Open Access Article
Open Access ArticleBQ-AurIPr: a redox-active anticancer Au(I) complex that induces immunogenic cell death†
Ravindra D.
Mule‡
ab,
Akhilesh
Kumar‡
c,
Shashank P.
Sancheti
e,
B.
Senthilkumar
 *a,
Himanshu
Kumar
*cd and
Nitin T.
Patil
*a,
Himanshu
Kumar
*cd and
Nitin T.
Patil
 *e
*e
aDivision of Organic Chemistry, CSIR-National Chemical Laboratory, Dr Homi Bhabha Road, Pune – 411008, India. E-mail: b.senthilkumar@ncl.res.in
bAcademy of Scientific and Innovative Research, Ghaziabad – 201 002, India
cLaboratory of Immunology and Infectious Diseases, Department of Biological Sciences, IISER Bhopal, Bhopal – 462 066, India
dImmunology Frontier Research Center (IFReC), Osaka University, Osaka – 565-0871, Japan. E-mail: hkumar@iiserb.ac.in
eDepartment of Chemistry, Indian Institute of Science Education and Research Bhopal, Bhopal Bypass Road, Bhauri, Bhopal – 462 066, India. E-mail: npatil@iiserb.ac.in
First published on 25th August 2022
Abstract
Immunogenic Cell Death (ICD) is a unique cell death mechanism that kills cancer cells while rejuvenating the anticancer immunosurveillance, thereby benefiting the clinical outcomes of various immuno-chemotherapeutic regimens. Herein, we report development of a library of benzo[a]quinolizinium-based Au(I) complexes through an intramolecular amino-auration reaction of pyridino-alkynes. We tested 40 candidates and successfully identified BQ-AurIPr as a novel redox-active Au(I) complex with potent anticancer properties. BQ-AurIPr efficiently triggered generation of DAMPs – the hallmarks of ICD – and was superior in terms of efficiency compared to FDA-approved drugs known to induce ICD. BQ-AurIPr significantly increased immunogenicity of cancer cells enhancing their phagocytosis when co-cultured with immune cells. Our investigation reveals that BQ-AurIPr induces oxidative stress inside mitochondria leading to mitophagy, as the mechanism for immunogenic cell death in A549 cells.
Our immune system is capable of discriminating and eliminating altered self or foreign cells, including cancer cells. However, owing to the high mutation and adaptability rate, cancer cells exhibit numerous mechanisms which aid evasion of the immune system.
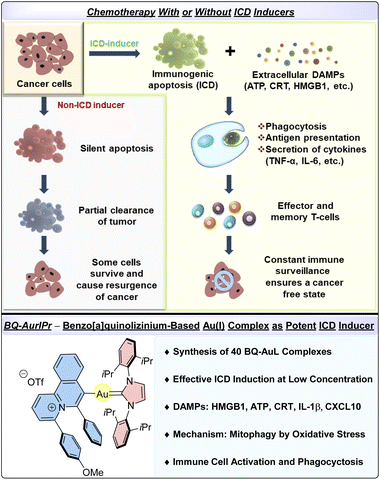
Neoplastic cells often overexpress various ‘don't eat me’ signals on their surface and secrete several factors that create an immunosuppressive microenvironment.1,2 Recent evidence suggests that some chemotherapeutic drugs actively help reinstate the host immunity against cancer cells, providing long-term protection against cancer and its recurrences.3,4 The cytotoxic effects of chemotherapeutic agents are primarily manifested through a cell death mechanism called apoptosis, which was until recently considered immunologically quiescent or tolerogenic. A recently identified subset, Immunogenic Cell Death (ICD), is a type of programmed cell death characterized by the release of various Damage-Associated Molecular Patterns (DAMPs) by dying cells that trigger a cascade of innate immune responses leading to long-lasting adaptive immunity. DAMPs are intracellular factors typically inaccessible to the immune system unless released from the cells at the tumor site. DAMPs help recruit immune cells at the tumor microenvironment and facilitate phagocytosis of cancer cells by antigen-presenting cells (APCs) like macrophages and dendritic cells.5 Upon phagocytosis, antigens of cancer origin are processed by APCs and presented to T cells, leading to activation and proliferation of effector and memory T cells, thereby providing long-lasting anticancer immunity (Scheme 1). Some of the characteristic DAMPs also referred to as hallmarks of ICD, are translocation of calreticulin (CRT) to the plasma membrane, release of ATP and high mobility group box 1 (HMGB1) protein, and secretion of type-I interferons, IL-1β, IL-17, and CXCL10.6 Interestingly, cancer cells that underwent ICD induction show protection in immunocompetent mice but not immunodeficient mice that received cancer xenografts, thus highlighting the importance of host immunity against cancer.7
Despite the increasing number of clinical studies in immuno-chemotherapy, the number of drugs identified to induce ICD is limited.8,9 While most of the ICD inducers are non-metallic drugs (doxorubicin,10–14 epirubicin,15 mitoxantrone,16 and cyclophosphamide,17etc.18), oxaliplatin19 is the only FDA approved metallic anticancer drugs reported to induce ICD. Owing to the highly immunosuppressive tumor microenvironment, at lower concentrations, many of these ICD inducers hardly produce satisfactory levels of DAMPs to trigger a significant antitumor immune response.20 As a result, the development of novel, more effective immuno-chemotherapeutics that could generate a robust immune response at comparatively lower concentrations is deemed necessary.
While there have been reports on metal complexes as ICD inducers,21 recent literature reports disclose some of the most potent ICD inducing metal complexes. Ang and co-workers designed a novel Pt–NHC complex which demonstrated excellent ICD and phagocytosis activity.22 Later, Babak and Ang reported an ER targeting complex: PlatinER, as a modified form of Pt–NHC, which performed superior to its previous version.23 Likewise, Zhou, Chen, and Liang reported an amino-phosphonate ester ligand-containing Pt-complex as a potent ICD inducer.24 Apart from Pt(II), Pt(IV),25 Cu(II),26 and Ru(III)27 based complexes have also been reported to induce ICD in mice and/or human cancer cell lines. In a recent report by Cui, Sessler, and Arambula, an Au-based complex was reported to induce ICD for the first time.28 Despite of all these developments, only two metal complexes have been reported to effectively induce ICD in lung cancers, which is the second most commonly diagnosed cancer.29 Recently, Zhu et al. reported an oxaliplatin-based and photocaged Pt(IV) complex: coumaplatin, which induced ICD in cisplatin-resistant non-small cell lung cancer (NSCLC) cells.30 Later, Chao and co-workers, for the first time, demonstrated the applicability of an Ir-complex in inducing ICD.31
Inspired by the growing importance of gold complexes as anticancer agents due to their distinct mode of action32–35 and banking on our ongoing interest in metal-mediated intramolecular cyclization reactions of pyridino-alkynes,36–38 we herein disclose a newly designed benzo[a]quinolizinium Au(I)–NHC complex–BQ-AurIPr as a potential ICD inducer in NSCLC A549 cells (Scheme 1). Moreover, we clearly demonstrate its effectiveness in activating immune cells and triggering phagocytosis of cancer cells. The mechanism of ICD induction for a gold complex has also been studied for the first time.
A library of 40 BQ-AuL complexes (3a–5f, Scheme 2) was synthesized and evaluated for their anticancer properties through cell proliferation assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and ATP release assays (see ESI† for details). After a systematic structure–activity study and rational designing of BQ core and ligands on Au, we found that BQ-AurIPr (4k) performed exceptionally well in eliminating different kinds of cancer cells (Table 1) and eliciting significant release of ATP, which is a key marker of ICD. To our delight, with a selectivity index (SI) of 5.28 (A549 vs. primary human small airway epithelial cells, hSAEC), BQ-AurIPr displayed potent anticancer activity in NSCLC A549 cells through apoptosis (Table 1 and ESI†). Therefore, we focused our study on NSCLC A549 cells. Next, we analysed other aspects of anticancer activity by in vitro cell mobility and invasion assays and found that 0.25 μM BQ-AurIPr doesn't just induce apoptosis in A549 but also decrease their migration and invasiveness (ESI†).
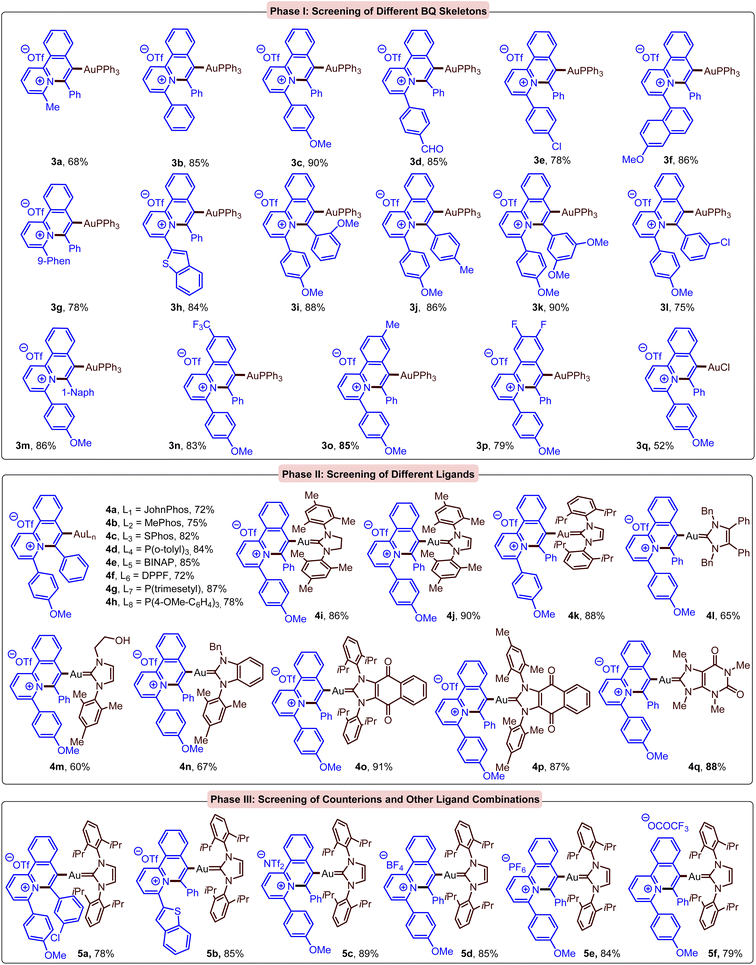 | ||
| Scheme 2 Synthesized BQ-AuL complexes and their phase-wise screening of IC50 values and ATP secretion. | ||
| Cell line | A549 | Caco-2 | MDA-MB-231 | HeLa | Primary hSAEC | SI in A549 |
|---|---|---|---|---|---|---|
| IC50 (μM) | 0.25 ± 0.08 | 0.45 ± 0.10 | 0.50 ± 0.06 | 0.78 ± 0.12 | 1.32 ± 0.21 | 5.28 |
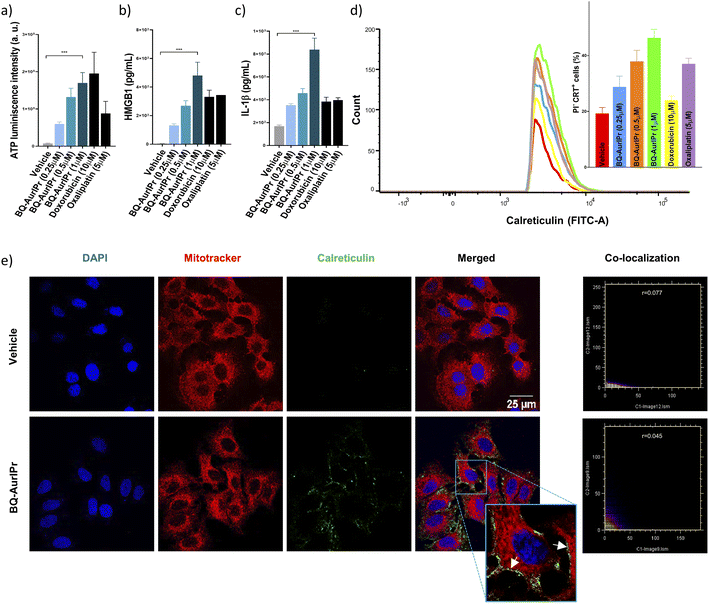
To study the effectiveness of BQ-AurIPr in inducing ICD in A549 cells we analysed the production of DAMPs. In this regard, ATP, HMGB1, IL-1β, and CXCL10 secretion in culture supernatant of BQ-AurIPr treated cells was analysed by luciferin-based assay and ELISA (Fig. 1a–c and ESI†), while translocation of CRT to the plasma membrane was analysed by flow cytometry and confocal microscopy (Fig. 1d and e). Interestingly, we found that BQ-AurIPr produced similar or higher levels of DAMPs in comparison to much higher doses of FDA-approved drugs doxorubicin and oxaliplatin. These findings suggest that cells undergoing apoptosis after BQ-AurIPr treatment are capable of producing DAMPs and might be immunogenic in nature, making BQ-AurIPr a putative ICD inducer.
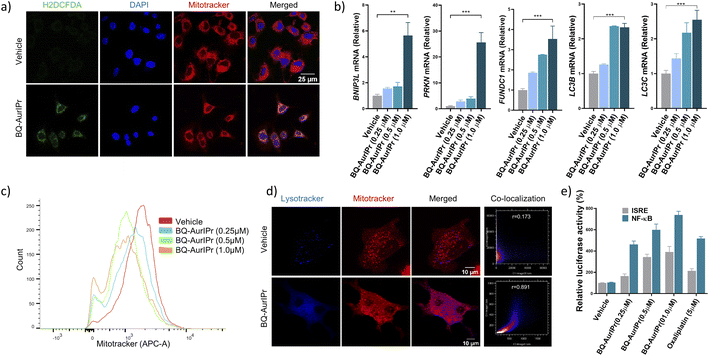
Redox-active gold(I) complexes have been reported to induce apoptosis and ICD in cancer cells, but the mechanism of action remains elusive.28 Gold complexes are known to exert anticancer activity primarily through oxidative stress.32 Therefore, to dissect the mechanism of action, we analyzed the level of ROS inside cancer cells upon treatment with BQ-AurIPr using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) probe and found a marked increase in intracellular ROS levels when cells were cultured in the presence of 0.25 μM BQ-AurIPr for 4 hours (ESI†). Interestingly, merged microscopic images of mitotracker red, DAPI and H2DCFDA indicate the accumulation of ROS in mitochondria (mtROS) (Fig. 2a and ESI†). We surmised that mtROS could oxidize mitochondrial DNA (mtDNA)39 and other contents triggering the degradation of mitochondria by mitophagy leading to DAMP production and ICD.20,40 To analyze mitophagy, we examined RNA expression of several mitophagy indicator genes by RT-PCR and probed mitochondrial mass by mitotracker red staining. To our delight, BQ-AurIPr treated cells showed higher expression of mitophagy indicator genes (Fig. 2b) and decreased mitotracker red staining in a dose-dependent manner, indicating degradation of mitochondria (Fig. 2c). Moreover, BQ-AurIPr also increased the colocalization of mitochondria and lysosomes (Fig. 2d), confirming that BQ-AurIPr induces mitophagy in cancer cells. Moreover, damaged mitochondria may release mtROS and oxidized mtDNA into cytosol which is sensed by innate immune sensors like NLRP3 and cGAS-STING, respectively.41,42 This triggers a cascade of innate immune responses through transcription factors IRF3 and NF-κB, leading to secretion of Type-I interferons and pro-inflammatory cytokines like IL-1β, CXCL10, and IL-17 which are highly potent DAMP signals. To confirm this hypothesis, we tested the activation of interferon stimulation response element (ISRE) and NF-κB by promoter-reporter assay and subsequent secretion of IL-1β by ELISA. Results indicate an increase in ISRE and NF-κB promoter activity (Fig. 2e) correlating to a marked increase in the secretion of IL-1β as shown previously, which was analogous to much higher concentrations of doxorubicin and oxaliplatin.
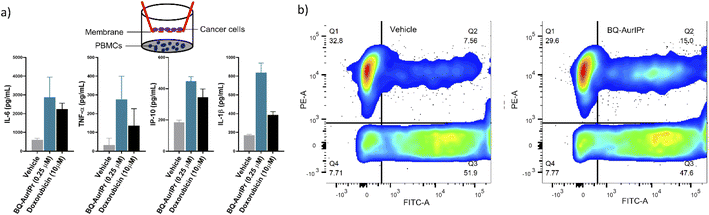
Noticeably, most ICD studies to date are conducted in cancer cell lines or animal models. However, in order to estimate the true potential of an ICD inducer in humans, it would be equally important to study their effect on activating the human immune system, however, the number of such studies remains fairly small.23 In this regard, we first analysed the response of human immune cells (peripheral blood mononuclear cells isolated from healthy donors, hPBMCs) to cancer cells treated with BQ-AurIPr. Cancer cells undergoing ICD should be able to activate immune cells like macrophages and dendritic cells, which are the first cells that encounter an antigen or DAMPs. Upon exposure, these cells become activated and start producing pro-inflammatory cytokines like TNF-α, IL-1β, CXCL10, IL-6, etc.43–45 to activate and invite other more specific types of immune cells at the local site. Hence, we performed indirect co-culture of BQ-AurIPr treated cancer cells with human hPBMCs using Boyden chamber method. Cancer cells were treated for 4 hours with BQ-AurIPr and cultured in the upper chamber, while hPBMCs were cultured in the lower chamber. This method allows soluble factors like DAMPs to cross through the membrane but does not allow direct contact between cancer cells and hPBMCs. Cytokines secreted by immune cells were analysed in culture supernatants after 6 hours of co-culture. Cells treated with 0.25 μM BQ-AurIPr induced markedly higher amounts of IL-6, CXCL10, IL-1β, and TNF-α from hPBMCs, indicating the DAMPs produced from cancer cells were sufficient for activating human immune cells. The amounts of cytokines produced were found to be analogous to that produced by much higher doses of doxorubicin (Fig. 3a).
Phagocytosis of immunogenic cancer cells by macrophage or dendritic cells initiates the anti-tumor immunity by presenting cancer antigens to adaptive immune cells. Therefore, to see if BQ-AurIPr treatment enhances phagocytosis of cancer cells by human macrophages we performed phagocytosis assay by directly culturing treated cancer cells with differentiated THP1 macrophages. EGFP expressing A549 cells were treated either with vehicle or 0.25 μM BQ-AurIPr for 4 hours followed by co-culture with PMA-differentiated THP1 macrophages (labelled red using mitotracker red). After 6 hours of co-culture, cells were analyzed by flow cytometry for the co-occurrence of green and red fluorescent signals. The rate of phagocytosis of cancer cells by macrophages doubled when cancer cells were treated with BQ-AurIPr compared to vehicle (Fig. 3b). Together these results demonstrate that while killing NSCLC A549 cells directly, BQ-AurIPr also strengthens host's anticancer immunity by instigating immunogenic apoptosis. Such immuno-chemotherapeutic agents might have several advantages over traditional chemotherapeutics like faster clearance of tumors, killing of resistant cells by the immune system, fewer side-effects due to high selectivity index, and most importantly fewer chances of relapse because of long-lasting immunological memory.
In summary, we have systematically identified a potential ICD inducer BQ-AurIPr from a rationally synthesized library of BQ-based Au(I) complexes through an amino-auration reaction of pyridino-alkynes. BQ-AurIPr, demonstrated excellent anticancer properties in various cancer cell lines and was studied to induce ICD in A549 cells. Important biomarkers of ICD studied include: (1) CRT translocation, (2) HMGB1 secretion, (3) ATP release, (4) IL-1b secretion, and (5) CXCL10 secretion. Experimental investigations suggest oxidative stress in mitochondria leading to mitophagy-dependent secretion of various DAMPs as the main mechanism for immunogenic cell death of A549 cells. Furthermore, BQ-AurIPr treatment enhanced the immunogenicity of A549 cells when co-cultured with hPBMCs. In addition to killing cancer cells directly, BQ-AurIPr also substantially enhances the phagocytosis of A549 cells by human macrophages. These findings strongly validate the competence of gold complexes as ICD inducers and are expected to instigate further development in this field of metal-based immune-chemotherapeutics.
Data availability
All data are available in the manuscript and in the ESI.†Author contributions
Ravindra Mule: conceptualization, investigation, validation, data curation, formal analysis, visualization. Akhilesh Kumar: conceptualization, investigation, validation, data curation, formal analysis, visualization. writing – original draft, Shashank P. Sancheti: investigation, validation, data curation, formal analysis, writing – original draft. B. Senthilkumar: resources. Himanshu Kumar: writing-review and editing, resources, supervision, funding acquisition, project administration. Nitin T. Patil: writing-review and editing, resources, supervision, funding acquisition, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Generous financial support by the CSIR, New Delhi [File No. 02(0336)/18/EMR-II], SERB, New Delhi (DIA/2018/000016 and CRG/2020/001417), and DBT, New Delhi (File No. BT/PR25052/NER/95/983/2017) is gratefully acknowledged. R. D. M. and A. K. thank the CSIR and UGC for the award of SRF. S. P. S. thanks IISERB for the SRF.References
- D. Hanahan and R. A. Weinberg, Cell, 2011, 144(5), 646 CrossRef CAS PubMed.
- M. P. Chao, S. Jaiswal, R. Weissman-Tsukamoto, A. A. Alizadeh, A. J. Gentles, J. Volkmer, K. Weiskopf, S. B. Willingham, T. Raveh, C. Y. Park, R. Majeti and I. L. Weissman, Sci. Transl. Med., 2010, 2(63), 63ra94 CAS.
- L. Galluzzi, L. Senovilla, L. Zitvogel and G. Kroemer, Nat. Rev. Drug Discovery, 2012, 11(3), 215 CrossRef CAS.
- L. Zitvogel, L. Galluzzi, M. J. Smyth and G. Kroemer, Immunity, 2013, 39(1), 74 CrossRef CAS.
- D. V. Krysko, A. D. Garg, A. Kaczmarek, O. Krysko, P. Agostinis and P. Vandenabeele, Nat. Rev. Cancer, 2012, 12(12), 860 CrossRef CAS.
- J. Fucikova, O. Kepp, L. Kasikova, G. Petroni, T. Yamazaki, P. Liu, L. Zhao, R. Spisek, G. Kroemer and L. Galluzzi, Cell Death Dis., 2020, 11(11), 1013 CrossRef CAS PubMed.
- N. Casares, M. O. Pequignot, A. Tesniere, F. Ghiringhelli, S. Roux, N. Chaput, E. Schmitt, A. Hamai, S. Hervas-Stubbs, M. Obeid, F. Coutant, D. Métivier, E. Pichard, P. Aucouturier, G. Pierron, C. Garrido, L. Zitvogel and G. Kroemer, J. Exp. Med., 2005, 202(12), 1691 CrossRef CAS PubMed.
- O. Kepp, L. Senovilla and G. Kroemer, Oncotarget, 2014, 5(14), 5190 CrossRef PubMed.
- E. Vacchelli, F. Aranda, A. Eggermont, J. Galon, C. Sautès-Fridman, I. Cremer, L. Zitvogel, G. Kroemer and L. Galluzzi, Oncoimmunology, 2014, 3(1), e27878 CrossRef PubMed.
- V. Berry, L. Basson, E. Bogart, O. Mir, J. Y. Blay, A. Italiano, F. Bertucci, C. Chevreau, S. Clisant-Delaine, B. Liegl-Antzager, E. Tresch-Bruneel, J. Wallet, S. Taieb, E. Decoupigny, A. Le Cesne, T. Brodowicz and N. Penel, Cancer, 2017, 123(12), 2294 CrossRef CAS PubMed.
- E. Choy, Y. Flamand, S. Balasubramanian, J. E. Butrynski, D. C. Harmon, S. George, G. M. Cote, A. J. Wagner, J. A. Morgan, M. Sirisawad, C. Mani, F. J. Hornicek, Z. Duan and G. D. Demetri, Cancer, 2015, 121(8), 1223 CrossRef CAS PubMed.
- P. G. Morris, N. M. Iyengar, S. Patil, C. Chen, A. Abbruzzi, R. Lehman, R. Steingart, K. C. Oeffinger, N. Lin, B. Moy, S. E. Come, E. P. Winer, L. Norton, C. A. Hudis and C. T. Dang, Cancer, 2013, 119(22), 3943 CrossRef CAS PubMed.
- R. Z. Orlowski, A. Nagler, P. Sonneveld, J. Bladé, R. Hajek, A. Spencer, T. Robak, A. Dmoszynska, N. Horvath, I. Spicka, H. J. Sutherland, A. N. Suvorov, L. Xiu, A. Cakana, T. Parekh and J. F. San-Miguel, Cancer, 2016, 122(13), 2050 CrossRef CAS PubMed.
- W. D. Tap, R. L. Jones, B. A. Van Tine, B. Chmielowski, A. D. Elias, D. Adkins, M. Agulnik, M. M. Cooney, M. B. Livingston, G. Pennock, M. R. Hameed, G. D. Shah, A. Qin, A. Shahir, D. M. Cronier, R. Ilaria Jr, I. Conti, J. Cosaert and G. K. Schwartz, Lancet, 2016, 388(10043), 488 CrossRef CAS.
- T. Hemdan, R. Johansson, S. Jahnson, P. Hellström, I. Tasdemir and P. U. Malmström, J. Urol., 2014, 191(5), 1244 CrossRef CAS PubMed.
- C. Li, H. Sun, W. Wei, Q. Liu, Y. Wang, Y. Zhang, F. Lian, F. Liu, C. Li, K. Ying, H. Huo, Z. Qi and B. Li, Cell. Oncol., 2020, 43(6), 1099 CrossRef CAS PubMed.
- A. D. Garg, S. More, N. Rufo, O. Mece, M. L. Sassano, P. Agostinis, L. Zitvogel, G. Kroemer and L. Galluzzi, Oncoimmunology, 2017, 6(12), e1386829 CrossRef PubMed.
- L. Menger, E. Vacchelli, S. Adjemian, I. Martins, Y. Ma, S. Shen, T. Yamazaki, A. Q. Sukkurwala, M. Michaud, G. Mignot, F. Schlemmer, E. Sulpice, C. Locher, X. Gidrol, F. Ghiringhelli, N. Modjtahedi, L. Galluzzi, F. André, L. Zitvogel, O. Kepp and G. Kroemer, Sci. Transl. Med., 2012, 4(143), 143ra99 Search PubMed.
- S. V. Hato, A. Khong, I. J. de Vries and W. J. Lesterhuis, Clin. Cancer Res., 2014, 20(11), 2831 CrossRef CAS PubMed.
- C. Chen, X. Ni, S. Jia, Y. Liang, X. Wu, D. Kong and D. Ding, Adv. Mater., 2019, 31(52), e1904914 CrossRef.
- B. Englinger, C. Pirker, P. Heffeter, A. Terenzi, C. R. Kowol, B. K. Keppler and W. Berger, Chem. Rev., 2019, 119(2), 1519 CrossRef CAS PubMed.
- D. Y. Q. Wong, W. W. F. Ong and W. H. Ang, Angew. Chem., Int. Ed., 2015, 54(22), 6483 CrossRef CAS PubMed.
- M. J. R. Tham, M. V. Babak and W. H. Ang, Angew. Chem., Int. Ed., 2020, 59(43), 19070 CrossRef CAS PubMed.
- K.-B. Huang, F.-Y. Wang, H.-W. Feng, H. Luo, Y. Long, T. Zou, A. S. C. Chan, R. Liu, H. Zou, Z.-F. Chen, Y.-C. Liu, Y.-N. Liu and H. Liang, Chem. Commun., 2019, 55(87), 13066 RSC.
- M. Sabbatini, I. Zanellato, M. Ravera, E. Gabano, E. Perin, B. Rangone and D. Osella, J. Med. Chem., 2019, 62(7), 3395 CrossRef CAS PubMed.
- P. Kaur, A. Johnson, J. Northcote-Smith, C. Lu and K. Suntharalingam, Chembiochem, 2020, 21(24), 3618 CrossRef CAS.
- D. Wernitznig, K. Kiakos, G. Del Favero, N. Harrer, H. Machat, A. Osswald, M. A. Jakupec, A. Wernitznig, W. Sommergruber and B. K. Keppler, Metallomics, 2019, 11(6), 1044 CrossRef CAS PubMed.
- S. Sen, S. Hufnagel, E. Y. Maier, I. Aguilar, J. Selvakumar, J. E. DeVore, V. M. Lynch, K. Arumugam, Z. Cui, J. L. Sessler and J. F. Arambula, J. Am. Chem. Soc., 2020, 142(49), 20536 CrossRef CAS.
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, Ca-Cancer J. Clin., 2021, 71(3), 209 CrossRef PubMed.
- Z. Deng, N. Wang, Y. Liu, Z. Xu, Z. Wang, T. C. Lau and G. A. Zhu, J. Am. Chem. Soc., 2020, 142(17), 7803 CrossRef CAS PubMed.
- L. Wang, R. Guan, L. Xie, X. Liao, K. Xiong, T. W. Rees, Y. Chen, L. Ji and H. Chao, Angew. Chem., Int. Ed., 2021, 60(9), 4657 CrossRef CAS.
- T. Zou, C. T. Lum, C. N. Lok, J. J. Zhang and C. M. Che, Chem. Soc. Rev., 2015, 44(24), 8786 RSC.
- S. Yue, M. Luo, H. Liu and S. Wei, Front. Chem., 2020, 8, 543 CrossRef CAS PubMed.
- M. Mora, M. C. Gimeno and R. Visbal, Chem. Soc. Rev., 2019, 48(2), 447 RSC.
- E. J. Anthony, E. M. Bolitho, H. E. Bridgewater, O. W. L. Carter, J. M. Donnelly, C. Imberti, E. C. Lant, F. Lermyte, R. J. Needham, M. Palau, P. J. Sadler, H. Shi, F. X. Wang, W. Y. Zhang and Z. Zhang, Chem. Sci., 2020, 11(48), 12888 RSC.
- A. C. Shaikh, D. S. Ranade, P. R. Rajamohanan, P. P. Kulkarni and N. T. Patil, Angew. Chem., Int. Ed., 2017, 56(3), 757 CrossRef CAS PubMed.
- R. D. Mule, A. C. Shaikh, A. B. Gade and N. T. Patil, Chem. Commun., 2018, 54(84), 11909 RSC.
- C. C. Chintawar, M. V. Mane, A. G. Tathe, S. Biswas and N. T. Patil, Org. Lett., 2019, 21(17), 7109 CrossRef CAS PubMed.
- I. Shokolenko, N. Venediktova, A. Bochkareva, G. L. Wilson and M. F. Alexeyev, Nucleic Acids Res., 2009, 37(8), 2539 CrossRef CAS PubMed.
- Z. Yu, J. Guo, M. Hu, Y. Gao and L. Huang, ACS Nano, 2020, 14(4), 4816 CrossRef CAS PubMed.
- C. Fang, F. Mo, L. Liu, J. Du, M. Luo, K. Men, F. Na, W. Wang, H. Yang and X. Wei, Cell. Mol. Immunol., 2020, 18, 2211 CrossRef.
- M. E. Heid, P. A. Keyel, C. Kamga, S. Shiva, S. C. Watkins and R. D. Salter, J. Immunol., 2013, 191(10), 5230 CrossRef CAS.
- G. Arango Duque and A. Descoteaux, Front. Immunol., 2014, 5, 491 Search PubMed.
- U. C. Müller-Quernheim, L. Potthast, J. Müller-Quernheim and G. Zissel, J. Interferon Cytokine Res., 2012, 32(4), 169 CrossRef.
- D. H. Chang, J. R. Rutledge, A. A. Patel, B. G. Heerdt, L. H. Augenlicht and R. J. Korst, PLoS One, 2013, 8(6), e64456 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental materials and methods. See https://doi.org/10.1039/d2sc03756d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |