Chromium-catalyzed transformations with Grignard reagents – new opportunities for cross-coupling reactions
Xiaoming
Zeng
* and
Xuefeng
Cong
Center for Organic Chemistry, Frontier Institute of Science and Technology, Xi'an Jiaotong University, Xi'an, Shaanxi 710054, P. R. China. E-mail: zengxiaoming@mail.xjtu.edu.cn
First published on 27th November 2014
Abstract
Beyond classic Nozaki–Hiyama–Kishi reactions, a low-cost and low-toxicity chromium(II) salt was recently shown to have the capability to catalyze the cross-couplings of C–X and C–H bonds with Grignard reagents. It is a remarkable progression and would point to a new direction for future development of chromium catalysis, as well as opening new opportunities for discovery of new cross-coupling reactions. This highlight focuses on these advances in chromium-catalyzed transformations using Grignard reagents.
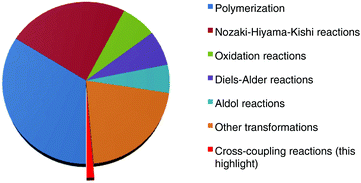
Chromium ranks among the most abundant elements on earth.1 As early as the 1910s, Hein performed a pioneering study on the treatment of chromium(III) chloride with a phenyl Grignard reagent for the synthesis of a bis(arene)chromium species.2 Moreover, up to the 1970s when Nozaki and Hiyama uncovered an interesting chromium-mediated addition of organic halides to aldehydes,3 the so-called “Nozaki–Hiyama–Kishi” reaction,4 the employment of this transition metal in organic synthesis has received increasing attention from chemists.5 Unfortunately, the studies at that stage mainly focused on the exploration of organochromium reagent-mediated stoichiometric reactions. A remarkable advance in the use of chromium salts as catalysts to promote organic transformations was achieved by the group of Fürstner.6 Since then, chromium catalysis has attracted broad interest and various useful synthetic strategies, including polymerization,7 oxidation,8 aldol9 and Diels–Alder reactions,10 have been developed. These transformations generally feature particular advantages, such as good compatibility with sensitive functional groups and high stereoselectivity, so that they are in widespread use in total synthesis as key steps for the preparation of sophisticated natural products.11 Despite these achievements, chromium-catalyzed organic reactions still remain undeveloped as compared to other transition metal catalysts, and considerable effort in the field could be dedicated to discover new transformations and broaden the reaction scope. Transition metal-catalyzed cross-coupling reactions have emerged as a powerful tool in organic synthesis that allows chemists to assemble basic chemical bonds for the build-up of valuable structural motifs of diversified interest.12 These reactions traditionally depended largely on precious palladium and toxic nickel and copper catalysts. Recent efforts have been devoted to the development of sustainable synthetic strategies, such as environmentally-benign iron-catalyzed transformations as an alternative.13 It is worth noting that, analogous to iron(II) chloride, chromium(II) chloride also exhibits a lower toxicity, although high-valent chromium(VI) salts are found to have high toxicity.14 Obviously, the employment of this attractive metal salt in uncovering new cross-coupling strategies would attract broad interest. It may open fascinating opportunities to discover new synthetic methodologies. Unfortunately, cross-coupling reactions enabled by chromium catalysts have rarely been investigated (Scheme 1). In this context, we want to highlight recent breakthroughs achieved by Knochel and co-workers on the chromium-catalyzed cross-coupling reactions of C–X(H) bonds with Grignard reagents, which allow for effective construction of C–C bonds with a fast conversion rate under ambient conditions.14,15
The challenge associated with chromium-promoted cross-coupling reactions may be ascribed to the difficulty in the insertion of unactivated chemical bonds into a high-valent chromium species. In contrast, a low-valent complex tends to undergo oxidative addition to generate an organochromium intermediate, which is thought to be produced during the assistance by nickel, iron or cobalt salts.16 Note that Fürstner and Shi demonstrated that the use of manganese powder as a reductant in combination with TMSCl allows the achievement of chromium-catalyzed Nozaki–Hiyama–Kishi reactions.6 In particular, insight from recent advances in low-valent iron and cobalt-catalyzed reactions, described by the groups of Fürstner, Nakamura, Yoshikai and Cook,17 shows that the treatment of a chromium salt with a Grignard reagent may allow the facile formation of a low-valent chromium complex as a catalytically active species, allowing the oxidative addition step to occur effectively leading to related cross-coupling products.18
Although the employment of Grignard reagents in chromium-promoted arylmagnesiation of alkynes was described by Oshima and Yorimitsu (Scheme 2),19 Knochel and co-workers revealed, for the first time, that chromium(II) chloride was found to have the capability to promote Kumada cross-coupling reactions between aromatic halides and Grignard reagents (Scheme 3).14 Interestingly, the reaction can proceed at room temperature and achieves satisfactory results in short reaction times. Notably, as compared with other first-row transition metal catalysts, the formation of homo-coupling side product was only observed in less than 1% yield in this transformation. It was proved that various nitrogen-containing heterocyclic and aromatic halides can be arylated effectively, while alkenyl iodides are suitable partners to couple efficiently with aromatic Grignard reagents. A variety of functionalities, such as chloride, fluoride, alkoxy, alkoxycarbonyl and amino, are compatible with the catalytic system.
 | ||
| Scheme 2 Chromium-catalyzed selective arylmagnesiation of alkynes (Oshima et al.).19 | ||
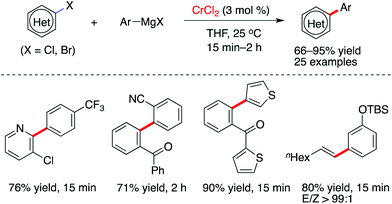 | ||
| Scheme 3 Chromium-catalyzed cross-coupling transformations of aromatic and alkenyl halides with Grignard reagents (Knochel et al.).14 | ||
Interestingly, by consecutive treatment of imino-protected 2-chlorobenzaldehyde with two different aryl Grignard reagents via a one-pot operation, the formation of a bisarylated compound was observed (Scheme 4).15 This reveals that the ortho-C–H bond on the aromatic motif can be successfully functionalized by chromium in combination with a stoichiometric amount of 2,3-dichlorobutane (DCB) oxidant. The authors demonstrated that benzo[h]quinolines, 2-(2-trimethylsilylphenyl)pyridines and 2-trimethylsilylphenyl-substituted oxazolines undergo this conversion smoothly, leading to the desired coupling products in good to excellent yields (Scheme 5). To our knowledge, this is the first example to demonstrate that chromium salts, like commonly effectively transition metal catalysts such as Pd, Rh, Ir, Ni, Co and Fe in C–H activation reactions,20 enable promotion of the functionalization of unactivated C–H bonds with the assistance of an auxiliary, although chromium-mediated C–H bond cleavage has been illustrated previously by Smith and co-workers in the achievement of an unexpected cyclometalated chromium complex.21 Further application of this transformation was described in the rapid construction of functionalized (terphenyl)pyridine scaffolds by the treatment of the resulting TMS-bearing arylated products with ICl, followed by a Negishi cross-coupling.
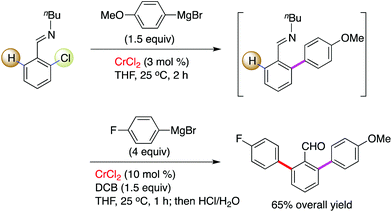 | ||
| Scheme 4 Chromium-promoted consecutive arylations for the introduction of two distinct aryl scaffolds. | ||
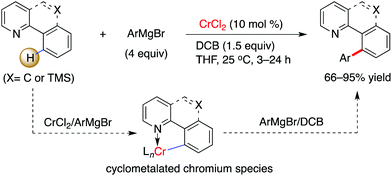 | ||
| Scheme 5 Cr-catalyzed arylation by use of Grignard reagents via chelation-assisted C–H activation (Knochel et al.).15 | ||
Despite the fact that the mechanism for the chromium-promoted C–H arylation has not been mentioned by the authors, analogous with cobalt and iron-catalyzed C–H transformations by use of Grignard reagents,17 a low-valent chromium complex can be considered to be involved in the transformation as a catalytically active species,18 which may allow coordination with the imino auxiliary followed by an ortho-C–H bond cleavage, leading to a five-membered metallacycle species. The role of the Grignard reagent in the reaction may not only be in serving as a nucleophilic partner to couple with the C–H bond, but is also likely to permit access to a low-valent chromium species in the reaction pathway.
Conclusions and perspectives
Taking into account the low cost and low-toxicity profiles of chromium(II) chloride, which are similar to those of iron salts, the use of this salt in developing efficient synthetic strategies would provide an alternative and beneficial complement to traditional transition metal catalysis. It may offer an opportunity for the discovery new reactions. In particular, as compared to cobalt and iron-promoted cross-couplings using Grignard reagents, these protocols have unique features such as forming only small amounts of homo-coupling products and proceeding with fast reaction rates. Despite these advances, chromium-catalyzed cross-coupling reactions are still in their infancy, and significant effort should be dedicated to shedding light on the mechanism to aid in uncovering new transformations and expanding the reaction scope. Without doubt, the exploration of efficient synthetic methods by use of a Grignard reagent as a reductant or base rather than a coupling partner will become important for future development.Acknowledgements
We thank the National Natural Science Foundation of China [no. 21202128] and XJTU for generous financial support.Notes and references
- W. F. McDonough, Science, 2011, 331, 1397 CrossRef CAS PubMed.
- (a) F. Hein, Ber. Dtsch. Chem. Ges., 1919, 52, 195 CrossRef; (b) H. H. Zeiss and M. Tsutsui, J. Am. Chem. Soc., 1957, 79, 3062 CrossRef CAS; (c) M. Tsutsui and H. Zeiss, J. Am. Chem. Soc., 1959, 81, 1367 CrossRef CAS; (d) W. Herwig and H. Zeiss, J. Am. Chem. Soc., 1959, 81, 4798 CrossRef CAS.
- Y. Okude, T. Hiyama and H. Nozaki, J. Am. Chem. Soc., 1977, 99, 3179 CrossRef CAS.
- For a recent review on Nozaki–Hiyama–Kishi reactions, see: (a) G. C. Hargaden and P. J. Guiry, Adv. Synth. Catal., 2007, 349, 2407 CrossRef CAS. For selected examples on Nozaki–Hiyama–Kishi reactions, see: (b) V. Coeffard, M. Aylward and P. J. Guiry, Angew. Chem., Int. Ed., 2009, 48, 9152 CrossRef CAS PubMed; (c) D. L. Usanov and H. Yamamoto, J. Am. Chem. Soc., 2011, 133, 1286 CrossRef CAS PubMed; (d) J. J. Miller and M. S. Sigman, J. Am. Chem. Soc., 2007, 129, 2752 CrossRef CAS PubMed.
- (a) A. Fürstner, Chem. Rev., 1999, 99, 991 CrossRef PubMed; (b) K. H. Dötz and P. Tomuschat, Chem. Soc. Rev., 1999, 28, 187 RSC; (c) K. H. Theopold, Acc. Chem. Res., 1990, 23, 263 CrossRef CAS.
- (a) A. Fürstner and N. Shi, J. Am. Chem. Soc., 1996, 118, 2533 CrossRef; (b) A. Fürstner and N. Shi, J. Am. Chem. Soc., 1996, 118, 12349 CrossRef.
- For a recent review on Cr-promoted ethylene oligomerization, see: (a) T. Agapie, Coord. Chem. Rev., 2011, 255, 861 CrossRef CAS PubMed. For selected recent examples on Cr-promoted polymerization, see: (b) Y. Shaikh, K. Albahily, M. Sutcliffe, V. Fomitcheva, S. Gambarotta, I. Korobkov and R. Duchateau, Angew. Chem., Int. Ed., 2012, 51, 1366 CrossRef CAS PubMed; (c) M. P. Conley, M. F. Delley, G. Siddiqi, G. Lapadula, S. Norsic, V. Monteil, O. V. Safonova and C. Copéret, Angew. Chem., Int. Ed., 2014, 53, 1872 CrossRef PubMed; (d) S. Liu, A. Motta, A. R. Mouat, M. Delferro and T. J. Marks, J. Am. Chem. Soc., 2014, 136, 10460 CrossRef CAS PubMed.
- For an early review on chromium-catalyzed oxidations, see: J. Muzart, Chem. Rev., 1992, 92, 113 CrossRef CAS.
- For a selected recent example, see: R. Kowalczyk, P. Kwiatkowski, J. Skarżewski and J. Jurczak, J. Org. Chem., 2009, 74, 753 CrossRef CAS PubMed.
- For selected examples, see: (a) A. Berkessel, E. Ertürk and C. Laporte, Adv. Synth. Catal., 2006, 348, 223 CrossRef CAS; (b) A. A. Boezio, E. R. Jarvo, B. M. Lawrence and E. N. Jacobsen, Angew. Chem., Int. Ed., 2005, 44, 6046 CrossRef CAS PubMed.
- For selected examples, see: (a) H. Guo, C.-G. Dong, D.-S. Kim, D. Urabe, J. Wang, J. T. Kim, X. Liu, T. Sasaki and Y. Kishi, J. Am. Chem. Soc., 2009, 131, 15387 CrossRef CAS PubMed; (b) W. Zhu, M. Jiménez, W.-H. Jung, D. P. Camarco, R. Balachandran, A. Vogt, B. W. Day and D. P. Curran, J. Am. Chem. Soc., 2010, 132, 9175 CrossRef CAS PubMed; (c) J. Pospíšil, C. Müller and A. Fürstner, Chem. – Eur. J., 2009, 15, 5956 CrossRef PubMed; (d) K. Kong, D. Romo and C. Lee, Angew. Chem., Int. Ed., 2009, 48, 7402 CrossRef CAS PubMed.
- (a) Cross-Coupling reactions. A Practical Guide, ed. N. Miyaura, Springer, Berlin, 2002 Search PubMed; (b) Organotransition Metal Chemistry, ed. J. F. Hartwig, University Science Books, Sausalito, CA, 2010 Search PubMed.
- (a) Iron Catalysis in Organic Chemistry, ed. B. Plietker, Wiley-VCH, Weinheim, 2008 Search PubMed; (b) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed; (c) C. Bolm, J. Legros, J. Le Paih and L. Zani, Chem. Rev., 2004, 104, 6217 CrossRef CAS PubMed; (d) B. D. Sherry and A. Fürstner, Acc. Chem. Res., 2008, 41, 1500 CrossRef CAS PubMed; (e) E. Nakamura and N. Yoshikai, J. Org. Chem., 2010, 75, 6061 CrossRef CAS PubMed.
- A. K. Steib, O. M. Kuzmina, S. Fernandez, D. Flubacher and P. Knochel, J. Am. Chem. Soc., 2013, 135, 15346 CrossRef CAS PubMed.
- O. M. Kuzmina and P. Knochel, Org. Lett., 2014, 16, 5208 CrossRef CAS PubMed.
- (a) H. Jin, J.-I. Uenishi, W. J. Christ and Y. Kishi, J. Am. Chem. Soc., 1986, 108, 5644 CrossRef CAS; (b) M. Kurosu, M.-H. Lin and Y. Kishi, J. Am. Chem. Soc., 2004, 126, 12248 CrossRef CAS PubMed.
- (a) A. Fürstner, A. Leitner, M. Méndez and H. Krause, J. Am. Chem. Soc., 2002, 124, 13856 CrossRef PubMed; (b) A. Fürstner, R. Martin, H. Krause, G. Seidel, R. Goddard and C. W. Lehmann, J. Am. Chem. Soc., 2008, 130, 8773 CrossRef PubMed; (c) M. Nakamura, K. Matsuo, S. Ito and E. Nakamura, J. Am. Chem. Soc., 2004, 126, 3686 CrossRef CAS PubMed; (d) K. Gao and N. Yoshikai, Acc. Chem. Res., 2014, 47, 1208 CrossRef CAS PubMed; (e) E. R. Fruchey, B. M. Monks and S. P. Cook, J. Am. Chem. Soc., 2014, 136, 13130 CrossRef CAS PubMed.
- The formation of a catalytically active low-valent chromium species by treating a Cr(III) complex with a vinyl Grignard reagent has been reported. See: K. Albahily, Y. Shaikh, E. Sebastiao, S. Gambarotta, I. Korobkov and S. I. Gorelsky, J. Am. Chem. Soc., 2011, 133, 6388 CrossRef CAS PubMed.
- K. Murakami, H. Ohmiya, H. Yorimitsu and K. Oshima, Org. Lett., 2007, 9, 1569 CrossRef CAS PubMed.
- For selected reviews on C–H activation reactions, see: (a) J. Wencel-Delord, T. Dröge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740 RSC; (b) D.-G. Yu, B.-J. Li and Z.-J. Shi, Tetrahedron, 2012, 68, 5130 CrossRef CAS PubMed; (c) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (d) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960 CrossRef CAS PubMed; (e) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS PubMed; (f) L. McMurray, F. O'Hara and M. J. Gaunt, Chem. Soc. Rev., 2011, 40, 1885 RSC; (g) O. Baudoin, Chem. Soc. Rev., 2011, 40, 4902 RSC.
- K. C. MacLeod, J. L. Conway, B. O. Patrick and K. M. Smith, J. Am. Chem. Soc., 2010, 132, 17325 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2015 |