Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Optical control of pH via chromoselective photodosimetry†
Amrita
Chaudhuri‡
 a,
Linda E.
Eijsink‡
a,
Linda E.
Eijsink‡
 ab and
Nadja A.
Simeth
ab and
Nadja A.
Simeth
 *ab
*ab
aInstitute for Organic and Biomolecular Chemistry, Department of Chemistry, University of Göttingen, Tammannstr. 2, 37077 Göttingen, Germany. E-mail: nadja.simeth@uni-goettingen.de
bCluster of Excellence “Multiscale Bioimaging: from Molecular Machines to Networks of Excitable Cells” (MBExC), University of Göttingen, 37075 Göttingen, Germany
First published on 14th August 2024
Abstract
The dynamic regulation of pH via an external stimulus is an attractive technique to gate chemical transformations. Applying photons of different energy, we preferentially address either a photoacid or a photobase donor in the same solutions and thus, present a technique to regulate the pH of a solution through light pulses.
The pH of a given system in both chemistry and biology is a crucial parameter to optimise the kinetics of a molecular transformation.1,2 Moreover, the absence or presence of protons can serve as a gatekeeper and facilitate or inhibit a (bio)chemical reaction from proceeding.3 Naturally, adjusting the pH became a straightforward approach to obtain stimulus-responsive control over natural and artificial systems. To additionally achieve a means of external control through a traceless reagent, the stimulation with photons via light-responsive small molecules became highly attractive.4,5 Consequently, photoacid and photobase generators have found their application there where physical access is restricted, such as in polymer chemistry and biomedicine.6–8
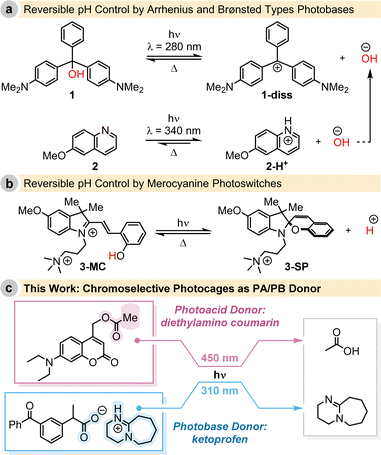
These advancements underscore the significance of light-triggered reversible pH modulation. As an example, Irie demonstrated the reversible change in pH using triphenylmethane leucohydroxide (1) under UV-light irradiation, followed by thermal reconciliation.9 Capitalizing on this fundamental work, Yucknovsky and Amdursky recently optimised the system, combining 1 with a second photobase generator 2, that serves as a hydroxide (OH−) ion donor, enabling reversible pH control with two excitation wavelengths (Fig. 1a).10
In parallel, the emergence of so-called photocages has provided an alternative means for the light-controlled, yet irreversible, release of small organic acids and bases.11 In contrast, photoswitchable systems allowed for reversible control. For example, in 2021, both the groups of Beves and Pezzato introduced merocyanine photoswitches capable of modulating the bulk pH upon exposure to 450 nm or 500 nm light irradiation, respectively (Fig. 1b).12–15
While the examples mentioned above showcase the reversible modulation of pH triggered by light irradiation, the reverse reaction occurs spontaneously, lacks temporal control, and the direction of pH change with respect to the initial state can only proceed in one direction due to the inherent reactivity of the molecule. Inspired by these advancements, our work aims to achieve complete spatiotemporal and reversible control of pH in a solution using chromoselective light irradiation, incorporating bimodality into our approach (Fig. 1c).
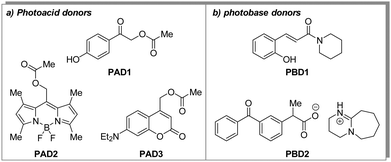
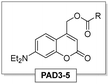
Thus, we selected a series of photoacid donors (PADs) and photobase donors (PBDs) to identify suitable PAD and PBD pairs, in which each component can be addressed chromoselectively to obtain a tool that allows to bidirectionally affect the pH from a given initial state. Specifically, we equipped UV- and visible-light sensitive photocages with an organic acid (PAD1, PAD2, and PAD3, Scheme 1a), and a UV-light sensitive photocage with an organic base (PBD1 and PBD2, Scheme 1b). Irradiation with UV- and/or visible light is expected to lower the observed pH, whereas irradiation with UV-light should increase the pH. Most visible-light sensitive photocages are also susceptible to UV-light, but since the quantum yield of release can vary, a global change in pH is still foreseen. The PADs and PBDs displayed in Scheme 1 were successfully synthesised following the procedures given in the ESI.†
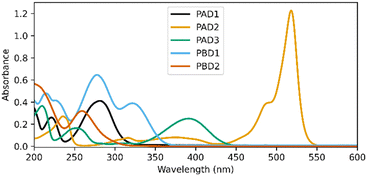
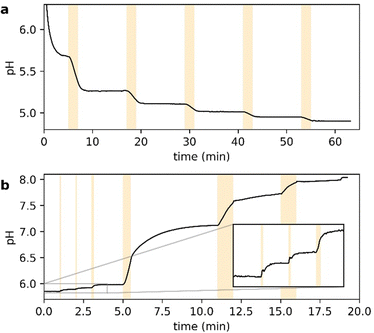
Fig. 2 shows the UV-vis spectra of the selected PADs and PBDs (30 μM, 50% methanol in water). However, to generate a pronounced, global change in pH, higher concentrations of both PAD and PBD are required (see ESI†). Successively, the photochemical release of cargo was assessed. Irradiation of PAD1 with 310 nm light for 5 minutes reduces the pH by 1 unit, from 6.25 to 5.25 (Fig. 3). Similarly, irradiation of PAD3 with 365 nm light in 5 steps of 2 minutes reduces the pH stepwise from 5.7 to 4.9 (Fig. 4).
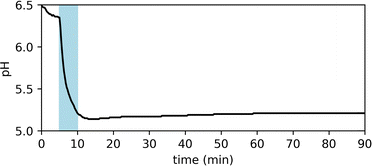 | ||
| Fig. 3 Observed pH of a solution of PAD1 (1.8 mM, 50% methanol in water) upon irradiation with 310 nm light for 5 min. | ||
Irradiation of PAD2 with 505 nm light did not result in an observed change in pH (Fig. S1, ESI†), potentially due to a combination of low solubility and low quantum yield of release, both of which are known issues for fluorinated BODIPY-photocages.16–18
Next, we turned towards the PBDs. Although the addition of piperidine increases the pH in a methanol/water mixture (Fig. S2, ESI†), photochemical release of piperidine from PBD1 (λ = 310 nm) did not result in a concerted increase in pH (Fig. S3, ESI†). However, irradiation of PBD2 in methanol/water at 365 nm does change the pH of the solution from pH 6 to ultimately pH 8 (Fig. 4). Addition of a small amount of buffer changes the initial pH but does not influence the observed behaviour (see ESI†). Furthermore, the dose-dependent response observed was encouraging.
We thus identified suitable molecules that allow us to both up- and downregulate the pH by irradiation with light in a dose-dependent manner, albeit both at 365 nm (Fig. 4). To obtain wavelength-selective preferential release of one over the other, the rate of release of acid at a selected wavelength must be higher than the release of base, and vice versa at a different wavelength.
Since PBD2 has an exceptionally high quantum yield of release (0.75) upon irradiation with UV-light,19,20 and the quantum yield of release of acid from PAD3 is only moderate at those wavelengths,21 a solution of PBD2 and PAD3 is expected to basify upon irradiation with UV-light. Moreover, the UV-vis absorption spectrum of PAD3 extends into the visible spectrum, while PBD2 does not absorb in this range. Hence, we expected that irradiation with visible light would decrease the pH. Furthermore, the activation maximum of a comparable photoactive base generator was recently shown to be close to 310 nm.22
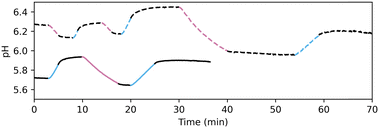
Indeed, when a solution of PAD3 and PBD2 (0.63 and 0.40 mM, respectively) is irradiated alternatively with 310 and 450 nm light, the observed pH fluctuated as expected (Fig. 5). In contrast to photoacids and photobases reported earlier, both up- and downregulation of pH is possible at a given starting point. Furthermore, pH recovery is not influenced by thermal backreactions, but is actively initiated by irradiation with the opposing wavelength of light.
Unfortunately, reduction of the pH below 5.5 was not yet accessible with PAD3/PBD2. For this reason, the same chromophore was equipped with more acidic leaving groups resulting in PAD4 and PAD5 (cf.Table 1).23 While PAD4 readily dissociates in water, PAD5 is stable and able to reduce the pH similar to PAD3. However, PAD5 does not reduce the pH further than 5.5, and due to the lower solubility compared to PAD3, in further experiments, PAD3 was employed. The inaccessibility of highly acidic solution was attributed to the buffering qualities of PBD2, which does not release at a pH below 5.5 (Fig S4, ESI†).
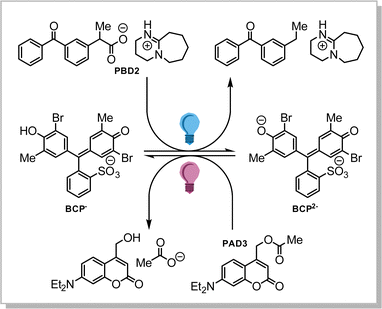
Many chemical reactions are governed by the pH of the medium, and thus we were interested to employ our PBD2/PAD3 pair to control the pH-dependent chemical equilibrium in a chemical system in solution. Specifically, we selected bromocresol purple (BCP) as the equilibrium between its singly (BCP−) and its doubly deprotonated form (BCP2−) is sensitive to pH and an optical read-out is feasible (Scheme 2).24
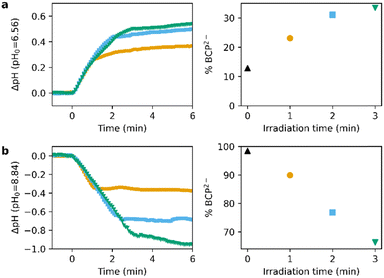
Addition of BCP (final concentration 13 μM) to a solution of PBD2 and PAD3 (0.56 and 0.91 mM resp.) irradiated with 310 nm light for 1–3 min shows an increase of BCP2−, as determined spectroscopically (Fig. 6a). Similarly, the amount of BCP2− drops linearly by up to 30% upon irradiation with 1–3 min of 445 nm light (Fig. 6b). Furthermore, Fig. 6 clearly shows the dose-dependency of the pH change.
Taken together, we could show the reversible up- and downregulation of pH using two individual chromophores and could use the global change in pH to affect the equilibrium of a chemical reacting in the same solution. A current limitation of this proof-of-principle system is, on the one hand, the necessity of UV-light to address PBD2. On the other hand, both photocages were not yet optimised towards full aqueous solubility. This will be the natural next step and could be achieved by, for instance, employing water-soluble chromophores previously described by the Southan and Tovar group.25
Despite these challenges, we demonstrated for the first time the chromoselective dynamic regulation of pH in the same solution. Transforming PAD/PBD into an all-visible-light-responsive, water-soluble system could enable its application in biochemical enzymatic reactions in the future.
We thank Milan Weitzel for fruitful discussion. This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy (EXC2067/1-390729940 to L. E. E. and N. A. S.) and by the Collaborative Research Center (CRC) 1190 to A. C. Generous support by the Fonds der Chemischen Industrie is gratefully appreciated.
Data availability
Experimental details and compound characterisation data can be found in the ESI.†Conflicts of interest
There are no conflicts to declare.References
- P. K. Robinson, Essays Biochem., 2015, 59, 1–41 CrossRef PubMed.
- C. J. Taylor, A. Pomberger, K. C. Felton, R. Grainger, M. Barecka, T. W. Chamberlain, R. A. Bourne, C. N. Johnson and A. A. Lapkin, Chem. Rev., 2023, 123, 3089–3126 CrossRef CAS PubMed.
- P. Swietach, R. D. Vaughan-Jones, A. L. Harris and A. Hulikova, Philos. Trans. R. Soc., B, 2014, 369, 20130099 CrossRef PubMed.
- S. Kohse, A. Neubauer, A. Pazidis, S. Lochbrunner and U. Kragl, J. Am. Chem. Soc., 2013, 135, 9407–9411 CrossRef CAS PubMed.
- H. Kagel, F. F. Bier, M. Frohme and J. F. Glökler, Sci. Rep., 2019, 9, 14372 CrossRef PubMed.
- N. Zivic, P. K. Kuroishi, F. Dumur, D. Gigmes, A. P. Dove and H. Sardon, Angew. Chem., Int. Ed., 2019, 58, 10410–10422 CrossRef CAS PubMed.
- T. Sun, L. Kang, H. Zhao, Y. Zhao and Y. Gu, Adv. Sci., 2024, 11, 2302875 CrossRef CAS PubMed.
- Y. Luo, C. Wang, P. Peng, M. Hossain, T. Jiang, W. Fu, Y. Liao and M. Su, J. Mater. Chem. B, 2013, 1, 997–1001 RSC.
- M. Irie, J. Am. Chem. Soc., 1983, 105, 2078–2079 CrossRef CAS.
- A. Yucknovsky and N. Amdursky, Chem. – Eur. J., 2024, 30, e202303767 CrossRef CAS PubMed.
- R. Weinstain, T. Slanina, D. Kand and P. Klán, Chem. Rev., 2020, 120, 13135–13272 CrossRef CAS PubMed.
- L. Wimberger, S. K. K. Prasad, M. D. Peeks, J. Andréasson, T. W. Schmidt and J. E. Beves, J. Am. Chem. Soc., 2021, 143, 20758–20768 CrossRef CAS PubMed.
- C. Berton, D. M. Busiello, S. Zamuner, R. Scopelliti, F. Fadaei-Tirani, K. Severin and C. Pezzato, Angew. Chem., Int. Ed., 2021, 60, 21737–21740 CrossRef CAS PubMed.
- V. J. Périllat, C. Berton and C. Pezzato, Mater. Today Chem., 2022, 25, 100918 CrossRef.
- V. J. Périllat, E. Del Grosso, C. Berton, F. Ricci and C. Pezzato, Chem. Commun., 2023, 59, 2146–2149 RSC.
- T. Slanina, P. Shrestha, E. Palao, D. Kand, J. A. Peterson, A. S. Dutton, N. Rubinstein, R. Weinstain, A. H. Winter and P. Klán, J. Am. Chem. Soc., 2017, 139, 15168–15175 CrossRef CAS PubMed.
- D. Kand, P. Liu, M. X. Navarro, L. J. Fischer, L. Rousso-Noori, D. Friedmann-Morvinski, A. H. Winter, E. W. Miller and R. Weinstain, J. Am. Chem. Soc., 2020, 142, 4970–4974 CrossRef CAS PubMed.
- P. Shrestha, D. Kand, R. Weinstain and A. H. Winter, J. Am. Chem. Soc., 2023, 145, 17497–17514 CrossRef CAS PubMed.
- L. L. Costanzo, G. D. Guidi, G. Condorelli, A. Cambria and M. Fama, Photochem. Photobiol., 1989, 50, 359–365 CrossRef CAS PubMed.
- K. Arimitsu and R. Endo, Chem. Mater., 2013, 25, 4461–4463 CrossRef CAS.
- V. R. Shembekar, Y. Chen, B. K. Carpenter and G. P. Hess, Biochemistry, 2005, 44, 7107–7114 CrossRef CAS PubMed.
- P. Utroša, J. A. Carroll, E. Žagar, D. Pahovnik and C. Barner-Kowollik, Chem. – Eur. J., 2024, e202400820 CrossRef PubMed.
- R. Williams, Bordwell pKa Table, https://organicchemistrydata.org/hansreich/resources/pka/, (accessed June 13, 2024).
- N. A. Gavrilenko, N. V. Saranchina, A. V. Sukhanov and D. A. Fedan, Mendeleev Commun., 2018, 28, 450–452 CrossRef CAS.
- K. K. Adatia, T. Halbritter, M. Reinfelds, A. Michele, M. Tran, S. Laschat, A. Heckel, G. E. M. Tovar and A. Southan, ChemPhotoChem, 2020, 4, 207–217 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc03076a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |