Hydrazone–palladium catalyzed annulation of 1-cinnamyloxy-2-ethynylbenzene derivatives†‡
Kohei
Watanabe
a,
Takashi
Mino
*ab,
Tatsuya
Ikematsu
a,
Chikako
Hatta
a,
Yasushi
Yoshida
ab and
Masami
Sakamoto
ab
aDepartment of Applied Chemistry and Biotechnology, Graduate School of Engineering, Chiba University, 1-33, Yayoi-cho, Inage-ku, Chiba 263-8522, Japan. E-mail: tmino@faculty.chiba-u.jp
bMolecular Chirality Research Center, Chiba University, 1-33, Yayoi-cho, Inage-ku, Chiba 263-8522, Japan. E-mail: tmino@faculty.chiba-u.jp
First published on 20th June 2016
Abstract
The annulation of 1-cinnamyloxy-2-ethynylbenzene derivatives using a hydrazone–palladium catalyst system proceeded smoothly and gave the corresponding 2-substituted-3-cinnamylbenzofurans in good-to-excellent yields.
Introduction
The benzofuran core structure is found in a wide variety of natural products and pharmacological activities.1 In particular, 2,3-disubstituted benzofuran derivatives are known as various bioactive compounds.2 Therefore, the synthetic methodologies for such a structure have attracted significant attention and subsequent development.3 The annulation of o-ethynylphenol derivatives is known as a simple protocol for the construction of the benzofuran core.4 However, this protocol afforded only a 2-substituted benzofuran derivative. Recently, some intermolecular annulations of o-ethynylphenol derivatives with electrophiles for the synthesis of 2,3-disubstituted benzofuran derivatives have been reported.5 On the other hand, the Cacchi group6 and Monteiro group7 independently reported that the palladium-catalyzed annulation of 1-allyloxy-2-ethynylbenzene derivatives afforded 2-substituted-3-allylbenzofuran.These protocols were attractive for the construction of 2,3-disubstituted benzofuran derivatives. However, they required high temperatures. Furthermore, the Liang group reported the palladium-catalyzed double annulation of 1-allyloxy-2-ethynylbenzene for the construction of 2-substituted-3-allylbenzofuran under relatively mild conditions.8 However, they did not attempt the reaction of 1-cinnamyloxy-2-ethynylbenzene. On the other hand, while the Fürstner group reported the platinum-catalyzed annulation of 1-allyloxy-2-ethynylbenzene for the construction of 2-substituted-3-allylbenzofuran, this annulation required a reaction to take place under a poisonous CO atmosphere.9
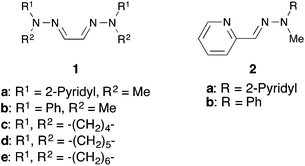
Previously, we demonstrated that easily prepared and air-stable hydrazones (Fig. 1) are effective ligands for palladium-catalyzed C–C bond formation reactions10 including the Tsuji–Trost type allylic arylation of allylic acetates with arylboronic acids.11 More recently, we also reported the intermolecular12 and intramolecular13 allylic arylation of cinnamyloxybenzene using a hydrazone–palladium catalyst system. Additionally, we demonstrated that a hydrazone–palladium catalyst system was effective for the annulation reaction forming naphthalene derivatives.14 Herein, we report that the hydrazone–palladium catalyzed annulation of 1-cinnamyloxy-2-(phenylethynyl)benzene was complete in only 1 hour under optimized conditions, and afforded the corresponding 2-substituted-3-cinnamylbenzofuran derivatives in good-to-excellent yields.
Results and discussion
Initially, we sought the optimum reaction conditions for the hydrazone–palladium catalyzed annulation of 1-cinnamyloxy-2-(phenylethynyl)benzene (3a) (Table 1). When the reaction of 3a was performed using Pd2(dba)3, the pyridine-methyl type bis-hydrazone 1a as a ligand and Et3N as a base in 1,4-dioxane/H2O (3/1) at 50 °C for 1 hour under an Ar atmosphere, 3-cinnamyl-2-phenylbenzofuran (4a) was obtained in 96% yield (Table 1, entry 1). On the other hand, only a trace amount of the product was detected using the phenyl-methyl type bishydrazone ligand 1b (entry 2). The reactions using bishydrazone ligands 1c–e bearing 5–7 member rings gave the corresponding product in 15%, 32%, and 15% yields, respectively (entries 3–5). We also tried to use pyridine-type monohydrazone ligands 2a and 2b (entries 6 and 7). However, these ligands were not effective for this annulation. The reaction in the absence of a ligand did not proceed (entry 8). When we tested a PPh3-palladium catalyst system, which was an effective catalyst system in Cacchi's report, the desired product was obtained in only 13% yield (entry 9). As a result, the pyridine-methyl type bishydrazone ligand 1a was the most suitable for this annulation (entry 1). Next, we checked the reactivity of various palladium sources (entries 1 and 10–13). We found that a reaction using a palladium(II) source, such as Pd(OAc)2, Pd(tfa)2, PdCl2 and PdCl2(MeCN)2, gave only a trace amount of the desired product (entries 10–13). Next, the effects of various bases were investigated (entries 1 and 14–18). Although the reaction using Ca(OH)2 afforded a good yield (entry 18), using Et3N led to the highest yield in this annulation (entry 1). Various solvents were also tested (entries 1 and 19–24). 1,4-Dioxane was the most suitable solvent for this annulation (entry 1). Additionally, the effect of H2O was investigated (entries 1 and 25–28). When we reduced the amount of water from 3/1 to 9/1, the yield of 4a was decreased to 33% (entry 25). Furthermore, the reaction without water gave the corresponding product in only a trace amount (entry 26). These results indicate that water is necessary to achieve this annulation. When we alternatively increased the amount of water from 3/1 to 2/1 and 1/1, the yield of 4a was also decreased to 82% and 71%, respectively (entries 27 and 28). Finally, we checked the reaction using 3a under Liang's condition8 (entry 29). This annulation was still not complete after 1 hour, despite using 10 mol% of palladium catalyst, and afforded the product 4a in only 48% yield. Therefore, we found that Liang's condition was not suitable for 1-cinnamyloxy-2-ethynylbenzene derivatives.| Entry | Ligand | Catalyst | Base | Solvent | Yield of 4a (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 3a, ligand (5.0 mol%), catalyst (Pd = 5.0 mol%), base (2.0 eq.), solvent (0.25 M) at 50 °C for 1 h under Ar. b Reaction conditions: 3a, PPh3 (20 mol%), Pd2(dba)3·CHCl3 (Pd = 10 mol%), DMF (0.05 M) at 60 °C for 1 h under Ar. | |||||
| 1 | 1a | Pd 2 (dba) 3 | Et 3 N | 1,4-Dioxane/H 2 O (3/1) | 96 |
| 2 | 1b | Pd2(dba)3 | Et3N | 1,4-Dioxane/H2O (3/1) | Trace |
| 3 | 1c | Pd2(dba)3 | Et3N | 1,4-Dioxane/H2O (3/1) | 15 |
| 4 | 1d | Pd2(dba)3 | Et3N | 1,4-Dioxane/H2O (3/1) | 32 |
| 5 | 1e | Pd2(dba)3 | Et3N | 1,4-Dioxane/H2O (3/1) | 15 |
| 6 | 2a | Pd2(dba)3 | Et3N | 1,4-Dioxane/H2O (3/1) | 46 |
| 7 | 2b | Pd2(dba)3 | Et3N | 1,4-Dioxane/H2O (3/1) | 11 |
| 8 | — | Pd2(dba)3 | Et3N | 1,4-Dioxane/H2O (3/1) | Trace |
| 9 | — | Pd(PPh3)4 | Et3N | 1,4-Dioxane/H2O (3/1) | 13 |
| 10 | 1a | Pd(OAc)2 | Et3N | 1,4-Dioxane/H2O (3/1) | Trace |
| 11 | 1a | Pd(tfa)2 | Et3N | 1,4-Dioxane/H2O (3/1) | Trace |
| 12 | 1a | PdCl2 | Et3N | 1,4-Dioxane/H2O (3/1) | Trace |
| 13 | 1a | PdCl2(MeCN)2 | Et3N | 1,4-Dioxane/H2O (3/1) | Trace |
| 14 | 1a | Pd2(dba)3 | DIEA | 1,4-Dioxane/H2O (3/1) | 29 |
| 15 | 1a | Pd2(dba)3 | NaOAc | 1,4-Dioxane/H2O (3/1) | 44 |
| 16 | 1a | Pd2(dba)3 | Cs2CO3 | 1,4-Dioxane/H2O (3/1) | 60 |
| 17 | 1a | Pd2(dba)3 | K3PO4 | 1,4-Dioxane/H2O (3/1) | 41 |
| 18 | 1a | Pd2(dba)3 | Ca(OH)2 | 1,4-Dioxane/H2O (3/1) | 94 |
| 19 | 1a | Pd2(dba)3 | Et3N | CPME/H2O (3/1) | 9 |
| 20 | 1a | Pd2(dba)3 | Et3N | DMA/H2O (3/1) | 82 |
| 21 | 1a | Pd2(dba)3 | Et3N | DMF/H2O (3/1) | 65 |
| 22 | 1a | Pd2(dba)3 | Et3N | DMSO/H2O (3/1) | 67 |
| 23 | 1a | Pd2(dba)3 | Et3N | NMP/H2O (3/1) | 62 |
| 24 | 1a | Pd2(dba)3 | Et3N | THF/H2O (3/1) | 85 |
| 25 | 1a | Pd2(dba)3 | Et3N | 1,4-Dioxane/H2O (9/1) | 33 |
| 26 | 1a | Pd2(dba)3 | Et3N | 1,4-Dioxane | Trace |
| 27 | 1a | Pd2(dba)3 | Et3N | 1,4-Dioxane/H2O (2/1) | 82 |
| 28 | 1a | Pd2(dba)3 | Et3N | 1,4-Dioxane/H2O (1/1) | 71 |
| 29b | PPh3 | Pd2(dba)3·CHCl3 | — | DMF | 48 |
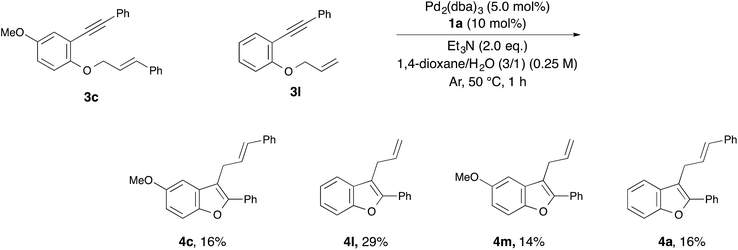
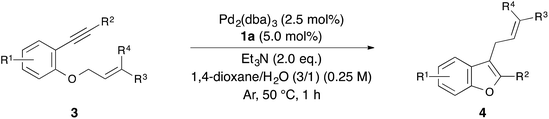
With the optimized reaction conditions obtained (Table 1, entry 1), we investigated the scope and limitation of this annulation using various substituted 1-allyloxy-2-ethynylbenzene derivatives 3 (Table 2). The reaction of 2-cinnamyloxy-1-(phenylethynyl)naphthalene (3b) as a starting material also proceeded and gave 1-cinnamyl-2-phenylnaphthofuran (4b) in good yield (entry 2). Although 10 mol% of palladium and the ligand were required in some cases, the reactions of substrates 3c–e bearing an electron-donating or withdrawing group on the aromatic ring were also tolerated (entries 3–5). On the other hand, the yield of the product was low when we employed methylbenzoate 3f as a starting material (entry 6). Next, we investigated the electronic effect of the cinnamyloxy group. We found that the annulations of 4-substituted cinnamyloxy benzenes 3g–j were also tolerated (entries 7–10). In particular, the corresponding benzofuran derivative 4h was obtained in a quantitative yield when we employed cinnamyloxybenzene 3h to this annulation (entry 8). Furthermore, we succeeded in synthesizing 2-alkyl-3-cinnamylbenzofuran 3k in 73% yield (entry 11). Allyloxybenzene derivatives 3l and 3m were allowed to react and 2-phenyl-3-allylbenzofurans 4l and 4m were obtained in 97% and 96% yields, respectively (entries 12 and 13). The reaction of crotyloxybenzene 3n afforded the mixture of 2-phenyl-3-crotylbenzofuran (4n) (E/Z = 91/9) and 3-(but-3-en-2-yl)-2-phenylbenzofuran (4n′) (4n/4n′ = 89/11) in 92% yield (entry 14). However, prenyloxybenzene 3o was not tolerated in this reaction because the oxidative addition was interrupted by two methyl groups at the allylic position in terms of steric hindrance and the electronic effect (entry 15). Finally, we attempted to use 4-substituted phenylethynylbenzene derivatives in this annulation. The reaction of substrates 3p–s afforded the corresponding products 4p–s in moderate-to-good yields (entries 16–19). All of the reactions, except that using 3o, proceeded for only 1 hour and gave the corresponding benzofuran derivatives in good-to-excellent yields. Next, in order to confirm whether this reaction is an intermolecular or intramolecular reaction, crossover reactions in the presence of 5.0 mol% of Pd2(dba)3 and 10 mol% of 1a were carried out using 3c and 3l as starting materials (Scheme 1). As a result, the corresponding products 4c and 4l were isolated. Furthermore, two crossover products 4m and 4a were also isolated. From this result, we concluded that this reaction occurred in an intermolecular manner.
| Entry | 3 | R1 | R2 | R3 | R4 | Yield of 4 (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1-cinnamyloxy-2-ethynylbenzene 3 (0.25 mmol), Pd2(dba)3 (2.5 mol%), 1a (5 mol%), Et3N (2.0 eq.), 1,4-dioxane/H2O (3/1) (0.25 M) at 50 °C for 1 h under Ar. b Pd2(dba)3 (5.0 mol%) and 1a (10 mol%) were used. c E/Z mixture of 3n (83/17) was used. d The E/Z isomer ratio was determined as 91/9 by 1H NMR spectroscopy. e The 4n/4n′ ratio was determined as 89/11 by 1H NMR spectroscopy. | ||||||
| 1 | 3a | H | Ph | Ph | H | 96 (4a) |
| 2 | 3b | –(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)2 (3,4) CH)2 (3,4) |
Ph | Ph | H | 91 (4b) |
| 3b | 3c | 4-OMe | Ph | Ph | H | 88 (4c) |
| 4b | 3d | 5-Cl | Ph | Ph | H | 95 (4d) |
| 5 | 3e | 5-CF3 | Ph | Ph | H | 94 (4e) |
| 6b | 3f | 5-COOMe | Ph | Ph | H | 39 (4f) |
| 7b | 3g | H | p-MeC6H4 | Ph | H | 81 (4g) |
| 8b | 3h | H | p-MeOC6H4 | Ph | H | 99 (4h) |
| 9b | 3i | H | p-CNC6H4 | Ph | H | 76 (4i) |
| 10b | 3j | H | p-FC6H4 | Ph | H | 95 (4j) |
| 11b | 3k | H | n Bu | Ph | H | 73 (4k) |
| 12 | 3l | H | Ph | H | H | 97 (4l) |
| 13 | 3m | 4-OMe | Ph | H | H | 96 (4m) |
| 14b |
3n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
H | Ph | Me | H | 92 (4n + 4n′)d,e |
| 15 | 3o | H | Ph | Me | Me | N.R. |
| 16b | 3p | H | Ph | p-MeC6H4 | H | 57 (4p) |
| 17b | 3q | H | Ph | p-MeOC6H4 | H | 77 (4q) |
| 18b | 3r | H | Ph | p-ClC6H4 | H | 70 (4r) |
| 19 | 3s | H | Ph | p-CF3C6H4 | H | 67 (4s) |
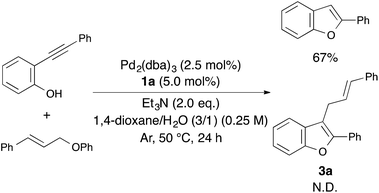
Therefore, we tried to carry out an intermolecular reaction using o-(phenylethynyl)phenol and cinnamyloxybenzene under optimized reaction conditions (Scheme 2). Unfortunately, the intermolecular reaction proceeded to afford only 2-phenylbenzofuran in 67% yield without producing 3a. Consequently, the use of 1-cinnamyloxy-2-ethynylbenzene derivatives as the starting material was found to be essential for constructing 2-phenyl-3-cinnamylbenzofuran.
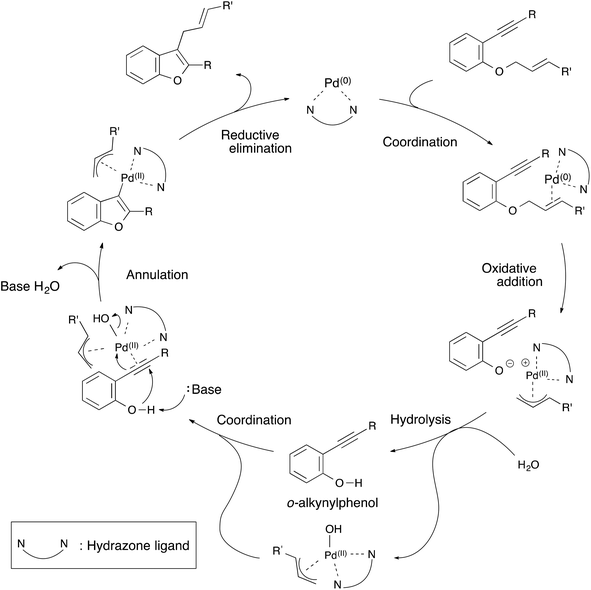
From these results, a plausible mechanism for the annulation of 1-cinnamyloxy-2-ethynylbenzene derivatives is illustrated in Scheme 3. First, the hydrazone–palladium(0) complex coordinates itself with the olefin of the starting material. An oxidative addition to the hydrazone–palladium(0) complex at the allylic position of the starting material takes place to generate an ion pair of a phenoxy anion and a π-allyl palladium(II) cation. Next, hydrolysis of the ion pair by water generates an o-(phenylethynyl)phenol15 and π-allyl-hydroxy palladium(II) complex. This complex coordinates with the triple bond of o-ethynylphenol. Then, the annulation of o-ethynylphenol occurs by the deprotonation of the hydroxyl group to generate a π-allyl palladium(II) benzofuran intermediate. Finally, reductive elimination occurs to produce a 2-substituted-3-allylbenzofuran product and to regenerate the hydrazone–palladium(0) complex. At this point, the catalytic cycle was complete. We assumed that these palladium complexes were stabilized by the hydrazone ligand, and this annulation proceeded smoothly in spite of using a cinnamyloxy substrate as a starting material.
Conclusions
In summary, we found that the palladium-catalyzed annulation of 1-cinnamyloxy-2-ethynylbenzene derivatives 3 using hydrazone 1a as a ligand, even at low temperature proceeded smoothly and gave the corresponding 2-substituted-3-cinnamylbenzofurans 4 in good-to-excellent yields in a very short reaction time.Acknowledgements
This work was supported by the COE Program of Chiba University.Notes and references
- (a) C. D. Poon and B. M. Fung, Synthesis, 1991, 2061 Search PubMed; (b) Z. Tomaszewski, M. P. Johnson, X. Huang and D. E. Nichols, J. Med. Chem., 1992, 35, 2061 CrossRef CAS PubMed; (c) M. Sauter and W. Adam, Acc. Chem. Res., 1995, 28, 289 CrossRef CAS; (d) O. Als, E. Sozialer and U. R. Institutionellen, Arbeitsmedizin, 1999, 14, 1700 Search PubMed; (e) P. Máximo, A. Lourenço, S. S. Feio and J. C. Roseiro, J. Nat. Prod., 2002, 65, 175 CrossRef; (f) T. Pacher, C. Seger, D. Engelmeier, S. Vajrodaya, O. Hofer and H. Greger, J. Nat. Prod., 2002, 65, 820 CrossRef CAS PubMed; (g) K. S. Song and I. Raskin, J. Nat. Prod., 2002, 65, 76 CrossRef CAS PubMed; (h) B. L. Flynn, E. Hamel and M. K. Jung, J. Med. Chem., 2002, 45, 2670 CrossRef CAS PubMed; (i) Y. L. Lin, Y. Y. Chang, Y. H. Kuo and M. S. Shiao, J. Nat. Prod., 2002, 65, 745 CrossRef CAS PubMed; (j) E. Tsuji, K. Ando, J. Kunitomo, M. Yamashita, S. Ohta, S. Kohno and Y. Ohishi, Org. Biomol. Chem., 2003, 1, 3139 RSC; (k) C. J. Hamilton, A. Saravanamuthu, A. H. Fairlamb and I. M. Eggleston, Bioorg. Med. Chem., 2003, 11, 3683 CrossRef CAS PubMed; (l) A. Katona, M. I. Toşa, C. Paizs and J. Rétey, Chem. Biodiversity, 2006, 3, 502 CrossRef CAS PubMed; (m) S. M. Bakunova, S. A. Bakunov, T. Wenzler, T. Barszcz, K. A. Werbovetz, R. Brun, J. E. Hall and R. R. Tidwell, J. Med. Chem., 2007, 50, 5807 CrossRef CAS PubMed; (n) K. Ando, Y. Kawamura, Y. Akai, J.-I. Kunitomo, T. Yokomizo, M. Yamashita, S. Ohta, T. Ohishi and Y. Ohishi, Org. Biomol. Chem., 2008, 6, 296 RSC; (o) K. Asoh, M. Kohchi, I. Hyoudoh, T. Ohtsuka, M. Masubuchi, K. Kawasaki, H. Ebiike, Y. Shiratori, T. A. Fukami, O. Kondoh, T. Tsukaguchi, N. Ishii, Y. Aoki, N. Shimma and M. Sakaitani, Bioorg. Med. Chem. Lett., 2009, 19, 1753 CrossRef CAS PubMed; (p) R. K. Thalji, N. Aiyar, E. A. Davenport, J. A. Erhardt, L. A. Kallal, D. M. Morrow, S. Senadhi, C. L. Burns-Kurtis and J. P. Marino, Bioorg. Med. Chem. Lett., 2010, 20, 4104 CrossRef CAS PubMed; (q) A. M. Venkatesan, O. Dos Santos, J. Ellingboe, D. A. Evrard, B. L. Harrison, D. L. Smith, R. Scerni, G. A. Hornby, L. E. Schechter and T. H. Andree, Bioorg. Med. Chem. Lett., 2010, 20, 824 CrossRef CAS PubMed; (r) C. Antczak, D. Shum, B. Bassit, M. G. Frattini, Y. Li, E. Stanchina, D. A. Scheinberg and H. Djaballah, Bioorg. Med. Chem. Lett., 2011, 21, 4528 CrossRef CAS PubMed; (s) J. Rangaswamy, H. V. Kumar, S. T. Harini and N. Naik, Bioorg. Med. Chem. Lett., 2012, 22, 4773 CrossRef CAS PubMed; (t) T. T. B. Nguyen, T. Lomberget, N. C. Tran, E. Colomb, L. Nachtergaele, S. Thoret, J. Dubois, J. Guillaume, R. Abdayem, M. Haftek and R. Barret, Bioorg. Med. Chem. Lett., 2012, 22, 7227 CrossRef CAS PubMed; (u) W. Eccles, J. M. Blevitt, J. N. Booker, C. C. Chrovian, S. Crawford, A. R. de Leon, X. Deng, A. M. Fourie, C. A. Grice, K. Herman, L. Karlsson, A. M. Kearney, A. Lee-Dutra, J. Liang, R. Luna, D. Pippel, N. Rao, J. P. Riley, A. Santillán, B. Savall, V. M. Tanis, X. Xue and A. L. Young, Bioorg. Med. Chem. Lett., 2013, 23, 811 CrossRef CAS PubMed; (v) X.-Y. Li, B.-F. He, H.-J. Luo, N.-Y. Huang and W.-Q. Deng, Bioorg. Med. Chem. Lett., 2013, 23, 4617 CrossRef CAS PubMed; (w) H. Watanabe, M. Ono, S. Iikuni, M. Yoshimura, K. Matsumura, H. Kimura and H. Saji, Bioorg. Med. Chem. Lett., 2014, 24, 4834 CrossRef CAS PubMed; (x) R. Naik, D. S. Harmalkar, X. Xu, K. Jang and K. Lee, Eur. J. Med. Chem., 2015, 90, 379 CrossRef CAS PubMed; (y) R. J. Nevagi, S. N. Dighe and S. N. Dighe, Eur. J. Med. Chem., 2015, 6, 561 CrossRef PubMed.
- (a) C. C. Felder, K. E. Joyce, E. M. Briley, M. Glass, K. P. Mackie, K. J. Fahey, G. J. Cullinan, D. C. Hunden, D. W. Johnson, M. O. Chaney, G. A. Koppel and M. Brownstein, J. Pharmacol. Exp. Ther., 1998, 284, 291 CAS; (b) M. Halabalaki, N. Aligiannis, Z. Papoutsi, S. Mitakou, P. Moutsatsou, C. Sekeris and A. L. Skaltsounis, J. Nat. Prod., 2000, 63, 1672 CrossRef CAS PubMed; (c) B. Carlsson, B. N. Singh, M. Temciuc, S. Nilsson, Y. L. Li, C. Mellin and J. Malm, J. Med. Chem., 2002, 45, 623 CrossRef CAS PubMed; (d) V. Wallez, S. Durieux-Poissonnier, P. Chavatte, J. A. Boutin, V. Audinot, J.-P. Nicolas, C. Bennejean, P. Delagrange, P. Renard and D. Lesieur, J. Med. Chem., 2002, 45, 2788 CrossRef CAS PubMed; (e) I. Hayakawa, R. Shioya, T. Agatsuma, H. Furukawa, S. Naruto and Y. Sugano, Bioorg. Med. Chem. Lett., 2004, 14, 4383 CrossRef CAS PubMed; (f) M. Ohno, M. Miyamoto, K. Hoshi, T. Takeda, N. Yamada and A. Ohtake, J. Med. Chem., 2005, 48, 5279 CrossRef CAS PubMed; (g) B. L. Flynn, G. S. Gill, D. W. Grobelny, J. H. Chaplin, D. Paul, A. F. Leske, T. C. Lavranos, D. K. Chalmers, S. A. Charman, E. Kostewicz, D. M. Shackleford, J. Morizzi, E. Hamel, M. K. Jung and G. Kremmidiotis, J. Med. Chem., 2011, 54, 6014 CrossRef CAS PubMed; (h) Y. He, J. Xu, Z.-H. Yu, A. M. Gunawan, L. Wu, L. Wang and Z.-Y. Zhang, J. Med. Chem., 2013, 56, 832 CrossRef CAS PubMed; (i) S. He, P. Jain, B. Lin, M. Ferrer, Z. Hu, N. Southall, X. Hu, W. Zheng, B. Neuenswander, C.-H. Cho, Y. Chen, S. A. Worlikar, J. Aubé, R. C. Larock, F. J. Schoenen, J. J. Marugan, T. J. Liang and K. J. Frankowski, ACS Comb. Sci., 2015, 17, 641 CrossRef CAS PubMed.
- (a) A. S. K. Hashmi, T. M. Frost and J. W. Bats, Org. Lett., 2001, 3, 3769 CrossRef CAS PubMed; (b) M. C. Willis, D. Taylor and A. T. Gillmore, Org. Lett., 2004, 6, 4755 CrossRef CAS PubMed; (c) C. Chen and P. G. Dormer, J. Org. Chem, 2005, 70, 6964 CrossRef CAS PubMed; (d) M. Carril, R. SanMartin, I. Tellitu and E. Domínguez, Org. Lett., 2006, 8, 1467 CrossRef CAS PubMed; (e) B. Zhao and X. Lu, Org. Lett., 2006, 8, 5987 CrossRef CAS PubMed; (f) B. L. Zhan, F. D. Wang and J. M. Yue, Synlett, 2006, 567 Search PubMed; (g) B. Lu, B. Wang, Y. Zhang and Y. D. Ma, J. Org. Chem., 2007, 72, 5337 CrossRef CAS PubMed; (h) J. Oppenheimer, W. L. Johnson, M. R. Tracey, R. P. Hsung, P.-Y. Yao, R. Liu and K. Zhao, Org. Lett., 2007, 9, 2361 CrossRef CAS PubMed; (i) A. S. K. Hashmi and M. Wölfle, Tetrahedron, 2009, 65, 9021 CrossRef CAS; (j) J. Hu, L.-Y. Wu, X.-C. Wang, Y.-Y. Hu, Y.-N. Niu, X.-Y. Liu, S. Yang and Y.-M. Liang, Adv. Synth. Catal., 2010, 352, 351 CrossRef CAS; (k) R. N. Nair, P. J. Lee, A. L. Rheingold and D. B. Grotjahn, Chem. – Eur. J., 2010, 16, 7992 CrossRef CAS PubMed; (l) Y. Liu and S. Ma, Org. Lett., 2012, 14, 720 CrossRef CAS PubMed; (m) B. Yin, C. Cai, G. Zeng, R. Zhang, X. Li and H. Jiang, Org. Lett., 2012, 14, 1098 CrossRef CAS PubMed; (n) H. Yuan, K.-J. Bi, B. Li, R.-C. Yue, J. Ye, Y.-H. Shen, L. Shan, H.-Z. Jin, Q.-Y. Sun and Q.-Y. W.-D. Zhang, Org. Lett., 2013, 15, 4742–4745 CrossRef CAS PubMed.
- (a) C. Koradin, W. Dohle, A. L. Rodriguez, B. Schmid and P. Knochel, Tetrahedron, 2003, 59, 1571 CrossRef CAS; (b) X. Li, A. R. Chianese, T. Vogel and R. H. Crabtree, Org. Lett., 2005, 7, 5437 CrossRef CAS PubMed; (c) K. Hiroya, S. Itoh and T. Sakamoto, Tetrahedron, 2005, 61, 10958 CrossRef CAS; (d) M. Yoshida, Y. Morishita, M. Fujita and M. Ihara, Tetrahedron, 2005, 61, 4381 CrossRef CAS; (e) Y. Zhang, Z.-J. Xin, J.-J. Xue and Y. Li, Chin. J. Chem., 2008, 26, 1461 CrossRef CAS; (f) A. Varela-Fernández, C. González-Rodríguez, J. A. Varela, L. Castedo and C. Saá, Org. Lett., 2009, 11, 5350 CrossRef PubMed; (g) W. Huang, J. H.-C. Liu, P. Alayoglu, Y. Li, C. A. Witham, C.-K. Tsung, F. D. Toste and G. A. Somorjai, J. Am. Chem. Soc., 2010, 132, 16771 CrossRef CAS PubMed; (h) A. S. K. Hashmi, T. D. Ramamurthi and F. Rominger, Adv. Synth. Catal., 2010, 352, 971 CrossRef CAS; (i) R. N. Nair, P. J. Lee and S. B. Grotjahn, Top. Catal., 2010, 53, 1045 CrossRef CAS; (j) I. R. Siddiqui, M. A. Waseem, S. Shamim, Shireen, A. Srivastava and A. Srivastava, Tetrahedron Lett., 2013, 54, 4154 CrossRef CAS; (k) M. Kim, S. Lee, K. Kim, D. Shin, H. Kim and H. Song, Chem. Commun., 2014, 50, 14938 RSC; (l) O. S. Morozov, A. V. Lunchev, A. A. Bush, A. A. Tukov, A. F. Asachenko, V. N. Khrustalev, S. S. Zalesskiy, V. P. Ananikov and M. S. Nechaev, Chem. – Eur. J., 2014, 20, 6162 CrossRef CAS PubMed; (m) S.-X. Sun, J.-J. Wang, Z.-J. Xu, L.-Y. Cao, Z.-F. Shi and H.-L. Zhang, Tetrahedron, 2014, 70, 3798 CrossRef CAS; (n) J. Liu, Z. Liu, P. Liao and X. Bi, Org. Lett., 2014, 4, 2 CrossRef PubMed; (o) A. Chakraborty, K. Jyothi and S. Sinha, Tetrahedron Lett., 2014, 55, 6795 CrossRef CAS.
- (a) Y. Kondo, F. Shiga, N. Murata, T. Sakamoto and H. Yamanaka, Tetrahedron, 1994, 50, 11803 CrossRef CAS; (b) A. Arcadi, S. Cacchi, M. Del Rosario, G. Fabrizi and F. Marinelli, J. Org. Chem., 1996, 61, 9280 CrossRef CAS; (c) M. Nakamura, L. Ilies, S. Otsubo and E. Nakamura, Org. Lett., 2006, 8, 2803 CrossRef CAS PubMed; (d) M. Nakamura, L. Ilies, S. Otsubo and E. Nakamura, Angew. Chem., Int. Ed., 2006, 45, 944 CrossRef CAS PubMed; (e) C. Martínez, R. Álvarez and J. M. Aurrecoechea, Org. Lett., 2009, 11, 1083 CrossRef PubMed; (f) A. Boyer, N. Isono, S. Lackner and M. Lautens, Tetrahedron, 2010, 66, 6468 CrossRef CAS; (g) S. Cacchi, G. Fabrizi and A. Goggiamani, Org. Biomol. Chem., 2011, 9, 641 RSC; (h) Z. Han, L. Zhang, Z. Li and R. Fan, Angew. Chem., Int. Ed., 2014, 53, 6805 CrossRef CAS PubMed; (i) J. Li, Z. Zhu, S. Yang, Z. Zhang, W. Wu and H. Jiang, J. Org. Chem., 2015, 80, 3870 CrossRef CAS PubMed; (j) X. Zhao, L. Zhang, X. Lu, T. Li and K. Lu, J. Org. Chem., 2015, 80, 2918 CrossRef CAS PubMed.
- S. Cacchi, G. Fabrizi and L. Moro, Synlett, 1998, 741 CrossRef CAS.
- N. Monteiro and G. Balme, Synlett, 1998, 746 CrossRef CAS.
- Z. Liang, S. Ma, J. Yu and R. Xu, J. Org. Chem., 2007, 72, 9219 CrossRef CAS PubMed.
- A. Fürstner and P. W. Davies, J. Am. Chem. Soc., 2005, 127, 15024 CrossRef PubMed.
- (a) T. Mino, Y. Shirae, M. Sakamoto and T. Fujita, Synlett, 2003, 882 CrossRef CAS; (b) T. Mino, Y. Shirae, M. Sakamoto and T. Fujita, J. Org. Chem., 2005, 70, 2191 CrossRef CAS PubMed; (c) T. Mino, Y. Shirae, Y. Sasai, M. Sakamoto and T. Fujita, J. Org. Chem., 2006, 71, 6834 CrossRef CAS PubMed; (d) T. Mino, Y. Shirae, T. Saito, M. Sakamoto and T. Fujita, J. Org. Chem., 2006, 71, 9499 CrossRef CAS PubMed; (e) T. Mino, H. Shindo, T. Kaneda, T. Koizumi, Y. Kasashima, M. Sakamoto and T. Fujita, Tetrahedron Lett., 2009, 50, 5358 CrossRef CAS; (f) T. Mino, S. Suzuki, K. Hirai, M. Sakamoto and T. Fujita, Synlett, 2011, 1277 CrossRef CAS; (g) T. Mino, M. Shibuya, S. Suzuki, K. Hirai, M. Sakamoto and T. Fujita, Tetrahedron, 2012, 68, 429 CrossRef CAS; (h) T. Mino, K. Watanabe, T. Abe, T. Kogure and M. Sakamoto, Heterocycles, 2013, 87, 2015 CrossRef CAS.
- (a) T. Mino, K. Kajiwara, Y. Shirae, M. Sakamoto and T. Fujita, Synlett, 2008, 2711 CrossRef; (b) T. Mino, T. Koizumi, S. Suzuki, S. K. Hirai, K. Kajiwara, M. Sakamoto and T. Fujita, Eur. J. Org. Chem., 2012, 678 CrossRef CAS.
- T. Mino, T. Kogure, T. Abe, T. Koizumi, T. Fujita and M. Sakamoto, Eur. J. Org. Chem., 2013, 1501 CrossRef CAS.
- K. Watanabe, T. Mino, T. Abe, T. Kogure and M. Sakamoto, J. Org. Chem., 2014, 79, 6695 CrossRef CAS PubMed.
- K. Watanabe, T. Mino, C. Hatta, S. Ito and M. Sakamoto, Org. Biomol. Chem., 2015, 13, 11645 Search PubMed.
- In the case of using NaOAc (Table 1, entry 15), a trace amount of 2-phenylbenzofuran16 was also obtained via the annulation of o-ethynylphenol.
- G. W. Kabalka, L. Wang and R. M. Pagni, Tetrahedron, 2001, 57, 8017 CrossRef CAS.
Footnotes |
| † Dedicated to Professor Barry M. Trost on the occasion of his 75th birthday. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00112b |
| This journal is © the Partner Organisations 2016 |