Modular preparation of functional bimetallic spinels from metal–organic frameworks: a deep exploration from macro and micro perspectives
Qi
Li
a,
Yuan
Zhu
*a,
Min
Cheng
 *a,
Li
Du
a,
Meihua
Zhao
*b,
Gaoxia
Zhang
c,
Guangfu
Wang
a,
Wenjun
Wang
d,
Hongda
Liu
a,
Yongxi
Chen
a and
Wenjun
Xiao
a
*a,
Li
Du
a,
Meihua
Zhao
*b,
Gaoxia
Zhang
c,
Guangfu
Wang
a,
Wenjun
Wang
d,
Hongda
Liu
a,
Yongxi
Chen
a and
Wenjun
Xiao
a
aCollege of Environmental Science and Engineering, Key Laboratory of Environmental Biology and Pollution Control (Ministry of Education), Hunan University, Changsha 410082, China. E-mail: zhuyuan@hnu.edu.cn; chengmin@hnu.edu.cn
bSchool of Civil Engineering, Guangzhou University, Guangzhou 510006, China. E-mail: zmhua@gzhu.edu.cn
cCarbon Neutrality Research Institute of Power China Jiangxi Electric Power Construction Co., Ltd, Nanchang 330001, China
dSchool of Resources and Environment, Hunan University of Technology and Business, Changsha 410205, China
First published on 21st November 2023
Abstract
In recent years, bimetallic spinels (AB2X4) have played significant roles in energy and environmental remediation due to their excellent redox capacity. However, the disadvantages of traditional bimetallic spinels (blocky structure, low specific surface area, and poor reaction kinetics) limit their performances. Noting the controllable chemical composition, high specific surface area and strong design capability of metal–organic frameworks (MOFs), bimetallic spinels synthesized using them as sacrificial templates inherit the structural advantages of the parent materials and improve the inherent defects of traditional bimetallic spinels. These provide great opportunities for the application and development of bimetallic spinels. Therefore, this review presents two synthetic strategies for MOF-derived bimetallic spinels. Special emphasis is placed on the performance advantages of MOF-derived bimetallic spinels due to their unique variations. Based on this, recent advances in the energy field and environmental remediation as well as the mechanism of action are systematically reviewed. Finally, some issues and outlooks on their design are presented. This review aims to provide a systematic reference for the design of MOF-derived bimetallic spinels.
 Top (from left to right): Li Du, Gaoxia Zhang, Guangfu Wang, Wenjun Wang; bottom (from left to right): Hongda Liu, Yongxi Chen, Wenjun Xiao |
1. Introduction
With the rapid development of the economy and science, the environment on which human beings rely for survival is continuously polluted and the required energy materials are decreasing.1,2 These phenomena seriously endanger human production and life.3–5 So far, researchers have developed a large number of functional materials based on these issues and applied them to low-carbon, environmentally friendly and sustainable energy storage and environmental remediation technology.6–8 However, the performance of some functional materials is constantly approaching their limiting value and is still insufficient to meet the needs.9,10 To address this challenge, it is necessary to further explore and commercialize functional materials that can meet performance requirements,11–13 thus solving environmental pollution and energy crises to a certain extent.14–16In recent years, bimetallic spinels consisting of the Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group17 have been rapidly developed as an important class of functional materials due to their special crystal structure.18–20 In detail, due to the different geometrical positions of two metals, the crystal structures of bimetallic spinels can be divided into three types (Fig. 1).21,22 (i) Normal spinels, which can be described as AB2X4 (X: O, S, N), where A and B denote divalent and trivalent metal cations respectively, occupying tetrahedral and octahedral sites, respectively. (ii) Inverse spinels (B(AB)X4),23 in which A and half of B occupy the octahedral sites, while the rest of B occupies the tetrahedral sites. (iii) Complex spinels, in which the tetrahedral and octahedral sites are occupied by A and B at random. These three kinds of bimetallic spinels with highly symmetric structures are rich in metal ions of different valence states and have synergistic effects, thus improving the electron transfer ability, stability and catalytic activity.24,25 However, traditional preparation methods lead to bimetallic spinels with larger sizes and more distorted morphologies, which make them prone to lattice distortion and agglomeration during the reaction process, greatly limiting their performance.26,27 To add insult to injury, these methods require an increase in pyrolysis temperature and time to refine the morphology of bimetallic spinels, which increases the cost. In addition, some traditional preparation methods are not conducive to practical applications due to their complex preparation steps and high energy consumption. Therefore, designing better preparation methods to obtain bimetallic spinels with excellent performances has become a current research focus.
m space group17 have been rapidly developed as an important class of functional materials due to their special crystal structure.18–20 In detail, due to the different geometrical positions of two metals, the crystal structures of bimetallic spinels can be divided into three types (Fig. 1).21,22 (i) Normal spinels, which can be described as AB2X4 (X: O, S, N), where A and B denote divalent and trivalent metal cations respectively, occupying tetrahedral and octahedral sites, respectively. (ii) Inverse spinels (B(AB)X4),23 in which A and half of B occupy the octahedral sites, while the rest of B occupies the tetrahedral sites. (iii) Complex spinels, in which the tetrahedral and octahedral sites are occupied by A and B at random. These three kinds of bimetallic spinels with highly symmetric structures are rich in metal ions of different valence states and have synergistic effects, thus improving the electron transfer ability, stability and catalytic activity.24,25 However, traditional preparation methods lead to bimetallic spinels with larger sizes and more distorted morphologies, which make them prone to lattice distortion and agglomeration during the reaction process, greatly limiting their performance.26,27 To add insult to injury, these methods require an increase in pyrolysis temperature and time to refine the morphology of bimetallic spinels, which increases the cost. In addition, some traditional preparation methods are not conducive to practical applications due to their complex preparation steps and high energy consumption. Therefore, designing better preparation methods to obtain bimetallic spinels with excellent performances has become a current research focus.
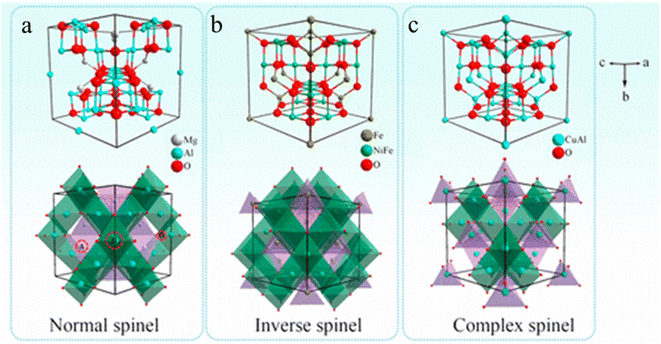 | ||
| Fig. 1 Schematic diagram of three crystal structures of bimetallic spinels: (a) normal spinels (MgAl2O4), (b) inverse spinels (NiFe2O4), and (c) complex spinels (CuAl2O4). Reproduced with permission from ref. 22. Copyright 2017 American Chemical Society. | ||
Metal–organic frameworks (MOFs) are layered functional materials consisting of organic ligands and open inorganic metal nodes (metal ions or metal clusters) that can obtain various morphologies by changing synthesis conditions.28–30 Pleasingly, special adjustability of MOFs allows them to be used as templates for synthesizing bimetallic spinels.31,32 This synthesis method brings some advantages, mainly reflected in the following points: firstly, the preparation strategy using MOFs as precursors is relatively simple, requires low energy consumption, and provides a rich variety of precursors compared to traditional preparation methods. Secondly, compared to other preparation methods, MOF-derived bimetallic spinels can obtain more stable metal sites, reducing the metal leaching rate during the reaction process.33 Thirdly, excellent bimetallic spinels with great dispersion, diverse morphology, rich crystal composition and high crystallinity can be obtained through simply designing MOF precursors and adjusting synthesis conditions.34,35 Lastly, particles can grow anisotropically on MOF precursors to form special crystal planes.36 These advantages have made bimetallic spinels a major breakthrough in energy and environmental fields.
Although significant progress has been made in the research related to MOF-derived bimetallic spinels, derived bimetallic spinels are typically summarized along with other derived metal compounds. For example, Li et al. comprehensively reviewed the energy applications of mixed metal oxides derived from MOFs, including MOF-derived bimetallic spinels.37 Nevertheless, as a special case of mixed metal compounds, the preparation strategy of derived bimetallic spinels and the relationship between the performance changes and structures brought about by MOF precursors have not been systematically reviewed. In addition, the review articles related to them have mainly focused on applications in the energy field, and their application in the environmental field has not been analyzed in depth. In order to promote the rapid development of this new species of functional materials, it is necessary to systematically analyze and summarize MOF-derived bimetallic spinels.
In this review, two preparation methods for MOF-derived bimetallic spinels are first investigated in depth, focusing on summarizing the influence of the synthetic conditions on their structure and crystalline phase composition. Special attention is also given to their unique advantages and a detailed analysis of the changes brought about by MOF precursors. Then, it summarizes the recent applications of MOF-derived bimetallic spinels in energy and environmental remediation and the mechanism of action. Finally, it briefly presents some of the problems and prospects for such functional materials.
2. Preparation of bimetallic spinels using MOF precursors
2.1 Effect of different types of MOF precursors on derived bimetallic spinels
Different types of MOFs can be used to construct bimetallic spinels with different compositions and structures. Although there are few experiments and contents related to the influence of different types of MOF precursors on derived bimetallic spinels up to now, it is evident from the large number of research results that different types of MOF precursors have a greater influence on derived bimetallic spinels. Consolidation of the large amount of relevant research shows that the MOF precursors used to derive bimetallic spinels are mainly focused on ZIFs (zeolitic imidazolate frameworks),38–40 MIL (Materials of Institut Lavoisier)41–43 and PBA (Prussian Blue Analogue).44–46 Among them, ZIF precursors are commonly used to prepare Co-based bimetallic spinels, and most of the bimetallic spinels formed by Cof ions occupying octahedral sites have relatively excellent catalytic activities. Similarly, MIL is mainly used for the preparation of Fe-based bimetallic spinels, because MIL as the source of Fe can increase the Fe content in the catalysts compared to other Fe sources. In addition, PBA is more feasible for the preparation of bimetallic spinels due to its lower synthesis cost and more stable modulation compared to other MOFs.More interestingly, it is confirmed that homogeneous bimetallic spinels prepared by using different types of MOF precursors have some differences according to extensive experiments. Firstly, MOF precursors can affect the morphology of bimetallic spinels. For example, MnCo2O4 derived from Mn Co–ZIF prepared by Dong et al. had a uniform rhombic dodecahedron.47 MnCo2O4 synthesized using MIL-53 by Yao et al. formed a special rod-like structure,48 while MnCo2O4 using the PBA prepared by Zhao et al. had a standard cubic structure.49 Nie et al. synthesized CoFe2O4 with a spindle shaped structure by using MIL(88A),50 while Long et al. synthesized CoFe2O4 with a cubic structure by using hybrid metal hexacyanoferrate (h-MHCFs) (Fig. 2a).51 However, although they prepared MnCo2O4 and CoFe2O4 with the same crystal form, porosity and exposed crystal surfaces of MnCo2O4 and CoFe2O4 were different. For example, in the above example, the BET specific surface area of MIL-derived MnCo2O4 was only 17 m2 g−1, which was smaller than that of ZIF-derived MnCo2O4 (117 m2 g−1). According to the selected area electron diffraction (SAED) pattern analysis, it was found that MIL-derived MnCo2O4 had (311), (220), (400) and (511) crystal planes, while ZIF-derived MnCo2O4 had (220) and (111) crystal planes. The primary factors for these differences might be due to the different organic ligands in the MOF precursors. The primary factors for these differences might be due to the different organic ligands in the MOF precursors.
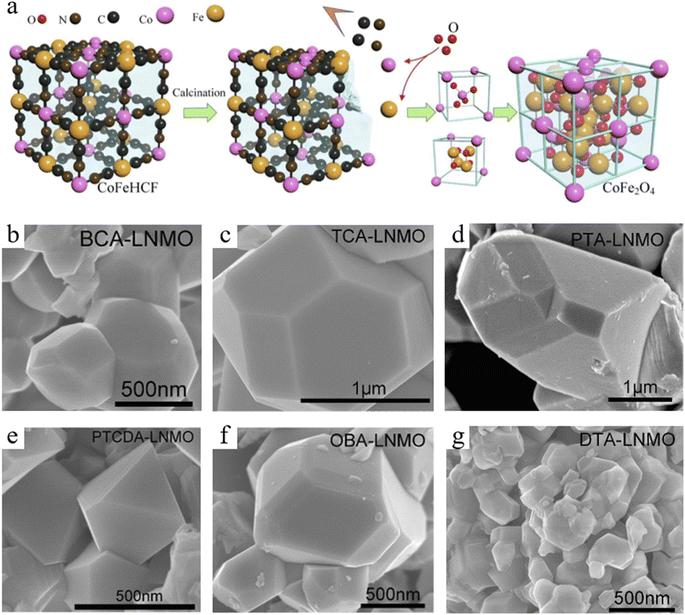 | ||
| Fig. 2 (a) Schematic illustration of the synthesis process of CoFe2O4. Reproduced with permission from ref. 51. Copyright 2021 Elsevier. SEM images of the prepared LNMO using different ligands, (b) BCA–LNMO, (c) TCA–LNMO, (d) PTA–LNMO, (e) PTCDA–LNMO, (f) OBA–LNMO, and (g) DTA–LNMO. Reproduced with permission from ref. 52. Copyright 2019 Elsevier. | ||
Based on the above discoveries, Yin et al. prepared different MOF precursors using different organic ligands and derived them into LiNi0.5Mn1.5O4 (LNMO).52 According to the experimental results, LNMO derived from MOFs using PTCDA (3,4,9,10-perylenetetracarboxylic acid) as an organic ligand was in the shape of an anti-double-triangular pyramid, while the other materials were in the shape of a truncated octahedron (Fig. 2b–g). Meanwhile, the size of LNMO decreased with an increase in the different chain lengths, number of coordination centers and steric hindrance between different ligands. Moreover, as metal elements needed to pass the long migration distance to form a homogeneous spinels phase, LNMO possessing a smaller particle size had a more uniform distribution of metal elements and was more conducive to the improvement of catalytic activity. Additionally, the author discovered that those LNMOs had different lattice spacings by comparing the lattice stripes of LNMOs. It could be due to the expansion of the lattice units in releasing CO2 generated by the organic decomposition and the ligand space effect. Therefore, TCA (trimeric acid)–LNMO and DTA (2,5-dihydroxyterephthalic acid)–LNMO had long-range ordered lattice stripes with a facet spacing of 0.47 nm, which improved the electrochemical rate and the catalytic activity. Therefore, by rationally modulating the functional structure of MOFs, different bimetallic spinels can be obtained so as to broaden their applications in various fields. Unfortunately, there are few studies to analyze and compare the electronic and coordination structures of bimetallic spinels derived from different types of MOF precursors. Future exploration of this part is important for the development of MOF-derived bimetallic spinels in various applications.
2.2 Preparation strategy of the MOF-derived bimetallic spinels
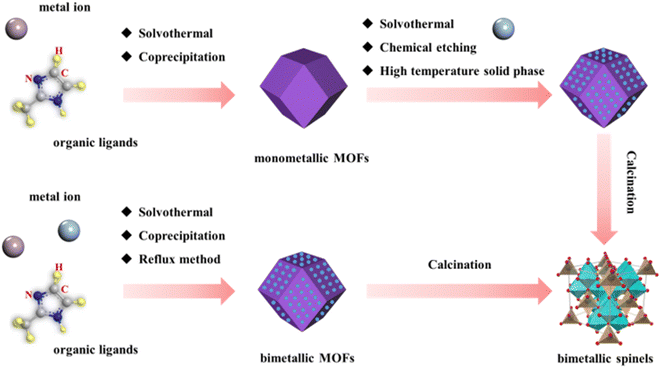
In bimetallic spinels, two metal ions with similar ionic radii and coordination structures are randomly located in the coordination centers of a tetrahedron and an octahedron.53 Due to their special architecture, the performances of bimetallic spinels depend strongly on their chemical composition and microstructure.54,55 However, for the commonly used preparation methods at present, two different metal salts or single metal oxides are usually used to synthesize bimetallic spinels by high-temperature solid state, hydrothermal and vapor deposition substitution. Some of these preparation methods may require high temperatures (over 900 °C),56 long preparation times (over 7 days)57 or more complex preparation steps,58 which increases the cost of preparing the materials and is not conducive to practical application. In addition, traditional preparation methods tend to cause agglomeration of the synthesized bimetallic spinels, which reduces the pore structure and specific surface area of the materials. To change this phenomenon, additional carbon-based materials need to be added to improve it.59,60Advantageously, in the preparation process where MOFs are used as precursors, the formation of bimetallic spinels is mainly by calcination under certain moderate conditions. Moreover, MOF-derived bimetallic spinels by calcination effectively inherit the advantages of parent MOFs and possess excellent morphological structures.61,62 More interestingly, compared with traditional preparation methods, because of the existence of nitrogen and carbon elements in the precursor of MOFs, the bimetallic spinel prepared by MOFs can form a closer association with N/C without an additional carbon source and nitrogen source in the preparation process.63,64 Such a preparation strategy not only obtains bimetallic spinels with excellent performances, but also simplifies the synthesis steps and reduces the cost. Therefore, the chemical composition and microstructure of bimetallic spinels can be effectively changed by reasonably designing MOF precursors and adjustment of the preparation process to obtain excellent materials.65,66 Based on this, this chapter discusses the preparation of bimetallic spinels by different precursors, which can be classified as monometallic MOFs and bimetallic MOFs. In addition, special attention is paid to the effect of some factors on the structure of the prepared spinels, such as metal ratios, annealing temperatures, annealing atmosphere and so on (Fig. 3).
The solvothermal method is one of the most adopted methods to introduce another metal. It mainly utilizes the dispersion of the solid in the solvent, allowing another metal to adhere onto the surface of MOFs. For example, Yu and co-workers successfully introduced a second metal ion (A+: Ni+, Co+, and Mn+) onto the surface of Fe4(Fe(CN)6)3 (Prussian Blue, PB) via the solvothermal method (Fig. 4a) which used inorganic metal compounds.45 In addition, grinding was performed to ensure uniform dispersion of A+ on the MOFs. According to the energy dispersive X-ray spectroscopy mapping of AFe2O4 cubic spinels after annealing at 700 °C, the elements Fe, A and O were uniformly distributed in AFe2O4. It was pointed out that AFe2O4 formed a hollow structure. This was due to the Kirkendall effect67 of the precursor during high temperature, namely the internal PB was subjected to high temperature diffusion outward faster than the oxygen diffusion inward, causing the internal PB to form a new oxide layer when it passed through the outside. In this way, the material formed a hollow nanocage with AFe2O4 as the shell which could improve the catalytic performance.
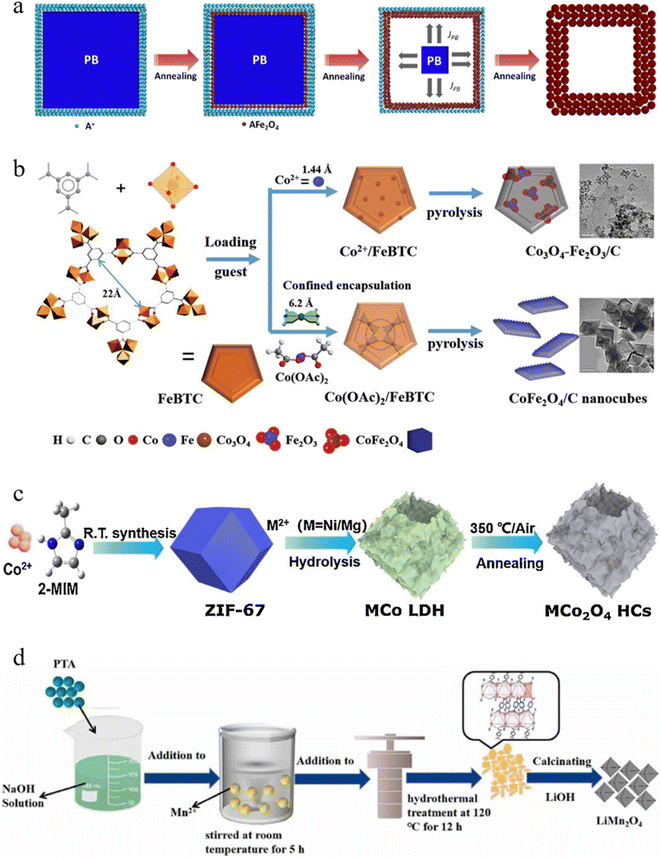 | ||
| Fig. 4 (a) Schematic illustration of the synthetic process of porous ZnFe2O4 hollow cubes. Reproduced with permission from ref. 45. Copyright 2015 American Chemical Society. (b) Schematic illustration of assembly of MOF-derived CoFe2O4. Reproduced with permission from ref. 68. Copyright 2020 WILEY-VCH. (c) Schematic illustration of the synthetic process of NiCo2O4 hollow cubes. Reproduced with permission from ref. 39. Copyright 2020 Elsevier. (d) Schematic illustration of the synthetic process of Mn–MOFs and LiMn2O4 sample. Reproduced with permission from ref. 74. Copyright 2022 Elsevier. | ||
However, inorganic metal ions tend to aggregate on the surface of monometallic MOFs to form large particles during the solvothermal reaction process, which makes it difficult for the inorganic metal ions to exchange ligands with the metal ions of monometallic MOFs. Moreover, the energy required for coordination exchange is high and not easy to control. Organometallic compounds as raw materials for introducing a second metal can achieve excellent crystalline phase transition and compositional reconstruction, leading to superior ligand exchange for the preparation of superior bimetallic spinels. Qin and co-workers used the solvothermal method to subject cobalt acetate molecules to a strong surface exchange–coordination reaction with FeBTC (BTC = 1,3,5-benzenetricarboxylic acid), followed by calcination in air to synthesize CoFe2O4/C nanocubes (Fig. 4b).68 For comparison, they performed the same steps using inorganic metal sources (CoCl2 and Co(NO3)2). Based on the X-ray diffraction (XRD) spectra and transmission electron microscope (TEM) mapping, it was confirmed that the inorganic metal sources did not alter the peaks of FeBTC, whereas the organometallic compounds altered the crystal structure of FeBTC due to strong restricted coordination, forming a large number of highly crystalline nanocrystals. The introduction of organometallic compounds not only overcame the particle sintering caused by the aggregation of inorganic metal ions on the surface of monometallic MOFs, but also obtained excellent interfacial interactions between the two metals to form highly crystalline bimetallic spinels. This preparation strategy had important research value. Furthermore, they also found that changing the solvent type during solvothermal processes could transform the exposed crystal surface of the bimetallic spinels. When using N,N-dimethylformamide (DMF) as a solvent, cobalt acetate formed a coordination complex with DMF, allowing for a reduction in the additional energy required for the Co ions to combine with the organic ligands in the MOFs to produce new coordination compounds. It altered the main exposure plane of the material ({112}). The {112} plane had a higher CO molecular bonding energy compared to the exposure plane ({110}) produced with water as the solvent.
In addition to the solvothermal method, a second metal can also be introduced into monometallic MOF precursors by chemical etching. This method can transform monometallic MOFs into bimetallic layered double hydroxides (LDHs) with adjustable layer thickness and structures, which is conducive to selectively adjusting the morphology of annealed bimetallic spinels.69 Beyond that, chemical etching can also lead to a hollow structure.70 Han et al. mixed cobalt 2-methylimidazole (ZIF-67) with Ni salt in ethanol (Fig. 4c).39 In this process, Co ions of ZIF-67 were hydrolyzed to produce protons capable of etching ZIF-67. These protons broke the coordination bonds between the metal ions and the organic linker of ZIF-67, resulting in Ni2+ and oxidized Co3+ ions to synthesize NiCo hydroxides (NiCo-LDHs). Then, NiCo2O4 was synthesized by heat treatment at 350 °C. It was noted that NiCo2O4 inherited the layered hollow nanocage morphology from NiCo-LDHs. This cleverly avoided the aggregation of bimetallic spinels that might be caused by high temperature calcination.
The above two methods are widely used to synthesize bimetallic spinels by introducing other metals into monometallic MOFs, which are applicable to the preparation of many bimetallic spinels. Recently, researchers found that the high-temperature solid phase process could be used to mix Li ions and MOFs to synthesize bimetallic spinels.71,72 However, Li ions could hinder mixing with oxides at high temperatures. Based on this, Yin et al. found that some organic ligands, such as pyromellitic acid (PMA), could be used as a Li+ adsorbent to promote the nucleation of bimetallic spinels and make them uniformly mixed.73 Additionally, Chen and co-workers reported a NaOH hydrothermal-assisted high-temperature solid phase method to successfully synthesize LiMn2O4 spinels (LMO-R) (Fig. 4d).74 Scanning electron microscopy (SEM) mapping revealed that LMO-3 showed an obvious truncated octahedral spinel shape. This was due to the addition of a proper alkaline environment, which could effectively promote the reaction of terephthalic acid (PTA) and produce enough organic ligands that could decompose at high temperatures to generate enough heat for the preferential growth of the (111), (110) and (100) facets. In addition, the Raman spectra and Mn 2p XPS (X-ray photoelectron spectroscopy) spectra of LMO-3 showed that the Mn4+/Mn3+ ratio of LMO-3 was low, indicating its weak lattice distortion. Therefore, rational design of the synthesis program to adjust the preparation structure of MOF precursors can effectively prevent the lattice distortion of bimetallic spinels and promote the growth of special crystalline surface advantages of bimetallic spinels, thus improving the structures and performances of bimetallic spinels.
| MOF precursors | Synthesis method | Metal combination | Calcination temperature (°C) | Gas | Derivatives | Ref. |
|---|---|---|---|---|---|---|
| Mn–Co MIL-53 | Solvothermal + calcination | Rod-like shape | 500 | Air | Mixed Mn–Co spinel oxides | 48 |
| PBA | Coprecipitation + calcination | Spherical shape | 500 | Air | FeCo2O4 | 44 |
| NiFe2 MOF | Coprecipitation + calcination | Mesh-like shape | 460, 500, 550 | Air | NiFe2O4 | 82 |
| Zn/Co–MOF | Coprecipitation + calcination | Polyhedral shape | 450 | N2/air | ZnCo2O4 | 83 |
| Zn–Co–ZIFs | Coprecipitation + calcination | Rhombic dodecahedral shape | 400 | N2/air | ZnxCo3−xO4 | 84 |
| Fe2Ni–MIL-88B | Solvothermal + calcination | Rod-like shape | 600, 800, 1000 | Air | NiFe2O4 | 89 |
| Co/Fe bi–MOFs | Solvothermal + calcination | Rod-like shape | 400 | Air | CoFe2O4 | 43 |
| [CoMn2{C6H3(COO)3}2] | Solvothermal + calcination | Nanocube shape | 400, 500, 550, and 600 | Air | CoMn2O4 | 62 |
| CoFeHCF NPs | Coprecipitation + calcination | Irregular polyhedron shape | 400, 500, 550, and 600 | Air | CoFe2O4 | 36 |
| Mn3[Co(CN)6]2·nH2O | Coprecipitation + calcination | Nanocube shape | 500, 450, 400, 350, and 300 | Air | CoMn2O4 | 90 |
| CoCu–ZIF | Coprecipitation + calcination | Petal-like shape | 700 | Air | CuCo2O4−σ | 91 |
| MIL-101(Fe/Co) | Solvothermal + calcination | Rod-like shape | 300, 400, 500, and 600 | Air | CoFe2O4 | 42 |
| CoωFe3−ω–MOFs | Solvothermal + calcination | Rod-like shape | 450 | Air | CoxFe3−xO4 | 80 |
| Mn3[Co(CN)6]2·nH2O | Coprecipitation + calcination | Nanocube shape | 450 | Air | MnxCo3−xO4 | 81 |
| Zn/Co-MOF | Solvothermal + calcination | Micro-rice like | 400 | Air | ZnCo2O4 | 92 |
| MCo–ZIFs (M = Zn, Ni, and Cu) | Coprecipitation + calcination | Yolk–shell polyhedral shape | 400 | N2/air | MxCo3−xO4 YSP | 93 |
| CoMn–MOF-74 | Solvothermal + calcination | Rod-like shape | 400 | N2/air | CoxMn3−xO4–C | 64 |
During the preparation process of bimetallic spinels, the ratio of A/B has the most direct influence on the synthesized bimetallic spinels.79 The low ratio of A/B may fail to synthesize bimetallic spinel structures after annealing, while the high ratio of A/B may result in the formation of mixed spinel structures.49 For this reason, Yao et al. discussed the relationship between the crystal phase structure of bimetals synthesized with different Co/Mn ratios and their corresponding derived metal oxides.48 They found that when Co/Mn was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the materials showed a pure cubic MnCo2O4 spinel structure (Fig. 5a). As the Co ions were decreased to a certain extent, other crystalline phases gradually replaced the cubic MnCo2O4 phase as the main phase, leading to the transformation of the crystalline phase structure of the materials from the cubic phase into the tetragonal phase structure. As a result, the Mn ions in the cubic phase gradually occupied the tetrahedral sites, which resulted in a higher ratio of Mn4+/Mn3+ than that of the other crystalline phases of materials, thereby providing a relatively excellent electrochemical activity. It is worth noting that different metal ratios can also affect the phase composition of bimetallic spinels, which may change the morphology of bimetallic spinels. Yang and co-workers prepared CowFe3−w–MOFs with different metal ratios via the solvothermal method and synthesized CoxFe3−xO4 with different morphologies by annealing CowFe3−w–MOFs, confirming the existence of this phenomenon.80 FESEM patterns showed that the rod-like shape of CoxFe3−xO4 became more homogeneous with the increase in Co content (Fig. 5b). Additionally, it could be clearly observed that the mixed spinels consisted of both rod and spherical shapes when the ratio of Co/Fe increased to 1.75 (Fig. 5c). According to the XRD mapping, the spherical structure was the second phase which was Co3O4. However, the poor redox performance of Co3O4 compared to that of CoFe2O4 negatively affected the performance of CoxFe3−xO4. For this reason, reasonable regulation of metal ratios in bimetallic MOFs is the key to the synthesis process.
1, the materials showed a pure cubic MnCo2O4 spinel structure (Fig. 5a). As the Co ions were decreased to a certain extent, other crystalline phases gradually replaced the cubic MnCo2O4 phase as the main phase, leading to the transformation of the crystalline phase structure of the materials from the cubic phase into the tetragonal phase structure. As a result, the Mn ions in the cubic phase gradually occupied the tetrahedral sites, which resulted in a higher ratio of Mn4+/Mn3+ than that of the other crystalline phases of materials, thereby providing a relatively excellent electrochemical activity. It is worth noting that different metal ratios can also affect the phase composition of bimetallic spinels, which may change the morphology of bimetallic spinels. Yang and co-workers prepared CowFe3−w–MOFs with different metal ratios via the solvothermal method and synthesized CoxFe3−xO4 with different morphologies by annealing CowFe3−w–MOFs, confirming the existence of this phenomenon.80 FESEM patterns showed that the rod-like shape of CoxFe3−xO4 became more homogeneous with the increase in Co content (Fig. 5b). Additionally, it could be clearly observed that the mixed spinels consisted of both rod and spherical shapes when the ratio of Co/Fe increased to 1.75 (Fig. 5c). According to the XRD mapping, the spherical structure was the second phase which was Co3O4. However, the poor redox performance of Co3O4 compared to that of CoFe2O4 negatively affected the performance of CoxFe3−xO4. For this reason, reasonable regulation of metal ratios in bimetallic MOFs is the key to the synthesis process.
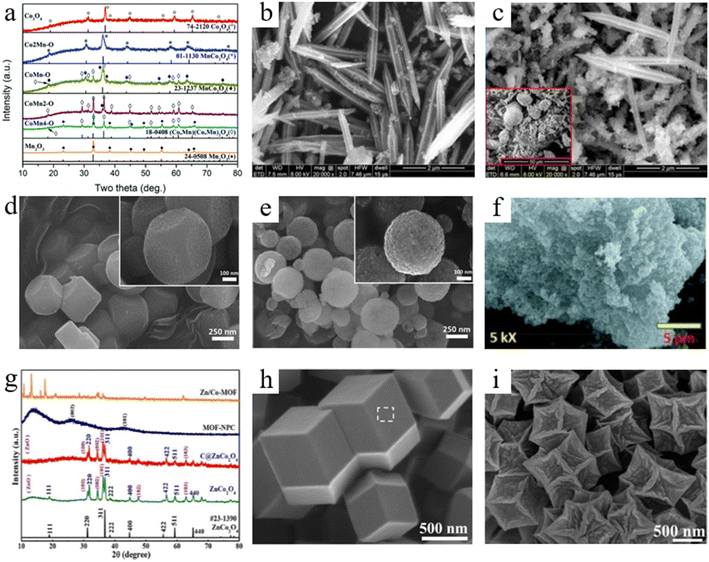 | ||
| Fig. 5 (a) XRD patterns of oxides derived from the relevant MOFs after annealing at 500 °C. Reproduced with permission from ref. 48. Copyright 2017 The Royal Society of Chemistry. (b and c) Morphological and elemental distribution of CoxFe3−xO4 MDCs: x = 1.51 and x = 1.79. Reproduced with permission from ref. 80. Copyright 2019 Elsevier. (d and e) SEM images of FCO-NCs and FCO-NSs. Reproduced with permission from ref. 44. Copyright 2015 The Royal Society of Chemistry. (f) SEM images of NFO550 at magnifications of 5k×. Reproduced with permission from ref. 82. Copyright 2021 Elsevier. (g) The XRD patterns of the as-prepared materials. Reproduced with permission from ref. 83. Copyright 2020 Frontiers. (h and i) High-magnification FESEM images of Zn–Co–ZIF-0.33 and ZnxCo3−xO4. Reproduced with permission from ref. 84. Copyright 2014 American Chemical Society. | ||
As mentioned above, the structure of bimetallic spinels can be effectively controlled by modulating the metal ratios. Not only that, during the process of synthesizing bimetallic MOFs, surfactants could be used as capping agents to promote the self-assembly of metals and organic ligands, resulting in the formation of bimetallic spinels with different morphological structures.81 Based on this, Liu et al. achieved the morphology control of bimetallic spinels (FexCo3−xO4 which are called FCO-NCs and FCO-NSs) by changing the surfactants during the synthesis of bimetals.44 According to SEM patterns (Fig. 5d and e), FCO-NCs which used sodium dodecyl-sulfate (SDS) as the surfactant, presented a dispersed cubic morphology due to the surfactant which promoted the preferred growth of crystal faces with lower surface energy. Furthermore, when sodium dodecyl benzene sulfonate (SDBS) was used as the surfactant, the surface of FCO-NSs was corroded because of the alkalinity of SDBS, resulting in the formation of the spherical structure of FCO-NSs. Although the catalytic effects of the two kinds of FexCo3−xO4 were relatively excellent, the pore structure of FCO NSs produced after calcination was excellent, and thus FCO-NSs showed better catalytic activity. Therefore, it can be believed that, in addition to regulating the ratio of metal ions, the different morphologies of bimetallic spinels can be obtained by adjusting the type of surfactant added during the synthesis of MOFs.
The influence of the synthesis of MOF precursors on bimetallic spinels is discussed earlier, while the pyrolysis process of MOF precursors also affects bimetallic spinels. First of all, changing the annealing temperature can be used to adjust the morphology of bimetallic spinels. Patil et al. first annealed the synthesized NiFe2 MOF precursors at 100 °C and then separately at different temperatures to investigate the effect of temperature on the morphology of the synthesized bimetallic spinels (NFO-X).82 SEM mappings showed that NFO460 exhibited an interwoven linear structure. With the temperature increasing, the thread diameter of the materials increased and was arranged randomly, which led to the porous network structure of NFO500. The porous structure could improve the catalytic ability of NFO500. Moreover, NFO550 possessed a spherical structure (Fig. 5f). Such morphological change might be due to the concentration of forces between nanoparticles caused by high temperature, resulting in the aggregation of materials to form large particles.42 Although this method could change the morphological structure of bimetallic spinels, it was difficult to control its impact on the bimetallic spinels, which could cause structural collapse of bimetallic spinels. The collapsed structure could lose the dominant morphology introduced by the MOF precursors, resulting in a reduction in the performances of the bimetallic spinels. Therefore, it is necessary to precisely adjust the annealing temperature to synthesize bimetallic spinels with an excellent structure.
Except for the annealing temperature, the choice of different annealing atmospheres for the calcination of MOF precursors could also change the crystal phase purity and microstructure of bimetallic spinels. He et al. synthesized Zn/Co MOFs via co-precipitation and then calcined the MOFs in three different ways.83 XRD mapping (Fig. 5g) showed that the purity of bimetallic spinels formed by calcination at 400 °C under N2 followed by calcination at 400 °C in air was higher than that of bimetallic spinels formed by primary calcination at 450 °C under N2. In addition, Wu and co-workers also used two calcination processes for MOF precursors to synthesize bimetallic spinels with large BET (Brunauer–Emmett–Teller) surface area.84 They thought that the carbon produced by the first calcination in N2 had a buffering effect, which could effectively slow down the further contraction of the precursors during the later calcination in air (Fig. 5h and i). Therefore, the above fully demonstrated that calcination with N2 for a period of time not only removed some organic ligands, but also basically stabilized the original structure and morphology of the MOFs due to the retention of the carbon structure. Subsequently, calcination in air burned the organic elements in the materials and took away more carbon, thereby forming bimetallic spinels with excellent crystallinity, high specific surface area and high porosity.
In summary, there are two different ways to synthesize MOF-derived bimetallic spinels: (i) using monometallic MOFs as templates, bimetallic spinels with high crystallinity are synthesized by introducing a second metal. This method can introduce more kinds of metal ions and has great ionic dispersion. However, it is relatively complex and needs to overcome the rotational kinematics between the two metals, which is relatively difficult to control. And (ii) bimetallic spinels are directly synthesized by using bimetallic MOFs as templates. Compared with the above methods, this method is easier to operate, and the reactions are more gentle and easier to control. However, there are few types of MOF precursors used to synthesize excellent bimetallic spinels in the current research.85,86 Meanwhile, in addition to the solvent method, co-precipitation and the reflux method, there are also some synthetic methods of bimetallic MOFs that have not been used in the preparation of bimetallic spinels.87,88 Furthermore, few researchers have explored the differences between the bimetallic spinels prepared by these different synthetic methods. More importantly, the current two synthetic routes have produced relatively few types of bimetallic spinels. Therefore, developing more types of MOF-derived bimetallic spinels to obtain better performances and structures is one of the hot topics for the future.
3. Variations in MOF-derived bimetallic spinels
Some inherent defects of traditional bimetallic spinels (large particle size, low stability, and few active sites) have hindered their large-scale application.94,95 Interestingly, the directed synthesis of bimetallic spinels with MOFs as templates not only improves the structure and some properties to compensate for the defects of traditional bimetallic spinels, but also unexpectedly brings some vacancy defects.96,97 These variations can enhance the advantages of MOF-derived bimetallic spinels for energy and environmental applications (Fig. 6).98,99 To date, few studies have specifically summarized these variations. Therefore, the variations caused by MOFs for derived bimetallic spinels (structural changes, property advantages, and vacancy defects) are elucidated in detail in this chapter, which are important for an in-depth exploration of these materials. Table 2 provides a brief summary of the advantages and disadvantages of bimetallic spinels derived from MOFs and traditional bimetallic spinels.| MOF-derived bimetallic spinels | Traditional bimetallic spinels | |
|---|---|---|
| Preparation | (i) Abundant precursors | (i) Abundant synthesis methods |
| (ii) Simple preparation | (ii) Rich variety of metals | |
| (iii) High designability | ||
| Structures | (i) High surface area | (i) Low specific surface area |
| (ii) High porosity | (ii) Low porosity | |
| (iii) Prevents aggregation | (iii) Easy to aggregate | |
| (iv) Stable framework structure | (iv) Easy to collapse | |
| (v) Rich morphology and structure | ||
| Performance | (i) High conductivity | (i) Low activity |
| (ii) Highly magnetic | (ii) Easy to deform during reaction | |
| (iii) Excellent catalytic activity | (iii) Low lifetime | |
| (iv) Rich electron transport pathways and rapid ion diffusion |
High specific surface area is one of the important features of MOFs.100 According to experience, MOFs have excellent thermal stability and maintain their original structure after calcination.89,101 Therefore, MOF-derived bimetallic spinels can inherit the advantage of their large specific surface area. Zhou and co-workers compared ZnCo2O4 prepared by traditional methods with ZnCo2O4 derived from ZnCo–ZIFs, and the results confirmed that the BET surface area of the derived ZnCo2O4 (100 m2 g−1) was much higher than that of traditional ZnCo2O4 (36 m2 g−1) (Fig. 7a).102 The higher specific surface area could provide more active sites, improving the catalytic performance of the materials. Moreover, the organic ligands of MOFs are converted into gases and spill outward during the calcination process, thus forming numerous pores in the MOF-derived bimetallic spinels, which can likewise increase their specific surface area.103,104 In MOF-74-derived ZnCo2O4 reported by Du et al., they found that due to a gas spill, MOF-74-derived ZnCo2O4 formed a large number of mesoporous structures (average diameter of about 18 nm), leading to a significant increase in its specific surface area (Fig. 7b).105 Porous ZnCo2O4 provided a larger reaction contact area, more diffusion channels, and abundant active sites, which shortened the length of ion insertion/extraction during the reaction process and improved the degree of electrolyte penetration.
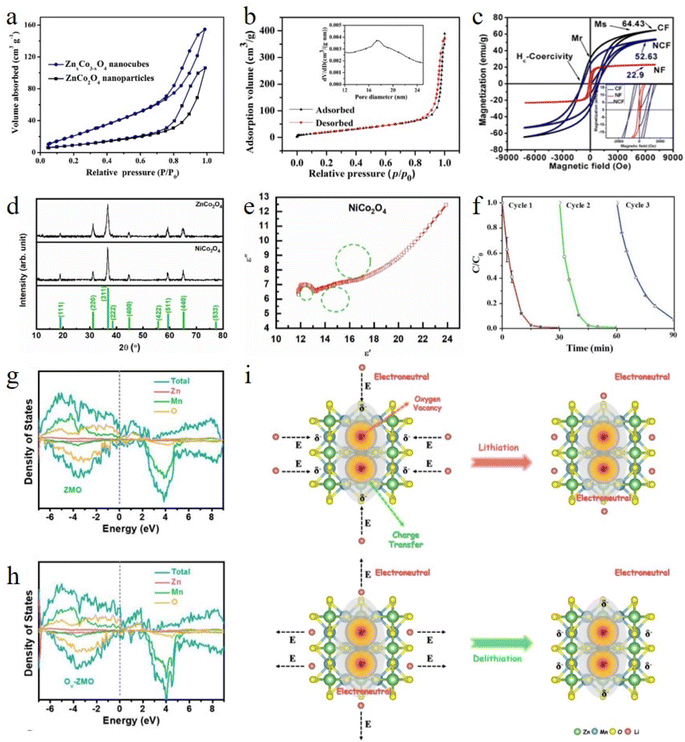 | ||
| Fig. 7 (a) The N2 adsorption–desorption isotherms of ZnxCo3−xO4 nanocubes and ZnCo2O4 nanoparticles. Reproduced with permission from ref. 102. Copyright 2019 Elsevier. (b) Nitrogen adsorption–desorption isotherms and the corresponding pore-size distribution curves of ZnCo2O4. Reproduced with permission from ref. 105. Copyright 2017 Elsevier. (c) Magnetic hysteresis curves for NF, CF and NCF. Reproduced with permission from ref. 107. Copyright 2018 IOP Publishing. (d) XRD patterns of the calcined NiCo and ZnCo MOFs. (e) Cole–Cole plot of NiCo2O4. Reproduced with permission from ref. 40. Copyright 2023 Elsevier. (f) SA degradation performances of the CoMn2O4 MD catalyst during consecutive reaction cycles. Reproduced with permission from ref. 33. Copyright 2018 Elsevier. (g and h) TDOS and PDOS profiles of ZMO and OV-ZMO. (i) Schematic illustration of the locally built-in electric field induced boosted Li ion diffusivity inside crystalline lattices around the OV sites. Reproduced with permission from ref. 111. Copyright 2022 Elsevier. | ||
Compared with traditional bimetallic spinels, MOF-derived bimetallic spinels not only undergo structural improvements, but also enhanced their own properties such as magnetism, electrical conductivity and metal stability. In terms of magnetic properties, the saturation magnetization strength and coercive force of bimetallic spinels are mainly determined by their sizes.106 However, traditional bimetallic spinels have large nanoparticles, which limit their magnetic properties. Remarkably, MOF-derived bimetallic spinels can inherit the advantages of parent MOFs to obtain superior nanosizes, significantly improving the saturation magnetization strength. Ni1−xCoxFe2O4 (x = 0.0, 0.5, 1) materials with grain sizes of only 9 nm ± 2 nm, much smaller than those of traditional bimetallic spinels, have been synthesized using Fe-templates as precursors reported by Pervaiz et al.107 It was interesting to note that thanks to the small size, a large number of grain boundaries were generated in the vicinity of the Ni1−xCoxFe2O4 nanoparticles, which could generate more magnetic domains. Therefore, NiCoFe2O4 showed the highest ferromagnetism compared to similar materials reported (Fig. 7c), which contributed to the subsequent separation and recovery of the materials from reactive substances. On the other hand, for the electrical conductivity, due to the low crystallinity of bimetallic spinels prepared by traditional methods, the electrical conductivity is consequently reduced, limiting the capacity of the battery and the charge transfer capability. Interestingly, bimetallic spinels synthesized by calcination at temperatures above the stable range of MOFs (>300 °C) have higher crystallinity. The high crystallinity can attenuate the dispersion of conducting electrons due to crystal defects, thereby imparting better electrical conductivity to the materials. Notably, Heidari et al. found that the grain boundaries of the derived NiCo2O4 were more defined due to their high crystallinity when exploring the microwave absorption performance of MOF-derived NiCo2O4 (Fig. 7d).40 Owing to the well-defined grain boundaries, charge carriers were susceptible to capture and produced electron accumulation, which led to interfacial polarization (Fig. 7e). The presence of interfacial polarization increased the dielectric constant, further improving the electrochemical properties of the materials. Additionally, it is well known that metal stability is particularly important for the evaluation of the possible environmental risks associated with the ion leaching of bimetallic spinels during practical applications.42 Surprisingly, MOFs with metal stability can be transformed into bimetallic spinels with stable and dispersed metal sites without destroying the original skeleton. In the experimental process, such excellent metal stability can effectively reduce the leaching rate of metals, thus stabilizing the catalytic activity of the materials and decreasing the toxicity to the environment. Li and co-workers used MOF-derived CoMn2O4 to degrade sulfanilamide (SA), and there was no significant change in the XPS (X-ray photon-electron spectroscopy) spectra of the three chemical compositions, namely Co, Mn, and O, before and after the reaction. After three cycles, the degradation effect of the materials was only reduced by 6% (Fig. 7f).33
The above research summarized the enhancement of structural and special properties of MOF-derived bimetallic spinels. More surprisingly, MOF-derived bimetallic spinels can also obtain a special feature, known as the generation of oxygen vacancies (OVs). Currently, there are two pathways to illustrate the generation of oxygen vacancies: (i) according to the law of equilibrium, the calcination process of MOFs causes oxygen detachment from the lattice under non-oxidizing conditions, leading to oxygen deficiency.108,109 And (ii) for two different metals, the easily broken metal atom will release the outer electrons due to the different stability of the bonds formed between them and oxygen, resulting in electron transfer and an unstable electron distribution. As a result, some of the oxygen atoms will escape to balance the internal charge, resulting in some OVs.110 Owing to the production of oxygen vacancies, the electronic structure of MOF-derived bimetallic spinels has changed positively, thus improving their catalytic activity. Lin and co-workers synthesized MOF-derived ZnMn2O4 with abundant oxygen vacancies (OV-ZMO) by using a simple annealing treatment.111 Density functional theory (DFT) calculations revealed that OV-ZMO had a greater density of states (DOS) near the Fermi level (Ef) compared with ZMO, which fully confirmed the effect of OVs on the electronic structure of bimetallic spinels (Fig. 7g and h). Benefiting from the change in the electronic structure of OV-ZMO, it led to a charge imbalance around OVs, which triggered the spontaneous charge transfer behavior during the reaction process (Fig. 7i). The formation of a built-in electric field could accelerate the migration of ions, improve the reaction kinetics, and reduce the energy barrier during repeated insertion/extraction. With the above advantages, the charge transfer resistance (Rct) of the OV-ZMO electrodes was lower than that of other ZMO electrodes, and the ion diffusion rates were higher than those of other ZMO electrodes. Meanwhile, the adsorption capacity of MOF-derived bimetallic spinels for reactive substances was also improved by introducing vacancy defects, thus optimizing the subsequent activation process. Wang and co-workers introduced Zn elements from ZIF-8 into ZIF-67 to construct ZnCo2O4 nanocages (OVs-ZnCo2O4 HNCs), which contained abundant OVs.112 According to the results of applied DFT, the adsorption energy of the OV-containing materials for peroxydisulfate (PDS) molecules was −4.50 eV, which was lower compared to the OV-free materials. The superior adsorption performance promoted the enrichment of PDS on the surface of OVs-ZnCo2O4 HNCs, thus facilitating the formation of a surface-active composite of PDS with the materials to enhance degradation. In addition, the presence of OVs could elongate the perovskite O–O bond length of bimetallic spinels to PDS molecules (1.529 eV). The longer O–O bond was unstable and susceptible to breakage, which further enhanced the catalytic performance of the materials.
From the above mentioned, we can conclude that the utilization of MOFs as sacrificial templates can obviously bring about some excellent structures, improve special properties and produce abundant oxygen vacancies. These excellent variations largely overcome the major drawbacks of traditional bimetallic spinels, resulting in a significant improvement in some performances such as the adsorption performance, stability, and electrochemical performance. Therefore, MOF-derived bimetallic spinels can fully meet the requirements for environmental remediation, energy storage and conversion, which are of great significance for the future development of bimetallic spinels.
4. Applications of MOF-derived bimetallic spinels
As mentioned above, bimetallic spinels utilizing MOFs as templates have excellent physical and chemical properties, which can be extensively used in energy, environmental and other major applications. Therefore, based on the above two parts of synthesis and variations, recent research studies on MOF-derived bimetallic spinels in energy and environmental fields are briefly discussed in this chapter. Most importantly, the mechanisms of their reactions are also detailed in order to provide new ideas for future research.4.1 Energy applications
With the development and application of catalysts in the energy field, MOF-derived bimetallic spinels show great promise for application due to their excellent electrochemical performances.113–115 Therefore, the following focuses on the application of MOF-derived bimetallic spinels in the energy field. In particular, the problems and solutions encountered with MOF-derived bimetallic spinels are discussed in detail.LIBs have been widely used in high-end electronics due to the rapid global development of rechargeable batteries over the past three decades. With the increasing demand for these products, the search for lithium-ion electrode materials with high charge/discharge capacity, high power and long life has become imperative.117–119 Over the past few years, bimetallic spinels have received much attention for their outstanding redox properties.120 However, the insertion/extraction of lithium ions may lead to certain volume changes in metal oxide, which is highly susceptible to cracking or even breaking of the materials, thus reducing the charge/discharge capacity and the service life of LIBs.121,122 To address these issues, the molecular and atomic modulation capabilities of MOF materials have entered the picture.111,123 The structural advantages of bimetallic spinels synthesized using MOFs as templates have been effectively improved, and superior electrochemical performances and structure stability have been obtained as a result (Table 3 summarizes in detail their application advantages in LIBs).124,125 Guo and co-workers126 demonstrated that an excellent hollow nanocage structure could provide richer electron transport pathways and enhanced ion diffusion, enabling the MOF-derived NiFe2O4 to achieve initial charge/discharge capacities of 1152/1245 mA h g−1 as the anode electrode for LIBs. Analogously, Wu and co-workers found that Ni–Co–BTC (BTC = 1,3,5-benzene tricarboxylate) solid microspheres underwent non-uniform shrinkage caused by non-equilibrium heat treatment during air calcination at medium temperature, thereby generating NixCo3−xO4 with multihull hollow microspheres (MS-HMSs) (Fig. 8a).127 Influenced by this special structure, the NixCo3−xO4 MS HMS gained more contact space with the electrolyte compared to traditional hollow structures. As a result, NixCo3−xO4 MS-HMSs acted as anode materials for LIBs, and the first charge and discharge capacities were as high as 1139.3 mA h g−1 and 1619.2 mA h g−1, respectively, with a coulombic efficiency (CE) of 70%. Moreover, after cycling many times, NixCo3−xO4 MS-HMSs still possessed excellent structural integrity that could exhibit excellent reversible discharge capacity and high capacity retention (1109.8 mA h g−1 reversible discharge capacity after the 100th cycle, with a capacity retention rate of 90%).
| MOF precursors | Derivatives | Application | Performance improvements | Ref. | ||
|---|---|---|---|---|---|---|
| Fe4(Fe(CN)6)3 | NiFe2O4 | LIBs | The initial discharge/charge capacities were 1629/1176 mA h g−1, maintained at 841 mA h g−1 after 100 cycles | 45 | ||
| Zn–Co–ZIFs | ZnxCo3−xO4 | LIBs | The initial discharge/charge capacities were 1272 and 969 mA h g−1, maintained at 990 mA h g−1 after 50 cycles | 84 | ||
| Ni/Mn-1,3,5-BTC BMOF | NiMn2O4 | LIBs | The initial discharge/charge capacities were 1738/1049 mA h g−1, maintained at 1257 mA h g−1 after 400 cycles | 123 | ||
| MnCo–MOF | MnCo2O4 | LIBs | The initial discharge/charge capacities were 1035.8/1360.7 mA h g−1, maintained at 827 mA h g−1 after 60 cycles | 124 | ||
| Mn–MOFs | LiMn2O4 | LIBs | The first specific capacity of 140.6 mA h g−1, and 102.82% capacity retention after 500 cycles | 74 | ||
| Co[Fe(CN)6]0.667 | CoFe2O4 | LIBs | The capacity was 815 mA h g−1 for 20C, and the specific capacity was 841 mA h g−1 for 1C after 100 cycles | 122 | ||
| Ni2Fe(CN)6 | NiFe2O4 | LIBs | The capacity was 652 mA h g−1 for 10C, and the specific capacity was 975 mA h g−1 for 1C after 200 cycles | 126 | ||
| ZnMn2–PTCDA MMOFs | ZnMn2O4 | LIBs | The capacities are 488 mA h g−1 for 600 mA g−1, 426 mA h g−1 for 900 mA g−1, and 324 mA h g−1 for 1800 mA g−1 | 65 | ||
| MOF-74–ZnCo | ZnCo2O4 | LIBs | The average discharge specific capacities of 1586.8, 994.6, 759.6 and 509.2 mA h g−1 at 20 0, 40 0, 60 0 and 80 0 mA g−1, maintained at 1243.2 mA h g−1 after 80 cycles | 105 | ||
| MIL-101 (Fe, Co) | CoFe2O4 | LIBs | The reversible capacity was maintained at 978 mA h g−1 after 300 cycles, and the specific capacities were maintained at 1118 mA h g−1 after 200 cycles | 125 | ||
| Ni–Co–MOFs | NiCo2O4 | LIBs | The initial discharge/charge capacities were 3158/1444 mA h g−1, maintained at 720 mA h g−1 after 1000 cycles | 113 | ||
| Zn–Co–MOFs | ZnCo2O4 | LIBs | The initial discharge/charge capacities were 1074.3/812.5 mA h g−1, maintained at 816.2 mA h g−1 after 100 cycles | 114 | ||
| MnCo–MOF | MnCo2O4 | LIBs | The initial discharge/charge capacities were 1035.8/1360.7 mA h g−1, maintained at 691.3 mA h g−1 after 500 cycles | 131 | ||
| CoMn–BDC | BDC–LCMO (LiCoMnO4) | LIBs | A reversible capacity of 151.6 mA h g−1, 94.75% capacity retention after 500 cycles | 71 | ||
| Ni–Co–BTC | NixCo3−xO4 | LIBs | The capacities of 832/673 mA h g−1 after 300 cycles at 1 and 2 A g−1, maintained at 1109.8 mA h g−1 after 100 cycles | 127 | ||
| Zn–Co–ZIF | ZnxCo3−xO4-0.1 | LIBs | A reversible capacity of 1141.7 mA h g−1, and an initial coulombic efficiency of 95.6% | 137 | ||
| NF-MIL-0.25 | Ni0.62Fe2.38O4 | LIBs | A capacity of 1184 mA h g−1, maintained at 0.25 A h g−1 after 200 cycles | 115 | ||
| CoxMn3−x–BTC | CoxMn3−xO4-400 | LIBs | The first discharge capacities of 1369.6 mA h g−1, and an initial coulombic efficiency of 73.06% | 77 | ||
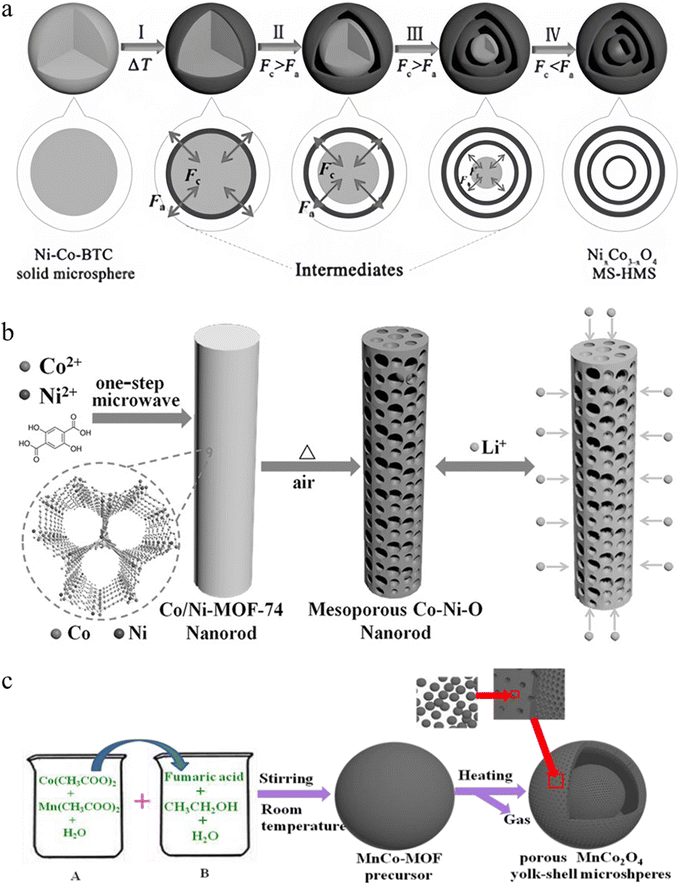 | ||
| Fig. 8 (a) Schematic illustration for the formation of NixCo3−xO4 MS-HMSs. Reproduced with permission from ref. 127. Copyright 2017 WILEY-VCH. (b) Synthesis of a mesoporous Co–Ni–O nanorod and schematic representation of their use for lithium storage. Reproduced with permission from ref. 130. Copyright 2016 WILEY-VCH. (c) Schematic illustration of the formation of porous MnCo2O4 yolk–shell microspheres. Reproduced with permission from ref. 131. Copyright 2019 The Royal Society of Chemistry. | ||
Although the unique structure of MOF-derived bimetallic spinels enhances electrochemical performance, an excessively high specific surface area may pose several drawbacks. It not only promotes side reactions between the materials and the electrolyte, but also generates high surface energy on the surface of the materials which leads to automatic aggregation of the materials during the reaction process, thus affecting the charge/discharge capacity.128,129 The preparation of bimetallic spinels with secondary nanodimensions by exploiting the tunability of MOFs is a feasible strategy to solve the above problems. Li et al. synthesized porous rod-like NixCo3−xO4 which was a sub-micron material connected by many nanoparticles (Fig. 8b).130 This special size not only retained the excellent electrochemical performances brought by the nanostructure, but also introduced the advantages brought by the micron-sized particles which could reduce secondary reactions between the electrode material and the electrolyte. Based on the above, the initial charging capacity of Ni0.3Co2.7O4 could be as high as 1189 mA h g−1 at low currents (100 mA g−1). Furthermore, Yang et al. found that the secondary nanostructure had some resistance to aggregation, ensuring the structural stability and dispersion of the materials and effectively adapting to volume changes during the cycling process (Fig. 8c).131 The MnCo2O4 egg-yolk–shell microspheres they prepared were able to remain stable over 500 cycles when used as a cathode material for LIBs. Therefore, MOF-derived bimetallic spinels with secondary nanodimensions have a promising application in LIBs, and the large-scale production of such materials can provide further developments in LIBs.
Except for the application of LIBs, MOF-derived bimetallic spinels have also been proven to be used as electrode materials for SIBs.132 SIBs are batteries that operate in the same mode as LIBs.133,134 However, the sodium metal has a larger atomic radius than lithium metal, which makes the space between its atomic layers insufficient, resulting in lower energy of SIBs. Moreover, large sodium ions also cause large strain in the lattice of the anode material during the reaction process, which destroys the electrochemical stability of SIBs.135,136 Wang and co-workers found that ZnxCo3−xO4 derived from MOFs could accommodate Na ions and achieve excellent reversible insertion and extraction as the anode material for SIBs due to its special crystalline phase structure.137 Owing to this, ZnxCo3−xO4 could achieve a reversible capacity of 381 mA h g−1 at a current of 0.1 mA g−1. In addition, the hollow nanobox structure of ZnxCo3−xO4 could provide sufficient space to reduce the volume strain during the reaction. Consequently, the reversible capacity of the material could reach 310 mA h g−1 after 100 cycles, with a capacity retention rate of 90.4%.
MIBs, in line with SIBs, are one of the potential alternatives to LIBs due to their abundant mineral resources and low cost.138,139 Compared to LIBs and SIBs, the process of Mg ion deposition does not produce dendritic crystals, so their charge/discharge capacity becomes higher. However, the strong interaction between Mg ions and the anions in the electrode weakens the diffusion rate of Mg ions, resulting in a lower electrochemical reaction rate for MIBs.140,141 Satisfactorily, bimetallic spinels with MOFs as sacrificial templates are materials consisting of ordered nano or micron sized particles with self-assembled ion diffusion pathways, greatly improving the electrochemical stability of the materials.142,143 Dong et al. confirmed this phenomenon in their study.144 The MOF-derived MgMn2O4 (called MOF-MMO) had a stable and ordered nanoparticle distribution and a cubic phase structure that effectively attenuated the geometrical distortion of the MgMn2O4 phase (Jahn–Teller effect) during the reaction, allowing the materials to maintain a capacity retention of 91.7% after 800 cycles. Although MOF-derived bimetallic spinels can provide some electrochemical stability for MIBs, it was inevitable that the charge/discharge capacity decreased gradually during cycling. This was due to the instability of the electrolyte in the reaction and the passivation of Mg at the anode. Therefore, exploring excellent MIB electrode materials and more stable battery components has become one of the key points in the development of the MIB application field.
| MOF precursors | Derivatives | Capacitance retention after charge–discharge cycles | Specific capacitance | Electrolyte | Ref. |
|---|---|---|---|---|---|
| MOF-74–NiCo2 | NiCo2O4 | 89.0% after 3000 cycles | 684 F g−1 at 0.5 A g−1 | 1 M KOH | 148 |
| ZIF-67 | NiCo2O4 | 81% after 5000 cycles | 2870 F g−1 at 1.5 A g−1 | 2 M KOH | 150 |
| Mn–Co–ZIF | MnCo2O4 | 95% after 4500 cycles | 1763 F g−1 at 1.0 A g−1 | 2 M KOH | 47 |
| JUC-155 | ZnCo2O4 | 97.9% after 1500 cycles | 457 F g−1 at 1.0 A g−1 | 6 M KOH | 78 |
| Mn/Ni–MOFs | MnNi2O4 | 93.25% after 5000 cycles | 2848 F g−1 at 1.0 A g−1 | 6 M KOH | 76 |
| MOF-74–NiCo | NixCo3−xO4 | 80% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
782 F g−1 at 2.0 A g−1 | 6 M KOH | 66 |
| NiFe2 MOF | NiFe2O4 | 74% after 3000 cycles | 833 F g−1 at 0.25 A g−1 | 1 M KOH | 82 |
| Co/Fe–MIL(88A) | CoxFe3−xO4 | 72.9% after 5000 cycles | 973.1 F g−1 at 1 A g−1 | 2 M KOH | 50 |
| Zn/Co–MOF | ZnCo2O4 | 87.2% after 5000 cycles | 420 F g−1 at 0.5 A g−1 | 6 M KOH | 83 |
Owing to these excellent redox properties and unique structural advantages, MOF-derived bimetallic spinels are also capable of serving as a conductive backbone for accelerated electron transfer to enhance the application advantages of SCs. Han and co-workers synthesized MOF-derived ZnCo2O4 with a two dimensional (2D) structure in situ on Ni foam (NF) and then deposited Ni(OH)2 nanosheets on the ZnCo2O4 surface using the hydrothermal method.149 It was confirmed that ZnCo2O4 with a layered and porous structure not only enhanced the dispersion of Ni(OH)2 and provided high electrical conductivity, but also formed a core–shell structure with Ni(OH)2 with excellent stability and provided sufficient space for close contact between the material and the electrolyte (Fig. 9b). As a result, thanks to the excellent core–shell structure and the synergy between the two substances, when used as a cathode material for SCs (Fig. 9a), the highest area specific capacitance of ZnCo2O4@Ni(OH)2/NF was 3063.2 mF cm−2 at a current density of 1 mA cm−2, which was twice as high as that of Ni(OH)2/NF (1367.3 mF cm−2) (Fig. 9c). Meanwhile, the composite retained a rate capacity of 55.3% at a current density of 10 mA cm−2, which was much higher compared to that of Ni(OH)2/NF (21.5%).
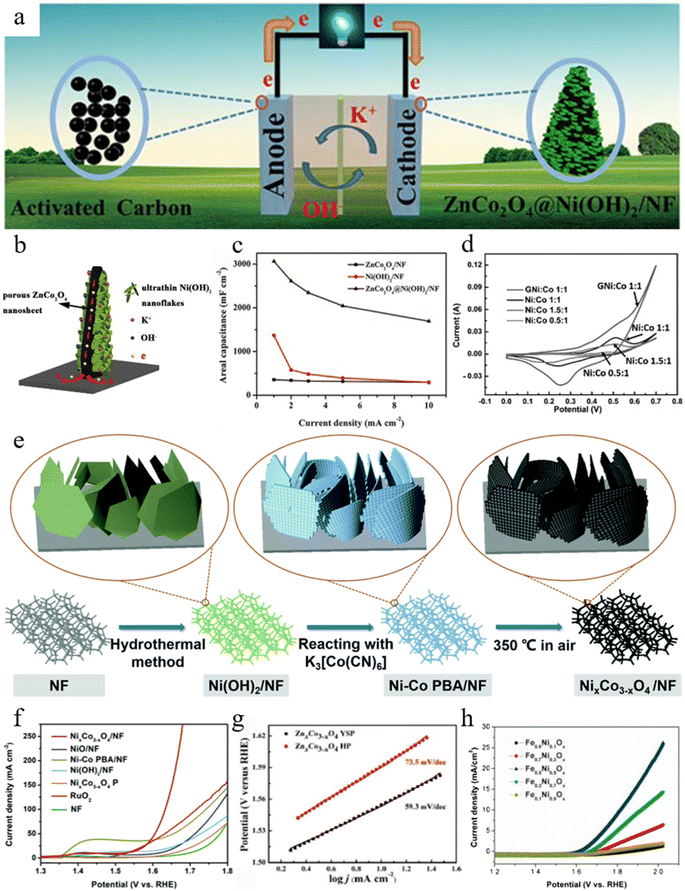 | ||
Fig. 9 (a) Schematic illustration of the assembled ASC of ZnCo2O4@Ni(OH)2/NF. (b) Schematic illustration of the advantages and charge storage mechanism of the 3D hierarchical ZnCo2O4@Ni(OH)2/NF electrode. (c) The areal capacitance of ZnCo2O4/NF, Ni(OH)2/NF and ZnCo2O4@Ni(OH)2/NF electrodes. Reproduced with permission from ref. 149. Copyright 2017 WILEY-VCH. (d) Overlap of GCD curves of all Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratios and GNi Co ratios and GNi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co at 1 mV s−1. Reproduced with permission from ref. 150. Copyright 2017 WILEY-VCH. (e) Schematic illustration of the preparation of the NixCo3−xO4/NF electrode. (f) LSV curves of NixCo3−xO4/NF, NiO/NF, Ni–Co PBA/NF, Ni(OH)2/NF, NixCo3−xO4 P, RuO2 and NF. Reproduced with permission from ref. 156. Copyright 2019 The Royal Society of Chemistry. (g) Tafel plots of ZnxCo3−xO4 YSP and ZnxCo3−xO4 HP in O2-saturated 1 M KOH electrolyte. Reproduced with permission from ref. 93. Copyright 2017 American Chemical Society. (h) Polarization curves of the Fe–Ni–Ox catalysts with a scan rate of 5 mV s−1 in 0.1 M KOH solution. Reproduced with permission from ref. 41. Copyright 2016 Elsevier. Co at 1 mV s−1. Reproduced with permission from ref. 150. Copyright 2017 WILEY-VCH. (e) Schematic illustration of the preparation of the NixCo3−xO4/NF electrode. (f) LSV curves of NixCo3−xO4/NF, NiO/NF, Ni–Co PBA/NF, Ni(OH)2/NF, NixCo3−xO4 P, RuO2 and NF. Reproduced with permission from ref. 156. Copyright 2019 The Royal Society of Chemistry. (g) Tafel plots of ZnxCo3−xO4 YSP and ZnxCo3−xO4 HP in O2-saturated 1 M KOH electrolyte. Reproduced with permission from ref. 93. Copyright 2017 American Chemical Society. (h) Polarization curves of the Fe–Ni–Ox catalysts with a scan rate of 5 mV s−1 in 0.1 M KOH solution. Reproduced with permission from ref. 41. Copyright 2016 Elsevier. | ||
Notably, despite the excellent structure of MOF-derived bimetallic spinels, there are still continuous ionic embedding and de-embedding phenomena that lead to nanoparticle aggregation. To address this issue, the use of carbon materials as carriers was found to be effective in reducing the agglomeration of active materials. Jayakumar and co-workers loaded highly optimized MOF-derived Ni–Co spinels on graphene in order to synthesize three dimensional (3D) composites.150 They found that the dispersion and the electrical conductivity brought by graphene with a porous network structure effectively facilitated the rapid charge movement generated by the reaction between the electrolyte and metal ions. Therefore, the 3D composites could achieve a capacitance of 2870 F g−1, significantly higher than that of the single materials (1931 F g−1) (Fig. 9d), and the capacitance of the materials remained at 81% after 5000 cycles.
The reaction kinetics of the OER is relatively slow, which mainly involves the transfer of four electrons and the formation of O bonds. Its reaction process under acidic and basic conditions can be expressed as:153
Acidic solution:
| 2H2O(l) → O2(g) + 4H+(aq) + 4e−, E0anode = 1.23 V (oxidation) | (1) |
Neutral and alkaline solutions:
| 4OH−(aq) → 2H2O(l) + O2(g) + 4e−, E0anode = 1.23 V (oxidation) | (2) |
Fe-Group elements (Fe, Co, and Ni) are considered to be one of the candidates for the preparation of excellent electrode materials for OER catalysts due to their rich resources and strong redox reactions. These elements can induce partial charge transfer mechanisms in MOF-derived bimetallic spinels, thereby enhancing electrical conductivity.154,155 Shen and co-workers confirmed this (Fig. 9e).156 They combined the high-resolution spectral analysis of Co and O and confirmed that electrochemically active CoOOH intermediates were formed on the surface of the materials during the OER. Meanwhile, density flooding calculations also corroborated that the adsorption of OH*, O*, and OOH* intermediates by MOF-derived Ni2CoO4 occurred mainly on Co ions. Therefore, the excellent adsorption intermediates possessed by Ni2CoO4 enhanced the OER, resulting in a significant decrease in the OER overpotential of Ni2CoO4, and the OER activity of Ni2CoO4 greatly exceeded that of similar materials and RuO2 (Fig. 9f).
Notably, the appearance of three-dimensional polyhedra and hollow structures of MOF-derived bimetallic spinels can significantly enhance the OER activity of the material. For example, Wang et al. synthesized dodecahedral ZnxCo3−xO4 with a hollow structure by calcining ZnCo–ZIFs precursors in air.38 They confirmed that the abundance of inner and outer planes provided more surface area as well as active sites, which facilitated the electron transfer capability during the OER. More importantly, the porous structure and high specific surface area of the materials also afford a large number of diffusion channels to release the oxygen generated on the electrode surface, effectively preventing oxygen from accumulating on the materials surface and corroding the materials. Benefiting from this, the starting potential of the obtained ZnxCo3−xO4 was only 1.63 V (vs. RHE) in an oxygen saturated alkaline solution. At a current density of 10 mA cm−2, the overvoltage value was only 435 mV, and the linear fitting slope of the Tafel curve was also only 66.3 mV dec−1. These values were significantly lower than those of ZnCo–ZIFs and other commercially available electrode materials. In contrast to this common hollow structure, Yu et al. found that ZnxCo3−xO4 with an egg-yolk–shell structure (ZnxCo3−xO4 YSP) synthesized by two-step calcination of bimetallic ZIF precursors under N2 and in air had more advantages in OER catalysts.93 Compared to the ZnxCo3−xO4 hollow polyhedron (ZnxCo3−xO4 HP) calcined only in air, the yolk–shell structure caused by N2 increased the specific surface area of the materials, which led to more catalytically active sites. As a result, the Tafel slope (59.3 mV dec−1) and overvoltage value of ZnxCo3−xO4 YSP were lower than those of ZnxCo3−xO4 HP (Fig. 9g). Additionally, the yolk–shell structure was more stable compared to the traditional hollow structure, which improved the structural stability of the materials during the reaction process. 12 hours of current measurements confirmed that the current density of ZnxCo3−xO4 YSP could still reach the initial 90%, while the current density of ZnxCo3−xO4 HP had decreased by 35%.
In general, spinel materials with mixed crystal composition may show better electrochemical performances due to the ratio of metals that affects the charge transfer mechanism of OER catalysts.157,158 For this, the adjustable structure of MOF precursors can be used to explore the mixed crystal composition to prepare electrode materials for OER catalysts with relatively excellent bimetallic spinel structures. Jiang et al. first prepared iron–nickel oxide (Fe–Ni–Ox) structures by calcination using the corresponding metal-amino terephthalate MOFs as sacrificial templates and then controlled the composition of the synthesized materials by adjusting the metal ratio of bimetallic spinels (Fe–Ni–Ox).41 When the Fe/Ni metal ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the OER activity of Fe0.5Ni0.5Ox was similar to that of commercial RuO2 and Pt/C and was significantly higher than that of nickel lean (Fe0.9Ni0.1Ox) and iron lean (Fe0.1Ni0.9Ox) catalysts (Fig. 9h). This might be due to the crystal structure of Fe0.5Ni0.5Ox which consisted of NiFe2O4 and NiO. Among them, NiO could undergo phase transformation into NiOOH, while NiFe2O4 did not change. Therefore, the high Ni and Fe valence states in NiOOH and NiFe2O4 promoted the catalytic reduction activity of the electrode material in the OER in alkaline media.
1, the OER activity of Fe0.5Ni0.5Ox was similar to that of commercial RuO2 and Pt/C and was significantly higher than that of nickel lean (Fe0.9Ni0.1Ox) and iron lean (Fe0.1Ni0.9Ox) catalysts (Fig. 9h). This might be due to the crystal structure of Fe0.5Ni0.5Ox which consisted of NiFe2O4 and NiO. Among them, NiO could undergo phase transformation into NiOOH, while NiFe2O4 did not change. Therefore, the high Ni and Fe valence states in NiOOH and NiFe2O4 promoted the catalytic reduction activity of the electrode material in the OER in alkaline media.
In summary, the charge/discharge capacity and battery operational performance of MOF-derived bimetallic spinels are undoubtedly improved due to the structure changes and the synergistic effect of the two metals. First of all, compared with traditional bimetallic spinels, a variety of core–shell structures (such as multiple core–shell structures and yolk–shell structures) of MOF-derived bimetallic spinels can significantly improve the electron transport capacity, electrocatalytic performance, and structural stability of bimetallic spinels. Secondly, MOF-derived bimetallic spinels can regulate the size to form secondary nanostructures which reduce the particle aggregation and volume expansion of bimetallic spinels during electrochemical reactions. Benefiting from the excellent structural advantages, MOF-derived bimetallic spinels can also serve as a conductive skeleton, which provides the composites with higher electrical conductivity and superior stability, broadening their applications in the energy field. Thirdly, a mixed crystal composition can be obtained by varying the ratio of the two metals, which contributes to the introduction of more high-valent cations to improve the redox capacity of the materials. However, MOF-derived bimetallic spinels still have some inherent drawbacks in energy storage and conversion that have not been addressed, affecting practical applications. This also requires further exploration to obtain excellent materials.
4.2 Environmental applications
With the rapid development of the global economy, a large amount of visible or invisible water pollution and gas pollution, such as dye pollution and NOx pollution,159–161 is inevitably generated in production and life. These not only destroy the ecological environment, but even pose a great threat to biological life.162,163 Based on the above, the treatment and monitoring of various pollutants has become an important part of the green and sustainable development of ecological environment protection.164–166 MOF-derived bimetallic spinels have become one of the dominant catalysts for the degradation and monitoring of pollutants due to their excellent size, structure, and tunable composition.43,90,91 Therefore, this section briefly summarizes the latest research on MOF-derived bimetallic spinels in the environmental field and provides insight into their main reaction mechanisms to broaden their practical applications in the environmental field.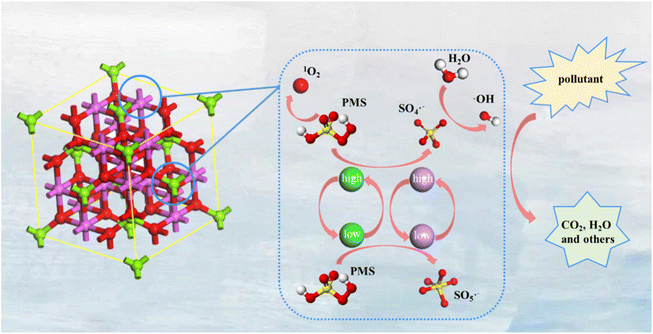 | ||
| Fig. 10 Schematic diagram of the general mechanism of organic pollutant degradation in a MOF-derived bimetallic spinels/PMS system. | ||
PMS:
| Mn+ + HSO5− → Mn+1 + SO4˙− + OH− | (3) |
| Mn+1 + HSO5− → Mn+ + SO5˙− + H+ | (4) |
PDS:
| Mn+ + S2O82− → Mn+1 + SO4˙− + SO42− | (5) |
| Mn+1 + S2O82− → Mn+ + S2O8˙− | (6) |
Long et al. prepared porous CuFe2O4 nanoparticles for the degradation of bisphenol A (BPA) in water.51 As illustrated in eqn (7)–(12), both cycle processes of Cu(II)–Cu(III)–Cu(II) and Fe(III)–Fe(II)–Fe(III) could activate PMS to produce a large amount of SO4˙− (Fig. 11a). More favorably, some of the SO4˙− reacted with the water and hydroxide ion (OH−) on the surface to produce part of the hydroxyl radical (˙OH). SO4˙− and ˙OH were the main reactive species in this system. Due to the combined action of the two active species, the degradation of 99.45% BPA could be accomplished in 10 min and a mineralization rate of 81.57% was obtained in 60 min under the optimal reaction conditions (Fig. 11b). Notably, the Co(OH)2 and Fe(OH)3 passivation films produced by CuFe2O4 during the reaction led to the blocking of the porous structure on the surface of the materials, reducing the catalytically active sites of the materials. Fortunately, drying could restore the porous structure and different drying temperatures might have different effects on the recovery performances of bimetallic spinels. Therefore, it was necessary to recover bimetallic spinels at appropriate drying temperature.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Co2+ + H2O → Co2+ + H2O → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Co2+–OH− + H+ Co2+–OH− + H+ | (7) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Co2+–OH− + HSO5− → Co2+–OH− + HSO5− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CoO+ + SO4− + H2O CoO+ + SO4− + H2O | (8) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CoO+ + 2H+ → CoO+ + 2H+ → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Co3+ + H2O Co3+ + H2O | (9) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Co3+ + HSO5− → Co3+ + HSO5− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Co2+ + SO5− + H+ Co2+ + SO5− + H+ | (10) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + HSO5− → Fe3+ + HSO5− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + SO5− + H+ Fe2+ + SO5− + H+ | (11) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + HSO5− → Fe2+ + HSO5− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + SO4− + OH− Fe3+ + SO4− + OH− | (12) |
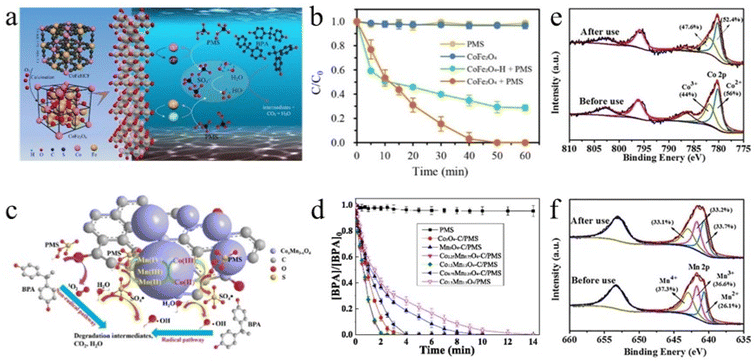 | ||
| Fig. 11 (a) Reaction mechanism of degradation of BPA in a CoFe2O4/PMS system. (b) Removal efficiency of BPA in different reaction systems within 60 min. Reproduced with permission from ref. 51. Copyright 2021 Elsevier. (c) Mechanism illustration of BPA degradation in the Co1.5Mn1.5O4–C/PMS system. (d) BPA removal of the Co1.5Mn1.5O4–C/PMS system. Reproduced with permission from ref. 64. Copyright 2022 Elsevier. (e) XPS spectra of Co 2p for CoMn2O4 MD before and after use. (f) XPS spectra of Mn 2p for CoMn2O4 MD before and after use. Reproduced with permission from ref. 33. Copyright 2018 Elsevier. | ||
The reaction of bimetallic spinels with the PMS system to degrade organic pollutants is basically dominated by radical pathways. However, the selectivity of radical active species is lower than that of non-radical active species, and it is susceptible to ionic interference. Wang et al. synthesized CoxMn3−xO4–C composites by two-step calcination of Co–Mn MOF-74 in N2 and air.64 The original carbon structure was retained after N2 calcination, and thus a large amount of CoxMn3−xO4 was uniformly loaded on the surface of the carbon materials. During the degradation of bisphenol A (BPA), the redox cycle of Co and Mn undoubtedly produced large amounts of ˙OH and SO4˙−. In addition, thanks to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group brought by the carbon materials, activated PMS to self-activate to generate part of the 1O2 (Fig. 11c). Both pathways (radical and non-radical) promoted the degradation efficiency of BPA (Fig. 11d). Therefore, Co1.5Mn1.5O4–C could degrade about 0.2 mM of BPA within 5 min, which was significantly higher than that of similar materials.
O group brought by the carbon materials, activated PMS to self-activate to generate part of the 1O2 (Fig. 11c). Both pathways (radical and non-radical) promoted the degradation efficiency of BPA (Fig. 11d). Therefore, Co1.5Mn1.5O4–C could degrade about 0.2 mM of BPA within 5 min, which was significantly higher than that of similar materials.
In MOF-derived bimetallic spinels, there is a certain degree of competition between the two different valence metals, with one of them playing a dominant role in PMS activation. Li et al. used redox potential values to analyze this.173 When MOF-derived Fe0.8Co2.2O4 was utilized to activate PMS for the degradation of BPA, the potential value of Co(II)/Co(III) was 1.8 V, which was located in the HSO5˙−/SO4˙− (2.5–3.1 V) and HSO5˙−/SO5˙− (1.1 V) between them. In contrast, the potential value of Fe(II)/Fe(III) was only 0.8 V, indicating that its redox potential was lower than that of HSO5˙−/SO5˙−, which was unfavorable for redox cycling reactions. Combined with the analysis of the 57Fe Mössbauer spectroscopy technique before and after the reaction, the iron ions did not change greatly in the PMS activation, which fully indicated the dominant role of Co in the material degradation process of BPA. However, the higher content of the dominant metal ion is not better. This phenomenon may be explained by the electron transfer process between the two metals. The non-dominant metal ion favors the return of the dominant metal ion to the reducible low valence state, thus maintaining the high catalytic activity of the system. Li and co-workers provided evidence for this.33 During the activation of PMS for the degradation of sulfanilamide (SA) with MOF-derived CoMn2O4 nanoplates (CoxMn3−xO4-MD), as illustrated in eqn (13) and (14), the lower standard reduction potentials of Mn3+/Mn2+ (E0 = 1.51 V) and Mn4+/Mn3+ (E0 = 0.15 V) compared to that of Co3+/Co2+ (E0 = 1.81 V). As a result, the presence of Mn which was essential for Co(II) regeneration was feasible. In addition, according to the XPS patterns (Fig. 11e and f), it could be found that the Co(II) of Co2.5Mn0.5O4-MD after the reaction was significantly less than that before the reaction, which laterally corroborated that the lower Mn(III) reduced the reduction of Co ions. Based on the above, the catalytic activity of CoMn2O4-MD containing lower Co ions for the degradation of SA (0.155 min−1) was higher than that of Co2.5Mn0.5O4-MD (0.145 min−1).
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Mn3+ + Mn3+ + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Co3+ → Co3+ → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Mn4+ + Mn4+ + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Co2+, E0 = 1.66 V Co2+, E0 = 1.66 V | (13) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Mn2+ + Mn2+ + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Co3+ → Co3+ → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Mn3+ + Mn3+ + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Co2+, E0 = 0.30 V Co2+, E0 = 0.30 V | (14) |
In conclusion, MOF-derived bimetallic spinels have great potential in the field of SR-AOPs due to their excellent structure and crystal composition. So far, MOF-derived bimetallic spinels are still being explored for persulfate oxidation, whereas in fact traditional bimetallic spinels have been shown to be useful for Fenton-like, photocatalytic, and ozone oxidation techniques. Therefore, the potential of MOF-derived bimetallic spinels for application in these technologies remains to be explored.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 at 140–290 °C (Fig. 12b and c).
000 h−1 at 140–290 °C (Fig. 12b and c).
 | ||
Fig. 12 (a) Gibbs free energy (400 K) diagram over NO-to-NO2 oxidation based on the MvK mechanism. (b) NO conversion in NH3-SCR of NO at different temperatures over FexMn3−xO4 NPs in 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 GHSV. (c) NO-to-NO2 oxidation rates over FexMn3−xO4 NPs in 200 000 h−1 GHSV. (c) NO-to-NO2 oxidation rates over FexMn3−xO4 NPs in 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 GHSV. Reproduced with permission from ref. 181. Copyright 2020 American Chemical Society. (d) The relationship curves of the response and gas concentration for different compounds. Reproduced with permission from ref. 195. Copyright 2018 The Royal Society of Chemistry. (e) Polar graphs and responses to 200 ppm acetone for ZnCo-0.27, ZnCo-0.37, ZnCo-0.48 at different temperatures ranging from 110 to 210 °C. Reproduced with permission from ref. 196. Copyright 2019 The Royal Society of Chemistry. (f) SEM images of ZnCo2O4 MFs. (g) Schematic illustration of the hollow spaces of ZnCo2O4 MFs acting as reaction chambers to confine gas molecules for gas-sensing reactions. (h) Responses of ZnCo2O4 MFs and ZnCo2O4 BMFs to 100 ppm acetone gas at different operating temperatures. Reproduced with permission from ref. 197. Copyright 2021 Elsevier. 000 h−1 GHSV. Reproduced with permission from ref. 181. Copyright 2020 American Chemical Society. (d) The relationship curves of the response and gas concentration for different compounds. Reproduced with permission from ref. 195. Copyright 2018 The Royal Society of Chemistry. (e) Polar graphs and responses to 200 ppm acetone for ZnCo-0.27, ZnCo-0.37, ZnCo-0.48 at different temperatures ranging from 110 to 210 °C. Reproduced with permission from ref. 196. Copyright 2019 The Royal Society of Chemistry. (f) SEM images of ZnCo2O4 MFs. (g) Schematic illustration of the hollow spaces of ZnCo2O4 MFs acting as reaction chambers to confine gas molecules for gas-sensing reactions. (h) Responses of ZnCo2O4 MFs and ZnCo2O4 BMFs to 100 ppm acetone gas at different operating temperatures. Reproduced with permission from ref. 197. Copyright 2021 Elsevier. | ||
It is noteworthy that during the oxidation of NH3, some SO2 is inevitably present in the gas, which can compete with NH3 for adsorption sites on the materials surface and react with reactive oxygen species, thereby reducing the degradability of the materials.182–184 Fortunately, MOF-derived bimetallic spinels are resistant to SO2 based on the Langmuir–Hinshelwood (L–H) mechanism and the E–Rideal (E–R) mechanism, which have a significant inhibitory effect on this phenomenon.185 Ko et al. confirmed this finding by preparing MnCO2O4 from MOFs for NH3-SCR.186 Based on in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy, it could be revealed that the monodentate nitrate and monodentate nitrite were gradually transformed into bidentate nitrate and bridging nitrate during the reaction process. Their presence fully confirmed that the MOF-derived bimetallic spinels have certain resistance to SO2 in the oxidation and reduction of NH3 as well as high sensing selectivity.
Based on the above, it can be concluded that MOF-derived bimetallic spinels have a great advantage in NOx degradation due to their adjustable metal ratio and structure. However, the advantage of certain metals in bimetallic spinels being enriched on the surface through the calcination process of MOF precursors has not been demonstrated so far in this application. Numerous experiments have confirmed that the enrichment of high valence metal ions on the surface of ordinary bimetallic spinels has an advantage in the degradation of NOx.187 Therefore, exploring such materials has the potential to facilitate the future development of MOF-derived bimetallic spinels for NOx degradation. In addition, the application scope of derived materials in inorganic pollutants is currently limited to NOx and formaldehyde,99 so it is necessary to explore the degradation process of more inorganic gaseous pollutants.
It is worth noting that the addition of different metal ratios affects the number of defects, specific surface area, and porous structure of MOF-derived bimetallic spinels, which may affect the sensing performance of the materials. In order to optimize the sensing performance of MOF-derived bimetallic spinels, Zhou et al. took advantage of this to prepare MOF-derived ZnxCo3−xO4 nanocages with different ratios of Zn (ZnCo-n, n is the ratio of Zn/Co) for studying the influence of the number of defects and the structure of the derived materials on the sensing performance.196 Bimetallic spinel nanoparticles gradually gathered with the addition of Zn. Their specific surface area and porous structure showed a trend of first increasing and then decreasing, reaching the maximum value when n was 0.37. Moreover, the number of oxygen vacancies increased with the addition of Zn, reaching a maximum value when n was 0.48. Essentially, the sensing performance of the materials was closely related to the oxygen adsorbed on the surface of the materials and the oxygen content of the vacancies. More oxygen vacancies provided more free electrons for better sensing performance. Shockingly, the response value of the sensor using ZnCo 0.37 to acetone could be as high as 35.6, which was superior to that of the sensor using ZnCo-0.48 or other similar materials (Fig. 12e). In addition, when cross reacting with 200 ppm mixed gas at 170 °C, ZnCo-0.37 had the best sensing response to acetone and the response value of other gases can be ignored, while the response value of other ZnCo-n sensors to all gases was low. This precisely explained that the excellent sensing properties of MOF derived bimetallic spinels were not only due to the influence of vacancy defects, but also to the influence of the high specific surface area and pore structure which played the leading role. Based on this, for MOF-derived bimetallic spinels, the synergy of the oxygen vacancy content, specific surface area and mesoporous structure of the materials makes the sensing material obtain different adsorption site contents and catalytic sites. It is possible to modulate these three variations by rationally designing the ratio of the two metals in order to improve the sensing ability of the sensor.
In addition to the above-mentioned changes, MOF-derived bimetallic spinels can effectively retain the original morphology of MOFs. Therefore, it can form bimetallic spinels with various microscopic morphologies, thus bringing more development prospects for this application. Li and co-workers used MOF precursors to synthesize hierarchical ZnCo2O4 flower-like spinels assembled using a 3D nano-slice (Fig. 12f).197 In the process of the gas sensing reaction, the microstructure composed of 3D nano-sheets could provide some special triangular or polygonal space, which could be used as an open reaction chamber to provide more contact area with the gas to complete the sensing reaction. More interestingly, there were some nano-junctions between the adjacent nano-sheets of the materials which could be used as a modulator for hole flow in the sensing process, thereby enhancing the response value of the gas sensor (Fig. 12g). In addition, the thin structure and small particle size brought about by the heat treatment of MOFs could make ZnCo2O4 show significant sensing selectivity for acetone (Fig. 12h).
All in all, the formation of bimetallic spinels using MOFs as templates opens up more attractive possibilities for gas sensor applications than for traditional bimetallic spinels. An excellent number of defects can be obtained by using a suitable metal ratio. Defects and special morphological structures together regulate the sensing response and selectivity of gaseous sensors, overcoming the shortcomings of poor selectivity of raw materials and high environmental conditions. In addition, the 3D structure introduced by MOFs provides a large amount of contact area for materials, achieving amazing sensing capabilities. However, the current applications of MOF-derived bimetallic spinels in gas sensors are mainly focused on acetone and formaldehyde. Compared to MOFs and original spinels, the application range is narrow, greatly limiting the practical application of derived materials. Therefore, it is necessary to further explore the sensing effects of MOF-derived bimetallic spinels on other gas pollutants.
5. Summary and outlook
Bimetallic spinels synthesized with MOFs as sacrificial templates repair the inherent structural defects of traditional bimetallic spinels and have achieved significant breakthroughs in applications. This review summarized the two different synthetic strategies for MOF-derived bimetallic spinels and their recent progress in energy and environmental applications. Owing to the tunable structure of MOFs, MOF-derived bimetallic spinels could obtain multiple morphologies, excellent porosity, large specific surface area, high crystallinity and different exposed crystal planes by adjusting the synthetic strategies. These structural changes, property advantages and vacancy defects greatly enhance the performance and application advantages of MOF-derived bimetallic spinels. Specifically, (i) the metal nodes of the derived bimetallic spinel are dispersed and stabilized, improving the metal leaching problem and metal aggregation. (ii) The core–shell structures formed, such as hollow layered nanocages, yolk–shell structures, and multi-shell hollow structures, improve the structural stability and ion transfer rate of bimetallic spinels. (iii) The high specific surface area and the large number of ordered mesopores or even hollow structures not only expose more active sites, but also provide extensive diffusion pathways, which promote electrochemical performances and the catalytic activity of the materials. More interestingly, based on their excellent specific surface area, the derived bimetallic spinels are able to be used as conductive substrates for loading other functional materials, which greatly enhances their application advantages. (iv) The presence of OVs accelerates the migration of ions, improves the reaction kinetics, and enhances the adsorption properties.Despite the many tantalizing results that have emerged, to date there are still some unresolved issues and challenges that limit the practical application of MOF-derived bimetallic spinels. Here are some thoughts we offer on their problems and future research directions.
(1) Typically, the structural features of MOF-derived bimetallic spinels are limited by MOF precursors. However, there are fewer methods to prepare bimetallic spinels using MOFs, and many of them have not yet been explored. In addition, annealing treatments are usually performed at higher temperatures during the preparation process, which increases the cost of material preparation. For this reason, it is necessary to explore methods with lower costs and better preparation strategies to produce such materials on a large scale.
(2) So far, although MOF-derived bimetallic spinels have achieved good results in morphology and structure, the combined types of two metals are limited to partial metals such as Fe, Ni, Co and Mn. The formation of compositional species diversity facilitates the application of such materials in other special fields. Therefore, more possibilities for metal composition need to be developed.
(3) To date, atomic change processes in the preparation of bimetallic spinels using MOFs as sacrificial templates have not been introduced in detail. The structural changes and crystal phase composition of MOF-derived bimetallic spinels can have a significant impact on the performances of materials. Therefore, in the future, we can focus on and explore the characteristic changes and sequencing of metal ions from the perspective of calculations (density functional theory and mixing enthalpy). Furthermore, it is possible to try to explore the derivative mechanisms using advanced characterization and other methods, which can provide guidance for the design of better materials.
(4) During the preparation process of traditional bimetallic spinels, it is possible to synthesize bimetallic spinels with cationic defects through reasonable design and preparation.198 This vacancy defect can greatly promote the sensing and electrochemical performances of materials. However, such materials have not been prepared using MOF-derived bimetallic spinels. Therefore, MOF-derived bimetallic spinels with cationic defects may become one of the research hotspots in the future.
(5) Currently, the application of MOF-derived bimetallic spinels in batteries is relatively hot, while their applications in fields such as environmental pollution and catalytic reduction are relatively rare. The current research on environmental pollution also focuses on persulfate oxidation. The application of catalytic reduction only studies NOx. Numerous studies have confirmed that the magnetic, electrochemical, optical and catalytic performances of MOF-derived bimetallic spinels have been improved to an excellent level. Expanding the range of applications of such materials is achievable.
(6) MOF-derived bimetallic spinels can be tried in other special fields, such as the biological field. Many studies have shown that MOFs and bimetallic spinels have a certain degree of biocompatibility and have been used in biologically related applications. For example, MOFs have recently been discovered to be used for the transportation and release of certain macromolecular drugs (proteins and nucleic acids).199 Additionally, bimetallic spinels have certain antibacterial activity against various pathogenic bacteria.200,201 Therefore, MOF-derived bimetallic spinels may also have great development potential in the biological field.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study was financially supported by the Program for the National Natural Science Foundation of China (51909084, 52000065, 51909085 and 52170128), the Natural Science Foundation of Hunan Province, China (2020JJ5069 and 2021JJ40105), and the Fundamental Research Funds for the Central Universities (531118010247).References
- L. Du, W. Xu, S. Liu, X. Li, D. Huang, X. Tan and Y. Liu, J. Colloid Interface Sci., 2020, 577, 419–430 CrossRef CAS
.
- L. Li, Z. Yin, M. Cheng, L. Qin, S. Liu, H. Yi, M. Zhang, Y. Fu, X. Yang, X. Zhou, G. Zeng and C. Lai, Chem. Eng. J., 2023, 454, 140126 CrossRef CAS
.
- B. Y. Guan, Y. Lu, Y. Wang, M. Wu and X. W. Lou, Adv. Funct. Mater., 2018, 28, 1706738 CrossRef
.
- K. Wang, C. Han, Z. Shao, J. Qiu, S. Wang and S. Liu, Adv. Funct. Mater., 2021, 31, 2102089 CrossRef CAS
.
- J. Li, P. M. Bhatt, J. Li, M. Eddaoudi and Y. Liu, Adv. Mater., 2020, 32, 2002563 CrossRef CAS
.
- M. Jia, Q. Liu, W. Xiong, Z. Yang, C. Zhang, D. Wang, Y. Xiang, H. Peng, J. Tong, J. Cao and H. Xu, Appl. Catal., B, 2022, 310, 121344 CrossRef CAS
.
- C. Tang, M. Cheng, C. Lai, L. Li, Z. Wei, D. Ma, L. Du, G. Wang, L. Yang and L. Tang, J. Environ. Chem. Eng., 2023, 11, 110395 CrossRef CAS
.
- H. Peng, W. Xiong, Z. Yang, J. Tong, M. Jia, Y. Xiang, S. Sun and Z. Xu, Chem. Eng. J., 2023, 457, 141317 CrossRef CAS
.
- H.-T. Zhang, T. Zhang and X. Zhang, Adv. Sci., 2023, 10, 2300193 CrossRef
.
- A. K. Chaudhari, H. J. Kim, I. Han and J.-C. Tan, Adv. Mater., 2017, 29, 1701463 CrossRef
.
- Y. Chen, M. Cheng, C. Lai, Z. Wei, G. Zhang, L. Li, C. Tang, L. Du, G. Wang and H. Liu, Small, 2023, 19, 2205902 CrossRef CAS
.
- H. Liu, M. Cheng, Y. Liu, J. Wang, G. Zhang, L. Li, L. Du, G. Wang, S. Yang and X. Wang, Energy Environ. Sci., 2022, 15, 3722–3749 RSC
.
- C. Guo, M. Cheng, G. Zhang, W. Xiong, C. Zhou, B. Song, L. Du, L. Li, C. Tang, G. Wang and H. Liu, Environ. Sci.: Nano, 2023, 10, 1528–1552 RSC
.
- K. Chen and L. Li, Adv. Mater., 2019, 31, 1901115 CrossRef
.
- W. Chaikittisilp, Y. Yamauchi and K. Ariga, Adv. Mater., 2022, 34, 2107212 CrossRef CAS
.
- Y. Chen, H. Kang, M. Cheng, G. Zhang, G. Wang, H. Liu, W. Xiao, Y. Liu and H. Jiang, Adv. Funct. Mater., 2023, 2309223 CrossRef
.
- J. Hemberger, P. Lunkenheimer, R. Fichtl, H.-A. Krug von Nidda, V. Tsurkan and A. Loidl, Nature, 2005, 434, 364–367 CrossRef CAS PubMed
.
- C. Li, X. Han, F. Cheng, Y. Hu, C. Chen and J. Chen, Nat. Commun., 2015, 6, 7345 CrossRef CAS PubMed
.
- S. Yang, F. Qi, S. Xiong, H. Dang, Y. Liao, P. K. Wong and J. Li, Appl. Catal., B, 2016, 181, 570–580 CrossRef CAS
.
- Y. Liao, S. Xiong, H. Dang, X. Xiao, S. Yang and P. K. Wong, J. Hazard. Mater., 2015, 299, 740–746 CrossRef CAS PubMed
.
- V. Kocevski, G. Pilania and B. P. Uberuaga, J. Mater. Chem. A, 2020, 8, 25756–25767 RSC
.
- Q. Zhao, Z. Yan, C. Chen and J. Chen, Chem. Rev., 2017, 117, 10121–10211 CrossRef CAS PubMed
.
- H. Zhao, Y. Dong, G. Wang, P. Jiang, J. Zhang, L. Wu and K. Li, Chem. Eng. J., 2013, 219, 295–302 CrossRef CAS
.
- M. Feng, Z. Xu, X. Bai, K. Lin and M. Zhang, Chem. Eng. J., 2022, 446, 137394 CrossRef CAS
.
- M. Shi, B. Wang, Y. Shen, J. Jiang, W. Zhu, Y. Su, M. Narayanasamy, S. Angaiah, C. Yan and Q. Peng, Chem. Eng. J., 2020, 399, 125627 CrossRef CAS
.
- P. Tang, P. Gao, X. Cui, Z. Chen, Q. Fu, Z. Wang, Y. Mo, H. Liu, C. Xu, J. Liu, J. Yan and S. Passerini, Adv. Energy Mater., 2022, 12, 2102053 CrossRef CAS
.
- L. Yin, Z. Zhang, Z. Li, F. Hao, Q. Li, C. Wang, R. Fan and Y. Qi, Adv. Funct. Mater., 2014, 24, 4176–4185 CrossRef CAS
.
- Q. Shi, S. Fu, C. Zhu, J. Song, D. Du and Y. Lin, Mater. Horiz., 2019, 6, 684–702 RSC
.
- S. Bhattacharyya and T. K. Maji, Coord. Chem. Rev., 2022, 469, 214645 CrossRef CAS
.
- W. Xiao, M. Cheng, Y. Liu, J. Wang, G. Zhang, Z. Wei, L. Li, L. Du, G. Wang and H. Liu, ACS Catal., 2023, 13, 1759–1790 CrossRef CAS
.
- Q. Chen, Y. Zhang, S. Ma, Y. Wang, P. Wang, G. Zhang, D. Gengzang, H. Jiao, M. Wang and W. Chen, J. Hazard. Mater., 2021, 415, 125662 CrossRef CAS
.
- B. Luo, S. Yan, H. Zhang, J. Zhou, F. Lan, B. Ying and Y. Wu, ACS Appl. Mater. Interfaces, 2021, 13, 34762–34772 CrossRef CAS
.
- C.-X. Li, C.-B. Chen, J.-Y. Lu, S. Cui, J. Li, H.-Q. Liu, W.-W. Li and F. Zhang, Chem. Eng. J., 2018, 337, 101–109 CrossRef CAS
.
- M. Abirami, S. M. Hwang, J. Yang, S. T. Senthilkumar, J. Kim, W.-S. Go, B. Senthilkumar, H.-K. Song and Y. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 32778–32787 CrossRef CAS PubMed
.
- T. Liu, W. Wang, M. Yi, Q. Chen, C. Xu, D. Cai and H. Zhan, Chem. Eng. J., 2018, 354, 454–462 CrossRef CAS
.
- X. Long, C. Feng, D. Ding, N. Chen, S. Yang, H. Chen, X. Wang and R. Chen, J. Hazard. Mater., 2021, 418, 126357 CrossRef CAS PubMed
.
- Y. Li, Y. Xu, W. Yang, W. Shen, H. Xue and H. Pang, Small, 2018, 14, 1704435 CrossRef
.
- L. Wang, T. Meng, C. Chen, Y. Fan, Q. Zhang, H. Wang and Y. Zhang, J. Colloid Interface Sci., 2018, 532, 650–656 CrossRef CAS PubMed
.
- B. Han, J. Song, S. Liang, W. Chen, H. Deng, X. Ou, Y.-J. Xu and Z. Lin, Appl. Catal., B, 2020, 260, 118208 CrossRef CAS
.
- P. Heidari, S. M. Masoudpanah and C. K. Ong, Ceram. Int., 2023, 49, 6678–6687 CrossRef CAS
.
- J. Jiang, C. Zhang and L. Ai, Electroanalysis, 2016, 208, 17–24 CAS
.
- L.-X. Yang, J.-C. E. Yang and M.-L. Fu, Chemosphere, 2021, 272, 129567 CrossRef CAS
.
- S. Yang, X. Qiu, P. Jin, M. Dzakpasu, X. C. Wang, Q. Zhang, L. zhang, L. Yang, D. Ding, W. Wang and K. Wu, Chem. Eng. J., 2018, 353, 329–339 CrossRef CAS
.
- L. Liu, Z. Hu, L. Sun, G. Gao and X. Liu, RSC Adv., 2015, 5, 36575–36581 RSC
.
- H. Yu, H. Fan, B. Yadian, H. Tan, W. Liu, H. H. Hng, Y. Huang and Q. Yan, ACS Appl. Mater. Interfaces, 2015, 7, 26751–26757 CrossRef CAS
.
- N. W. Kim, H. Yu and J. Oh, RSC Adv., 2022, 12, 12371–12376 RSC
.
- Y. Dong, Y. Wang, Y. Xu, C. Chen, Y. Wang, L. Jiao and H. Yuan, Electrochim. Acta, 2017, 225, 39–46 CrossRef CAS
.
- L. Yao, W. Yang, H. Liu, J. Jia, G. Wu, D. Liu, T. Liu, T. Tan and C. Wang, Dalton Trans., 2017, 46, 15512–15519 RSC
.
- X. Zhao, F. Li, B. Li, T. Zhang, Q. Teng, L. Wang, H. Wang and Y. Zhang, J. Phys. Chem. Solids, 2018, 113, 134–141 CrossRef CAS
.
- H. Nie, T. Xu, K. Mi, Y. Yu, L. Song and M. Xia, Ionics, 2021, 27, 3995–4002 CrossRef CAS
.
- X. Long, S. Yang, X. Qiu, D. Ding, C. Feng, R. Chen, J. Tan, X. Wang, N. Chen and Q. Lei, Chem. Eng. J., 2021, 404, 127052 CrossRef CAS
.
- C. Yin, Z. Bao, H. Tan, H. Zhou and J. Li, Chem. Eng. J., 2019, 372, 408–419 CrossRef CAS
.
- J. Wu, X. Wang, W. Zheng, Y. Sun, Y. Xie, K. Ma, Z. Zhang, Q. Liao, Z. Tian, Z. Kang and Y. Zhang, J. Am. Chem. Soc., 2022, 144, 19163–19172 CrossRef CAS PubMed
.
- J. Zhang, D. Zhang, Y. Yang, J. Ma, S. Cui, Y. Li and B. Yuan, RSC Adv., 2016, 6, 92699–92704 RSC
.
- S. Wu, X. Li, Y. Xu, J. Wu, Z. Wang, Y. Han and X. Zhang, Ceram. Int., 2018, 44, 19390–19396 CrossRef CAS
.
- Y.-L. Kuo, W.-M. Hsu, P.-C. Chiu, Y.-H. Tseng and Y. Ku, Ceram. Int., 2013, 39, 5459–5465 CrossRef CAS
.
- P. G. Radaelli, Y. Horibe, M. J. Gutmann, H. Ishibashi, C. H. Chen, R. M. Ibberson, Y. Koyama, Y.-S. Hor, V. Kiryukhin and S.-W. Cheong, Nature, 2002, 416, 155–158 CrossRef CAS PubMed
.
- C. C. Agrafiotis, C. Pagkoura, A. Zygogianni, G. Karagiannakis, M. Kostoglou and A. G. Konstandopoulos, Int. J. Hydrogen Energy, 2012, 37, 8964–8980 CrossRef CAS
.
- W. R. P. Barros, Q. Wei, G. Zhang, S. Sun, M. R. V. Lanza and A. C. Tavares, Electrochim. Acta, 2015, 162, 263–270 CrossRef CAS
.
- T. Chen, Y. Tang, W. Guo, Y. Qiao, S. Yu, S. Mu, L. Wang, Y. Zhao and F. Gao, Electrochim. Acta, 2016, 212, 294–302 CrossRef CAS
.
- Y. Feng, D. Xiang, Y. Qiu, L. Li, Y. Li, K. Wu and L. Zhu, Electroanalysis, 2020, 32, 571–580 CrossRef CAS
.
- P. Mahata, D. Sarma, C. Madhu, A. Sundaresen and S. Natarajan, Dalton Trans., 2011, 40, 1952–1960 RSC
.
- F. Zhao, J. Tian and B. Wang, Mater. Lett., 2015, 161, 104–107 CrossRef CAS
.
- L. Wang, Q. Jin, Y. Xiang, L. Gan, L. Xu, Y. Wu, X. Fang, H. Lu, S. Han and J. Cui, Chem. Eng. J., 2022, 435, 134877 CrossRef CAS
.
- J. Zhao, F. Wang, P. Su, M. Li, J. Chen, Q. Yang and C. Li, J. Mater. Chem., 2012, 22, 13328–13333 RSC
.
- S. Chen, M. Xue, Y. Li, Y. Pan, L. Zhu and S. Qiu, J. Mater. Chem. A, 2015, 3, 20145–20152 RSC
.
- S. Peng and S. Sun, Angew. Chem., Int. Ed., 2007, 46, 4155–4158 CrossRef CAS PubMed
.
- L. Qin, Z. Xu, Y. Zheng, C. Li, J. Mao and G. Zhang, Adv. Funct. Mater., 2020, 30, 1910257 CrossRef CAS
.
- Y. Feng and J. Yao, Coord. Chem. Rev., 2022, 470, 214699 CrossRef CAS
.
- W. Wang, H. Yan, U. Anand and U. Mirsaidov, J. Am. Chem.
Soc., 2021, 143, 1854–1862 CrossRef CAS
.
- J.-E. Zhou, J. Chen, X. Zhang, A. Zeb and X. Lin, J. Energy Chem., 2022, 75, 216–228 CrossRef CAS
.
- Y.-L. Huo, Y.-J. Gu, Z.-L. Chen, X.-Y. Ma, F.-Z. Wu and X.-Y. Dai, New J. Chem., 2023, 47, 14068–14077 RSC
.
- C. Yin, H. Zhou, Z. Yang and J. Li, ACS Appl. Mater. Interfaces, 2018, 10, 13625–13634 CrossRef CAS PubMed
.
- Z.-L. Chen, Y.-J. Gu, Y.-L. Huo, X.-Y. Ma and F.-Z. Wu, J. Alloys Compd., 2022, 917, 165485 CrossRef CAS
.
- S. Liu, Y. Qiu, Y. Liu, W. Zhang, Z. Dai, D. Srivastava, A. Kumar, Y. Pan and J. Liu, New J. Chem., 2022, 46, 13818–13837 RSC
.
- M. Lan, X. Wang, R. Zhao, M. Dong, L. Fang and L. Wang, J. Alloys Compd., 2020, 821, 153546 CrossRef CAS
.
- X. Hu, C. Li, X. Lou, X. Yan, Y. Ning, Q. Chen and B. Hu, RSC Adv., 2016, 6, 54270–54276 RSC
.
- S. Chen, M. Xue, Y. Li, Y. Pan, L. Zhu, D. Zhang, Q. Fang and S. Qiu, Inorg. Chem. Front., 2015, 2, 177–183 RSC
.
- J. H. Li, M. Y. Liu, Y. Li, L. Yuan, P. Zhang, Z. Cai, H. Chen and J. L. Zou, Mater. Today Energy, 2021, 19, 100574 CrossRef CAS
.
- S. Yang, X. Guo, Z. Wang, M. Dzakpasu, X. Dai, D. Ding, K. Wu, Y. huang, Q. Zhang, P. Jin and X. C. Wang, Chem. Eng. J., 2019, 359, 552–563 CrossRef CAS
.
- L. Zhang, L. Shi, L. Huang, J. Zhang, R. Gao and D. Zhang, ACS Catal., 2014, 4, 1753–1763 CrossRef CAS
.
- P. D. Patil, S. R. Shingte, V. C. Karade, J. H. Kim, T. D. Dongale, S. H. Mujawar, A. M. Patil and P. B. Patil, J. Energy Storage, 2021, 40, 102821 CrossRef
.
- D. He, Y. Gao, Y. Yao, L. Wu, J. Zhang, Z.-H. Huang and M.-X. Wang, Front. Chem., 2020, 8, 719 CrossRef CAS PubMed
.
- R. Wu, X. Qian, K. Zhou, J. Wei, J. Lou and P. M. Ajayan, ACS Nano, 2014, 8, 6297–6303 CrossRef CAS PubMed
.
- L. J. Wang, H. Deng, H. Furukawa, F. Gándara, K. E. Cordova, D. Peri and O. M. Yaghi, Inorg. Chem., 2014, 53, 5881–5883 CrossRef CAS PubMed
.
- Z. Song, R. Li, D. Wen, J. Pei, H. Zhu and S. Ruan, ACS Mater. Lett., 2023, 5, 473–479 CrossRef CAS
.
- S. Abednatanzi, P. G. Derakhshandeh, H. Depauw, F.-X. Coudert, H. Vrielinck, P. V. D. Voort and K. Leus, Chem. Soc. Rev., 2019, 48, 2535–2565 RSC
.
- S. Li, Y. Gao, N. Li, L. Ge, X. Bu and P. Feng, Energy Environ. Sci., 2021, 14, 1897–1927 RSC
.
- B. Iqbal, A. Laybourn, A. ul-Hamid and M. Zaheer, Ceram. Int., 2021, 47, 12433–12441 CrossRef CAS
.
- L. Luo, R. Huang, W. Hu, Z. Yu, Z. Tang, L. Chen, Y. Zhang, D. Zhang and P. Xiao, ACS Appl. Nano Mater., 2022, 5, 8232–8242 CrossRef CAS
.
- C. Xu, J. Tan, X. Zhang and Y. Huang, Sep. Purif. Technol., 2022, 291, 120933 CrossRef CAS
.
- D. Zhang, Z. Wang, J. Li, C. Hu, X. Zhang, B. Jiang, Z. Cao, J. Zhang and R. Zhang, RSC Adv., 2020, 10, 9063–9069 RSC
.
- Z. Yu, Y. Bai, Y. Liu, S. Zhang, D. Chen, N. Zhang and K. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 31777–31785 CrossRef CAS PubMed
.
- N. Kamyar, Y. Khani, M. M. Amini, F. Bahadoran and N. Safari, Int. J. Hydrogen Energy, 2020, 45, 21341–21353 CrossRef CAS
.
- X. Zhang, D. Li, G. Zhu, T. Lu and L. Pan, J. Colloid Interface Sci., 2017, 499, 145–150 CrossRef CAS PubMed
.
- T. Huang, Z. Lou, Y. Lu, R. Li, Y. Jiang, G. Shen and D. Chen, ChemElectroChem, 2019, 6, 5836–5843 CrossRef CAS
.
- Y. Liu, Z. Wang, Y. Zhong, M. Tade, W. Zhou and Z. Shao, Adv. Funct. Mater., 2017, 27, 1701229 CrossRef
.
- X. Wang, J. Ying, Y. Mai, J. Zhang, J. Chen, M. Wen and L. Yu, Catal. Sci. Technol., 2019, 9, 5845–5854 RSC
.
- S. Tu, Y. Chen, X. Zhang, J. Yao, Y. Wu, H. Wu, J. Zhang, J. Wang, B. Mu, Z. Li and Q. Xia, Appl. Catal., A, 2021, 611, 117975 CrossRef CAS
.
- G. Wang, D. Huang, M. Cheng, S. Chen, G. Zhang, L. Du, L. Lei, Y. Chen, R. Li and Y. Liu, Environ. Sci.: Nano, 2022, 9, 4393–4410 RSC
.
- W. Qu, H. Wen, X. Qu, Y. Guo, L. Hu, W. Liu, S. Tian, C. He and D. Shu, Chemosphere, 2022, 303, 135301 CrossRef CAS PubMed
.
- Q. Zhou, Q. Zhao, W. Xiong, X. Li, J. Li and L. Zeng, J. Colloid Interface Sci., 2018, 516, 76–85 CrossRef CAS PubMed
.
- J. Wei, Y. Feng, Y. Liu and Y. Ding, J. Mater. Chem. A, 2015, 3, 22300–22310 RSC
.
- H. Song, L. Zhang, G. Xu, C. Zhang, X. Ma, L. Zhang and D. Jia, RSC Adv., 2018, 8, 24805–24811 RSC
.
- M. Du, D. He, Y. Lou and J. Chen, J. Energy Chem., 2017, 26, 673–680 CrossRef
.
- V. S. Kirankumar and S. Sumathi, Mater. Today Chem., 2020, 18, 100355 CrossRef CAS
.
- E. Pervaiz, M. S. A. Virk, A. K. Tareen, B. Zhang, Q. Zhao, Z. Wang and M. Yang, Nanotechnology, 2018, 29, 215710 CrossRef PubMed
.
- X. Hou, J. Wang, B. Mousavi, N. Klomkliang and S. Chaemchuen, Dalton Trans., 2022, 51, 8133–8159 RSC
.
- J. Sun, Y. Wang, J. Zhou, K. Chen, K. Tao, W. Zhao and L. Han, Inorg. Chem., 2022, 61, 4283–4291 CrossRef CAS PubMed
.
- Z. Lu, B. Wang, Y. Hu, W. Liu, Y. Zhao, R. Yang, Z. Li, J. Luo, B. Chi, Z. Jiang, M. Li, S. Mu, S. Liao, J. Zhang and X. Sun, Angew. Chem., Int. Ed., 2019, 58, 2622–2626 CrossRef CAS PubMed
.
- J. Lin, T. Huang, M. Lu, X. Lin, R. C. K. Reddy and X. Xu, Chem. Eng. J., 2022, 433, 133770 CrossRef CAS
.
- M.-M. Wang, L.-J. Liu, J.-R. Xi, Y. Ding, P.-X. Liu, L. Mao, B.-J. Ni, W.-K. Wang and J. Xu, Chem. Eng. J., 2023, 451, 138605 CrossRef CAS
.
- K. Chu, Z. Li, S. Xu, G. Yao, Y. Xu, P. Niu and F. Zheng, Dalton Trans., 2020, 49, 10808–10815 RSC
.
- Y. Kang, H. Shi, Y.-H. Zhang and F.-N. Shi, Chem. Commun., 2021, 57, 10723–10726 RSC
.
- Y. Xia, B. Wang, G. Wang, X. Liu and H. Wang, ChemElectroChem, 2016, 3, 299–308 CrossRef CAS
.
- Z. Wang, W. Jin, X. Huang, G. Lu and Y. Li, Chem. Rec., 2020, 20, 1198–1219 CrossRef CAS PubMed
.
- Y. Deng, Y. Zhou, Z. Shi, X. Zhou, X. Quan and G. Chen, J. Mater. Chem. A, 2013, 1, 8170–8177 RSC
.
- S. Luo, P. Zhang, T. Yuan, J. Ruan, C. Peng, Y. Pang, H. Sun, J. Yang and S. Zheng, J. Mater. Chem. A, 2018, 6, 15755–15761 RSC
.
- L. Zhang, X. Qin, S. Zhao, A. Wang, J. Luo, Z. L. Wang, F. Kang, Z. Lin and B. Li, Adv. Mater., 2020, 32, 1908445 CrossRef CAS PubMed
.
- C.-M. Park, J.-H. Kim, H. Kim and H.-J. Sohn, Chem. Soc. Rev., 2010, 39, 3115–3141 RSC
.
- L. Hu and Q. Chen, Nanoscale, 2014, 6, 1236–1257 RSC
.
- H. Guo, T. Li, W. Chen, L. Liu, X. Yang, Y. Wang and Y. Guo, Nanoscale, 2014, 6, 15168–15174 RSC
.
- S. Maiti, A. Pramanik, T. Dhawa, M. Sreemany and S. Mahanty, J. Mater. Sci. Eng. B, 2018, 229, 27–36 CrossRef CAS
.
- F. Zheng, D. Zhu, X. Shi and Q. Chen, J. Mater. Chem. A, 2015, 3, 2815–2824 RSC
.
- L. Ding, M. Zeng, H. Wang and X. Jiang, J. Mater. Sci.: Mater. Electron., 2021, 32, 1778–1786 CrossRef CAS
.
- H. Guo, T. Li, W. Chen, L. Liu, J. Qiao and J. Zhang, Sci. Rep., 2015, 5, 13310 CrossRef PubMed
.
- L.-L. Wu, Z. Wang, Y. Long, J. Li, Y. Liu, Q.-S. Wang, X. Wang, S.-Y. Song, X. Liu and H.-J. Zhang, Small, 2017, 13, 1604270 CrossRef PubMed
.
- A. S. Aricò, P. Bruce, B. Scrosati, J.-M. Tarascon and W. van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef PubMed
.
- L. Wu, J. Lang, P. Zhang, X. Zhang, R. Guo and X. Yan, J. Mater. Chem. A, 2016, 4, 18392–18400 RSC
.
- H. Li, M. Liang, W. Sun and Y. Wang, Adv. Funct. Mater., 2016, 26, 1098–1103 CrossRef CAS
.
- H. Yang, Y. Xie, M. Zhu, Y. Liu, Z. Wang, M. Xu and S. Lin, Dalton Trans., 2019, 48, 9205–9213 RSC
.
- Y. Guo, Y. Zhu, C. Yuan and C. Wang, Mater. Lett., 2017, 199, 101–104 CrossRef CAS
.
- J.-Y. Hwang, S.-T. Myung and Y.-K. Sun, Chem. Soc. Rev., 2017, 46, 3529–3614 RSC
.
- J. Chai, N. Han, S. Feng, X. Huang, B. Tang and W. Zhang, Chem. Eng. J., 2023, 453, 139768 CrossRef CAS
.
- K. Chayambuka, G. Mulder, D. L. Danilov and P. H. L. Notten, Adv. Energy Mater., 2020, 10, 2001310 CrossRef CAS
.
- H. Tan, D. Chen, X. Rui and Y. Yu, Adv. Funct. Mater., 2019, 29, 1808745 CrossRef
.
- M. Wang, Y. Huang, Y. Zhu, N. Zhang, J. Zhang, X. Qin and H. Zhang, Electrochim. Acta, 2020, 335, 135694 CrossRef CAS
.
- R. Mohtadi, O. Tutusaus, T. S. Arthur, Z. Zhao-Karger and M. Fichtner, Joule, 2021, 5, 581–617 CrossRef CAS
.
- J. Han, S. Yagi, H. Takeuchi, M. Nakayama and T. Ichitsubo, J. Mater. Chem. A, 2021, 9, 26401–26409 RSC
.
- X. Xue, R. Chen, X. Song, A. Tao, W. Yan, W. Kong and Z. Jin, Adv. Funct. Mater., 2021, 31, 2009394 CrossRef CAS
.
- K. Shimokawa, T. Atsumi, M. Harada, R. E. Ward, M. Nakayama, Y. Kumagai, F. Oba, N. L. Okamoto, K. Kanamura and T. Ichitsubo, J. Mater. Chem. A, 2019, 7, 12225–12235 RSC
.
- C. Dong, A. Li, H. Kobayashi, Y. Chang, R. Li, X.-B. Chen and W. Dong, Mater. Today Nano, 2021, 16, 100141 CrossRef CAS
.
- Y. Wang, X. Zhu, D. Liu, H. Tang, G. Luo, K. Tu, Z. Xie, J. Lei, J. Li, X. Li and D. Qu, J. Appl. Electrochem., 2019, 49, 1103–1112 CrossRef CAS
.
- C. Dong, H. Kobayashi and I. Honma, Mater. Today Energy, 2022, 30, 101143 CrossRef CAS
.
- H. Guo, M. Qiao, J. Yan, L. Jiang, J. Yu, J. Li, S. Deng and L. Qu, Adv. Funct. Mater., 2023, 33, 2213514 CrossRef CAS
.
- D. Zhang, C. Tan, W. Zhang, W. Pan, Q. Wang and L. Li, Molecules, 2022, 27, 716 CrossRef CAS PubMed
.
- D. Ghosh, A. Pal, S. Ghosh, A. Gayen, M. M. Seikh and P. Mahata, Eur. J. Inorg. Chem., 2019, 2019, 3076–3083 CrossRef CAS
.
- L. Gong, M. Xu, R. Ma, Y. Han, H. Xu and G. Shi, Sci. China: Technol. Sci., 2020, 63, 1470–1477 CrossRef CAS
.
- X. Han, Y. Yang, J.-J. Zhou, Q. Ma, K. Tao and L. Han, Chem.–Eur. J., 2018, 24, 18106–18114 CrossRef CAS PubMed
.
- A. Jayakumar, R. P. Antony, R. Wang and J.-M. Lee, Small, 2017, 13, 1603102 CrossRef PubMed
.
- B. Weng, F. Xu, C. Wang, W. Meng, C. R. Grice and Y. Yan, Energy Environ. Sci., 2017, 10, 121–128 RSC
.
- S. Sanati, A. Morsali and H. García, Energy Environ. Sci., 2022, 15, 3119–3151 RSC
.
- J. O. Olowoyo and R. J. Kriek, Small, 2022, 18, 2203125 CrossRef CAS PubMed
.
- X. Lu and C. Zhao, Nat. Commun., 2015, 6, 6616 CrossRef CAS PubMed
.
- J. Geng, L. Kuai, E. Kan, Q. Wang and B. Geng, ChemSusChem, 2015, 8, 659–664 CrossRef CAS PubMed
.
- Y. Shen, S.-G. Guo, F. Du, X.-B. Yuan, Y. Zhang, J. Hu, Q. Shen, W. Luo, A. Alsaedi, T. Hayat, G. Wen, G.-L. Li, Y. Zhou and Z. Zou, Nanoscale, 2019, 11, 11765–11773 RSC
.
- L. Kuai, J. Geng, C. Chen, E. Kan, Y. Liu, Q. Wang and B. Geng, Angew. Chem., Int. Ed., 2014, 53, 7547–7551 CrossRef CAS PubMed
.
- C. Zhu, D. Wen, S. Leubner, M. Oschatz, W. Liu, M. Holzschuh, F. Simon, S. Kaskel and A. Eychmüller, Chem. Commun., 2015, 51, 7851–7854 RSC
.
- Y. Li, H. Dong, J. Xiao, L. Li, D. Chu, X. Hou, S. Xiang, Q. Dong and H. Zhang, J. Hazard. Mater., 2023, 442, 130014 CrossRef CAS PubMed
.
- L. Rodríguez-Lado, G. Sun, M. Berg, Q. Zhang, H. Xue, Q. Zheng and C. A. Johnson, Science, 2013, 341, 866–868 CrossRef PubMed
.
- V. Turan, S. A. Khan, M. ur-Rahman, M. Iqbal, P. M. A. Ramzani and M. Fatima, Ecotoxicol. Environ. Saf., 2018, 161, 409–419 CrossRef CAS PubMed
.
- C. Li, R. Guo, Z. Zhang, T. Wu and W. Pan, Small, 2023, 19, 2207875 CrossRef CAS PubMed
.
- H. Weng, Y. Yang, C. Zhang, M. Cheng, W. Wang, B. Song, H. Luo, D. Qin, C. Huang, F. Qin and K. Li, Chem. Eng. J., 2023, 453, 139812 CrossRef CAS
.
- R. Xiao, D. Huang, L. Du, B. Song, L. Yin, Y. Chen, L. Gao, R. Li, H. Huang and G. Zeng, Sci. Total Environ., 2023, 869, 161855 CrossRef CAS PubMed
.
- C. Qin, X. Wu, L. Tang, X. Chen, M. Li, Y. Mou, B. Su, S. Wang, C. Feng, J. Liu, X. Yuan, Y. Zhao and H. Wang, Nat. Commun., 2023, 14, 5238 CrossRef CAS PubMed
.
- B. Li, S. Chen, D. Huang, M. Cheng, C. Du, C. Feng, Y. Yang, L. Lei, Y. Chen, W. Xue and R. Deng, J. Phys. Chem. C, 2021, 125, 18693–18707 CrossRef CAS
.
- W.-D. Oh and T.-T. Lim, Chem. Eng. J., 2019, 358, 110–133 CrossRef CAS
.
- B. Ning and N. J. Graham, J. Environ. Eng., 2008, 134, 944–953 CrossRef CAS
.
- M. Li, X. Yuan, Y. Lai, C. Qin, L. Jiang, A. Duan and H. Wang, J. Hazard. Mater., 2023, 460, 132316 CrossRef CAS PubMed
.
- G. Zhang, D. Huang, M. Cheng, L. Lei, S. Chen, R. Wang, W. Xue, Y. Liu, Y. Chen and Z. Li, J. Mater. Chem. A, 2020, 8, 17883–17906 RSC
.
- Q. Shi, S. Deng, Y. Zheng, Y. Du, L. Li, S. Yang, G. Zhang, L. Du, G. Wang, M. Cheng and Y. Liu, Environ. Res., 2022, 212, 113340 CrossRef CAS PubMed
.
- D. Huang, L. Du, M. Cheng, L. Yin, R. Xiao, S. Chen, L. Lei, Y. Chen, G. Wang, W. Xu and Y. Liu, J. Colloid Interface Sci., 2023, 629, 685–696 CrossRef CAS PubMed
.
- X. Li, Z. Wang, B. Zhang, A. I. Rykov, M. A. Ahmed and J. Wang, Appl. Catal., B, 2016, 181, 788–799 CrossRef CAS
.
- P. Wu, X. Tang, Z. He, Y. Liu and Z. Wang, Energy Fuels, 2022, 36, 5622–5646 CrossRef CAS
.
- T. Andana, K. G. Rappé, F. Gao, J. Szanyi, X. Pereira-Hernandez and Y. Wang, Appl. Catal., B, 2021, 291, 120054 CrossRef CAS
.
- S. Xiong, J. Chen, H. Liu, X. Chen, W. Si, Z. Gong, Y. Peng and J. Li, Environ. Sci. Technol., 2022, 56, 3739–3747 CrossRef CAS PubMed
.
- S. Deng, T. Meng, B. Xu, F. Gao, Y. Ding, L. Yu and Y. Fan, ACS Catal., 2016, 6, 5807–5815 CrossRef CAS
.
- J. Yang, S. Ren, T. Zhang, Z. Su, H. Long, M. Kong and L. Yao, Chem. Eng. J., 2020, 379, 122398 CrossRef
.
- S. Yang, C. Wang, J. Li, N. Yan, L. Ma and H. Chang, Appl. Catal., B, 2011, 110, 71–80 CrossRef CAS
.
- S. Xiong, Y. Peng, D. Wang, N. Huang, Q. Zhang, S. Yang, J. Chen and J. Li, Chem. Eng. J., 2020, 387, 124090 CrossRef CAS
.
- Z. Liu, G. Sun, C. Chen, K. Sun, L. Zeng, L. Yang, Y. Chen, W. Wang, B. Liu, Y. Lu, Y. Pan, Y. Liu and C. Liu, ACS Catal., 2020, 10, 6803–6809 CrossRef CAS
.
- X. Yi, J. Wang, Y. Liu, Y. Chen and J. Chen, Catal. Sci. Technol., 2022, 12, 4169–4180 RSC
.
- A. Zhang, Z. Zhang, H. Lu, Z. Liu, J. Xiang, C. Zhou, W. Xing and L. Sun, Ind. Eng. Chem. Res., 2015, 54, 2930–2939 CrossRef CAS
.
- X.-T. Wang, T. Ouyang, L. Wang, J.-H. Zhong, T. Ma and Z.-Q. Liu, Angew. Chem., Int. Ed., 2019, 58, 13291–13296 CrossRef CAS PubMed
.
- F. Gao, X. Tang, H. Yi, J. Li, S. Zhao, J. Wang, C. Chu and C. Li, Chem. Eng. J., 2017, 317, 20–31 CrossRef CAS
.
- S. Ko, X. Tang, F. Gao, C. Wang, H. Liu and Y. Liu, J. Solid State Chem., 2022, 307, 122843 CrossRef CAS
.
- H. Zhao, Z. Qu and H. Sun, Appl. Surf. Sci., 2020, 529, 147044 CrossRef CAS
.
- T. Wagner, S. Haffer, C. Weinberger, D. Klaus and M. Tiemann, Chem. Soc. Rev., 2013, 42, 4036–4053 RSC
.
- C. Zhang, P. Chen and W. Hu, Chem. Soc. Rev., 2015, 44, 2087–2107 RSC
.
- Y. Jian, W. Hu, Z. Zhao, P. Cheng, H. Haick, M. Yao and W. Wu, Nano-Micro Lett., 2020, 12, 71 CrossRef CAS PubMed
.
- A. Afzal, N. Cioffi, L. Sabbatini and L. Torsi, Sens. Actuators, B, 2012, 171–172, 25–42 CrossRef CAS
.
- R. Zhang, L. Lu, Y. Chang and M. Liu, J. Hazard. Mater., 2022, 429, 128321 CrossRef CAS PubMed
.
- Y. M. Choi, S.-Y. Cho, D. Jang, H.-J. Koh, J. Choi, C.-H. Kim and H.-T. Jung, Adv. Funct. Mater., 2019, 29, 1808319 CrossRef
.
- V. Schroeder, S. Savagatrup, M. He, S. Lin and T. M. Swager, Chem. Rev., 2019, 119, 599–663 CrossRef CAS PubMed
.
- X.-Z. Song, F.-F. Sun, S.-T. Dai, X. Lin, K.-M. Sun and X.-F. Wang, Inorg. Chem. Front., 2018, 5, 1107–1114 RSC
.
- T. Zhou, S. Cao, R. Zhang, T. Fei and T. Zhang, Inorg. Chem. Front., 2019, 6, 3177–3183 RSC
.
- X. Li, Y. Zhang, Y. Cheng, X. Chen and W. Tan, Ceram. Int., 2021, 47, 9214–9224 CrossRef CAS
.
- K. Li, R. Zhang, R. Gao, G.-Q. Shen, L. Pan, Y. Yao, K. Yu, X. Zhang and J.-J. Zou, Appl. Catal., B, 2019, 244, 536–545 CrossRef CAS
.
- H. D. Lawson, S. P. Walton and C. Chan, ACS Appl. Mater. Interfaces, 2021, 13, 7004–7020 CrossRef CAS PubMed
.
- A. B. Abou Hammad, B. A. Hemdan and A. M. El Nahrawy, J. Environ. Manage., 2020, 270, 110816 CrossRef CAS PubMed
.
- A. Rabbani, R. Haghniaz, T. Khan, R. Khan, A. Khalid, S. S. Naz, M. Ul-Islam, F. Vajhadin and F. Wahid, RSC Adv., 2021, 11, 1773–1782 RSC
.
| This journal is © The Royal Society of Chemistry 2024 |