO2 cathode to inhibit the dissolution of cobalt and enhance the cycling stability. Finally, the proposed electrolyte enabled Li‖LiCoO2 cells with an initial capacity of 211.6 mAh g−1 at 1C and a high-capacity retention of 81.6% after 200 cycles at a high upper cut-off voltage of 4.6 V.
Results and discussion
Characterization of the electrolyte
The solvation structure of the electrolyte TFDT (1 M LiPF6 dissolved in a blend of bis(2,2,2-trifluoroethyl) carbonate (TFEC), fluoroethylene carbonate (FEC), DFEC and TMSPi (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05
0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05, vol%)) was revealed using molecular dynamics (MD) simulation. The selected snapshots and locally enlarged structures (Fig. 1a) show that the Li+ cations are closely coordinated with TFEC, FEC, and PF6− anions. Additionally, the snapshot results show that the additives DFEC and TMSPi are not involved in the solvation structure. This conclusion was further supported by the subsequent nuclear magnetic resonance (NMR) characterization. In the 1H NMR spectra (Fig. 1b), no peak shift was observed between the spectra of TFDT with and without the additives DFEC and TMSPi, indicating that these two additives are not involved in the solvation structure. The local coordination environment around the Li+ cations was obtained by analyzing the radial distribution function (RDF) (Fig. 1d). The average coordination number of the Li+ cations with PF6−, TFEC and FEC was 3.3, 0.97, and 0.95, respectively. However, the average coordination number with the additives DFEC and TMSPi is negligible, indicating that they are not involved in the solvation structure.27–29
0.05, vol%)) was revealed using molecular dynamics (MD) simulation. The selected snapshots and locally enlarged structures (Fig. 1a) show that the Li+ cations are closely coordinated with TFEC, FEC, and PF6− anions. Additionally, the snapshot results show that the additives DFEC and TMSPi are not involved in the solvation structure. This conclusion was further supported by the subsequent nuclear magnetic resonance (NMR) characterization. In the 1H NMR spectra (Fig. 1b), no peak shift was observed between the spectra of TFDT with and without the additives DFEC and TMSPi, indicating that these two additives are not involved in the solvation structure. The local coordination environment around the Li+ cations was obtained by analyzing the radial distribution function (RDF) (Fig. 1d). The average coordination number of the Li+ cations with PF6−, TFEC and FEC was 3.3, 0.97, and 0.95, respectively. However, the average coordination number with the additives DFEC and TMSPi is negligible, indicating that they are not involved in the solvation structure.27–29
To investigate the redox reactivity of the included solvents and additives in the electrolytes, the corresponding HOMO and LUMO were calculated and analyzed using frontier molecular orbital theory (Fig. 1c). Due to the introduction of fluorine, the fluorinated organic solvents TFEC and FEC show higher LUMO levels and lower HOMO levels than the commercial carbonate solvent, indicating their superior oxidation stability. Notably, the additive DFEC possesses a lower LUMO level than the main solvents TFEC and FEC, indicating that DFEC can be preferentially reduced on the lithium metal anode before TFEC. Additionally, the HOMO level of TMSPi (−5.84139 eV) is higher than that of TFEC and FEC (−7.66346 eV), indicating that TMSPi is preferentially oxidized before TFEC and FEC on the LiCoO2 cathode. Thus, the additives DFEC and TMSPi could potentially be decomposed to produce an interface on the lithium metal anode and LiCoO2 cathode simultaneously. To evaluate the electrochemical stability of the electrolytes, LSV was conducted at a scanning rate of 0.1 mV s−1 on the anodic side in a coin cell containing stainless steel as the working electrode and Li metal as the counter and reference electrode. 1 M LiPF6 dissolved in a blend of ethylene carbonate (EC), diethyl carbonate (DEC) and vinylene carbonate (VC) (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, vol%)—denoted as EDV—was chosen as the reference electrolyte. The results in Fig. 1e show that the current rises sharply in EDV when the potential is higher than 3 V (vs. Li/Li+). In comparison, for TFDT, the current remains relatively stable until the potential is higher than 5.0 V, demonstrating its prominent anodic electrochemical stability.30,31
0.1, vol%)—denoted as EDV—was chosen as the reference electrolyte. The results in Fig. 1e show that the current rises sharply in EDV when the potential is higher than 3 V (vs. Li/Li+). In comparison, for TFDT, the current remains relatively stable until the potential is higher than 5.0 V, demonstrating its prominent anodic electrochemical stability.30,31
Electrochemical performance and interface characterization of the LiCoO2 cathode
To further evaluate the effect of the electrolytes on the electrochemical performance, the cycle performance of Li‖LiCoO2 cells with the two electrolytes was measured with an upper cut-off voltage 4.6 V at 1C after it had been activated at 0.1C for the first three cycles (Fig. 2a and b). An initial discharge capacity of 195.9 mAh g−1 was delivered by Li‖LiCoO2 cells with the electrolyte EDV, while the current rapidly decreased to 108.57 mAh g−1 after the 100th cycle. In comparison, Li‖LiCoO2 cells with TFDT exhibited an initial capacity of 211.6 mAh g−1 and maintained a specific capacity of 190.37 mAh g−1 after the 100th cycle. The corresponding coulombic efficiency for the Li‖LiCoO2 cells with the electrolytes TFDT and EDV is 95.94% and 87.8%, respectively. Notably, TFDT endowed the cell with a high-capacity retention of 81.6% after 200 cycles, which was much higher than that achieved using EDV (7%) (Fig. 2c). Throughout the entire 200 cycles, the coulombic efficiency of the cell with the TFDT was consistently higher than that of the EDV cell. The reason behind the quite different cycling performances of the electrolytes EDV and TFDT was elucidated using XRD (Fig. S1†). Although the LiCoO2 electrode cycled in the EDV has similar characteristic peaks to the pristine LiCoO2 electrode, after cycling in TFDT, the peak intensities of the 003 and 012 crystal planes of the LiCoO2 were reduced to a much lesser extent compared to those cycled in EDV (Table S1†), indicating that the CEI layer generated in TFDT maintains the structural stability of LiCoO2. In addition, the first cycle of the cell with TFDT shows obvious fluctuations in the curve when the voltage is around 4.0 V, while these disappear in the second cycle. This phenomenon can likely be attributed to the decomposition of the electrolyte additive in the first cycle (Fig. S2†) and is not present in EDV (Fig. S3†). However, the charging and discharging curves suffer dramatic variations. A high average discharge potential of 4.0 V can be delivered by Li‖LiCoO2 cells with TFDT throughout 200 cycles, while the value drops from 4.0 V to 3.5 V with EDV (Fig. S4†).The rate performance of Li‖LiCoO2 cells using EDV and TFDT was evaluated under cycling from 0.1C to 5C in the range of 3.0–4.6 V (Fig. 2d). The Li‖LiCoO2 cell with TFDT can attain capacities of 214.2, 209.4, 206.1, 201.9, 195.7, and 187.3 mAh g−1 at 0.1, 0.2, 0.33, 0.5, 1, 2, and 5C, respectively. When the current density is returned to 0.1C, the cell can recover to 202.9 mAh g−1 (94.7% retention rate) and maintain stable cycling performance in subsequent cycles. In comparison, the capacity of the Li‖LiCoO2 cell with EDV was much lower than that of the TFDT at the corresponding current density, indicating the enhanced lithium-ion transport dynamics endowed by TFDT. Furthermore, a constant current intermittent titration test (GITT) was conducted to measure the Li+ diffusion coefficient when using the electrolytes TFDT and EDV (Fig. S5†). The results indicate that the Li+ diffusion coefficient in the Li‖LiCoO2 cell with TFDT is higher than that in the Li‖LiCoO2 cell with EDV.
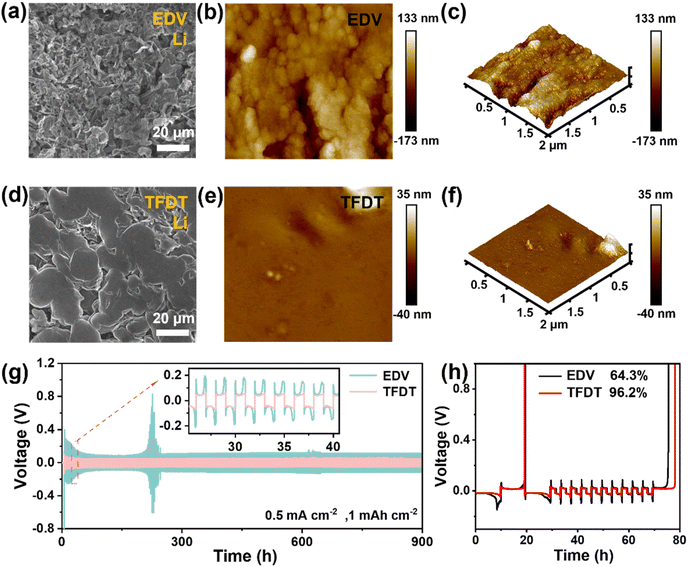
The surface morphology of the LiCoO2 cathode was probed using SEM after 50 cycles in the different electrolytes at 3–4.6 V, as shown in Fig. 2e–g. The results show that large cracks appear on the surface of the LiCoO2 particles after 50 cycles in EDV (Fig. 2f), which are not found for the fresh LiCoO2 electrode (Fig. 2e) or the LiCoO2 electrode cycled in TFDT (Fig. 2g). To further inspect the LiCoO2 electrode interface, transmission electron microscopy (TEM) was performed on the LiCoO2 cathodes cycled in EDV and TFDT. It was found that an inhomogeneous CEI layer with a varying thickness (5–10 nm) was formed on the LiCoO2 cathode in EDV (Fig. 2h), whereas a dense and thin (3–4 nm) CEI layer was generated on the LiCoO2 cathode in TFDT (Fig. 2i). The above results could indicate that the severe degradation of LiCoO2 particles in EDV is related to the absence of a stable CEI layer, while the structural stability of LiCoO2 particles in TFDT benefits from the protection of a robust CEI layer.
Li/Li and Li/Cu cells were assembled and measured to evaluate the effect of the electrolyte TFDT on lithium plating and stripping. In the symmetric Li/Li cells setup at a current density of 1 mAh cm−2, TFDT endowed the cell with a relatively low polarization voltage (∼51 mV) and a long cycle life of 900 h (Fig. 3g), while the commercial electrolyte EDV resulted in a high polarization voltage (∼118 mV) with an unstable phenomenon. The Auerbach method was used to detect the stability of electrolytes, and the average coulomb efficiency (CE) during plating/stripping was recorded for Li/Cu asymmetric cells, as shown in Fig. 3h.32 TFDT endowed the Li/Cu cells with a higher average CE (96.2%) than EDV (64.3%), indicating that TFDT is more compatible with lithium metal. The exchange current density (J0) of Li‖LiCoO2 cells with the electrolytes TFDT and EDV was assessed using Tafel curves during the plating/stripping process at 20 °C. The results show that TFDT generates a higher J0 than EDV (0.116 vs. 0.007 mA cm−2) (Fig. S6†), indicating faster kinetics and better reversibility for lithium plating/stripping in TFDT.33
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak at 289.3 eV originate from Li2CO3 as a result of the decomposition of the organic carbonate solvents. In the F 1s spectra (Fig. 4b), the lithium metal anode in TFDT reveals a stronger signal of inorganic LiF (284.8 eV) and LixPOyFz (284.8 eV) than that of EDV. The intensity of all elements was normalized to evaluate their relative contents in the SEI layer (Fig. 4c). The relative proportions of F and P in the SEI formed in TFDT are significantly higher than those in the SEI formed in EDV; in turn, the proportions of C and O in the SEI formed in TFDT were lower. This indicates that the SEI layer formed on the lithium metal anode in TFDT contains inorganic-rich substances (LiF and LixPOyFz), thereby enhancing the interface stability of the lithium metal anode.
O peak at 289.3 eV originate from Li2CO3 as a result of the decomposition of the organic carbonate solvents. In the F 1s spectra (Fig. 4b), the lithium metal anode in TFDT reveals a stronger signal of inorganic LiF (284.8 eV) and LixPOyFz (284.8 eV) than that of EDV. The intensity of all elements was normalized to evaluate their relative contents in the SEI layer (Fig. 4c). The relative proportions of F and P in the SEI formed in TFDT are significantly higher than those in the SEI formed in EDV; in turn, the proportions of C and O in the SEI formed in TFDT were lower. This indicates that the SEI layer formed on the lithium metal anode in TFDT contains inorganic-rich substances (LiF and LixPOyFz), thereby enhancing the interface stability of the lithium metal anode.
To probe the interface chemistry on the LiCoO2 cathodes cycled in the electrolytes TFDT and EDV, the XPS spectra for the LiCoO2 cathode in a charged state (4.6 V) are presented in Fig. 4d–g. In the C 1s spectra (Fig. 4d), the spectra peak at 285.6 eV, 287.1 eV, and 289.3 eV are ascribed to C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and O–C
O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups, respectively. The peaks at 290.1 eV and 291.4 eV were potentially due to Li2CO3 and polyvinylidene fluoride (PVDF).4 It is speculated that the CEI on the LiCoO2 surface in EDV is much richer in organic species than the TFDT one. Regarding the O 1s spectra in Fig. 4e, the peaks at 531.8 eV and 533.0 eV are assigned to C
O functional groups, respectively. The peaks at 290.1 eV and 291.4 eV were potentially due to Li2CO3 and polyvinylidene fluoride (PVDF).4 It is speculated that the CEI on the LiCoO2 surface in EDV is much richer in organic species than the TFDT one. Regarding the O 1s spectra in Fig. 4e, the peaks at 531.8 eV and 533.0 eV are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O functional groups, respectively.34 When comparing the inorganic fluorine-containing species in the CEIs (Fig. 4f), the CEI formed in TFDT shows a higher signal for LixPOyFz, which is mainly due to the decomposition of the additive TMSPi during the cycling process. Furthermore, the Si–O peak at 103 eV for the CEI on the LiCoO2 cycled in TFDT is ascribed to the decomposition of the additive TMSPi (Fig. 4g). The Si–O bond has a relatively high bond energy, which can form a stable chemical adsorption layer on the electrode surface and enhance the mechanical strength of the interface, maintaining the stability of the interface.35 The XPS spectra were normalized to calculate the relative proportions of different substances in the CEI (Fig. 4h). The relative proportions of C, F, P and Si in the CEI on the LiCoO2 cathode in TFDT were significantly higher than those on LiCoO2 cathode with EDV, while the proportion of O was lower for the LiCoO2 with TFDT. These collective findings indicate that the CEI formed in TFDT mainly contains inorganic substances, which is beneficial for stabilizing the material structure of the LiCoO2 cathode.36 In addition, the effective influence of the CEI interface on the LiCoO2 was investigated using inductively coupled plasma optical emission spectroscopy (ICP), as shown in Fig. 4i. It was found that the amount of cobalt leached in TFDT was 45.5 μg L−1, which was just 23.9% the amount leached in EDV (190.3 μg L−1), indicating the enhanced structural stability of the LiCoO2 cathode in TFDF.
O and C–O functional groups, respectively.34 When comparing the inorganic fluorine-containing species in the CEIs (Fig. 4f), the CEI formed in TFDT shows a higher signal for LixPOyFz, which is mainly due to the decomposition of the additive TMSPi during the cycling process. Furthermore, the Si–O peak at 103 eV for the CEI on the LiCoO2 cycled in TFDT is ascribed to the decomposition of the additive TMSPi (Fig. 4g). The Si–O bond has a relatively high bond energy, which can form a stable chemical adsorption layer on the electrode surface and enhance the mechanical strength of the interface, maintaining the stability of the interface.35 The XPS spectra were normalized to calculate the relative proportions of different substances in the CEI (Fig. 4h). The relative proportions of C, F, P and Si in the CEI on the LiCoO2 cathode in TFDT were significantly higher than those on LiCoO2 cathode with EDV, while the proportion of O was lower for the LiCoO2 with TFDT. These collective findings indicate that the CEI formed in TFDT mainly contains inorganic substances, which is beneficial for stabilizing the material structure of the LiCoO2 cathode.36 In addition, the effective influence of the CEI interface on the LiCoO2 was investigated using inductively coupled plasma optical emission spectroscopy (ICP), as shown in Fig. 4i. It was found that the amount of cobalt leached in TFDT was 45.5 μg L−1, which was just 23.9% the amount leached in EDV (190.3 μg L−1), indicating the enhanced structural stability of the LiCoO2 cathode in TFDF.
To further investigate the heat-related behavior associated with these electrolytes, a cone calorimeter was used to collect some parameters of the combustion process, such as the self-extinction time (SET), total heat release (THR), and maximum heat release rate (MHRR). All samples were exposed to a constant thermal radiation value of 25 kW m−2 until fully ignited. The TFDT electrolyte has a lower MHRR (15.4 W g−1vs. 58.1 W g−1), lower SET (5 s vs. 19 s) and lower THR (6.54 kJ g−1vs. 18.78 kJ g−1) than EDV electrolyte, as shown in Fig. 5c. In addition, the gas produced during the combustion of TFDT has a higher CO concentration and lower CO2 content than that of EDV, which indicates the incomplete combustion and notable flame retardance of TFDT (Table S3†). To quantify the flammability of the electrolytes, the limiting oxygen index (LOI) test was used to further measure their flammability. As shown in Fig. 5d, TFDT has a much higher LOI (32%) than TFDT (16%). Based on the classification zones in Fig. 5d, TFDT has flame- retardance characteristics, while EDV is flammable. In summary, TFDT has better flame retardance and self-extinguishing characteristics compared with EDV. Additionally, the lower heat release during combustion may be related to the higher concentration of fluorine-containing solvents in TFDT.
Conclusions
In summary, a novel electrolyte containing dual additives was designed, and its corresponding solvation structure was studied. The electrolyte has properties that optimize the formation of electrolyte interfaces. The TMSPi additive enhances the interface stability of the LiCoO2 cathode by forming stable CEI layers, thereby further preventing the structural collapse of LiCoO2 and the leaching of cobalt under high operation potential. Additionally, the additive DFEC generates an inorganic-rich SEI layer on the surface of the lithium metal anode and inhibits the growth of lithium dendrites. Therefore, the tailored electrolyte endowed Li/LiCoO2 cells with an initial capacity of 211.6 mAh−1 (846.4 W h kg−1) and a high-capacity retention of 81.6% after 200 cycles at a high cut-off voltage of 4.6 V. This work broadens the perspective on electrolyte development for high-voltage Li/LiCoO2 cells through the use of electrolyte additives.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Y. Y. and J. H. conceived and supervised the project; J. D. designed the experiments; J. D., W. H., and D. P. conducted the experiments; X. L. and A. Z. conducted the DFT calculations and MD simulations. J. D. and C. Y. conducted the characterizations and analysis. J. D. drafted the manuscript with the help of Y. Y. and J. H.; all authors agreed on the manuscript and approved submission.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Key Research and Development Program of China (2022YFB3803400).Notes and references
- E. Cha, J. H. Yun, R. Ponraj and D. K. Kim, Mater. Chem. Front., 2021, 5, 6294–6314 RSC.
- S. Awasthi, J. Energy Storage, 2024, 100, 113439 CrossRef.
- Z. Chang, C. Ma, R. Wang, B. Wang, M. Yang, B. Li, T. Zhang, Z. Li, P. Zhao, X. Qi and J. Wang, ACS Appl. Mater. Interfaces, 2024, 16, 18980–18990 CrossRef CAS PubMed.
- K. Guo, C. Zhu, H. Wang, S. Qi, J. Huang, D. Wu and J. Ma, Adv. Energy Mater., 2023, 13, 2204272 CrossRef CAS.
- M. Ma, Z. Zhu, D. Yang, L. Qie, Z. Huang and Y. Huang, ACS Appl. Mater. Interfaces, 2024, 16, 12554–12562 CrossRef CAS PubMed.
- C. Luo, Q. Liu, X. Wang, Y. Tian, Z. Liu, F. Kang and B. Li, Nano Energy, 2023, 109, 108323 CrossRef CAS.
- X.-B. Cheng, R. Zhang, C.-Z. Zhao and Q. Zhang, Chem. Rev., 2017, 117, 10403–10473 CrossRef CAS PubMed.
- J.-F. Ding, Y.-T. Zhang, R. Xu, R. Zhang, Y. Xiao, S. Zhang, C.-X. Bi, C. Tang, R. Xiang, H. S. Park, Q. Zhang and J.-Q. Huang, Green Energy Environ., 2023, 8, 1509–1530 CrossRef CAS.
- H. Kang, H. Kang, M. Lyu and E. Cho, EcoMat, 2024, 6, e12498 CrossRef CAS.
- X. Duan, J. Sun, L. Shi, S. Dong and G. Cui, Interdiscip. Mater., 2024, 4, 217–234 Search PubMed.
- J. Xu, X. Cai, S. Cai, Y. Shao, C. Hu, S. Lu and S. Ding, Energy Environ. Mater., 2023, 6, e12450 CrossRef CAS.
- O. B. Chae and B. L. Lucht, Adv. Energy Mater., 2023, 13, 2203791 CrossRef CAS.
- Y. Yan, S. Weng, A. Fu, H. Zhang, J. Chen, Q. Zheng, B. Zhang, S. Zhou, H. Yan, C.-W. Wang, Y. Tang, H. Luo, B.-W. Mao, J. Zheng, X. Wang, Y. Qiao, Y. Yang and S.-G. Sun, ACS Energy Lett., 2022, 7, 2677–2684 CrossRef CAS.
- H. Yang, T. Zhao, K. Huang, Y. Shen, Y. Huang and W. Luo, Adv. Funct. Mater., 2023, 33, 2302203 CrossRef CAS.
- J. Yang, X. Liu, Y. Wang, X. Zhou, L. Weng, Y. Liu, Y. Ren, C. Zhao, M. Dahbi, J. Alami, D. A. Ei-Hady, G. L. Xu, K. Amine and M. Shao, Adv. Energy Mater., 2021, 11, 2101956 CrossRef CAS.
- Y. You, A. Dolocan, W. Li and A. Manthiram, Nano Lett., 2018, 19, 182–188 CrossRef PubMed.
- Q. Ding, Z. Jiang, K. Chen, H. Li, J. Shi, X. Ai and D. Xia, Carbon Energy, 2024, 6, e602 CrossRef CAS.
- S. Chen, J. Zheng, D. Mei, K. S. Han, M. H. Engelhard, W. Zhao, W. Xu, J. Liu and J. G. Zhang, Adv. Mater., 2018, 30, 1706102 CrossRef PubMed.
- S. Dai, W. Fang, T. Wang, Y. Gao, T. Zhang, Z. Qin, G. Chen and X. Zhou, Chem. Eng. J., 2024, 500, 157269 CrossRef CAS.
- M. Ma, R. Huang, M. Ling, Y. S. Hu and H. Pan, Interdiscip. Mater., 2023, 2, 833–854 CAS.
- Z. Wang, L. P. Hou, Z. Li, J. L. Liang, M. Y. Zhou, C. Z. Zhao, X. Zeng, B. Q. Li, A. Chen, X. Q. Zhang, P. Dong, Y. Zhang, J. Q. Huang and Q. Zhang, Carbon Energy, 2022, 5, e283 CrossRef.
- A. Fu, C. Xu, J. Lin, Y. Su, H. Zhang, D.-Y. Wu, X. Zhang, M. Xia, Z. Zhang, J. Zheng and Y. Yang, J. Mater. Chem. A, 2023, 11, 3703–3716 RSC.
- E. Zhang, H. Tian, M. Li, S. Le, B. Li, L. Wu, Q. Zhang and L. Fan, Chem. Commun., 2023, 59, 10996–10999 RSC.
- A. Zhang, Z. Bi, G. Wang, S. Liao, P. Das, H. Lin, M. Li, Y. Yu, X. Feng, X. Bao and Z.-S. Wu, Energy Environ. Sci., 2024, 17, 3021–3031 RSC.
- C. Zhang, S. Gu, D. Zhang, J. Ma, H. Zheng, M. Zheng, R. Lv, K. Yu, J. Wu, X. Wang, Q.-H. Yang, F. Kang and W. Lv, Energy Storage Mater., 2022, 52, 355–364 CrossRef.
- S. D. Zhang, M. Y. Qi, S. J. Guo, Y. G. Sun, X. X. Tan, P. Z. Ma, J. Y. Li, R. Z. Yuan, A. M. Cao and L. J. Wan, Small Methods, 2022, 6, 2200148 CrossRef CAS PubMed.
- H. Zhu, J. Ma, H. Ding, H. Wu, C. Zhang, L. Ni, X. Fang, L. Lao and X. Wang, Appl. Surf. Sci., 2024, 677, 161100 CrossRef CAS.
- Y. Wang, Z. Li, Y. Hou, Z. Hao, Q. Zhang, Y. Ni, Y. Lu, Z. Yan, K. Zhang, Q. Zhao, F. Li and J. Chen, Chem. Soc. Rev., 2023, 52, 2713–2763 RSC.
- J. Wu, Z. Gao, Y. Wang, X. Yang, Q. Liu, D. Zhou, X. Wang, F. Kang and B. Li, Nano-Micro Lett., 2022, 14, 147 CrossRef CAS PubMed.
- Y. Wu, Z. Zeng, H. Zhang, M. Liu, S. Lei, W. Zhong, S. Cheng and J. Xie, Energy Storage Mater., 2024, 70, 103499 CrossRef.
- W. Xi, X. Xia, J. Zhu, D. Yang and S. Xie, Nano Res., 2024, 17, 7230–7241 CrossRef CAS.
- F. Li, J. Liu, H. Wang, Y. Ren, X. Tang and G. Li, ACS Energy Lett., 2025, 10, 779–787 CrossRef CAS.
- C.-C. Su, M. He, M. Cai, J. Shi, R. Amine, N. D. Rago, J. Guo, T. Rojas, A. T. Ngo and K. Amine, Nano Energy, 2022, 92, 106720 CrossRef CAS.
- Z. Zhang, C. Qin, K. Wang, X. Han, J. Li, M. Sui and P. Yan, J. Energy Chem., 2023, 81, 192–199 CrossRef CAS.
- R. Tian, O. Seitz, M. Li, W. Hu, Y. J. Chabal and J. Gao, Langmuir, 2010, 26, 4563–4566 CrossRef CAS PubMed.
- Z. Zhang, C. Qin, X. Cheng, J. Li, Y. Zhang, W. Zhao, L. Wang, Y. Du, M. Sui and P. Yan, Electron, 2023, 1, e9 CrossRef.
- A. Yang, C. Yang, K. Xie, S. Xin, Z. Xiong, K. Li, Y.-G. Guo and Y. You, ACS Energy Lett., 2023, 8, 836–843 CrossRef CAS.
- Z.-H. Wu, Y. Wu, Y. Tang, J.-C. Jiang and A.-C. Huang, Process Saf. Environ. Prot., 2023, 169, 285–292 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, test parameters and additional figures. See DOI: https://doi.org/10.1039/d5sc03120f |
| This journal is © The Royal Society of Chemistry 2025 |