Zinc-mediated allylation of carbon dioxide—the LiOAc effect†
Bukeyan
Miao
a and
Shengming
Ma
*bc
aShanghai Key Laboratory of Green Chemistry and Chemical Process, Department of Chemistry, East China Normal University, 3663 North Zhongshan Lu, Shanghai 200062, P. R. China
bState Key Laboratory of Organometallic Chemistry and Shanghai Institute of Organic Chemistry and Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, P. R. China. E-mail: masm@sioc.ac.cn
cDepartment of Chemistry, Fudan University, 220 Handan Lu, Shanghai 200433, P. R. China
First published on 2nd December 2014
Abstract
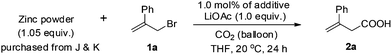
A direct carboxylation reaction of allylic bromides mediated by zinc powder has been developed. The reaction occurred smoothly at room temperature with 1 atm of CO2 provided by a balloon. The reaction was conducted in the presence of LiOAc giving excellent yields. An excellent branched regioselectivity was observed and the reaction was easily scaled-up to 10 gram-scale.
Introduction
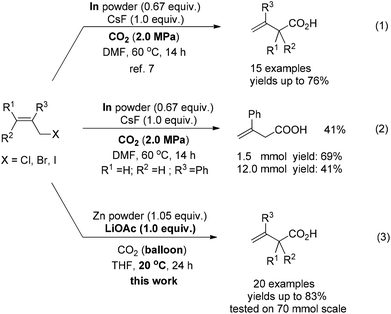
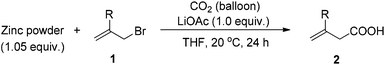
With growing concern over the greenhouse effect, much attention has been paid to the different issues relating CO2. For example, carbon dioxide itself is becoming a more and more popular C1 starting material in C–C bond formation reactions owing to its non-toxicity and abundance as well as its advantages for the syntheses of carbonyl compounds.1 As we know the allylation reaction of carbon dioxide would be the most efficient approach for the synthesis of the not readily available 3-alkenoic acids. Early attempts with allylic lithium or Grignard reagents afforded the products with poor regioselectivity and functional group tolerance.2 Transition metal-catalyzed reactions of pre-prepared toxic allylic stannanes and boranes, in some cases, yielded a mixture of 2- and 3-alkenoic acids.3–6 CO2 allylation reactions starting directly from allylic halides in the presence of an appropriate metal is no doubt the most promising and straightforward approach. Early this year, we reported an indium-mediated allylation of carbon dioxide, which requires a 2.0 MPa pressure of CO2 at 60 °C [Scheme 1, eqn (1)]; in addition, while it is a reaction with a very high regioselectivity, it is difficult to run the reaction on a synthetically practical scale: the yield dropped to 41% when the reaction was conducted on 12 mmol scale [Scheme 1, eqn (2)].7 Thus, we wish to further develop a protocol using atmospheric pressure of CO2 with cheap metal, which may be easily run on a large scale for practical application. Here we report a room temperature zinc-mediated carboxylation reaction of allylic bromides under a CO2 balloon atmosphere with the help of LiOAc [Scheme 1, eqn (3)].Results and discussion
To address the issue of high pressure, we run the reaction with the CO2 provided by a balloon (1 atm) and screened all the commercially available metal powders at room temperature for the purpose of energy saving. Treatment of 2-phenylallyl bromide (1a) with indium in DMF led to a complicated mixture after 24 h (Table 1, entry 1). Screening of other metal powders including Al, Fe, Bi, Mn didn't give any better results (Table 1, entries 2–5). Interestingly, when Zn powder was used, the expected product 2a was formed in 73% yield (Table 1, entry 6). Replacing DMF with THF gave the same yield but THF is beneficial in the workup for the reason of its low boiling point (entry 8). Other solvents led to poor results (Table 1, entries 7, 9 and 10). Then many additives were screened and it is exciting to observe that some lithium salts may accelerate the reaction with a much higher yield: When 1.0 equivalent of LiOAc was added, the yield reached 84% and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C isomerization was not observed as judged by the NMR analysis of the crude product (Table 1, entry 14, see also entries 3 and 5 of Table 4; entry 3 of Table 5)!8
C isomerization was not observed as judged by the NMR analysis of the crude product (Table 1, entry 14, see also entries 3 and 5 of Table 4; entry 3 of Table 5)!8
| Entry | Metal powder (x equiv.) | Solvent | Additive | Yield of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|---|---|
| a The reaction was conducted with 1.0 mmol of allylic halide and metal powder in 5 mL of an anhydrous solvent with a CO2 balloon. b NMR yield. | |||||
| 1 | In (0.70) | DMF | — | Complicated | — |
| 2 | Al (0.70) | DMF | — | — | 76 |
| 3 | Fe (0.70) | DMF | — | — | 74 |
| 4 | Bi (0.70) | DMF | — | Complicated | — |
| 5 | Mn (1.05) | DMF | — | — | 68 |
| 6 | Zn (1.05) | DMF | — | 73 | — |
| 7 | Zn (1.05) | DMSO | — | 58 | — |
| 8 | Zn (1.05) | THF | — | 73 | — |
| 9 | Zn (1.05) | Dioxane | — | 25 | — |
| 10 | Zn (1.05) | Et2O | — | — | 87 |
| 11 | Zn (1.05) | THF | LiCl | 81 | 5 |
| 12 | Zn (1.05) | THF | LiBr | 74 | 6 |
| 13 | Zn (1.05) | THF | LiOCOCF3 | 34 | 1 |
| 14 | Zn (1.05) | THF | LiOAc | 84 | — |
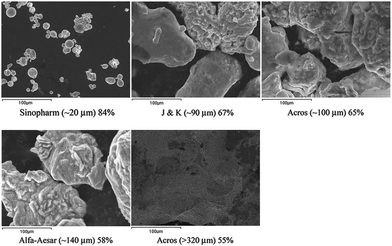
In order to check whether any impurity in the zinc powder may have an impact on the yield of the reaction, zinc powders from different manufacturers with different purities were tested. The results in Table 2 show that different zinc powders gave the product in different yields.
However, we did consider that the difference was not caused by the purity but by the particle size of the zinc powder. The EDX (energy dispersive X-ray spectroscopy) results for the different zinc powders are shown in Fig. 1. It is obvious that the smaller the zinc powder size, the higher the yield.
In order to further confirm the above conclusion, the purity of the zinc powder purchased from Sinopharm Chemical Reagent Co., Ltd was measured by XRF (X-ray fluorescence) after washing and drying sequentially with 3 M HCl, acetone and Et2O (each 3 times). The purity was measured to be 99.94% and the confirmed impurities including Fe (0.03%) and Cr (0.03%) were caused by background information from the XRF studies. To further eliminate the possible catalytic effect of these impurities, 1 mol% each of these salts was added to the reactions with zinc powder purchased from J&K (purity: 98%) (Table 3). We didn't observe any obvious improvement in the yield.
| Entry | Additive (1 mol%) | Yield of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|
| a The reaction was conducted with 0.01 mmol of additive, 1.0 mmol of 1a and 1.05 mmol of zinc powder in 5 mL of THF with a CO2 balloon. b NMR yield. | ||
| 1 | — | 68 |
| 2 | Fe powder | 61 |
| 3 | FeCl2 | 54 |
| 4 | FeCl3 | 53 |
| 5 | Fe2O3 | 70 |
| 6 | Cr powder | 65 |
| 7 | CrCl3 | 41 |
| 8 | Cr2O3 | 68 |
| 9 | CrCl3–FeCl3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 |
The scope of the reaction was then investigated under the optimized conditions with zinc powder from Sinopharm. Reactions with 2-aryl, 2-benzyl- and 2-alkyl-substituted allylic bromides 1a–g were attempted and the corresponding acids were afforded in decent yields (Table 4, entries 1–5). Interestingly, substrates containing an enyne fragment 1f and 1g underwent the reaction smoothly with no C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond isomerization observed although the yields were a little bit lower (Table 4, entries 6 and 7).
C bond isomerization observed although the yields were a little bit lower (Table 4, entries 6 and 7).
| Entry | R | Yieldb (%) |
|---|---|---|
| a The reaction was conducted with 1.0 mmol of allylic halide and 1.05 mmol of zinc powder in 5 mL of anhydrous THF at room temperature with a CO2 balloon. b Isolated yield. c The reaction was conducted in the absence of LiOAc. | ||
| 1 | Ph (1a) | 82 (2a) |
| 2 | α-naphthyl (1b) | 79 (2b) |
| 3 | p-tol (1c) | 80 (61)c (2c) |
| 4 | Bn (1d) | 80 (2d) |
| 5 | n C6H13 (1e) | 83 (68)c (2e) |
| 6 |
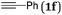
|
49 (2f) |
| 7 |
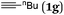
|
65 (2g) |
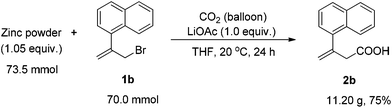
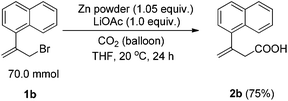
The reaction was then conducted on a 70 mmol scale successfully to afford 11.20 g of 2b, showing its practicality (Scheme 2).
To further investigate the generality of this reaction, the reaction of allylic bromides bearing halogen atoms at ortho-, meta- and para-positions of the 2-phenyl group was studied providing the corresponding carboxylic acids in good yields with an excellent chemoselectivity (Table 5, entries 1–5); a CF3- group did not affect the reaction (Table 5, entry 6); gratifyingly, ester and cyano functionalities, which are not compatible with organolithium or Grignard reagents, have been preserved under the standard reaction conditions (Table 5, entries 7–8); although a ketone carbonyl group is not directly applicable, the reaction proceeded successfully after a simple protection with glycol (Table 5, entry 9).
| Entry | R | Yieldb (%) |
|---|---|---|
| a The reaction was conducted with 1.0 mmol of allylic halide and 1.05 mmol of zinc powder in 5 mL of anhydrous THF at room temperature with a CO2 balloon. b Isolated yield. c The reaction was conducted in the absence of LiOAc. d The reaction was conducted in 5 mL of DMF. | ||
| 1 | o-F (1h) | 78 (2h) |
| 2 | m-F (1i) | 74 (2i) |
| 3 | p-F (1j) | 83 (45)c (2j) |
| 4 | m-Cl (1k) | 73 (2k) |
| 5 | p-Cl (1l) | 73 (2l) |
| 6 | p-CF3 (1m) | 71 (2m) |
| 7 | p-COOEt (1n) | 55 (2n) |
| 8 | m-CN (1o) | 55 (2o)d |
| 9 |
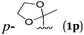
|
71 (2p) |
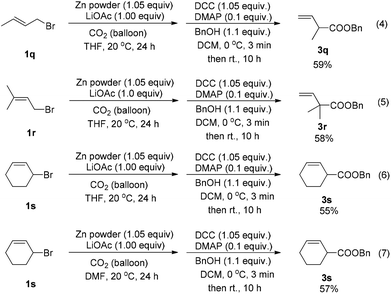
The reaction was attempted with several commercially available differently substituted allylic bromides followed by esterification with benzyl alcohol. For the 3-substituted allylic bromides such as crotyl bromide, the branched product 3q was formed exclusively with no regioisomer detected as judged by 1H NMR analysis of the crude reaction mixture [Scheme 3, eqn (4)]; an all-carbon quaternary center could even be formed in a moderate yield when starting from 3-methyl-2-butenyl bromide [Scheme 3, eqn (5)]; 2-cyclohexenyl bromide also worked [Scheme 3, eqn (6)]. Replacing THF with DMF as the solvent gave a similar yield [Scheme 3, eqn (7)].
Conclusions
In summary, a direct carboxylation reaction of allylic bromides mediated by zinc powder with 1 atm of carbon dioxide provided by a balloon has been developed. A wide range of functional groups such as halogen, CF3, ester, cyano, ketal are well tolerated and a specific regioselectivity for the formation of the branched products has been observed. The reaction could easily be conducted on 70 mmol scale, which indicates its practicality. Further studies on the reaction mechanism and the role of LiOAc are still on-going in our group.8Experimental section
Synthesis of 3-(1-naphthyl)-3-butenoic acid (2b)
To an oven-dried 1 L three-neck flask equipped with a magnetic stirring bar were added zinc powder (4.81 g, 73.5 mmol), LiOAc (4.62 g, 70.0 mmol), 1a (17.30 g, 70.0 mmol) and 350 mL of THF under an argon atmosphere. The mixture was then frozen with a liquid nitrogen bath and the argon inside was completely replaced by a balloon of CO2. The flask was then allowed to stand until the mixture thawed with the CO2 balloon and was stirred at 20 °C for 24 h. The mixture was poured into 500 mL of 3 M aq. HCl and the aqueous layer was extracted with ethyl acetate (150 mL × 5). The combined organic layer was washed with 500 mL of brine, dried over anhydrous Na2SO4, filtrated and concentrated. The crude product was purified by column chromatography on silica gel to afford 11.20 g of 2b7 (75%, eluent: petroleum ether–ethyl acetate = 5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → dichloromethane–methanol = 20
1 → dichloromethane–methanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow solid: 1H NMR (400 MHz, CDCl3) δ 9.03 (bs, 1 H, COOH), 8.04–7.97 (m, 1 H, ArH) 7.87–7.80 (m, 1 H, ArH), 7.80–7.73 (m, 1 H, ArH), 7.50–7.32 (m, 4 H, ArH), 5.61 (s, 1 H, one proton of CH2
1) as a yellow solid: 1H NMR (400 MHz, CDCl3) δ 9.03 (bs, 1 H, COOH), 8.04–7.97 (m, 1 H, ArH) 7.87–7.80 (m, 1 H, ArH), 7.80–7.73 (m, 1 H, ArH), 7.50–7.32 (m, 4 H, ArH), 5.61 (s, 1 H, one proton of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 5.34 (s, 1 H, one proton of CH2
), 5.34 (s, 1 H, one proton of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 3.55 (s, 2 H, CH2).
), 3.55 (s, 2 H, CH2).
Acknowledgements
Financial support from Shanghai Municipal Committee of Science and Technology (12JC1-403700) and National Basic Research Program of China (973 Program) (no. 2015CB856600) is greatly appreciated. We thank Jianxin Dai in this group for reproducing the results of entry 6 in Table 2, entry 5 in Table 3 and eqn(5) in Scheme 3.Notes and references
- For recent reviews on CO2 activation, see: (a) J. Louie, Curr. Org. Chem., 2005, 9, 605 CrossRef CAS; (b) T. Sakakura, J. C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365 CrossRef CAS PubMed; (c) M. Aresta and A. Dibenedetto, Dalton Trans., 2007, 36, 2975 RSC; (d) A. Correa and R. Martin, Angew. Chem., Int. Ed., 2009, 48, 6201 CrossRef CAS PubMed; (e) S. N. Riduan and Y. Zhang, Dalton Trans., 2010, 39, 3347 RSC; (f) C. Federsel, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 6254 CrossRef CAS PubMed; (g) K. Huang, C. Sun and Z. Shi, Chem. Soc. Rev., 2011, 40, 2435 RSC; (h) M. Cokoja, C. Bruckmeier, B. Rieger, W. A. Herrmann and F. E. Kuhn, Angew. Chem., Int. Ed., 2011, 50, 8510 CrossRef CAS PubMed.
- (a) R. T. Sanderson, J. Am. Chem. Soc., 1955, 77, 4531 CrossRef CAS; (b) L. E. Friedrich and R. Cormier, J. Org. Chem., 1971, 36, 3011 CrossRef CAS; (c) G. Courtois and L. Miginiac, J. Organomet. Chem., 1974, 69, 1 CrossRef CAS.
- M. Shi and K. M. Nicholas, J. Am. Chem. Soc., 1997, 119, 5057 CrossRef CAS.
- (a) R. Johansson and O. F. Wendt, Dalton Trans., 2007, 36, 488 RSC; (b) M. T. Johnson, R. Johansson, M. V. Kondrashov, G. Steyl, M. S. G. Ahl-quist, A. Roodt and O. F. Wendt, Organometallics, 2010, 29, 3521 CrossRef CAS.
- (a) J. Wu, J. C. Green, N. Hazari, D. P. Hruszkewycz, C. D. Incarvito and T. J. Schmeier, Organometallics, 2010, 29, 6369 CrossRef CAS PubMed; (b) D. P. Hruszkewycz, J. Wu, N. Hazari and C. D. Incarvito, J. Am. Chem. Soc., 2011, 133, 3280 CrossRef CAS PubMed; (c) J. Wu and N. Hazari, Chem. Commun., 2011, 47, 1069 RSC; (d) N. Hazari, D. P. Hruszkewycz and J. Wu, Synlett, 2011, 13, 1793 CrossRef; (e) D. P. Hruszkewycz, J. Wu, J. C. Green, N. Hazari and T. J. Schmeier, Organometallics, 2012, 31, 470 CrossRef CAS.
- H. A. Duong, P. B. Huleatt, Q. Tan and E. L. Shuying, Org. Lett., 2013, 15, 4034 CrossRef CAS PubMed.
- B. Miao and S. Ma, Chem. Commun., 2014, 50, 3285 RSC.
- For a brief discussion on the role of LiOAc, see ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4qo00300d |
| This journal is © the Partner Organisations 2015 |