Defect-containing metal–organic framework materials for sensor applications
Dahui
An
a,
Long
Chen
 *a,
Yun
Liang
a,
Juan
Hou
*a,
Yun
Liang
a,
Juan
Hou
 *a and
Jiangzhao
Chen
*a and
Jiangzhao
Chen
 *bc
*bc
aSchool of Chemistry and Chemical Engineering/State Key Laboratory Incubation Base for Green Processing of Chemical Engineering, Shihezi University, Shihezi, 832003, China. E-mail: arnoan@qq.com; 2669347572@qq.com; chenlong2012@sinano.ac.cn; hjuan05@shzu.edu.cn
bKey Laboratory of Optoelectronic Technology & Systems (Ministry of Education), College of Optoelectronic Engineering, Chongqing University (CQU), Chongqing 400044, China. E-mail: jiangzhaochen@cqu.edu.cn
cFaculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming 650093, China
First published on 11th December 2023
Abstract
Defective metal–organic framework (MOF) materials exhibit remarkable potential across diverse fields such as adsorption, electrocatalysis, and electrochemical sensors due to their exposed massive active sites, defective microstructure, exceptional porosity, substantial specific surface area, and unoccupied metal ligand sites. Herein, the working principles of defect construction, defect mechanism, and defect application of MOFs are first briefly summarized. Therefore, this review enables researchers to be inspired to design defective MOFs for electrochemical sensor applications, which is analyzed to establish a relationship between defect construction, electronic structures, and electrocatalytic performance. To address the current challenging issues of electrochemical sensors, various emerging defect strategies are finally prospected and potential avenues for utilizing defects to enhance sensor technologies are pointed out, providing valuable references for future research.
1. Introduction
In recent years, MOFs have gained widespread attention for their distinctive mechanical, electrochemical, and optical properties, which make them highly promising for various applications, including water electrolysis for hydrogen production, electrochemical sensors, and supercapacitors.1–4 Since Yaghi et al.5 discovered MOFs in 1999 (Fig. 1b), MOFs have garnered widespread attention among researchers due to their outstanding characteristics, including large specific surface area, tunable pores, designable crystal structures, and abundant active sites.6 Despite their remarkable properties, MOFs face numerous limitations in electrochemical sensor applications due to their ordered crystalline framework, consisting of metal ions and organic ligands, which can limit MOF adaptability to diverse analytes and sensing conditions, reducing their versatility.7 Furthermore, pristine MOFs lack intrinsic active sites and constrain the electrochemical sensitivity, particularly in detecting low-concentration analytes or achieving rapid responses.29 To address these limitations and enhance the sensing capabilities of MOFs, researchers have explored various modification methods, including functionalization with different organic groups, metal doping, incorporation of guest molecules, and defect engineering strategies.8 Functionalization with organic groups, such as carboxylic acids or amine groups, can introduce additional functional sites on surfaces of MOFs, thereby improving their interactions with target analytes. Metal doping involves the substitution of some metal ions within the MOF framework with others, resulting in alterations to the electronic structure and enhanced reactivity. The incorporation of guest molecules into MOF pores creates specific binding sites for analytes, thereby enhancing selectivity. Among these strategies, defect engineering,20 as depicted in Fig. 1a, has increasingly gained researchers' favor over other modification methods, which not only provides much more active sites compared with alternative approaches,9 but also stands out in electrochemical performance due to its precision, controllability, and wide applicability.10 Defect engineering has attracted increasing attention from researchers in various applications such as adsorption, electrocatalysis, and electrochemical detection and has gradually become the preferred choice for MOF modification.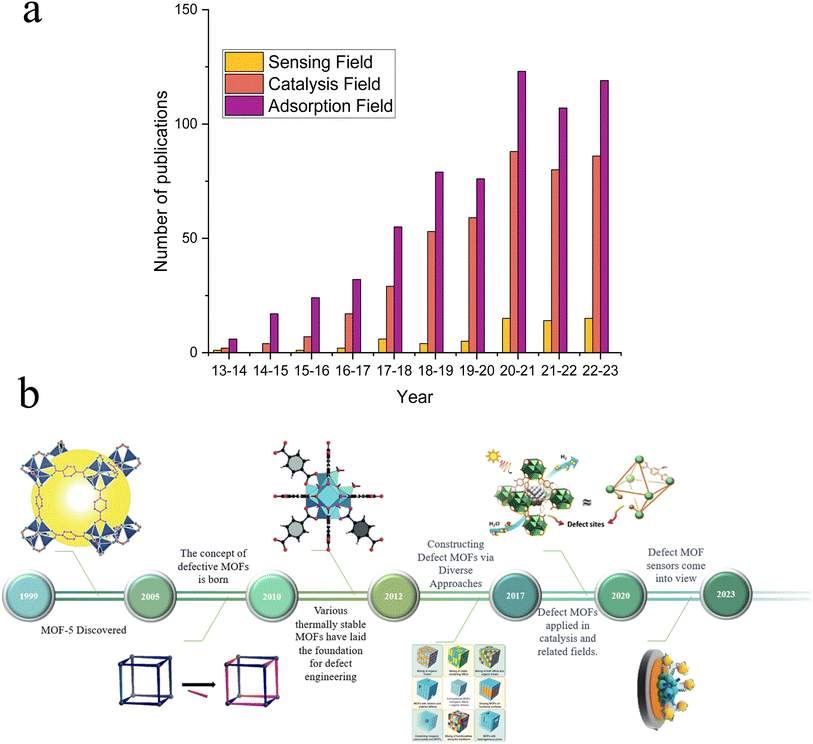 | ||
| Fig. 1 (a) The number of published research articles for the application of defective MOFs in sensing, catalysis, and adsorption fields (data source: Web of Science, retrieved: August 20, 2023). (b) Development of defective MOFs, MOF-5, reproduced with permission.5 Copyright, 1999, Nature. Defect schematic, reproduced with permission.106 Copyright, 2023, Elsevier. Defective UIO-66, reproduced with permission.112 Copyright, 2015, Wiley-VCH. Constructing defect MOFs via diverse approaches, reproduced with permission.113 Copyright, 2017, Elsevier. Catalytic applications of defective MOFs, reproduced with permission.114 Copyright, 2019, Wiley-VCH. And a defective MOF sensor, reproduced with permission.93 Copyright, 2023, Elsevier. | ||
Active defects in MOFs are distinct from the reconstruction process caused by external factors, or internal structural instability in the coordinated environment. These defects are intentionally introduced into MOFs through deliberate and precise design and control. Defective MOFs can be categorized into two main types: metallic defects, which involve the absence of a metal cluster or the substitution of certain metal ions with others in the framework,25 and conversely, ligand defects,11,12 which occur when organic ligands are absent or replaced by other small molecules.26–28 This approach revolves around introducing controlled defects, which play a pivotal role in finely tuning structural,33 electronic,34 and various other characteristics of MOFs.35–37 Defective MOFs present several advantages: (1) expanded surface area and reactivity: defect engineering significantly provides additional adsorption and active sites for target ions to interact with due to the enhanced surface area, which facilitates a more efficient adsorption process;13–15 (2) abundance of anchoring sites: these additional anchoring sites enable the loading of a greater variety and quantity of MOFs, contributing significantly to enhanced sensing performance;16,17 (3) expanded sensing range: defects in MOFs significantly alter atomic-level structure–property relationships.18,19 The presence of defect sites dramatically enhances the permeability of guest molecules,27,28 including large organic species, through the MOF framework.21–24 Furthermore, defects enhance charge transport properties of MOFs, further improving their sensing capabilities.30–32
Researchers have increasingly employed defect engineering strategies to enhance MOFs' performance across various applications.134–136 However, compared with gas adsorption and electrocatalytic reactions, the use of defect MOFs in sensor applications has received less attention.136–139 In this review, we focus on the recent developments in using defective MOFs for optical and electrochemical sensors. The definition, advantages and creation of defectives are summarized. In addition, a comprehensive survey of defective MOF applications for sensors is provided. The design and the primary limitations of defective MOFs for sensor applications, as well as potential avenues for future resolution are analyzed and studied. This review intends to explore potential development prospects of defective MOFs and their derivatives in the field of sensors.
2. Construction and characterization of defective MOFs
Defect engineering can lead to increased adsorption sites,38,39 improved catalytic performance,40 and enhanced sensor sensitivity,41 and there are many ways to introduce defects into the MOF lattice.With the continuous development of defect engineering for MOFs, the theory of defect construction has become more and more refined. These classifications can be broadly categorized into “de novo synthesis (DNS)25,42” and “post-synthetic treatment (PST).26,43” Recent cases of using DNS and PST to construct defective MOFs are shown in Table 1.
| Defect name | Typical strategies | Defect construction methods | Application areas | References |
|---|---|---|---|---|
| UIO-66 | DNS | Mixed linker | Electrode | 120 |
| MOF-74 | DNS | Mixed linker | CO2 capture | 123 |
| UIO-67 | DNS | Modulation method | Gas adsorption | 119 |
| MOF-801 | PST | Solvent-assisted linker exchange | Hydrogen restore | 121 |
| ZIF-8 | DNS | Modulation method | Sensor | 122 |
| HKUST-1 | PST | Post-synthetic heat treatment | Catalytic conversion | 118 |
2.1 DNS
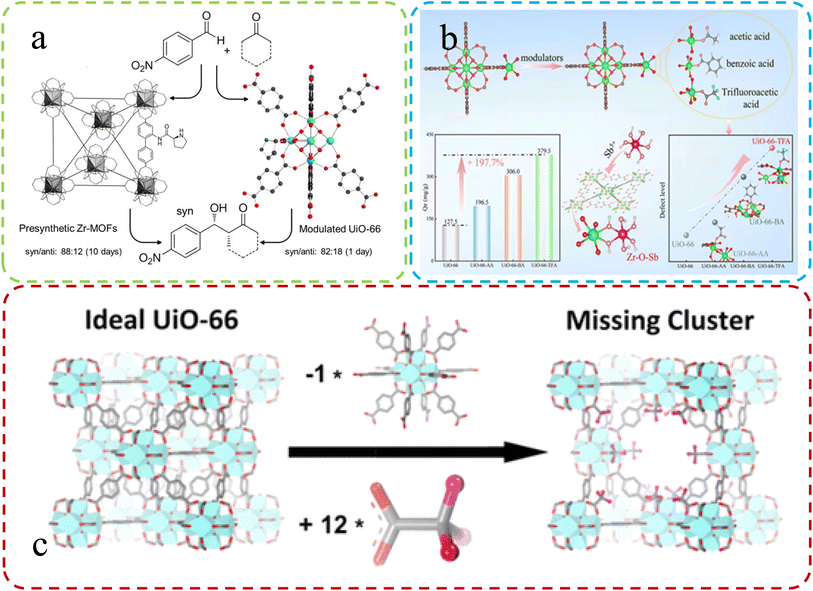 | ||
| Fig. 2 (a) Synthesis of UiO-66 using L-proline as a modulator. Reproduced with permission.52 Copyright, 2018, Elsevier. (b) Varied adsorption in defect MOFs with different modifiers. Reproduced with permission.53 Copyright, 2022, Elsevier. (c) Differences in ideal vs. defective UiO-66 unit cells. Reproduced with permission.47 Copyright 2016, American Chemical Society. | ||
Feng et al.53 team corroborated the previous research on defect synthesis using a modulator approach, employing L-proline to synthesize UiO-66 and introduce chiral active sites. Fig. 2b illustrates this process. The modified Zr-MOFs displayed enhanced catalytic activity and diastereoselectivity in aldol reactions, partially attributed to the introduced defects within the MOFs. Peng et al.47 emphasized the vital role of the modulator approach. They successfully synthesized highly defective UiO-66 by incorporating monocarboxylic acids, partially replacing linkers. Subsequent high-temperature treatment removed modulators, creating multiple open sites within the MOF structure. These defect-induced enhanced adsorption properties effectively removed antimony from real water samples (Fig. 2c).
Using a mixture of ligands, Epp et al.60 synthesized Ru-based MOFs and subsequently explored defect formation in MOFs initiated by crystal growth rates during different synthesis procedures. They systematically investigated the impact of these defects on the structure, morphology, and properties, including catalytic performance, while also studying how various synthesis methods influence the morphologies of MOFs. The change in catalytic properties after the introduction of defects is shown in Fig. 3a. Fan et al.54 applied a mixed-linker defect engineering technique to synthesize Cu(I)-enriched MOFs of the Cu-BTC type. During synthesis, they substituted a portion of the parent linker with truncated pyridine-3,5-dicarboxylate, creating Lewis basic pyridyl sites and reducing carboxyl coordination sites. This modification resulted in the formation of mixed-valence Cu(I)–Cu(II) paddlewheels within the defect-engineered CuBTC structure (Fig. 3b). In another study, Yu and colleagues61 used a mixing ligands strategy with Zn2+ ions, yielding MOF-derived ZnO nanoclusters with tunable surface oxygen vacancies through annealing. Their findings suggest that coordinate competition can introduce additional unsaturated metal sites in MOFs, resulting in more oxygen vacancies in derived materials. Creating defects in MOFs by mixing the original linker with a second lower-topology linker is a common construction method. Villajos J. A., et al.62 used this approach to synthesize MOFs-74. Higher pKa and an increased modulator concentration resulted in more defects, providing a simple way to introduce functional sites. As shown in Fig. 3c, a combination of three ligands effectively bound Co ions in the synthesized Co-MOFs-74, generating numerous defects within the material. Therefore, it is an effective means to improve the catalytic and detection performance of MOFs by optimizing ligands.
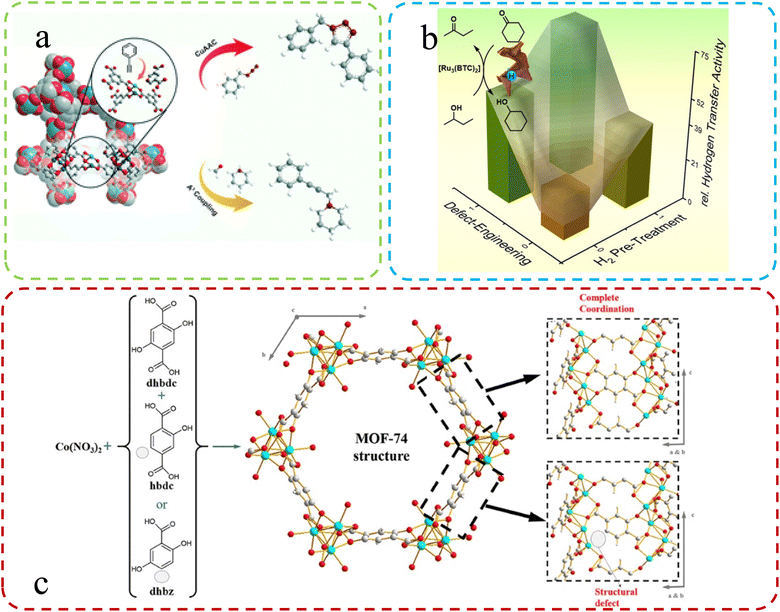 | ||
| Fig. 3 (a) Schematic illustration of the mixed-linker approach. Reproduced with permission.60 Copyright, 2021, Royal Society of Chemistry. (b) Defective HKUST-1 synthesized via the mixed-linker approach. Reproduced with permission.54 Copyright, 2020, Wiley-VCH. (c) Generation of structural defects in a MOFs-74 structure by partial linker substitution. Reproduced with permission.62 Copyright, 2019, Frontiers. | ||
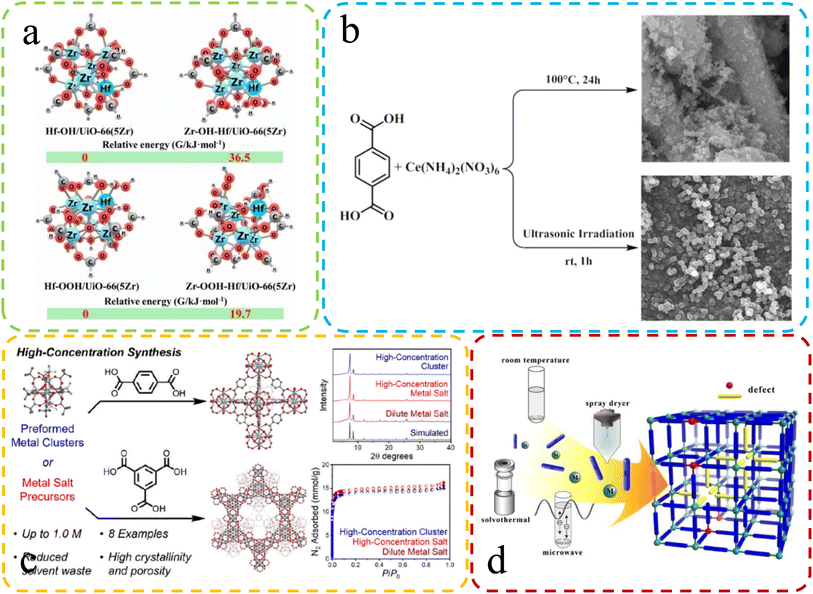 | ||
| Fig. 4 (a) The difference in Gibbs free energy at different synthesis speeds. Reproduced with permission.67 Copyright, 2021, Elsevier. (b) Differential SEM characterization of Ce-UiO-66 synthesized via reflux and sonochemical methods. Reproduced with permission.65 Copyright, 2021, Elsevier. (c) Varied characterization and performance in high-concentration synthesis. Reproduced with permission.104 Copyright 2022, American Chemical Society. (d) Schematic: fast microwave synthesis of defective MOFs. Reproduced with permission.64 Copyright, 2017, Elsevier. | ||
Karimi et al.65 recently harnessed ultrasonic radiation to synthesize Ce-UiO-66 MOFs with substantial defects, a key factor in enhancing their catalytic performance. Fig. 4b offers a concise overview of these two distinct synthesis methods. The selection of a specific synthesis technique significantly impacts crystal size and defect formation. Crystallization from a quiescent solution tends to produce more extensive crystals, while agitation can result in smaller crystals with increased defect density. These factors underscore the importance of method choice in tailoring MOF properties for optimal performance.66
Rapid synthesis of defective MOFs using solvent-free methods has been favored due to their greenness and rapidity. Ye et al.67 introduced an environmentally friendly method for rapidly synthesizing solvent-free bimetallic UiO-66(Hf–Zr) MOFs, which exhibited highly dispersed Hf/Zr–OH defect sites. Theoretical calculations showed that rapid synthesis led to an increase in defects (Fig. 4a). The high-concentration, solvent-free synthesis conditions could also be one of the contributing factors to the increased defect formation. Jerozal et al.104 demonstrated that UIO-66 based on Zr or Hf can autonomously assemble in high-concentration solutions (Fig. 4c). Moreover, in comparison to traditional synthesis methods, the resulting MOFs exhibited a higher prevalence of valeric acid defects. In addition, the fast crystallization time may lead to the assembly of incomplete MOF products, which are inherently more defective than MOF crystals synthesized by slow reactions.68
2.2 PST
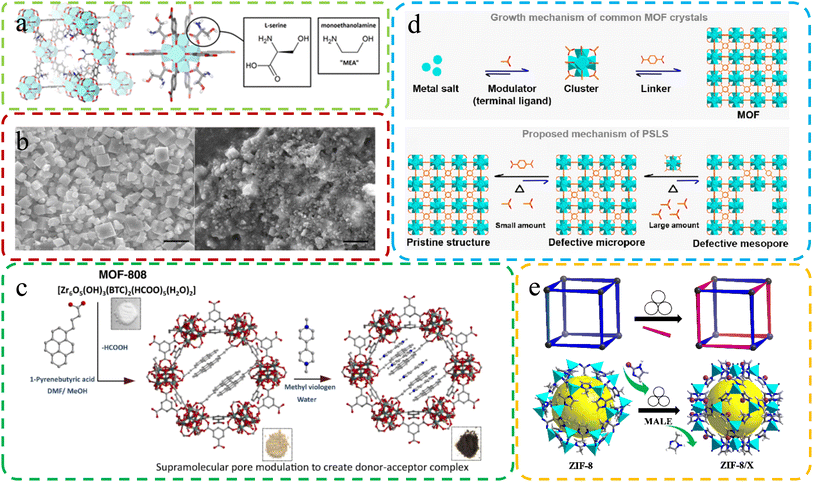 | ||
| Fig. 5 (a) Molecular structure of defective MOFs with L-serine ligand substitution. Reproduced with permission.73 Copyright 2016, American Chemical Society. (b) Differences in SEM images of MOFs under different synthesis conditions. Reproduced by permission.72 Copyright, 2019, Nature. (c) Constructing MOF-808-PBA as the donor, exchanging linkers, and adding MV as the electron acceptor for MOF-808-PBA-MV. Reproduced with permission.105 Copyright, 2023, Nature. (d) Crystal generation: common MOFs (above) vs. SALE method (below). Reproduced with permission.115 Copyright 2022, American Chemical Society. (e) Schematic diagram of the MALE method. Reproduced with permission.106 Copyright, 2023, Elsevier. | ||
Sanchita Karmakar et al.105 utilized the SALE method to synthesize defective MOF-808-PBA. This intricate integration of methyl viologen generated a sophisticated donor–acceptor composite, akin to an artificial “special pair” at the molecular level (Fig. 5c). This approach allowed for the creation of a unique and advanced molecular structure. Jiang et al.106 introduced a groundbreaking solvent-free and environmentally friendly approach called mechanochemistry-assisted linker exchange (MALE) for post-synthetic modification of MOFs. This innovative method, conducted at room temperature, offers a sustainable alternative to the commonly employed SALE technique. Demonstrated by the efficient exchange of linkers in ZIF-8 using the imidazole-2-formaldehyde (Im-CHO) ligand (Fig. 5e), MALE showcased remarkably faster and more efficient reaction rates compared to traditional SALE. The versatility of the MALE method extended to UiO-66 and diverse functional ZIF-8 analogs, exemplified by ZIF-8/Br, ZIF-8/CF3, ZIF-8/CH2OH, ZIF-8/Cl, and ZIF-8/Et. Notably, ZIF-8/CF3 exhibited enhanced kinetic gas separation performance for C3H6/C3H8 mixtures.
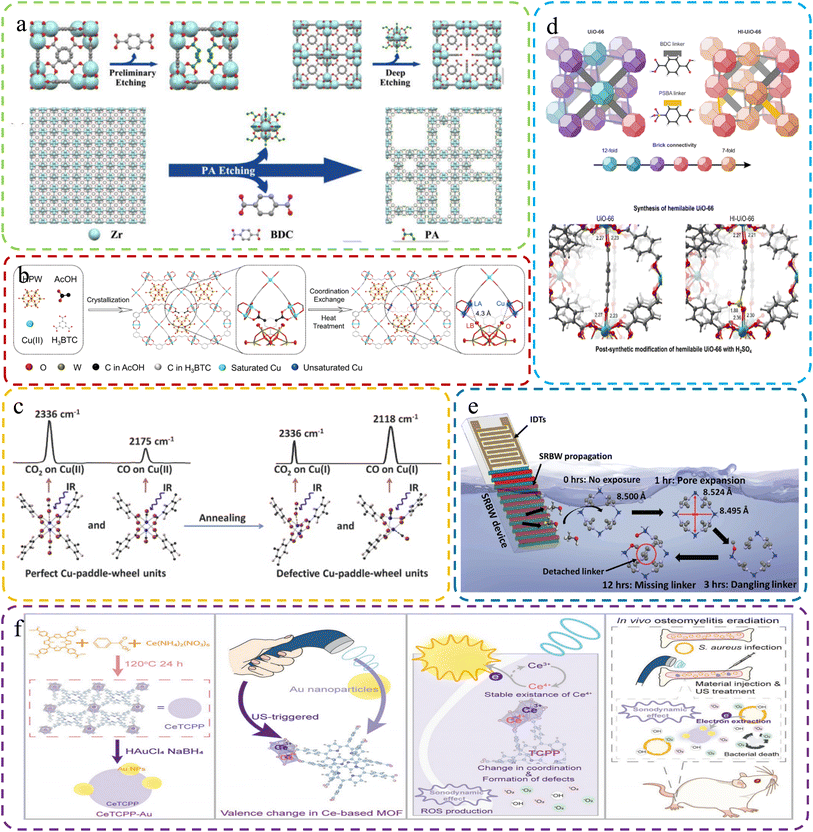 | ||
| Fig. 6 (a) Creating hierarchical porous Zr-based MOFs via monocarboxylic acid etching with propionic acid etching. Reproduced with permission.74 Copyright, 2018, Wiley-VCH. (b) Creating Dx-NENU-3 with FLP sites via coordination defect engineering. Reproduced with permission.107 Copyright, 2023, Wiley-VCH. (c) Structural and characterization changes of MOFs and defective MOFs after heat treatment. Reproduced with permission.76 Copyright, 2015, Elsevier. (d) Top: UiO-66 and Hl-UiO-66 with varied brick configurations, creating unsaturated Zr sites. Bottom: UiO-66 with one missing linker and Hl-UiO-66 with one missing linker and one PSBA linker. Reproduced with permission.108 Copyright 2020, American Chemical Society. (e) Schematic of the setup for acoustomicrofluidic defect creation. Reproduced with permission.109 Copyright, 2023, Wiley-VCH. (f) CeTCPP-Au for US-triggered Au NP electron trapping, enhancing SDT for bacterial eradication and osteomyelitis detection. Reproduced with permission.116 Copyright, 2023, Wiley-VCH. | ||
DNS is the method that involves the incorporation of active sites directly into the MOFs' structure during the synthesis process. This is achieved by introducing unstable small molecule ligands that can be easily removed before catalysis, leaving behind coordinated unsaturated metal ion centers. DNS is known for its convenience, as it streamlines the process of introducing defects during MOF formation. It is particularly useful when specific defect types or configurations are desired. However, one limitation of DNS is that it may lack precise control over the final configuration of the defects, and the outcome can sometimes be unpredictable.77
In contrast, PST is an approach that aims to introduce defects or functional groups after the MOFs' formation. This is achieved by employing various techniques under extreme conditions, such as harsh washing, ball milling, acid treatments, or plasma treatment. PST provides a high degree of control over the type and location of defects introduced into the MOFs. It allows for tailored defect engineering while preserving the original MOF structure and stability. PST is particularly effective when precise control over defect characteristics is essential for specific applications.
Both DNS and PST have their merits and considerations. The choice between them depends on the specific application and the desired level of control over defect characteristics in the MOFs. DNS offers convenience during MOF synthesis, while PST provides precision in defect engineering. Understanding the mechanisms underlying these synthesis methods is crucial for advancing research on MOF synthesis.
2.3 Characterisation of defective MOFs
To attain a comprehensive understanding of the defect coordination environment within MOFs, it is imperative to provide a detailed overview of characterization techniques. These techniques play a crucial role in revealing valuable insights into various aspects of defect sites, including their type, location, and quantity.124 Various techniques have been employed to detect defects in MOFs, primarily including X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), thermal gravimetric analysis (TG), nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy (FTIR), electron paramagnetic resonance (EPR), and more.XRD is a key technology for identifying lattice defects in MOFs, used to detect anomalies such as vacancies, dislocations, or other lattice irregularities. This method hinges on analyzing the diffraction patterns arising from X-ray interactions with the crystalline matrix of MOFs. Perturbations in these patterns due to lattice defects are discernible through meticulous analysis. Zhang et al., employed XRD to detect missing linker defects in modified UiO-66, which was evidenced by the deviation in diffraction patterns, most notably a broad peak at 2θ = 5–7 °C, divergent from that of standard UiO-66, as elucidated in Fig. 7A.125
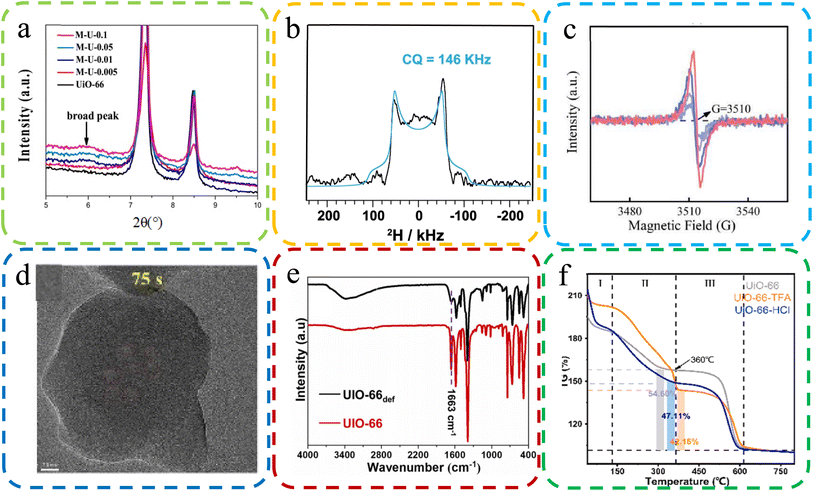 | ||
| Fig. 7 (a) XRD images with some missing linker defective UiO-66. Reproduced with permission.125 Copyright, 2019, Elsevier. (b) An NMR map of MOF-74 in the presence of a formate deficiency. Reproduced with permission.127 Copyright, 2023, Nature Communications. (c) EPR spectra of the oxygen-vacancy content of UiO-66-TFA. Reproduced with permission.47 Copyright, 2022, Elsevier. (d) HRTEM was applied to visualize the ZIF-7 sample defects. Reproduced with permission.126 Copyright, 2023, Elsevier. (e) FT-IR spectra of UIO-66 and cluster defects UIO-66 def. Reproduced with permission.128 Copyright, 2023, Elsevier. (f) TGA plots of body weight loss by UiO-66, UiO-66-TFA, and UiO-6-6-HCl. Reproduced with permission.129 Copyright, 2023, Elsevier. | ||
NMR spectroscopy serves as a robust tool for detecting and quantifying molecular-level defects in MOFs, particularly in discerning variations in functional groups and linkers. Anomalies in the NMR spectrum, such as shifts in peak positions, indicate the presence of defects. In recent research, Fu et al. leveraged solid-state 2H NMR on MOF-74, uncovering formate defects originating from DMF. This was inferred from a distinct broad 2H quadrupolar pattern, as delineated in Fig. 7B.127
EPR spectroscopy is instrumental in the identification of paramagnetic defects, such as unpaired electrons or oxygen vacancies within MOFs. EPR mainly determines the formation of defects via the interaction strength between magnetic moment and electromagnetic radiation. Peng et al. utilized EPR to probe oxygen vacancies in MOFs, observing symmetrical signals around 3510 G in UiO-66-TFA, the hallmark of oxygen vacancies, as depicted in Fig. 7C.47
HRTEM offers a profound visualization of the microstructure of MOFs, including explicit characterization of lattice defects. It provides high-resolution imagery that elucidates the location, morphology, and density of defects. Chen et al. utilized HRTEM to examine ZIF-7 samples, unearthing voids indicative of structural defects, as shown in Fig. 7D.126
FT-IR spectroscopy is a pivotal method for detecting structural defects in MOFs by scrutinizing vibrational modes. Alterations in the FT-IR spectrum, such as the broadening or shifting of peaks, are suggestive of defects. Kar et al. compared the FT-IR spectra of UIO-66 and UIO-66def, which revealed marked discrepancies, particularly in the –CO stretching peak at 1663 cm−1, indicative of structural defects, as evidenced in Fig. 7E.128
TGA is an essential technique for identifying defects in MOFs, achieved by analyzing weight loss patterns correlated with defect density. It methodically tracks weight loss during thermal treatment, with deviations from expected patterns signaling the presence of defects. Peng et al. employed TGA to evaluate the thermal stability of MOFs, which indicated the higher incidence of ligand defects in UiO-66-TFA compared to UiO-66-HCl, as illustrated in Fig. 7F.129
Beyond the primary characterization techniques outlined earlier, an array of supplementary methods stands to augment our comprehensive understanding of defects in MOFs. These additional techniques include high-resolution neutron powder diffraction (HRNPD),130 electrospray ionization mass spectrometry (ESI-MS),131 atomic force microscopy (AFM),132 and confocal fluorescence microscopy (CFM),133 alongside the strategic integration of multiple approaches. Each of these methods provides distinct and invaluable insights into the nature, distribution, and typology of defects within MOFs, thereby enriching our understanding of their structural complexities.
At its core, the characterization of structural defects in MOFs is a multifaceted task that demands the deployment of a diverse spectrum of analytical tools, each grounded in unique operational principles. By harnessing the strengths of these primary and supplementary techniques, researchers are equipped to construct a holistic view of defective MOF architectures. This enriched perspective is pivotal in demystifying the intricate ways in which defects influence the properties and applications of MOFs, thereby catalyzing progress in the realm of MOF research.
3. Defective MOFs: advancing sensor innovations
Introducing defects into MOFs has become an effective strategy for improving their detection performance. This seemingly counterintuitive approach is underpinned by a range of intricate mechanisms that collectively contribute to the improved sensing capabilities of these materials.78 Defect sites can introduce additional active sites that increase the material's capacity for adsorption and reactivity with the target molecule, thereby enhancing its performance. However, it is important to acknowledge that these defect sites may also lead to structural instability in the material, which can reduce its overall durability and long-term viability.79 Researchers must carefully balance the trade-off between the performance improvements gained through defect engineering and the potential consequences of structural instability.Defects in MOFs can create localized disruptions in the regular crystalline structure, leading to a myriad of effects that synergistically amplify detection performance.80 One key mechanism is the increased availability of active sites. Defects often create vacant sites or unsaturated metal centers, serving as preferred binding sites for target analytes. This enhanced availability of binding sites facilitates a higher degree of interaction between MOFs and analyte molecules, leading to heightened sensitivity in detection. Furthermore, defects can significantly alter the surface properties of MOFs. These structural irregularities translate into a larger surface area, effectively providing more room for molecules to adsorb and participate in chemical reactions. As a result, MOFs with defects can accommodate a larger quantity of analytes, leading to amplified signals and improved detectability.81
The introduction of defects can also influence the diffusion kinetics of molecules within the MOF framework. Defects can create more accessible diffusion pathways, enabling faster and more efficient movement of analytes to active sites.82 This streamlined diffusion enhances the response time of the sensing process, allowing for rapid and accurate detection. Another critical aspect is the modulation of electronic properties.83 Defects can introduce energy levels within the MOFs' electronic band structure, leading to altered charge transfer and electronic interactions.84 These changes can enhance the affinity of the MOFs for specific analytes, resulting in increased selectivity and sensitivity.85
However, it's essential to note that defects can induce structural strain, which imparts mechanical flexibility to MOFs. While this flexibility can enable conformational changes upon analyte binding, leading to a measurable signal change that is essential for certain sensing mechanisms, it can also contribute to the overall structural instability of the material. This potential instability may limit the material's long-term viability and durability.86
Importantly, defects can be tailored and engineered to elicit specific responses to target analytes.87–89 Through deliberate defect design, researchers can customize MOFs for diverse applications, spanning environmental monitoring to medical diagnostics. In conclusion, the introduction of defects in MOFs represents a sophisticated approach to boost detection performance, but it should be approached with careful consideration of the potential trade-offs. Through mechanisms such as increased active sites, enhanced surface area, altered diffusion kinetics, and modified electronic properties, defects synergistically enhance the interactions between MOFs and analytes. This results in improved sensitivity, selectivity, and response time, making defect-engineered MOFs highly promising for advanced sensing technologies.
3.1 Advanced biosensing
Biological molecules are fundamental building blocks that underpin the complexity and functioning of living organisms. Understanding and analyzing these molecules are crucial for various scientific disciplines, from medicine and biotechnology to environmental monitoring and food safety. As the intricacies of biological processes continue to be unraveled, the need for sensitive and specific detection methods becomes increasingly apparent. Biosensing, which accurately detects and quantifies biological molecules, is now vital in modern research and applications. Defective MOFs have garnered significant attention due to their substantial specific surface area, rendering them promising candidates as carriers for nanocrystal immobilization, thus emerging as prospective materials for the detection of small biological molecules.Tian et al.93 utilized a diverse ligand synthesis approach to create an efficient electrochemical autosensing method for Staphylococcus aureus detection. They employed mixed ligands to synthesize copper-based metal–organic frameworks encapsulated with Cu2O nanocrystals (ML-Cu2O@Cu-MOFs). These nanospheres, featuring multiple Cu valence states and a defect-rich crystal structure, exhibited high catalytic activity for thiamine hydrochloride and hydrogen peroxide (H2O2) assays. The sensor demonstrated low detection limits for thiamine hydrochloride and H2O2, along with robust stability and reproducibility (Fig. 8d). This biosensor based on ML-Cu2O@Cu-MOFs offers a promising approach for foodborne bacteria analysis and food safety assessment. Lu et al.102 developed a fluorescence-based method for quantitative chloramphenicol (CAP) detection using Cu/UiO-66, a bimetallic organic framework nanomaterial. Fig. 8a illustrates the concept of performance improvement before and after defect introduction. They employed double-labeled aptamers of CAP with phosphate and the fluorescent dye 6-carboxy-x-rhodamine (ROX). In the absence of CAP, Cu/UiO-66 quenched ROX fluorescence via photoinduced electron transfer. However, the presence of CAP induced a change in the aptamers' spatial structure, leading to ROX fluorescence recovery. This method demonstrated high sensitivity, selectivity, and a low detection limit of 0.09 nmol L−1, enabling precise CAP quantification. Enzyme immobilization enhances enzyme stability and activity by affixing them to a carrier. This technique improves biosensor sensitivity in complex environments, expanding its effectiveness in detecting a wider range of analytes (Fig. 8c depicts common methods of immobilizing enzymes using defective MOFs). However, conventional carrier materials might encounter limitations in certain scenarios, where in this interdisciplinary field, defect-engineered MOFs are emerging as a novel class of materials. In a related study, Feng et al.110 present an innovative dynamic strategy for enzyme immobilization within MOFs to address challenges in industrializing bioactive molecules. This approach leverages enzyme-mediated MOF dissociation, with enzymes acting as “macro ligands,” competing with original ligands. This competition triggers metal cluster release within MOFs, generating defects and facilitating gradual enzyme transport from surface to interior. Consequently, various enzymes can efficiently immobilize within MOFs, creating composites with robust enzymatic activities, protection, and high reusability (Fig. 8b). This method revolutionizes enzyme immobilization and enables efficient multienzyme bioreactors for cascade reactions. Meanwhile, in another sensor study that investigated enzyme concentration, Shao and colleagues111 demonstrated a novel approach to tackle the challenges of conductivity and emission efficiency in MOFs, which often limit their effectiveness in electrogenerated chemiluminescence (ECL) sensing applications. They achieved this by ingeniously combining an electroactive linker (H2-TCPP) with an ECL-active electrogenerated chemiluminescence linker (H4-TBAPy), resulting in the creation of mixed-linker defective ML-MOFs with significantly enhanced photoelectrochemical activity (Fig. 8e). Notably, the leading ML-MOF variant, denoted as M6-MOFs, exhibits a remarkable 15.4-fold improvement in ECL performance compared to single-linker MOFs. Equally impressive is the M6-MOFs' ECL efficiency, which boasts a remarkable 73-fold enhancement over commercial Ru(bpy)32+. This exceptional enhancement is attributed to the synergistic interplay between the two distinct linkers. Remarkably, researchers leverage M6-MOFs as a potent ECLphore, showcasing their capacity for sensitive and selective α-glucosidase detection. The applicability of this system extends to human serum samples, offering promising practicality. This work not only pioneers the advancement of MOFs' conductivity and ECL efficiency through strategic linker amalgamation and bandgap manipulation but also underscores the immense potential of defect-engineered MOFs for advanced ECL sensing applications.
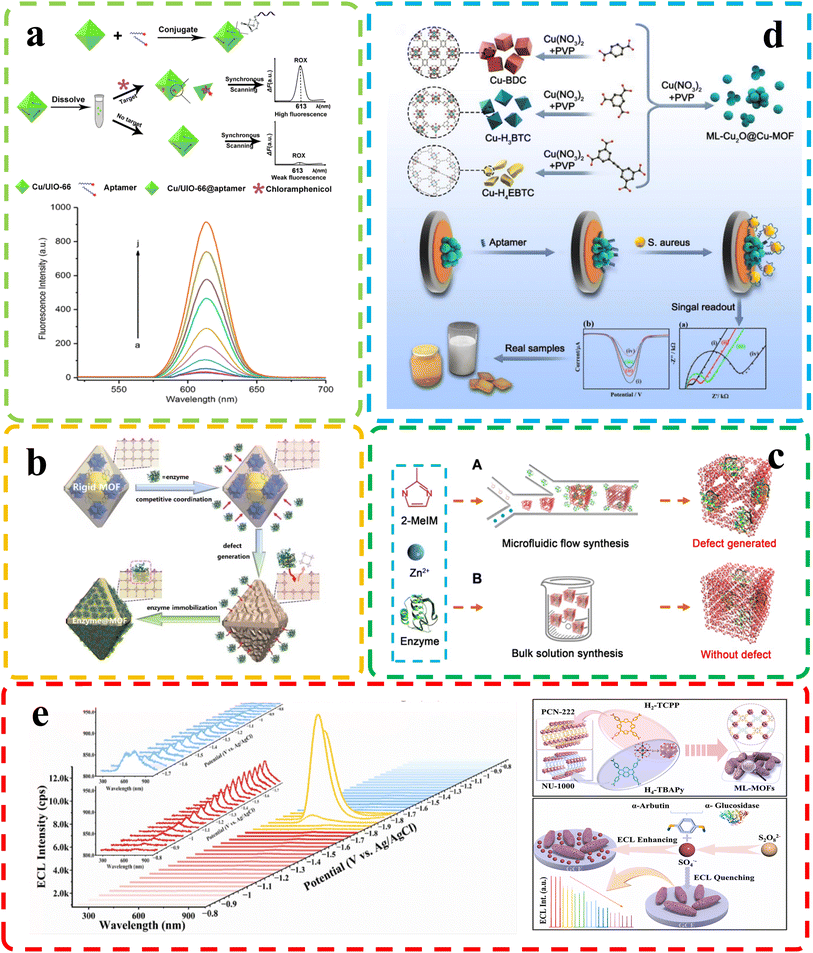 | ||
| Fig. 8 (a) Top: chloramphenicol detection principle; bottom: fluorescence of chloramphenicol at different concentrations. Reproduced with permission.102 Copyright, 2020, Nature. (b) Dynamic defect generation for enzyme immobilization via MOF dissociation equilibrium. Reproduced with permission.110 Copyright, 2023, Wiley-VCH. (c) Enzyme-MOF composite synthesis. Reproduced with permission.117 Copyright, 2020, Science. (d) Top: Cu-MOF-based Staphylococcus aureus biosensor; bottom: electrochemical biosensor with ML-Cu2O@Cu-MOFs for Staphylococcus aureus. Reproduced with permission.93 Copyright, 2023, Elsevier. (e) Top: ECL spectra of M6-MOFs on a GCE with K2S2O8; insets show spooled ECL spectra: red for evolution and blue for devolution. Bottom: M6-MOF preparation. Reproduced with permission.111 Copyright, 2023, Elsevier. | ||
3.2 Enhanced chemical sensing
Chemical molecules, both natural and synthetic, surround us in our daily lives and profoundly impact human health and the environment. The potential hazards posed by these molecules demand vigilant monitoring and accurate detection methods. Analyzing and identifying harmful chemical species is critical for ensuring public safety, environmental preservation, and the quality of various products. As scientific knowledge continues to evolve, the need for enhanced chemical sensing technologies becomes increasingly apparent. These innovative sensing methods hold the key to more effectively and efficiently detecting hazardous chemicals, leading to improved risk assessment, timely interventions, and better mitigation strategies.Sha and his team92 (Fig. 9b) focused on constructing defect-engineered MOFs with histidine-functionalized active sites (His-MIL-101) by the missing linker strategy. His-MIL-101 with a histidine unit in the iron active site exhibited double the peroxidase-like activity of parent MOFs. The introduction of histidine increased the specific surface area and provided an optimized electronic structure for active sites, promoting the generation of active intermediates (hydroxyl radicals). A His-MIL-101-based colorimetric biosensing platform was designed to accurately detect metallothioneins, demonstrating a wide detection range from 20 nM to 50 μM and a low detection limit of 10.49 nM.
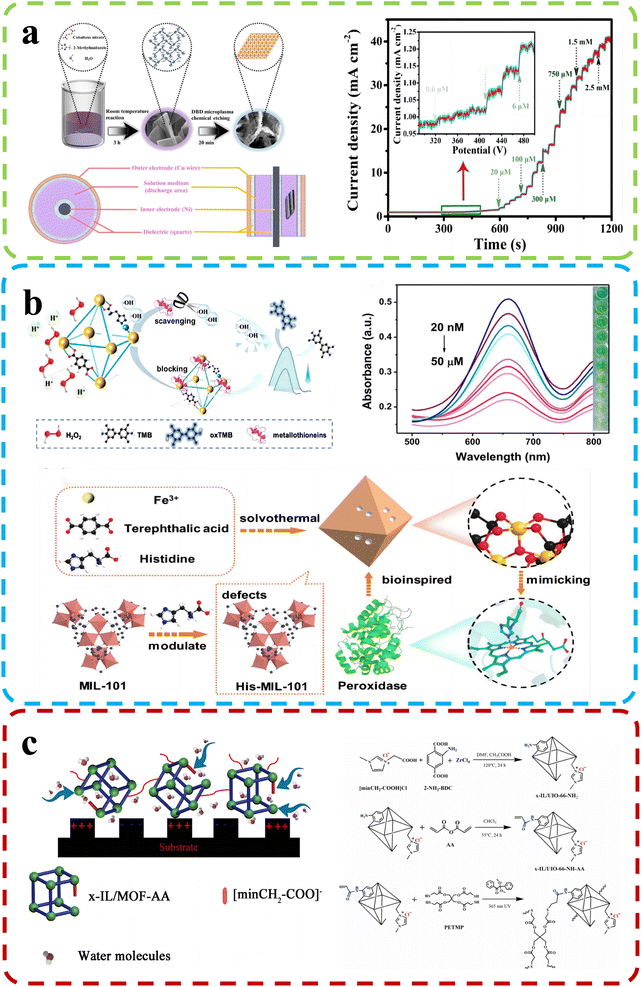 | ||
| Fig. 9 (a) Top: D-Co(OH)2 synthesis schematic and DBD reactor device structure. Bottom: Amperometric (i–t) curve of D-Co(OH)2/CC (applied potential: −0.2 V). Reproduced with permission.92 Copyright, 2022, Elsevier. (b) Top: the His-MIL-101 mechanism and performance for metallothionein detection. Bottom: Synthesis of His-MIL-101. Reproduced with permission.91 Copyright, 2022, Elsevier. (c) Top: Humidity sensing mechanism. Bottom: MOF-based polyelectrolyte synthesis. Reproduced with permission.95 Copyright, 2022, Elsevier. | ||
Researchers led by Wu developed high-performance humidity sensors using ionic liquid-modified MOF-derived polymer films.95
The preparation of the material and the mechanism of humidity detection are shown in Fig. 9c. By introducing defect engineering, they uniformly incorporated the ionic liquid ligand into the frameworks of UIO-66, resulting in a significant enhancement of hydrophilicity in the sensing materials. These defects enhanced adsorption and desorption properties, enabling the synthesis of functional MOF-based films via in situ photoinduced thiol–ene click reactions. The humidity sensor excelled in the 5% RH – 30% RH range, showing minimal hysteresis (approximately 0.2% RH) and rapid response/recovery times (0.6 s/1.7 s). Missing-linker defects facilitate analyte desorption kinetics, resulting in faster sensor recovery. Zhang et al.99 developed a method to fabricate microporous MOFs as efficient chemical sensors. They assembled UiO-66 crystals with controlled sizes and introduced missing-linker defects to create MOF optical sensors with adjustable optical properties. The sensors displayed rapid response (2.00 s) and short recovery times (3.00 s) to ethanol vapor. Mesoporous features significantly improved sensitivity (∼24.6% for saturated ethanol vapor), response speed (∼42.9%), and recovery speed (∼59.7%) compared to dense counterparts. Crystal size mildly influenced response speed but had a more profound effect. Crystal sizes mildly influence response speed but profoundly affect it. Similarly, under the guidance of defect engineering, Kang and their colleagues97 successfully synthesized highly stable 3D MOFs, namely DUT-67(Hf) and DUT-67(Zr), through a solvothermal reaction. These defect-rich MOFs demonstrated excellent proton conductivities and were employed as highly sensitive room temperature sensors for detecting ammonia. Under various relative humidity conditions (68%, 85%, and 98% RH), the sensors exhibited remarkable stability and sensitivity in the detection of volatile amine gases, with impressive detection limits of 0.5 ppm for methylamine, dimethylamine, and trimethylamine. Furthermore, derivatives of defect-rich MOFs have also exhibited exceptional sensing performance. For instance, Hu et al.91 created a defect-rich Co(OH)2 nanoenzyme sensor from MOF-derived cobalt via a room-temperature reaction and chemical etching with dielectric barrier discharge microplasma (Fig. 9a). It displayed superior catalytic activity for thiamine hydrochloride and hydrogen peroxide (H2O2) assays, with detection ranges of 0.0006 mM to 2.75 mM for thiamine hydrochloride and 0.001 mM to 5.5 mM for H2O2. The sensor also achieved low limits of detection at 14 nM for thiamine hydrochloride and 93 nM for H2O2. Its long-term durability exceeded 25 days, maintaining high sensitivity for TCL and H2O2 assays.
3.3 Environmental sensing
The environment, with its intricate web of ecosystems and natural processes, serves as the life-supporting foundation of our planet's biosphere. It sustains an incredible diversity of living organisms and provides vital resources essential for human well-being. However, in recent times, escalating anthropogenic activities have posed unprecedented challenges to environmental integrity, necessitating a deeper understanding and effective monitoring of environmental changes. By harnessing innovative sensing technologies, we can gain invaluable insights into environmental parameters, assess ecosystem health, and devise informed strategies for conservation and sustainable resource management. Defect-rich MOFs may help mitigate pesticide residue's environmental impact on human health.Yan et al.90 conducted a study involving the creation of a hierarchically porous enzyme@peptide-directed metal–organic framework nanoarchitecture (Fig. 10a). This unique nanostructure was achieved through the introduction of a γ-poly(L-glutamic acid) (PLGA) peptide surface modifier, resulting in both morphological evolution and the formation of defect structures. This modified MOFs-P exhibited expanded pore sizes, providing ample space for enzyme immobilization and facilitating improved substrate diffusion and material communication. These enzyme@peptide-directed MOFs-P played a pivotal role in the development of a plasmonic immunosensor designed for the highly sensitive detection of the imidacloprid pesticide. The enzyme@peptide-directed plasmonic immunosensor exhibited an impressive response and unparalleled selectivity, surpassing traditional immunoassays by a factor of 1000 in terms of sensitivity. Furthermore, the researchers employed a color-based detection method for rapid and precise concentration determination, with the imidacloprid pesticide as the model target. This innovative approach yielded a distinct blue-to-red color shift, enabling precise quantitative analysis through a smartphone-based homemade device and image-processing algorithm. This investigation has significant implications for the advancement of size-controlled nanoarchitectures in cutting-edge biosensors. Fu et al.94 reported their findings on the synthesis of defective copper-based metal–organic frameworks (Cu-MOFs) achieved by reacting Cu2+ ions with 1,3,5-benzene dicarboxylate linkers in the presence of acetic acid (Fig. 10c). These defective Cu-MOFs exhibited enhanced pesticide adsorption kinetics and greater adsorption capacities compared to their pristine counterparts. The researchers developed an efficient dispersive solid-phase extraction method known as Cu-MOFs-6, designed specifically for the rapid extraction of pesticides from food samples. This method allowed for wide-range pesticide detection, boasting low detection limits (ranging from 0.0067 to 0.0164 μg L−1) and impressive recovery rates (ranging from 81.03% to 109.55%) in spiked samples. Their innovative approach addresses the pressing need for efficient pesticide detection in food safety applications. Li and his team96 conducted comprehensive research on two distinct MOFs, denoted as MOF 1 and MOF 2, each containing an excess of either formic acid or benzoic acid, respectively (Fig. 10b). These MOFs served as versatile platforms with multifaceted functionalities, including sensing, adsorption, and catalytic reduction of chromium Cr(VI) ions. MOF 1, characterized by its fluorescence sensing and impressive Cr(VI) adsorption capabilities, exhibited an ultralow detection limit of 0.0176 ppm and a robust saturated adsorption capacity of 145.77 mg g−1. On the other hand, MOF 2 excelled in the photochemical removal of Cr(VI), achieving a remarkable reduction efficiency of 98.05% within just 70 minutes, maintaining an impressive 92.21% efficiency even after five consecutive photocatalytic cycles. This research showcases the adaptability of defect-rich MOFs for diverse environmental applications, offering a promising solution to environmental pollution challenges.
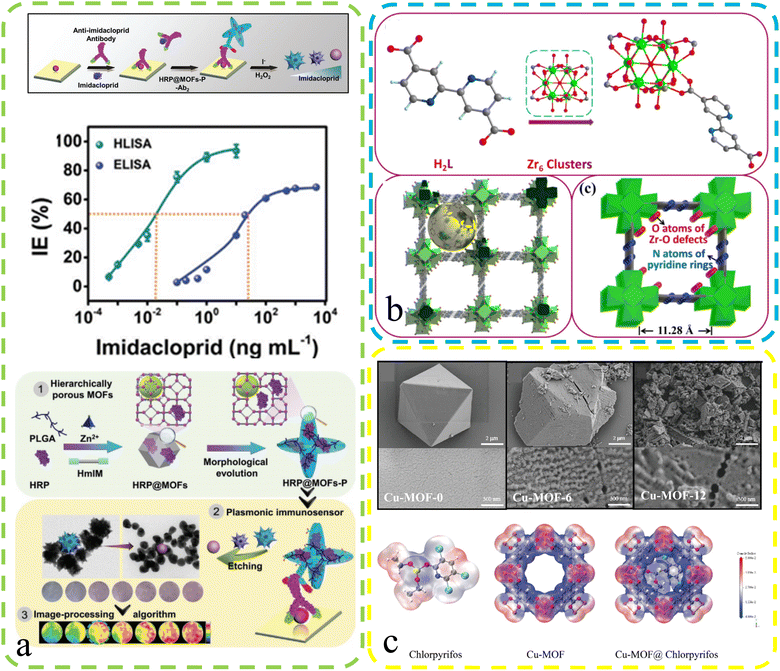 | ||
| Fig. 10 (a) Enzyme@MOFs-P plasma immunosensor: defects, mechanism, and performance. Reproduced with permission.90 Copyright, 2023, Wiley-VCH. (b) Schematic: Zr MOFs 1 assembly via repeated units with uncoordinated bipyridyl nitrogen. Reproduced with permission.96 Copyright, 2021, Royal Society of Chemistry. (c) Top: SEM images for Cu-MOF-0, Cu-MOF-6, and Cu-MOF-12. Bottom: Electron density maps for chlorpyrifos, Cu-MOF and Cu-MOF@chlorpyrifos. Reproduced with permission.94 Copyright, 2023, Elsevier. | ||
3.4 Biomedical sensing and health monitoring
The human body is a remarkable ecosystem consisting of complex biological molecules, each playing a crucial role in maintaining health and homeostasis. From DNA and proteins to hormones and metabolites, these biomolecules orchestrate intricate physiological processes essential for our well-being. As the understanding of human biology advances, so does the recognition of the importance of continuous health monitoring. Timely detection of changes in biomolecular profiles can serve as a valuable indicator of potential health issues, allowing for early interventions and personalized healthcare approaches.Biomedical sensing is crucial for precise biomolecular analysis. The use of defect-rich MOFs to load photosensitive materials for small biomolecule detection has gained recent attention. For instance, Wang et al.103 introduced a turn-on photoelectrochemical biosensor for highly sensitive protein kinase activity analysis and inhibitor evaluation, relying on MOFs' surface defect recognition and multiple signal amplification (Fig. 11b). They employed Zr-based UiO-66 MOFs with surface defects, accommodating [Ru(bpy)3]2+ photoactive dyes in their pores. These MOFs were linked to a phosphorylated Kemptide-modified TiO2/ITO electrode through chelation. Under visible light irradiation, excited electrons from [Ru(bpy)3]2+ in UiO-66 pores are injected into the TiO2 conduction band, generating photocurrent for kinase activity detection. Defective UiO-66's large surface area and high porosities increased [Ru(bpy)3]2+ and enhanced the photocurrent, enabling sensitive photoelectrochemical analysis with a detection limit of 0.0049 U mL−1 (S/N = 3). It was used for kinase inhibitor evaluation and PKA activity detection in MCF-7 cell lysates, promising clinical diagnosis and drug discovery. A carbon dot (CD) material derived through MOF calcination is also widely used for biosensors. Li et al.101 developed CDs@MOFs composites via MOF self-carbonization using UiO-66 type MOFs (UiO-66N) as platforms. They obtained near defect-free and defect-containing UiO-66N through the solvothermal reaction, with defects favoring self-carbonization. CDs@MOFs composites exhibited exceptional luminescence sensing for picric acid, with a high quenching constant (KSV = 4.0 × 105 M−1) and a low limit of detection (LOD: 6.54 × 10−7 M).
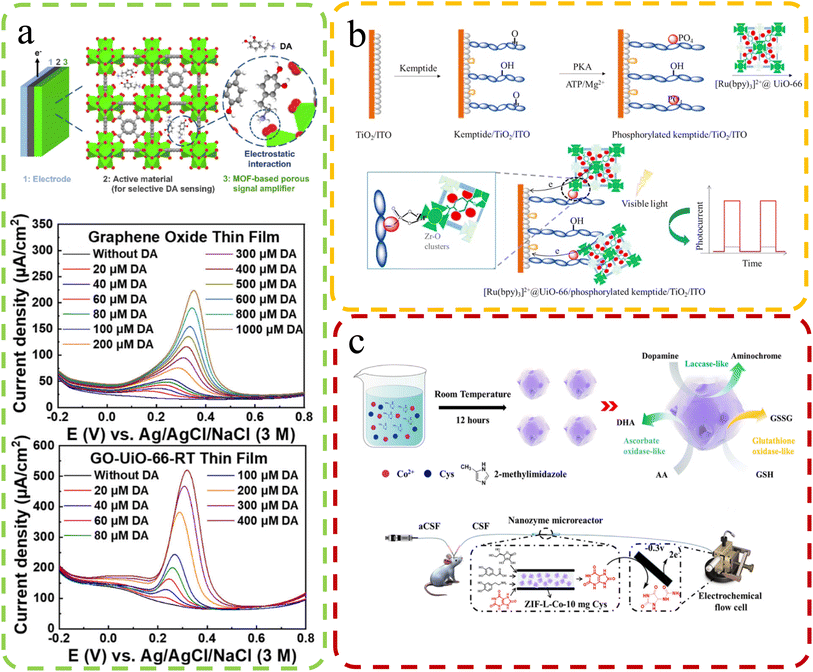 | ||
| Fig. 11 (a) Top: the electrochemical DA sensor structure – Zr (green), O (red), and C (grey) in UiO-66. Bottom: DPV curves of GO-modified and GO-UiO-66-RT-modified electrodes in 0.5 M KCl with varying DA concentrations. Reproduced with permission.100 Copyright 2023, American Chemical Society. (b) The configuration of a photoelectrochemical biosensor for kinase activity detection. Reproduced with permission.103 Copyright, 2020, Nature. (c) Synthesis & microdialysis-electrochemical UA detection with ZIF-L-Co-10 mg cysteine. Reproduced with permission.98 Copyright 2021, American Chemical Society. | ||
Electrodeposition is an important method for enhancing the sensing capabilities of defect-rich MOFs. Chang et al.100 designed electrode materials for electrochemical dopamine (DA) sensing, even in the presence of interferents such as AA and uric acid (UA). They synthesized nanoporous zirconium-based MOFs, UiO-66, with tunable missing-linker defects. A thin film of defective UiO-66 deposited on the electrode surface significantly amplifies the electrochemical sensing signal for DA. This signal enhancement is attributed to the hopping-based electrochemical process of irreversibly adsorbed DA in the defective MOFs. By using defective UiO-66 as a signal amplifier cast on a graphene oxide (GO) modified electrode, they achieved higher sensitivity (6.4-fold), lower limit of detection (0.23-fold), and better selectivity towards DA against AA and UA (2.6-fold and 1.2-fold, respectively) compared to a GO thin film (Fig. 11a). This approach enhances the sensing performance of electrochemical systems for DA detection. In the study conducted by Guoyuan Ren and colleagues98 (Fig. 11c), the focus is on enhancing the sensing performance of artificial nanozymes through the manipulation of structural defects in MOFs. The goal is to enhance the catalytic efficiency of nanozymes for accurate biomolecule analysis, addressing limitations such as the high cost and poor stability associated with natural enzymes. Their approach involves using structural defects in MOFs to regulate the catalytic efficiency of nanozymes. By introducing defects into a Co-containing zeolitic imidazolate framework (ZIF-L-Co) through cysteine doping, catalytic properties are significantly enhanced. Activities such as ascorbate oxidase-like, glutathione oxidase-like, and laccase-like improve over 5, 2, and 3 times, respectively. This improvement can be attributed to sulfur doping disrupting the cobalt–nitrogen equilibrium, enhancing oxygen adsorption and oxidase-like activity. The significance lies in potential sensing applications. Advanced ZIF-L-Co nanozymes are employed in a microreactor integrated into an online electrochemical system. This system eliminates interfering compounds, enabling continuous monitoring of uric acid changes in rat brains after ischemia-reperfusion injury. This demonstrates the practical utility of defect-tuned nanozymes in biomedical sensing applications.
4. Conclusion and outlook
Defect engineering in MOFs has emerged as a transformative strategy to enhance material properties and tailor their applications across diverse fields. The deliberate introduction of defects offers remarkable opportunities for tuning the structural, electronic, and surface characteristics of MOFs. Controlling defects in MOFs can effectively enhance their adsorption, catalytic conversion, and degradation performance toward pollutants. This paper provides an overview of the distinctions between defective MOFs and regular MOFs and further discusses synthesis methods, specific classifications, and reasons for application, as well as the pros and cons of the DNS and PST for defect engineering in MOFs. Meanwhile, the characterisation of defects in some MOFs is presented. Additionally, the study analyzes synthesis methods and performance modulation strategies for defect-engineered MOFs, along with the current applications of defect-rich MOF sensors in areas such as biosensing, chemical sensing, environmental monitoring, and health diagnostics.In summary, the development of defect-engineered MOFs has ushered in a new era of understanding regarding the types and concentrations of defects in these materials. This progress is driven by a combination of precise synthesis techniques and cutting-edge characterization tools. Advanced methods such as HRTEM and HRNPD have allowed us to examine defects in unprecedented detail. Emerging techniques, including AFM, CFM, and others, are expanding our ability to study beam-sensitive MOF materials. These advancements in characterization techniques are poised to enhance our comprehension of defects and enable precise quantification.
Controlled synthesis techniques, exemplified by the development of ordered defects in specific MOFs, have brought us closer to the prospect of a universal approach for generating defects. However, it's essential to recognize that when defect engineering offers ways to adjust structural and functional properties, it often involves a trade-off with structural stability. Striking the balance between performance enhancement and stability remains a critical challenge in the design and customization of materials through defect engineering.
The complexity of defects, including their spatial distribution and the proportions of different defect types, presents a central challenge in defect engineering. Herein, computational chemistry emerges as a valuable tool for deciphering the intricate relationship between defects and material properties. Computational methods, such as density functional theory (DFT), are likely to play a more targeted role in the design of defective MOFs for specific applications, guiding experimental synthesis and characterization. The future of defect engineering may see a deliberate integration of computational tools to predict mechanical stability, catalytic mechanisms, and other aspects related to defective MOFs.
To effectively tailor defect-rich MOFs for specific sensing tasks, achieving precision in controlling defect formation and concentration stands as a challenging yet indispensable objective. Fine-tuning these attributes can yield MOFs with unparalleled sensitivity and selectivity, enhancing their effectiveness as sensors. Looking ahead, the synthesis landscape is poised to evolve. Ongoing exploration into advanced synthesis methods, combined with a refined understanding of how structural intricacies correlate with material properties, will undoubtedly lead to groundbreaking progress in defect-based MOFs. The integration of advanced materials such as defective MOFs and sensor technologies holds the promise of reshaping sensing paradigms. By strategically combining the distinctive attributes of defect-rich MOFs with synergistic components, we are on the path to engineering sensors that exhibit exceptional accuracy, responsiveness, and robustness across diverse applications. Furthermore, as researchers continue to forge ahead with determination, pioneering novel synthesis pathways and unraveling the underpinnings of MOFs' functionality, it appears that this field is destined for transformative leaps.
Data availability
The raw data cannot be made public at the request of the affiliated institute.Author contributions
DA designed the review and prepared the manuscript. YL edited the manuscript and investigated the conceptualization. LC revised the manuscript. All authors contributed to the work and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of China (52302105 and 51962032), the program for Strong Youth Technology Leading Talents (2023CB008-11), the Youth Innovative Top Talents Fund, Shihezi University (CXBJ202203), the Youth Science and Technology Innovation Leading Talent Fund, Bashi Shihezi (2023RC02), and the Youth Innovation Promotion Association CAS (2021433).References
- K. Sun, J. Pang, Y. Zheng, F. Xing, R. Jiang, J. Min, J. Ye, L. Wang, Y. Luo, T. Gu and L. Chen, J. Alloys Compd., 2022, 923, 166470 CrossRef CAS.
- C. Fan, X. Zhang, L. Chen, H. Fu, H. Li, J. Hou, F. Yu, H. Li, Y. Shi and X. Guo, New J. Chem., 2019, 43(38), 15066–15071 RSC.
- J. Pang, H. Fu, W. Kong, R. Jiang, J. Ye, Z. Zhao, J. Hou, K. Sun, Y. Zheng and L. Chen, Chem. Eng. J., 2022, 433, 133854 CrossRef CAS.
- H. Li, L. Chen, P. Jin, H. Lv, H. Fu, C. Fan, S. Peng, G. Wang, J. Hou, F. Yu and F. Shi, Dalton Trans., 2020, 49(20), 6587–6595 RSC.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402(6759), 276–279 CrossRef CAS.
- C. Ni, H. Zheng, W. Liu, L. Wu, R. Li, K. Zhou and W. Zhang, Adv. Funct. Mater., 2023, 2301075 CrossRef CAS.
- M. Xiao, C. Wu, J. Zhu, C. Zhang, Y. Li, J. Lyu, W. Zeng, H. Li, L. Chen and S. Mu, Nano Res., 2023, 1–8 Search PubMed.
- D. Wu, W. Yan, H. Xu, E. Zhang and Q. Li, Inorg. Chim. Acta, 2017, 460, 93–98 CrossRef CAS.
- K. D. Nguyen, N. T. Vo, K. T. M. Le, K. V. Ho, N. T. S. Phan, P. H. Ho and H. V. Le, New J. Chem., 2023, 47(13), 6433–6447 RSC.
- G. Qu, P. Jia, T. Zhang, Z. Li, C. Chen and Y. Zhao, Chemosphere, 2022, 288, 132594 CrossRef CAS PubMed.
- C. Zhang, C. Han, D. S. Sholland and J. R. Schmidt, J. Phys. Chem. Lett., 2016, 7(3), 459–464 CrossRef CAS PubMed.
- H. Park, S. Kim, B. Jung, M. H. Park, Y. Kim and M. Kim, Inorg. Chem., 2018, 57(3), 1040–1047 CrossRef CAS PubMed.
- Z. Wang, J. Huang, W. Wang, X. Wang, Y. Wang, B. Gao and Q. Li, Chem. Eng. J., 2023, 466, 143194 CrossRef CAS.
- Y. Song, M. Xu, X. Liu, Z. Li, C. Wang, Q. Jia, Z. Zhang and M. Du, Electrochim. Acta, 2021, 368, 137609 CrossRef CAS.
- K. Sun, Y. Shen, J. Min, J. Pang, Y. Zheng, T. Gu, G. Wang and L. Chen, Chem. Eng. J., 2023, 454, 140394 CrossRef CAS.
- Y. An, S. Dong, Z. He, Q. Xie and T. Huang, Microchem. J., 2022, 172, 106942 CrossRef CAS.
- J. Long, Q. Yao, X. Zhang, H. Wu and Z. Lu, Appl. Catal., B, 2023, 320, 121989 CrossRef CAS.
- B. Yeh, S. Chheda, S. D. Prinslow, A. S. Hoffman, J. Hong, J. E. Perez-Aguilar, S. R. Bare, C. C. C. Lu, L. Gagliardi and A. Bhan, J. Am. Chem. Soc., 2023, 145(6), 3408–3418 CrossRef CAS PubMed.
- Y. Wu, H. Wang, J. Peng, J. Zhang and M. Ding, Chem. Eng. J., 2023, 454, 140156 CrossRef CAS.
- O. Basu, S. Mukhopadhyay, S. Laha and S. K. Das, Chem. Mater., 2022, 34(15), 6734–6743 CrossRef CAS.
- Y. Meng, Z. Ma, Y. Huang and Y. Song, Anal. Sci., 2023, 1–9 Search PubMed.
- I. Ahmed, H. J. Lee and S. H. Jhung, J. Mol. Liq., 2021, 344, 117765 CrossRef CAS.
- A. K. Kar, R. Sarkar, A. K. Manal, R. Kumar, S. Chakraborty, A. Rajeev and R. Srivastava, Appl. Catal., B, 2023, 325, 122385 CrossRef CAS.
- L. Pukdeejorhor, S. Wannapaiboon, J. Berger, K. Rodewald, S. Thongratkaew, S. Impeng, J. Warnan, S. Bureekaew and R. A. Fischer, J. Mater. Chem. A, 2023, 11(16), 9143–9151 RSC.
- Z. Fang, B. Bueken, D. E. De Vos and P. D. R. A. Fischer, Angew. Chem., Int. Ed., 2015, 54(25), 7234–7254 CrossRef CAS PubMed.
- S. Dissegna, K. Epp, W. R. Heinz, G. Kieslich and R. A. Fischer, Adv. Mater., 2018, 30(37), 1704501 CrossRef PubMed.
- C. H. Shim, S. Oh, S. Lee, G. Lee and M. Oh, RSC Adv., 2023, 13(12), 8220–8226 RSC.
- Y. Wang and R. Yu, J. Environ. Chem. Eng., 2023, 110422 CrossRef CAS.
- J. Li, K. K. Lu, L. H. Xu, Y. X. Li, H. Li, G. Shu, X. J. Zhang, R. S. Marks, S. Cosnier and D. Shan, Analyst, 2022, 147(1), 72–79 RSC.
- C. Zhang, X. Zhang, Z. Tao, B. Li, D. Zhao, H. Gao, Z. Zhu, G. Wang and X. Shu, Chem. Eng. J., 2023, 455, 140487 CrossRef CAS.
- N. Prasetya and K. Li, Sep. Purif. Technol., 2022, 301, 122024 CrossRef CAS.
- Y. Feng, X. Cao, L. Zhang, J. Li, S. Cui, Y. Bai, K. Chen and J. Ge, Chem. Eng. J., 2022, 439, 135736 CrossRef CAS.
- Y. Gu, B. A. Anjali, S. Yoon, Y. Choe, Y. G. Chung and D. W. Park, J. Mater. Chem. A, 2022, 10(18), 10051–10061 RSC.
- L. Duan, H. Jiang, W. Wu, D. Lin and K. Yang, J. Hazard. Mater., 2023, 445, 130426 CrossRef CAS PubMed.
- C. Yang, H. Wu, J. Yun, J. Jin, H. Meng, J. Caro and J. Mi, Adv. Mater., 2023, 2210235 CrossRef CAS PubMed.
- F. Yan, F. Cheng, C. Guo, G. Liang, S. Zhang, S. Fang and Z. Zhang, J. Colloid Interface Sci., 2023, 641, 59–69 CrossRef CAS PubMed.
- H. Chen, J. Wan, Z. Yan, Y. Ma, Y. Wang, Y. Xie and J. Hou, J. Cleaner Prod., 2022, 348, 131367 CrossRef CAS.
- H. An, W. Tian, X. Lu, H. Yuan, L. Yang, H. Zhang, H. Shen and H. Bai, Chem. Eng. J., 2023, 144052 CrossRef CAS.
- X. Liu, W. Qi, Y. Wang, R. Su and Z. He, Nanoscale, 2017, 9(44), 17561–17570 RSC.
- I. Abánades Lázaro, C. J. R. Wells and R. S. Forgan, Angew. Chem., 2020, 132(13), 5249–5255 CrossRef.
- H. Yuan, S. A. Alateeqi Aljneibi, J. Yuan, Y. Wang, H. Liu, J. Fang, C. Tang, X. Yan, H. Cai and Y. Gu, Adv. Mater., 2019, 31(11), 1807161 CrossRef PubMed.
- M. J. Cliffe, J. A. Hill, C. A. Murray, F. X. Coudert and A. L. Goodwin, Phys. Chem. Chem. Phys., 2015, 17(17), 11586–11592 RSC.
- G. Zhang, L. Jin, R. Zhang, Y. Bai, R. Zhu and H. Pang, Coord. Chem. Rev., 2021, 439, 213915 CrossRef CAS.
- Y. N. Chang, C. H. Shen, C. W. Huang, M. D. Tsai and C. W. Kung, ACS Appl. Nano Mater., 2023, 6(5), 3675–3684 CrossRef CAS.
- V. V. Butova, O. A. Burachevskaya, I. V. Ozhogin, G. S. Borodkin, A. G. Starikov, S. Bordiga, A. Damin, K. P. Lillerud and A. V. Soldatov, Microporous Mesoporous Mater., 2020, 305, 110324 CrossRef CAS.
- E. Moumen, L. Bazzi and S. El Hankari, Coord. Chem. Rev., 2022, 455, 214376 CrossRef CAS.
- M. Peng, D. You, H. Shi, P. Shao, W. Ren, L. Yang, X. Sheng, J. Shao, X. Ding and L. Ding, Chem. Eng. J., 2022, 448, 137612 CrossRef CAS.
- P. Wang, L. Sun, J. Ye, Q. Liu, Z. Fei, X. Chen, Z. Zhang, J. Tang, M. Cui and X. Qiao, Microporous Mesoporous Mater., 2021, 312, 110778 CrossRef CAS.
- A. A. Tereshchenko, V. V. Butova, A. A. Guda, O. A. Burachevskaya, A. L. Bugaev, A. N. Bulgakov, A. A. Skorynina, Y. V. Rusalev, I. V. Pankov and V. A. Volochaev, Inorg. Chem., 2022, 61(9), 3875–3885 CrossRef CAS PubMed.
- Y. Shan, G. Zhang, Y. Shi and H. Pang, Cell Rep. Phys. Sci., 2023, 4(3), 101301 CrossRef CAS.
- F. Vermoortele, B. Bueken, G. L. Bars, B. V. d. Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier and V. V. Speybroeck, J. Am. Chem. Soc., 2013, 135(31), 11465–11468 CrossRef CAS PubMed.
- G. C. Shearer, S. Chavan, S. Bordiga, S. Svelle, U. Olsbye and K. P. Lillerud, Chem. Mater., 2016, 28(11), 3749–3761 CrossRef CAS.
- X. Feng, H. S. Jena, K. Leus, G. Wang, J. Ouwehand and P. V. D Voort, J. Catal., 2018, 365, 36–42 CrossRef CAS.
- Z. Fan, Z. Wang, M. Cokoja and R. A. Fischer, Catal. Sci. Technol., 2021, 11(7), 2396–2402 RSC.
- B. N. Truong, D. D. Borges, J. Park, J. S. Lee, D. Jo, J. S. Chang, S. J. Cho, M. Guillaume, K. H. Cho and U. H. Lee, Adv. Sci., 2023, 2301311 CrossRef CAS PubMed.
- J. Zhou, X. Zeng, L. Dong, L. Chen, X. Wei, Y. Liu, L. Shi, L. Yu and J. Fu, Chem. Eng. J., 2023, 466, 143243 CrossRef CAS.
- X. C. Zhou, C. Liu, J. Su, Y. F. Liu, Z. Mu, Y. Sun, Z. M. Yang, S. Yuan, M. Ding and J. Zuo, Angew. Chem., Int. Ed., 2023, 62(10), e202211850 CrossRef CAS PubMed.
- W. Tang, Z. Yu, H. Chen, R. Jiang, J. Huang, S. Li, Y. Hou, M. Wang, H. Pang and J. Liu, J. Colloid Interface Sci., 2023, 644, 358–367 CrossRef CAS PubMed.
- Y. Cao, X. Mi, X. Li and B. Wang, Front. Chem., 2021, 9, 673738 CrossRef CAS PubMed.
- K. Epp, I. Luz, W. R. Heinz, A. Rapeyko, X. Li and R. A. Fischer, ChemCatChem, 2020, 12(6), 1720–1725 CrossRef CAS.
- S. Yu, J. Dong, H. Wang, S. Li, H. Zhu and T. Yang, J. Mater. Chem. A, 2022, 10(48), 25453–25462 RSC.
- J. A. Villajos, N. Jagorel, S. Reinsch and F. Emmerling, Front. Mater., 2019, 6, 230 CrossRef.
- A. F. Sapnik, D. N. Johnstone, S. M. Collins, G. Divitini, A. M. Bumstead, C. W. Ashling, P. A. Chater, D. S. Keeble, T. Johnson and D. A. Keen, Dalton Trans., 2021, 50(14), 5011–5022 RSC.
- S. Chaemchuen, Z. Luo, K. Zhou, B. Mousavi, S. Phatanasri, M. Jaroniec and F. Verpoort, J. Catal., 2017, 354, 84–91 CrossRef CAS.
- M. Karimi, H. Mohebali, S. Sadeghi, S. Vahid, A. Mahjoub and A. Heydari, Microporous Mesoporous Mater., 2021, 322, 111054 CrossRef CAS.
- R. J. Davey, S. L. M. Schroeder and J. H. Ter Horst, Angew. Chem., Int. Ed., 2013, 52(8), 2166–2179 CrossRef CAS.
- G. Ye, H. Wang, X. Zeng, L. Wang and J. Wang, Appl. Catal., B, 2021, 299, 120659 CrossRef CAS.
- F. N. Shi, Y. Hu, X. Wang, X. Sun, M. Lu, G. Shia and G. Xun, Dalton Trans., 2017, 46(6), 1936–1942 RSC.
- O. Karagiaridi, N. A. Vermeulen, R. C. Klet, T. C. Wang, P. Z. Moghadam, S. S. Al-Juaid, J. F. Stoddart, J. T. Hupp and O. K. Farha, Inorg. Chem., 2015, 54(4), 1785–1790 CrossRef CAS PubMed.
- C. T. Lollar, J. S. Qin, J. Pang, S. Yuan, B. Becker and H. C. Zhou, Langmuir, 2018, 34(46), 13795–13807 CrossRef CAS PubMed.
- H. G. T. Nguyen, M. H. Weston, O. K. Farha, J. T. Hupp and S. T. Nguyen, CrystEngComm, 2012, 14(12), 4115–4118 RSC.
- X. Kang, K. Lyu, L. Li, J. Li, L. Kimberley, B. Wang, L. Liu, Y. Cheng, M. D. Frogley and S. Rudić, Nat. Commun., 2019, 10(1), 4466 CrossRef PubMed.
- G. C. Shearer, J. G. Vitillo, S. Bordiga, S. Svelle, U. Olsbye and K. P. Lillerud, Chem. Mater., 2016, 28(20), 7190–7193 CrossRef CAS.
- P. Yang, F. Mao, Y. Li, Q. Zhuang and J. Gu, Chem. –Eur. J., 2018, 24(12), 2962–2970 CrossRef CAS PubMed.
- J. Szanyi, M. Daturi, G. Clet, D. R. Baerc and C. H. F. Peden, Phys. Chem. Chem. Phys., 2012, 14(13), 4383–4390 RSC.
- Z. Wang, H. Sezen, J. Liu, C. Yang, S. E. Roggenbuck, K. Peikert, M. Fröba, A. Mavrandonakis, B. Supronowicz and T. Heine, Microporous Mesoporous Mater., 2015, 207, 53–60 CrossRef CAS.
- A. F. Zorzo, H. C. Nunes, R. C. Lunardi, R. A. Michelin and S. S. Kanhere, 2018 Eighth Latin-American Symposium on Dependable Computing (LADC), IEEE, 2018, pp. 1–9 Search PubMed.
- D. K. Yoo and S. H. Jhung, Appl. Catal., A, 2023, 659, 119170 CrossRef CAS.
- Y. Zhang, S. Lv, L. Jiang, F. Liu, J. Wang, Z. Yang, B. Wang, R. You, C. Wang and X. Yan, ACS Sens., 2021, 6(12), 4435–4442 CrossRef CAS.
- L. Han, P. Qin, M. Li, D. Li, M. Mu, Y. Gao, S. Zhu, M. Lu and Z. Cai, Chem. Eng. J., 2023, 456, 140969 CrossRef CAS.
- A. Melillo, M. Cabrero, S. Navalón, M. Álvaro, B. Ferrer and H. García, Appl. Catal., B, 2020, 278, 119345 CrossRef CAS.
- M. Fu, X. Deng, S.-Q. Wang, F. Yang, L.-C. Lin, M. J. Zaworotko and Y. Dong, Sep. Purif. Technol., 2022, 288, 120620 CrossRef CAS.
- H. Sun, G. Song, W. Gong, W. Lu, S. Cong and Z. Zhao, Nano Res., 2022, 15(6), 5347–5354 CrossRef CAS.
- Z. Liu, J. Xu, F. Zhang, L. Ji and Z. Shi, Int. J. Hydrogen Energy, 2023, 48(39), 14622–14632 CrossRef CAS.
- G. Ye, L. Wan, Q. Zhang, H. Liu, J. Zhou, L. Wu, X. Zeng, H. Wang, X. Chen and J. Wang, Inorg. Chem., 2023, 62(10), 4248–4259 CrossRef CAS PubMed.
- C. Guo, T. Wang, L. Zhang, T. Chen, C. Guo, A. Hassan, N. Akram, Y. Koua and J. Wang, Nanoscale, 2022, 14(41), 15316–15326 RSC.
- S. Gong, Y. Niu, X. Teng, X. Liu, M. Xu, C. Xu, T. J. Meyer and Z. Chen, Appl. Catal., B, 2022, 310, 121333 CrossRef CAS.
- M. Islamov, P. Boone, H. Babaei, A. J. H. McGaughey and C. E. Wilmer, Chem. Sci., 2023, 14, 6592–6600 RSC.
- I. G. Koryakina, S. V. Bachinin, E. N. Gerasimova, M. V. Timofeeva, S. A. Shipilovskikh, A. S. Bukatin, A. Sakhatskii, A. S. Timin, V. A. Milichko and M. V. Zyuzin, Chem. Eng. J., 2023, 452, 139450 CrossRef CAS.
- X. Yan, D. Su, H. Li, X. Zhao, X. Liu, F. Liu, P. Sun and G. Lu, Adv. Funct. Mater., 2023, 2215192 CrossRef CAS.
- P. Hu, H. Qin, K. Hu, R. Dai, Z. Wang and K. Huang, J. Colloid Interface Sci., 2022, 628, 597–606 CrossRef CAS.
- M. Sha, W. Xu, Y. Wu, L. Jiao, Y. Chen, J. Huang, Y. Tang, W. Gu and C. Zhu, Sens. Actuators, B, 2022, 366, 131927 CrossRef CAS.
- J.-Y. Tian, X. Liu, S. Zhang, K. Chen, L. Zhu, Y. Song, M. Wang, Z. Zhang and M. Du, Food Chem., 2023, 402, 134357 CrossRef CAS PubMed.
- Q. Fu, X. Jia, S. Zhang, J. Zhang, D. Sun-Waterhouse, C. Wang, G. I. N. Waterhouse and P. Wu, Food Chem., 2023, 423, 136319 CrossRef CAS PubMed.
- K. Wu, Y. Yu, Z. Hou, X. Guan, H. Zhao, S. Liu, X. Yang, T. Fei and T. Zhang, Sens. Actuators, B, 2022, 355, 131136 CrossRef CAS.
- Q. Li, X. Cai, L.-H. Chen, B.-B. Guan, Z.-L. Fan, W. Zhu and D.-X. Xue, Inorg. Chem., 2021, 60(11), 8143–8153 CrossRef CAS PubMed.
- L.-L. Kang, C. Xing, Y.-X. Jin, L.-X. Xie, Z.-F. Li and G. Li, Inorg. Chem., 2023, 62(7), 3036–3046 CrossRef CAS PubMed.
- G. Ren, F. Dong, Z. Zhao, K. Li and Y. Lin, ACS Appl. Mater. Interfaces, 2021, 13(44), 52987–52997 CrossRef CAS PubMed.
- R. Zhang, D. Zhang, Y. Yao, Q. Zhang, Y. Xu, Y. Wu, H. Yu and G. Lu, ACS Appl. Mater. Interfaces, 2019, 11(23), 21010–21017 CrossRef CAS PubMed.
- Y.-N. Chang, C.-H. Shen, C.-W. Huang, M.-D. Tsai and C.-W. Kung, ACS Appl. Nano Mater., 2023, 6(5), 3675–3684 CrossRef CAS.
- L.-X. Li, S. He, S. Zeng, W.-T. Chen, J.-W. Ye, H.-L. Zhou and X.-C. Huang, J. Mater. Chem. C, 2023, 11(1), 321–328 RSC.
- Z. Lu, Y. Jiang, P. Wang, W. Xiong, B. Qi, Y. Zhang, D. Xiang and K. Zhai, Anal. Bioanal. Chem., 2020, 412, 5273–5281 CrossRef CAS PubMed.
- Z. Wang, Z. Yan, F. Wang, J. Cai, L. Guo, J. Su and Y. Liu, Biosens. Bioelectron., 2017, 97, 107–114 CrossRef CAS PubMed.
- R. T. Jerozal, T. A. Pitt, S. N. MacMillan and P. J. Milner, J. Am. Chem. Soc., 2023, 145(24), 13273–13283 CrossRef CAS PubMed.
- S. Karmakar, S. Barman, F. A. Rahimi, D. Rambabu, S. Nath and T. K. Maji, Nat. Commun., 2023, 14(1), 4508 CrossRef CAS PubMed.
- Z. Jiang, W. Xue, H. Huang, H. Zhu, Y. Sun and C. Zhong, Chem. Eng. J., 2023, 454, 140093 CrossRef CAS.
- Y. Liang, Z. Zhang, X. Su, X. Feng, S. Xing, W. Liu, R. Huang and Y. Liu, Angew. Chem., 2023, e202309030 CAS.
- X. Feng, J. Hajek, H. S. Jena, G. Wang, S. K. P. Veerapandian, R. Morent, N. De Geyter, K. Leyssens, A. E. J. Hoffman and V. Meynen, J. Am. Chem. Soc., 2020, 142(6), 3174–3183 CrossRef CAS PubMed.
- E. Massahud, H. Ahmed, R. Babarao, Y. Ehrnst, H. Alijani, C. Darmanin, B. J. Murdoch, A. R. Rezk and L. Y. Yeo, Small Methods, 2023, 2201170 CrossRef CAS.
- Y. Feng, R. Shi, M. Yang, Y. Zheng, Z. Zhang and Y. Chen, Angew. Chem., 2023, 135(20), e202302436 CrossRef.
- M. Shao, Y. Sun, Y. Li, Z. Wu, X. Li, R. Zhang and L. Zhang, Biosens. Bioelectron., 2023, 237, 115530 CrossRef CAS PubMed.
- C. A. Trickett, K. J. Gagnon, S. Lee, F. Gándara, H.-B. Bürgi and O. M. Yaghi, Angew. Chem., Int. Ed., 2015, 54(38), 11162–11167 CrossRef CAS PubMed.
- J. Ren, M. Ledwaba, N. M. Musyoka, H. W. Langmi, M. Mathe, S. Liao and W. Pang, Coord. Chem. Rev., 2017, 349, 169–197 CrossRef CAS.
- X. Ma, L. Wang, Q. Zhang and H.-L. Jiang, Angew. Chem., Int. Ed., 2019, 58(35), 12175–12179 CrossRef CAS PubMed.
- G. Cai, X. Ma, M. Kassymova, K. Sun, M. Ding and H.-L. Jiang, ACS Cent. Sci., 2021, 7(8), 1434–1440 CrossRef CAS PubMed.
- Q. Zheng, X. Liu, S. Gao, Z. Cui, S. Wu, Y. Liang, Z. Li, Y. Zheng, S. Zhu and H. Jiang, Small, 2023, 2207687 CrossRef CAS PubMed.
- C. Hu, Y. Bai, M. Hou, Y. Wang, L. Wang, X. Cao, C.-H. Chan, H. Sun, W. Li, J. Ge and K. Ren, Sci. Adv., 2020, 6(5), eaax5785 CrossRef CAS PubMed.
- M. Bagheri, A. Melillo, B. Ferrer, M. Y. Masoomi and H. Garcia, ACS Appl. Mater. Interfaces, 2021, 14(1), 978–989 CrossRef PubMed.
- Q. Zhao, Q. Du, Y. Yang, Z. Zhao, J. Cheng, F. Bi, X. Shi, J. Xu and X. Zhang, Chem. Eng. J., 2022, 433, 134510 CrossRef CAS.
- X. Zhang, D. Zhang, L. Liu, K. Zhang, Y. Zhang, J. Zhao, L. Han, M. Jing, J. Liu and C. Yan, Chem. Eng. J., 2023, 467, 143360 CrossRef CAS.
- F. Pambudi, N. Pratiwi and U. Chusnawati, Mater. Today Commun., 2023, 35, 105967 CrossRef CAS.
- C. Tong, G. Cai, Y. Yang, T. Wang, Y. Yang, Y. Chen, S. Shi, L. Wu and Y. Guo, J. Mater. Chem. C, 2023, 11(5), 1872–1878 RSC.
- C. Koschnick, R. Stäglich, T. Scholz, M. W. Terban, A. von Mankowski, G. Savasci, F. Binder, A. Schökel, M. Etter and J. Nuss, Nat. Commun., 2021, 12(1), 3099 CrossRef CAS PubMed.
- X. Zhang, X. Shi, J. Chen, Y. Yang and G. Lu, J. Environ. Chem. Eng., 2019, 7(5), 103405 CrossRef CAS.
- X. Zhang, Y. Yang, X. Lv, Y. Wang, N. Liu, D. Chen and L. Cui, J. Hazard. Mater., 2019, 366, 140–150 CrossRef CAS PubMed.
- J. Chen, M. Zhang, J. Shu, S. Liu, X. Dong, C. Li, L. He, M. Yuan, Y. Wu and J. Xu, J. Am. Chem. Soc., 2023, 145(43), 23651–23658 CrossRef CAS PubMed.
- Y. Fu, Y. Yao, A. C. Forse, J. Li, K. Mochizuki, J. R. Long, J. A. Reimer, G. De Paëpe and X. Kong, Nat. Commun., 2023, 14(1), 2386 CrossRef CAS PubMed.
- A. K. Kar, R. Sarkar, A. K. Manal, R. Kumar, S. Chakraborty, R. Ahuja and R. Srivastava, Appl. Catal., B, 2023, 325, 122385 CrossRef CAS.
- M. Peng, D. You, Z. Jin, C. Ni, H. Shi, J. Shao, X. Shi, L. Zhou, P. Shao, L. Yang and X. Luo, Environ. Res., 2023, 236, 116752 CrossRef CAS.
- A. Hartl, S.-H. Park, M. Hoelze, N. Paul and R. Gilles, J. Solid State Chem., 2019, 277, 290–302 CrossRef CAS.
- C. Tian, C. Feng and Q. Wang, Sci. Total Environ., 2021, 754, 142154 CrossRef CAS PubMed.
- M. Gu, M. S. Hyun, M. Han, G. Kim and Y. J. Chang, Curr. Appl. Phys., 2023 Search PubMed.
- S. Guthrie, L. Huelsenbeck, A. Salahi, W. Varhue, N. Smith, X. Yu, L. U. Yoon, J. J. Choi, N. Swami and G. Giri, Nanoscale Adv., 2019, 1(8), 2946–2952 RSC.
- M. Ostermann and R. Haubner, Tungsten, 2023, 5(1), 136–144 CrossRef.
- B. Dou, H. Zhang, T. Ye, Q. S. Ma and S. Guo, Tungsten, 2023, 5(1), 189–198 CrossRef.
- K. Sun, Z. Xiao and Y. Shen, et al. , Nano Res., 2023, 1–9 Search PubMed.
- Y. Shen, Y. Liu and K. Sun, et al. , J. Mater. Sci. Technol., 2024, 169, 137–147 CrossRef.
- J. B. Dong, K. Li, L. X. Gao and C. S. Li, Tungsten, 2023, 5(4), 512–521 CrossRef.
- Y. Han, Z. H. Liu, C. B. Wu, Y. Zhao, G. Q. Zu, W. W. Zhu and X. Ran, Tungsten, 2023, 5(4), 419–439 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |





