Open Access Article
Open Access ArticleThe sequential structural transformation of a heptanuclear zinc cluster towards hierarchical porous carbon for supercapacitor applications†
Tian
Li
a,
Yi-Fan
Wang
a,
Zheng
Yin
 c,
Jian
Li
d,
Xu
Peng
c,
Jian
Li
d,
Xu
Peng
 *ad and
Ming-Hua
Zeng
*ad and
Ming-Hua
Zeng
 *ab
*ab
aCollaborative Innovation Center for Advanced Organic Chemical Materials Co-constructed by the Province and Ministry, Ministry-of-Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules, College of Chemistry & Chemical Engineering, Hubei University, Wuhan, 430062, P. R. China. E-mail: pengxu@hubu.edu.cn
bDepartment of Chemistry and Pharmaceutical Sciences, Guangxi Normal University, Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources, Guilin, 541004, P. R. China. E-mail: zmh@mailbox.gxnu.edu.cn
cCollege of Chemistry and Chemical Engineering, Shaanxi University of Science and Technology, Xi'an 710021, P. R. China
dAnHui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials, Anhui University, Hefei 230601, P. R. China
First published on 19th August 2022
Abstract
The peripheral N/O chelating of Schiff base ligands, inner bridges, counterions, and metal centers gave rise to a brucite disk cluster [Zn7L6(OCH3)6](NO3)2 (Zn7, (HL = 2-methoxy-6-((methylimino)-methyl)phenolate)) which crystallized into hexagonal prismatic plates. The combination of crystallographic studies, in situ TG-MS, and other characterization techniques showed that with a fixed metal and ligand composition in the precursors, weak correlative interactions (e.g., electrostatic interactions) and shape matching between the cluster core and counterions determine the cluster packing modes in the crystals and affect their phase and morphological changes during pyrolysis. The tracking of the pyrolysis process showed that the peripheral ligands, inner bridge, and counterion decompose first, followed by the Zn7O6 core merging with cubic ZnO, which was then reduced by carbon and eventually evaporated, leaving behind a porous carbon structure. In this process, the solid material composition change was in the sequence {Zn7}-{Zn–O core@C}-{ZnO@C}-{Zn@C}-{C}, which was accompanied by a porosity change from micropores to hierarchical pores, and then to micropores again. The core structure and packing modes of Zn7 evolved into micropores and mesopores, respectively. Micro-mesoporous carbon Zn7-1000 featured a capacitance of 1797 F g−1 at 1 A g−1, where the BET specific surface area was 3119.18 m2 g−1, which, to the best of our knowledge, is the highest value reported for a porous carbon electrode. This work represents an important benchmark for the analysis of dynamic chemical processes involving coordination clusters at high temperatures, and it could lead to important applications in high-performance devices.
Introduction
Time-dependent evolutions of composite solid materials at elevated temperatures are highly complex processes that involve both chemical decomposition and condensation reactions. Understanding these processes by tracking the dynamic evolution of the solid-state material is both intriguing and challenging.1 Usually, the entire pyrolysis reaction involves a synergistic effect applied to solid-state chemistry, including decomposition, agglomeration, carbonization and hydrocarbons by dry distillation.2 However, these four processes are often interrelated and disordered, and it is difficult to find obvious and insightful rules, which significantly aggravates the analysis of the pyrolysis process. If the pyrolysis process can be controlled and idealized through the design of precursor molecules, for example, precursor templates, decomposition and ordering, quantification, and product porosity, it will greatly improve the efficiency of our analysis of the pyrolysis process. Designing multi-component materials at different levels that are used as precursors in controllable pyrolysis has successfully lead to various nanocomposite materials, especially functional carbon materials.3 Some of these carbon materials have exceptional physical and chemical properties and can be used for energy storage and conversion devices, or as electrode materials for electrocatalysis.4–6 However, designing hierarchical porous carbon materials with specific pore size distributions that are advantageous for specific applications remains both a challenge for material design and an opportunity for advancing the applications of these materials. Metal organic framework (MOF)-derived carbons inheriting the porous structure of the parent materials have been shown to have various pore distributions and high surface areas, and, thus, represent a promising route in this regard and its derivatives.7–9 On the other hand, the controllable pyrolysis of predesigned coordinative molecular clusters is emerging as an effective new way of achieving carbon derivatives. However, derivation from non-porous coordinated molecular clusters to obtain hierarchical porous structures and high-performance carbon materials is a remarkable challenge. We, therefore, studied the solid–liquid and solid–solid structural correlation of self-assembled coordination clusters and elucidated the mechanism of their assembly, where significant effort was made in exploring simple solid-state transformations in the pyrolysis process. For example, by selecting two ligands with different shapes but similar coordination reactivities, we revealed the mechanism of competition and transformations between the different species in Co4.10 The rational introduction of a μ3-CN inner bridge into Co7+1 clusters followed by pyrolysis created Co@NC core–shell nano materials that achieved a higher OER performance.11 Also, the co-crystalline zinc clusters of Zn7+1+1 are completely pyrolyzed to a microporous carbon material with excellent capacitance performance.12 The abovementioned studies provide a meticulous analysis of changes at the molecular and morphological level during the pyrolysis processes and constructs a preliminary structure–activity relationship between the coordination clusters and their derivatives, however, the sequential structural transformation has not yet been elucidated. In this report, we used elaborately designed multi-component precursors to systematically study the correlation between molecular level chemical reactions and solid material morphological changes in the thermolysis process. We employed a “molecule-to-condensed-matter” perspective in the design and characterization of the coordination clusters thermolysis reaction, where we established the time-dependent correlation between the material structures on molecular, cluster packing and crystal morphology levels.Results and discussions
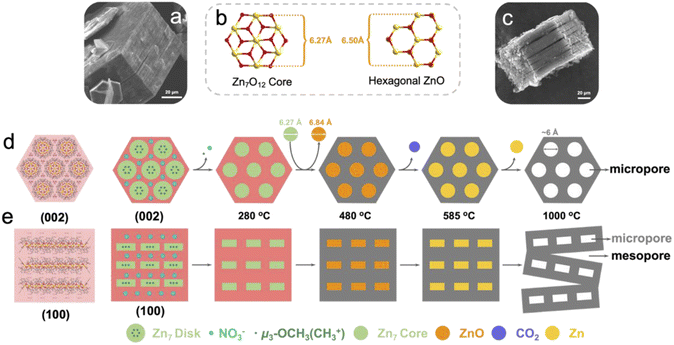
Based on the above understandings, a heptanuclear zinc cluster, [Zn7L6(OCH3)6](NO3)2 (Zn7), was synthesized as hexagonal-prismatic-plate crystals from Zn(NO3)2·6H2O and the N/O chelated Schiff base ligand 2-methoxy-6-((methylimino)-methyl)phenolate in CH3OH solution, where the μ3-OCH3 plays a crucial role for the formation of the heptanucleated cubic Zn–O core. The Zn7 disk cations are tightly arranged in layers parallel to the ab plane, which are then packed along the c axis. The NO3− counterions are located in the 1D channel formed by three adjacent columns of clusters. We use the zinc cluster Zn7 as the precursor, and the products after pyrolysis in an Ar atmosphere are designated as Zn7-T (where T represent the annealing temperature). Notably, a carbon material with a micro-mesoporous hierarchical structure is carbonized at 1000 °C under an argon atmosphere (Zn7-1000), presenting the highest Brunauer–Emmet–Teller (BET) specific surface area of 3119.18 m2 g−1 and a pore volume of 1.82 cm3 g−1, which exhibits excellent capacitance performance. The specific capacitance value is 1797 F g−1 when the current density reaches 1 A g−1. We carried out a systematic analysis of the crystal structure of Zn7 and its evolution, which unravels the formation mechanism of the hierarchical porous carbon (Scheme 1).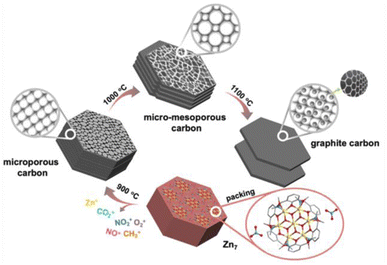 | ||
| Scheme 1 Progressive phase transformation from a heptanuclear zinc cluster (Zn7) to hierarchical porous carbon (Zn7-1000). The microporous carbon samples Zn7-900 and Zn7-1100 are also illustrated. | ||
X-ray single crystal diffraction analysis showed that Zn7 crystallized in the trigonal system P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c1 space group. It is composed of a [Zn7L6(OCH3)6]2+ cation cluster and two counter anions of NO3− but without any lattice solvents, indicating highly condensed packing (Fig. S2a and Table S1a†). The peripheral Schiff base ligands adopting a η1:η2:η1:μ2 coordination and six μ3-OCH3 as inner bridges grasp seven Zn2+ ions in to a cluster with a disk core. All ZnII atoms are in a six-coordinated octahedral configuration, with the central ZnO6 octahedron surrounded by six outer ZnNO5 octahedrons. The core of the disk is similar to brucite-like fragments and all seven Zn2+ ions are located in a coplanar fashion, with a Zn–OCH3 bond length of 2.036 to 2.127 Å, and a Zn–O–Zn angle of 94.6–101.3°. The benzene ring based peripheral ligands endow the cluster duple open hemispheres at two sides of the disk. Three inner –OCH3– motifs coordinate vertically to the Zn7 plane, which sit inside the bowl and occupy the void space that could potentially host solvent molecules. As a result, weak intermolecular interactions induce a cluster packing mode, where eight Zn7 disks are located at the vertex of a quadrilateral prism with four additional disks at the middle position of the pillar. Two 1D channels with triangular windows are generated in each cell to host the NO3−. The regular vertical arrangement of the disk with duple open hemispheres and the hydrophobic –OCH3– sitting inside the bowl facilitates the close packing of the heptanuclear cations and the facile removal of NO3− trapped in the 1D inter-cluster channel during pyrolysis. The nearest intercluster distance is 11.64 and 14.12 Å along the plane and vertical direction, respectively, considering the center Zn⋯Zn distance for adjacent disks (Fig. S2c†).
c1 space group. It is composed of a [Zn7L6(OCH3)6]2+ cation cluster and two counter anions of NO3− but without any lattice solvents, indicating highly condensed packing (Fig. S2a and Table S1a†). The peripheral Schiff base ligands adopting a η1:η2:η1:μ2 coordination and six μ3-OCH3 as inner bridges grasp seven Zn2+ ions in to a cluster with a disk core. All ZnII atoms are in a six-coordinated octahedral configuration, with the central ZnO6 octahedron surrounded by six outer ZnNO5 octahedrons. The core of the disk is similar to brucite-like fragments and all seven Zn2+ ions are located in a coplanar fashion, with a Zn–OCH3 bond length of 2.036 to 2.127 Å, and a Zn–O–Zn angle of 94.6–101.3°. The benzene ring based peripheral ligands endow the cluster duple open hemispheres at two sides of the disk. Three inner –OCH3– motifs coordinate vertically to the Zn7 plane, which sit inside the bowl and occupy the void space that could potentially host solvent molecules. As a result, weak intermolecular interactions induce a cluster packing mode, where eight Zn7 disks are located at the vertex of a quadrilateral prism with four additional disks at the middle position of the pillar. Two 1D channels with triangular windows are generated in each cell to host the NO3−. The regular vertical arrangement of the disk with duple open hemispheres and the hydrophobic –OCH3– sitting inside the bowl facilitates the close packing of the heptanuclear cations and the facile removal of NO3− trapped in the 1D inter-cluster channel during pyrolysis. The nearest intercluster distance is 11.64 and 14.12 Å along the plane and vertical direction, respectively, considering the center Zn⋯Zn distance for adjacent disks (Fig. S2c†).
The simulation of the crystal morphology of Zn7 using the Bravais–Friedel–Donnay–Harker (BFDH) method showed a hexagonal prism-shaped morphology (Fig. S3†),13 which was consistent with that of the obtained sample (Fig. 1a). The [Zn7] units parallel the hexagonal plane of the crystal, and the NO3− anions sit above and below the individual heptanuclear complexes, which interact with the ligand of the Zn7 cluster through C–H⋯O hydrogen bonding interactions. When the observing direction is along the (002) plane and the (100) plane, the surface where the nitrate is located is parallel to the (002) surface while being perpendicular to the (100) surface of [Zn7L6(OCH3)6]2+ (Fig. S3d–f†). For such hexagonal prism crystals constituting the disks arranged in a compact manner, the horizontal and vertical intercluster interactions contribute to the maintenance of the hexagonal morphology during the high temperature carbonization process. When the pyrolysis temperature is 1000 °C, the crystal still maintains a hexagonal prism shape, but shows a slit when viewed from the side of the crystal; slits can be observed parallel to the ab plane (Fig. 1c). This macroscopic observation is strongly related to the thermal decomposition of microscopic molecules.
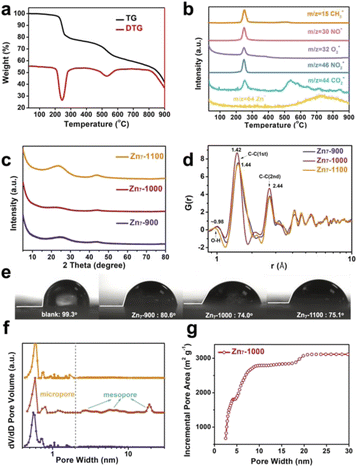
In the first stage of decomposition (205–280 °C), CH3+, NO+, NO2+, O2+ fragments are detected by TG-MS (Fig. 2b). Among them, the methyl group is attributed to the cracking of μ3-OCH3 and the methoxy group of L, which with the C–O bond in Zn7 of similar strength (Table S1b†) and the O2+ is ascribed to the decomposition of nitrate and its further reactions (2NO3− = 2NO2 + O2, NO2 + C = 2NO + CO2) (Table S2†). It should be noted that the first stage of decomposition is completed at 280 °C, where Zn7 collapses to form an amorphous structure, yet the Zn7 core still remains independent (Fig. S4a†). At the same time, due to the removal of nitrate “planes”, the force between the “planes” where the Zn7 core is located is significantly reduced, and a tendency to separate would start to develop. A heptanuclear [Zn7O12] core with a diameter of about 6.27 Å transform into hexagonal ZnO with a diameter of about 6.50 Å in the temperature range of 280–480 °C (Fig. S4a†). In this process, the elaborately designed cluster with a precise spatial arrangement of ligands, counterions and the Zn7 core enables the coherent decomposition and departure of the nitrate group with methoxy species, which helps to maintain the regular morphology and ordered mesoscopic structure of the resulting ZnO@carbon structure. In the second stage (480–585 °C), the primary chemical reaction is 7ZnO + 7/2C = 7Zn + 7/2CO2 (Table S3†). The stoichiometric number 7 is due to the fact that there are seven Zn atoms in a Zn7 molecule. In the third stage of decomposition (>585 °C), Zn+ is detected by TG-MS because the liquid zinc element began to evaporate and escape (Fig. 2b), and left micropores with a diameter of about 6 Å at 900 °C (Fig. 1d). Moreover, at 1000 °C, the abovementioned separation between the “planes” where the Zn7 cores are located results in a large number of slit-type mesopores at 1000 °C (Fig. 1e).14 In brief, inorganic metal nodes in molecular clusters can be converted into metal oxides by pyrolysis, and further reduced to elemental metals by a carbothermal reduction process, while Zn molecular clusters can generate pore structures of specific sizes in pyrolyzed carbon-based materials during this process. The phase evolution process can be summarized as Zn7[C6H3(CH3O)(O)(NCH3)]6 (OCH3)6(NO3)2 → [Zn7O12]@N,O-doped carbon → [ZnO]@N,O-doped carbon → [Zn]@N,O-doped carbon → N,O-doped hierarchical carbon. Obviously, choosing clusters with specific morphologies for pyrolysis can further change the pore structure of the pyrolysis products and increase the porosity of the derived carbon materials.
A series of characterizations, including PXRD, ex situ SAXS, PDF, TEM, Raman and BET were conducted to further illustrate the thermal decomposition process of Zn7 and the pore structure changes during pyrolysis. The PXRD patterns of 280–480 °C pyrolysis samples show a clear hexagonal ZnO peak (JCPDS card 65-3411), indicating that the Zn7 core is crystallized into hexagonal ZnO. Meanwhile, the PXRD patterns of 600–800 °C pyrolysis samples also exhibit a strong diffraction peak of ZnO, which is derived from the remaining elemental zinc in the samples oxidized by oxygen in air after the annealing process (Fig. S4†). It should be noted that the PXRD pattern of the Zn7-900 sample has a broad and diffuse diffraction peak near 2θ = 23° and a weak diffraction peak near 2θ = 44°, which is a distinct characteristic of amorphous carbon.15 Also, among the diffraction peak at 2θ = 23°, the diffraction peak of Zn7-1000 is the weakest, which indicates that the degree of graphitization is the lowest (R = 1.36), while the diffraction peak of Zn7-1100 is narrowed to a certain extent, demonstrating an increased degree of graphitization (R = 2.19).16 Furthermore, experimental atomic pair distribution function (PDF) analysis also proves that the peak is centered at 1.42 Å of Zn7-1000 (slightly smaller than other samples, ∼1.44 Å) corresponding to the sp2 C–C bond in graphitic carbon, which is also consistent with the sequential phase evolution process deduced by TG-MS and other structural characterization17 (Fig. 2c and d). Ex situ SAXS patterns of Zn7-900, Zn7-1000, and Zn7-1100 are shown in Fig. S5.† In addition, high-resolution transmission electron microscopy (HRTEM) mapping displays the disordered carbon layer in Zn7-900 and Zn7-1000 samples, while Zn7-1100 contains lattice fringes corresponding to graphitic carbon (002), indicating that amorphous carbon has crystallized into graphitic carbon (Fig. S6†). Similarly, in Raman spectra, the ID/IG value of the pyrolysis products (T = 900, 1000, 1100 °C) remained approximately the same, with an intensity ratio of 0.94, essentially indicating no apparent changes in the degree of carbon defects in the resulting material (Fig. S7†). ICP data of the Zn residual within the sample is shown in Table S4.† According to the analysis of the XPS spectrum, there are predominantly noticeable peaks of C and O elements in the sample (Fig. S8–S10†). The presence of the element O confirms that the surface of the material has oxygen-containing functional groups, which can enhance the hydrophilicity of the surface of the material to the water-based electrolyte and encourage the transfer of electrolyte ions; it can also introduce certain Faraday pseudo capacitors to enhance the capacitor performance.27 The presence of hydrophilic functional groups can also be verified from the PDF data, where a peak at 0.98 Å is ascribed to the considerable O–H bond distance in the sample. Therefore, we used the polyurethane mixed sample and coated the film to conduct the contact angle test. In comparison with the blank, the contact angles of the three samples after high-temperature pyrolysis are θ < 90°, and they all show hydrophilicity. Among them, the contact angle of Zn7-1000 is the smallest, indicating that better hydrophilic characteristics are more conducive to the contact between the ions in the electrolyte solution and the material (Fig. 2e).
In the gas adsorption measurements, a high gas uptake at a low pressure range corresponds to the presence of micropores and the hysteresis loop suggests mesoporous adsorption (Fig. S12†). The 900 °C pyrolysis product has microporous vacancies left by the evaporation and escape of zinc, with a pore size of about 0.6 nm.12,18 In addition, there are other micropores that provide more adsorption sites during energy storage (Fig. 2f).19–22 After being carbonized at a high temperature of 1000 °C, the pore size distribution shows that the Zn7-1000 sample contains mesopores, making the product show a micro-mesoporous hierarchical structure. These pores can become channels for the diffusion of electrolyte ions and accelerating ionization. The diffusion and transfer are rapid, and the surface area utilization rate has significantly improved. Compared with Zn7-900 and Zn7-1100, the adsorption capacity of Zn7-1000 is significantly increased to 1295.16 cm3 g−1 (Fig. S12b†) with a more significant proportion of mesopores. Calculation results of the density functional theory (DFT) method23–25 show that the pore structure of Zn7-1000 primarily consists of pore sizes of 0.6 nm, 0.8 nm, ∼1.2 nm, 3–8 nm, and 20 nm, of which 20 nm is attributed to the gap between the crystal blocks (Fig. 2f). Zn7-1000 has a maximum BET specific surface area, as high as 3119.18 m2 g−1, and a maximum total pore volume of 1.82 cm3 g−1 (Fig. S12d†). According to the curve of the cumulative specific surface area with the pore diameter calculated by the NLDFT method, it can be seen that the increase of the Zn7-1000 specific surface area is mainly due to the contribution of mesopores (Fig. 2g). The ultra-high specific surface area of Zn7-1000 and the extensive micropore–mesoporous hierarchical structure can facilitate more electrolyte ion adsorption sites.26 Briefly, we have discovered the distinct characteristics of Zn7-1000 and other pyrolyzed samples, and these differences are destined to have a significant impact on its electrochemical performance.
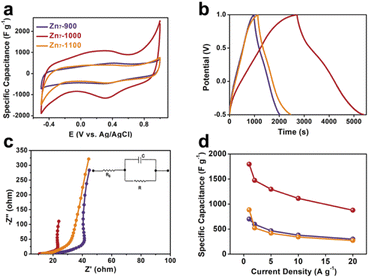
Compared with Zn7-900 and Zn7-1100, which only have a single micropore size, Zn7-1000 combines micropores and mesopores to form a hierarchical porous structure that uses not only mesopores to provide efficient diffusion channels, but also micropores or smaller mesopores that leads to a larger active area, thereby achieving a high capacitance in supercapacitors. In order to verify our above analysis of the pyrolyzed samples from the zinc cluster on the electrochemical performance, we determined their specific capacitance by cyclic voltammetry (CV), galvanostatic charge and discharge (GCD) and electrochemical impedance spectroscopy (EIS) measurements, using a three-electrode configuration with Ag/AgCl as the reference electrode. The CV curves of Zn7-900, Zn7-1000, and Zn7-1100 samples range from −0.6 to 1.0 V at a scan rate of 5 to 100 mV s−1. The shape of the CV curves under different scanning rates is not ideally rectangular-like; a prominent redox peak is especially located at ca. 0.3–0.4 V. This phenomenon indicates that a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group exists in the pyrolyzed samples, which is consistent with XPS analyses.27 In addition, the slight difference in the position of the redox peak is due to the difference in the content of the pyridine nitrogen and the pyrrole nitrogen in the resulting materials (Fig. 4b, S11 and S13†).28
O group exists in the pyrolyzed samples, which is consistent with XPS analyses.27 In addition, the slight difference in the position of the redox peak is due to the difference in the content of the pyridine nitrogen and the pyrrole nitrogen in the resulting materials (Fig. 4b, S11 and S13†).28
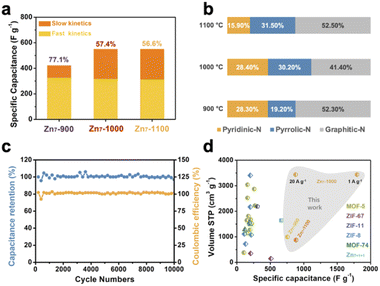
Moreover, GCD curves of Zn7-1000 electrode were tested, as shown in Fig. 3b and S14,† and exhibit a specific capacitance of 1797, 1473, 1297, 1113, and 877 F g−1 at current densities of 1, 2, 5, 10, and 20 A g−1, respectively. This value records the best value among porous carbons as electrodes from MOFs or MOFs-derived materials as precursors (Fig. 4d and Table S5†). Also, the electrochemical impedance spectroscopy test was performed using a three-electrode configuration from 100 kHz to 100 mHz (Fig. 3c). A relatively low equivalent series resistance of only 10.71 Ω is exhibited by Zn7-1000, reflected at the high-frequency region by the intersection of the curve at the real part. Meanwhile, the slope of Zn7-1000 is also larger than that of Zn7-900 and Zn7-1100, revealing a better transport and diffusion of ions in Zn7-1000 samples (Fig. 3d). Zn7-1000 still has a superior specific capacitance retention compared with other samples under a current density of 2 A g−1 to 20 A g−1, which is due to the reduced ion transfer resistance assisted by its microporous–mesoporous hierarchical structure.
Furthermore, we calculated the contribution of fast kinetics from the CV curves of Zn7-900, Zn7-1000 and Zn7-1100 samples, giving 77.1%, 57.4% and 56.6%, respectively (Fig. 4a and S15†).29,30 It should be noted that the value of slow kinetics increases obviously from Zn7-900 to Zn7-1000, which may be due to the introduction of mesopores and the increase of slow kinetics in Zn7-1000 contributing to the higher capacitance. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles at a current density of 10 A g−1, the capacitance retention remained above 96%, indicating that Zn7-1000 has an excellent cycle stability as a supercapacitor material and a good coulombic efficiency which was kept at ∼100% (Fig. 4c).
000 charge/discharge cycles at a current density of 10 A g−1, the capacitance retention remained above 96%, indicating that Zn7-1000 has an excellent cycle stability as a supercapacitor material and a good coulombic efficiency which was kept at ∼100% (Fig. 4c).
Conclusions
In summary, a heptanuclear zinc disk, Zn7, was rational designed via a judicious choice of peripheral N/O-chelating Schiff base ligands, inner bridges, counteracting ions, and metal centers, where a predesigned ligand and metal ion ratio, weak interactions, and shape matching have been dominant in determining the cluster structure and packing mode. The structure and chemical composition of Zn7 were studied via TG-MS, which showed that pyrolysis leads to a sequential structural transformation that turns from crystal to MO/M@C and then to carbon. The study elucidated the formation mechanism of pores, both in terms of the decomposition–condensation reaction mechanism and the cooperative bonding and packing in the cluster structure. This is, thus, the first time that the effects of the main ligand, inner bridge, and counterions on the formation, morphology, packing, and decomposition of coordination clusters have been thoroughly studied. It was noted that the precisely arranged cluster core, counterion ligands, hierarchical bonds in the crystal, and sequential derivative as a function of temperature and time lead to a coherent decomposition and departure of species and a structural transformation into an ordered porous structure. Zn7-1000 with a hierarchical pore structure exhibits excellent supercapacitor performance with a capacitance of 1797 F g−1 at 1 A g−1, which, to the best of our knowledge, corresponds to the best value among reported porous carbon electrodes. To summarize, we established a new route to study the complex pyrolysis reaction of coordination clusters, which could inspire the rational design of functional nanomaterials from coordination compounds. We have provided a new vision for condensed matter chemistry, based on molecular, cluster packing, and crystal morphology levels, that unravels time-dependent changes in a high-temperature solid-state reaction and the relationship with the material properties.Data availability
All experimental data associated with this article have been included in the main text and ESI.†Author contributions
Xu Peng and Ming-Hua Zeng conceived the idea, co-wrote the paper and supervised the whole experimental procedure and data analysis. Tian Li, Yi-Fan Wang, Zheng Yin and Jian Li performed the experiments, analyzed the data and wrote the manuscript. All the authors discussed the results, commented on and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the NSFC (Grants 22171075), the NSFC of Hubei (Grants 2021CFB420), and the BAGUI talent program (2019AC26001).Notes and references
- Y. Jia, Z. Xue, J. Yang, Q. Liu, J. Xian, Y. Zhong, Y. Sun, X. Zhang, Q. Liu, D. Yao and G. Li, Angew. Chem., Int. Ed., 2022, 61, e202110838 CAS
.
-
P. Basu, in Biomass Gasification Design Handbook, Elsevier, 2010, pp. 65–96 Search PubMed
.
- Y.-Z. Chen, C. Wang, Z.-Y. Wu, Y. Xiong, Q. Xu, S.-H. Yu and H.-L. Jiang, Adv. Mater., 2015, 27, 5010–5016 CrossRef CAS PubMed
.
- H. Shao, Y.-C. Wu, Z. Lin, P.-L. Taberna and P. Simon, Chem. Soc. Rev., 2020, 49, 3005–3039 RSC
.
- Y.-S. Hu, P. Adelhelm, B. M. Smarsly, S. Hore, M. Antonietti and J. Maier, Adv. Funct. Mater., 2007, 17, 1873–1878 CrossRef CAS
.
- W. Tian, H. Zhang, X. Duan, H. Sun, G. Shao and S. Wang, Adv. Funct. Mater., 2020, 30, 1909265 CrossRef CAS
.
- J. Hou, C. Cao, F. Idrees and X. Ma, ACS Nano, 2015, 9, 2556–2564 CrossRef CAS PubMed
.
- S. Dutta, A. Bhaumik and K. C.-W. Wu, Energy Environ. Sci., 2014, 7, 3574–3592 RSC
.
- B. Fang, J. H. Kim, M.-S. Kim and J.-S. Yu, Acc. Chem. Res., 2013, 46, 1397–1406 CrossRef CAS PubMed
.
- H.-L. Zheng, X.-L. Chen, T. Li, Z. Yin, Y. Zhang, M. Kurmoo and M.-H. Zeng, Chem.–Eur. J., 2018, 24, 7906–7912 CrossRef CAS PubMed
.
- J.-Q. Zhao, D. Cai, J. Dai, M. Kurmoo, X. Peng and M.-H. Zeng, Sci. Bull., 2019, 64, 1667–1674 CrossRef CAS
.
- Y. Wang, Y. Liang, Y. Wu, J. Yang, X. Zhang, D. Cai, X. Peng, M. Kurmoo and M. Zeng, Angew. Chem., Int. Ed., 2020, 59, 13232–13237 CrossRef CAS PubMed
.
- R. Docherty, G. Clydesdale, K. J. Roberts and P. Bennema, J. Phys. D: Appl. Phys., 1991, 24, 89–99 CrossRef CAS
.
- Z. Li, W. Lv, C. Zhang, B. Li, F. Kang and Q.-H. Yang, Carbon, 2015, 92, 11–14 CrossRef CAS
.
- B. Liu, H. Shioyama, T. Akita and Q. Xu, J. Am. Chem. Soc., 2008, 130, 5390–5391 CrossRef CAS PubMed
.
- Y. Liu, J. S. Xue, T. Zheng and J. R. Dahn, Carbon, 1996, 34, 193–200 CrossRef CAS
.
- V. Petkov, Y. Ren, S. Kabekkodu and D. Murphy, Phys. Chem. Chem. Phys., 2013, 15, 8544 RSC
.
- M. J. Wang, Z. X. Mao, L. Liu, L. Peng, N. Yang, J. Deng, W. Ding, J. Li and Z. Wei, Small, 2018, 14, 1804183 CrossRef PubMed
.
- J. Chmiola, Science, 2006, 313, 1760–1763 CrossRef CAS PubMed
.
- J. Chmiola, C. Largeot, P.-L. Taberna, P. Simon and Y. Gogotsi, Angew. Chem., 2008, 120, 3440–3443 CrossRef
.
- D. T.
L. Galhena, B. C. Bayer, S. Hofmann and G. A. J. Amaratunga, ACS Nano, 2016, 10, 747–754 CrossRef CAS PubMed
.
- E. Raymundo-Piñero, K. Kierzek, J. Machnikowski and F. Béguin, Carbon, 2006, 44, 2498–2507 CrossRef
.
- M. Thommes and K. A. Cychosz, Adsorption, 2014, 20, 233–250 CrossRef CAS
.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS
.
- A. V. Neimark, Y. Lin, P. I. Ravikovitch and M. Thommes, Carbon, 2009, 47, 1617–1628 CrossRef CAS
.
- P. Hao, Z. Zhao, J. Tian, H. Li, Y. Sang, G. Yu, H. Cai, H. Liu, C. P. Wong and A. Umar, Nanoscale, 2014, 6, 12120–12129 RSC
.
- H. A. Andreas and B. E. Conway, Electrochim. Acta, 2006, 51, 6510–6520 CrossRef CAS
.
- T. Lin, I.-W. Chen, F. Liu, C. Yang, H. Bi, F. Xu and F. Huang, Science, 2015, 350, 1508–1513 CrossRef CAS PubMed
.
- H.-S. Kim, J. B. Cook, H. Lin, J. S. Ko, S. H. Tolbert, V. Ozolins and B. Dunn, Nat. Mater., 2017, 16, 454–460 CrossRef CAS PubMed
.
- C. Choi, D. S. Ashby, D. M. Butts, R. H. DeBlock, Q. Wei, J. Lau and B. Dunn, Nat. Rev. Mater., 2020, 5, 5–19 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC [2020712]. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc03987g |
| This journal is © The Royal Society of Chemistry 2022 |