Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
From lead–acid batteries to perovskite solar cells – efficient recycling of Pb-containing materials†
Jiajia
Suo
 *ab,
Bowen
Yang
*ab,
Bowen
Yang
 a,
Sonja
Prideaux
b,
Henrik
Pettersson
b and
Lars
Kloo
a,
Sonja
Prideaux
b,
Henrik
Pettersson
b and
Lars
Kloo
 c
c
aDepartment of Chemistry, Ångström Laboratory, Uppsala University, SE-75120 Uppsala, Sweden
bDyenamo AB, Greenhouse Labs, Teknikringen 38A, SE-114 28 Stockholm, Sweden. E-mail: jiajia.suo@dyenamo.se
cDepartment of Chemistry, Applied Physical Chemistry, KTH Royal Institute of Technology, SE-100 44 Stockholm, Sweden
First published on 16th January 2025
Abstract
The most efficient and stable perovskite solar cells typically contain lead compounds as a key component in the light-absorbing layer. To advance the commercialization of perovskite photovoltaics, it is crucial to address sustainability concerns regarding the use of toxic lead. In this work, we have developed a straightforward lead recycling pathway that converts lead compounds from lead–acid batteries into lead iodide. Purity analyses of the resulting lead iodide and the direct fabrication of perovskite solar cells demonstrate that the recycled lead iodide matches the quality of commercially available products. Most importantly, establishing this efficient lead recycling process not only supports sustainable recycling and resource utilization in a circular materials flow but also promotes the future development of perovskite photovoltaics.
Sustainability spotlightLead compounds are critical for fabricating efficient perovskite solar cells (PSCs), despite their toxicity. Rather than exploring new lead sources, it is crucial to develop efficient recycling pathways for lead from waste products to minimize waste generation as well as protect both the environment and human health. In this work, we have established a straightforward lead recycling method using widely available lead–acid batteries to produce lead iodide, a key component in the light-absorbing layer of PSCs. Our work aligns with the UN Sustainable Development Goal (SDG) 12: Responsible Consumption and Production by minimizing waste and improving resource efficiency. Moreover, by advancing the sustainable development of renewable energy technologies, this work contributes to the SDG 7: Affordable and Clean Energy. |
Introduction
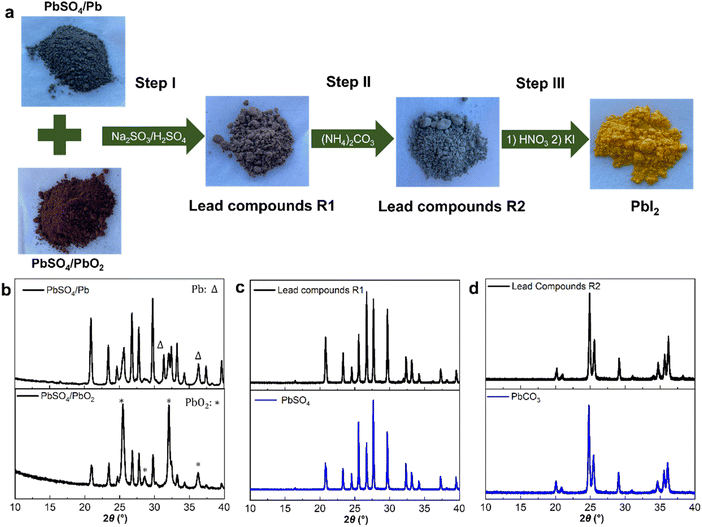
Perovskite solar cells (PSCs) have emerged as one of the most promising technologies of the next-generation photovoltaics (PVs) due to their excellent device performance, unique defect tolerance, straightforward fabrication process, low manufacturing costs, and other advantages.1–6 Over the past decade, advancements in materials and device engineering have significantly improved the efficiency and stability of PSCs. Recently, single-junction PSCs have shown power conversion efficiencies (PCEs) exceeding 26%.7–9 Additionally, the stability of PSCs has markedly improved, with devices passing different industry standard tests, such as the damp heat test (85% relative humidity, 85 °C) and the temperature cycling test (−40 to 85 °C).10–12 These accomplishments have brought the perovskite PV technology closer to commercialization.2,13,14In perovskite PVs, toxic lead compounds are key components of the light-absorbing materials, playing a crucial role in the generation of highly efficient PSCs. For instance, producing one gigawatt of solar PV capacity with perovskite modules at 20% efficiency would require approximately 3.5 tons of lead, based on a perovskite film thickness of 500 nm. Extrapolating to a broader context, if perovskite photovoltaics would comprise 20% of the anticipated 8500 gigawatt PV market by 2050, the total lead content in these modules could amount to about 6000 tons.15 To realize future commercialization, exploring new lead resource for perovskite PVs is highly demanded.16 In industry, extracting lead from ores such as galena, cerussite, and anglesite typically involves multiple steps including mining, crushing, roasting, smelting, and refining.17 These traditional extraction processes demand high energy input and generate substantial waste products. In addition, these industrial activities produce different pollutants, such as sulfur dioxide (SO2) and lead-containing dust, leading to significant environmental and health concerns. Therefore, the identification of alternative lead sources for PSCs is highly necessary. This also offers the possibility to make a conceptual change in the materials flow of lead-containing compounds, from a linear flow generating large amounts of waste products to a circular flow with minimal waste generation. Recycling lead compounds from used lead–acid batteries to manufacture PSCs offers a promising solution to this challenge. In a lead–acid battery, highly pure lead chemicals (lead and lead dioxide) as well as sulfuric acid (H2SO4) requiring a purity of at least 99.9%, are crucial for the performance, longevity, and safety of the batteries. Lead–acid batteries are widely used in a multitude of applications, such as automotive, uninterruptible power supplies, and backup power systems. To avoid environmental pollution and health hazards, over 95% of lead–acid batteries in the world are recycled to fabricate new batteries. In some regions, especially in Europe, the recycling yield is over 99%. However, the growth in lead–acid battery demand may be moderated with the development of competing and superior lithium-ion and other advanced battery technologies, as well as the widespread PV materials. Therefore, the current lead recycling process for manufacturing new lead–acid batteries might be disrupted in the future, necessitating the exploration of alternative reuse pathways.18–20 With the expected future commercialization of perovskite PVs, using recycled lead materials from the surplus of “end-of-life” lead–acid batteries to produce perovskite PVs offers an ideal solution. Furthermore, the establishment of this new approach will not only reduce the potential environmental impact of industrial activities by developing an alternative lead source but also promote the widespread application of perovskite PVs.
Although some recycling studies have been reported, most of previous studies involve high-temperature calcination (above 500 °C), which require a significant energy input.17–19 In addition, the use of hazardous chemicals, such as hydrogen peroxide, limits the industrial application of the recycling processes suggested.20 In this study, we have developed a simple and efficient recycling method suitable for future industrial use. The current process integrates lead compounds, including lead dioxide, lead, lead sulfate, and other mixed lead-based materials, collected from both the positive and negative electrodes of lead–acid batteries. These recycled lead compounds are utilized to synthesize lead iodide (PbI2), a crucial material for efficient and stable PSCs, as summarized in Fig. 1a. Different purity analysis techniques confirm that the composition of the recycled PbI2 is similar to that of commercially available PbI2. Importantly, perovskite devices fabricated based on the recycled PbI2 following the procedures outlined in this work show comparable performance to those made from commercial PbI2. This indicates that the recycled PbI2 meets the high purity standards required for PSCs and matches the quality of commercially available PbI2.
Results and discussions
Lead recycling process
Ideally, during the discharging process of lead–acid batteries, the lead dioxide (PbO2) from the positive electrode (cathode) and the lead (Pb) from the negative electrode (anode) both convert to lead sulfate (PbSO4). When the battery is recharged, the lead sulfate on the electrodes reverts back to lead and lead dioxide, respectively. Thus, Pb, PbO2, and PbSO4 are the primary lead compounds in a lead–acid battery, although different Pb-containing compounds will accumulate during ageing of the battery. To investigate the recycling methods for these three compounds, we discharged a lead–acid battery from 12 V to 5 V, as depicted in Fig. S1.† After disassembling the battery, we collected the lead-containing compounds from the negative and positive electrodes separately. X-ray diffraction (XRD) measurements were then used to confirm the main chemical composition of the lead-based compounds.As depicted in Fig. 1b and S2,† when comparing the XRD patterns of pure PbSO4 (peaks at 2θ 31.4° and 36.4°) suggest the presence of lead metal in combination with PbSO4 originating from the negative electrode. Meanwhile, 2θ peaks detected at 25.5°, 28.5°, 32.0° and 36.2° indicate a mixture of PbSO4 and PbO2 within the lead-containing compounds collected from the positive electrode. In order to streamline the recycling process, we mixed the lead-based compounds from both the positive and negative electrodes for subsequent treatment. Sodium sulfite (Na2SO3) was used as the reducing agent under acidic (H2SO4) conditions to facilitate the reduction of Pb(IV) in PbO2 to Pb(II) compounds. According to the following reaction (1), PbO2 reacts with Na2SO3 to yield lead oxide (PbO), which promptly undergoes a reaction with H2SO4 to form PbSO4. Simultaneously, lead reacts slowly with dilute H2SO4 to generate PbSO4, as shown in reaction (2). Thus, after this reaction, both PbO2 and Pb from the different electrodes have converted into PbSO4. The composition of the product from this step was confirmed by the XRD results, which showed the disappearance of peaks corresponding to Pb and PbO2, with only signals from PbSO4 remaining in Fig. 1c.
 | (1) |
 | (2) |
Due to the low reactivity of PbSO4, a desulfurization reaction is necessary. To achieve this, excess ammonium carbonate ((NH4)2CO3) was added in the second step to react with PbSO4, producing lead carbonate (PbCO3), as shown in reaction (3). XRD results (Fig. 1d) of the product displayed 2θ peaks corresponding to PbCO3, confirming its formation. In addition, energy dispersive spectrometry (EDS) measurements (Fig. S3†) showed the complete disappearance of sulfur from the carbonate product, indicating a successful conversion of PbSO4 to PbCO3.
 | (3) |
In the final step, as shown in reaction (4), the lead-based compounds were added to a dilute nitric acid solution, where PbCO3 reacted to form lead nitrate (Pb(NO3)2). Subsequently, potassium iodide (KI) was added, producing the desired end product lead iodide (PbI2) as a precipitate. To further enhance the chemical purity of PbI2, the product was re-crystallized. XRD measurements were then conducted to confirm the composition of the final product, as shown in Fig. 2a, which matches the reference pattern for PbI2.
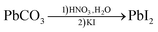 | (4) |
It is worth mentioning that we used the mixture of lead compounds originating from both electrodes in the first recovery reaction, which only allows an approximate estimate of the recovery yield. However, due to the limited solubility of these lead compounds in aqueous solution, as summarized in Table S1,† more than 99% of the lead species can be successfully recycled during this initial step, allowing nearly all of the lead compounds to be collected for the production of lead iodide. Moreover, to minimize lead contamination during the recycling process and reduce waste, we also recycled all reaction solutions from the previous steps, repeating the reactions under the same conditions. The same products were obtained, as confirmed by the XRD results shown in Fig. S4.† This approach also effectively mitigates the environmental impact of the other chemicals used.
In addition, we estimated the costs associated with the recycling process to approximately USD 1.3 per gram lead iodide. The details of our estimate can be found in Table S2.† The obtained cost is significantly lower than the commercial price of lead iodide, which exceeds USD 3 per gram lead iodide.
Purity assessment of recycled PbI2
The quality of PbI2 is critical for the performance of PSCs. Therefore, we systematically compared the quality of the recycled PbI2 with that of pure, commercially available PbI2. The XRD patterns of the pure PbI2 (Fig. S5a†) showed differences in peak width and peak ratios as compared to the recycled PbI2, indicating differences in crystallinity, crystal size and possibly preferential crystal orientation. These differences were further confirmed by scanning electron microscopy (SEM) experiments on the two PbI2 powders, as observed in Fig. S5b and c.† Furthermore, EDS was performed to analyze the chemical elements present and their relative abundances. EDS analyses (Fig. 2b and c) showed only lead and iodine to be present in both samples; with identical ratios, indicating the same material composition. To further investigate the elemental composition at the surface of the PbI2 films, we employed X-ray photoelectron spectroscopy (XPS). Results from XPS measurements in Fig. 2d and e showed identical peak positions for Pb (4f) and I (3d) in both samples, confirming that the elements in recycled PbI2 experience the same chemical environment as in the commercial PbI2. In other words, the recycled PbI2 exhibits similar purity as the pure, commercially obtainable PbI2.Perovskite film and solar cell fabrication
To assess the quality of recycled PbI2 for use in PSCs, we selected a formamidinium lead triiodide (FAPbI3) perovskite composition to fabricate perovskite films and subsequent solar cells. XRD results (Fig. 3a) show no presence of PbI2 or δ-FAPbI3 peaks in the films generated, indicating the formation of a pure α-FAPbI3 phase. Furthermore, comparable ratios of the (001) and (002) XRD peaks in the peaks from the two sources of PbI2 suggest that both perovskite films exhibited similar crystallization properties.21 SEM images in Fig. 3b and c show compact, pinhole-free polycrystalline perovskite films with similar grain sizes and surface morphologies, consistent with the XRD findings. UV-vis spectra (Fig. 3d) further demonstrate that both films display similar light absorption properties.Lastly, as shown in Fig. 3e, carbon-based PSCs (C-PSCs) with the architecture of fluorine-doped tin oxide coated glass (FTO glass)/tin oxide (SnO2)/FAPbI3/n-hexyl trimethyl ammonium bromide (HTMABr)/P3HT/carbon were fabricated to investigate the effect of recycled PbI2 on device performance. The JV-curve in Fig. 3f shows that the device fabricated with recycled PbI2 generates a champion power conversion efficiency (PCE) of 18.38%, demonstrating a performance comparable to C-PSCs made from commercially available PbI2. The parameters of the champion devices are detailed in Table 1. Moreover, statistical box charts for various photovoltaic parameters (photocurrent density (Jsc), open-circuit voltage (VOC), fill factor (FF), PCE) from 15 separate devices, as summarized in Fig. S6,† also reveal that PSCs fabricated with recycled PbI2 exhibit a performance comparable to those made from pure, purchased PbI2.
| J sc (mA cm−2) | V oc (V) | FF | PCE (%) | |
|---|---|---|---|---|
| C-PSCs (ref.) | 24.50 | 1.039 | 0.725 | 18.45 |
| C-PSCs (recycled) | 24.46 | 1.042 | 0.721 | 18.38 |
Conclusions
Recycling of lead to replace the exploration of new sources is essential for minimizing lead pollution and protecting both the environment and human health. In this study, we have established a straightforward recycling pathway to convert lead compounds from combined positive and negative electrodes of lead–acid batteries into PbI2, a key component for efficient and stable PSCs. This opens a new circular resource flow pathway for Pb-containing compounds. The recycled PbI2 was characterized in detail to demonstrate that it meets the required purity standards and comparable quality to commercially available PbI2. Implementing this recycling strategy will mitigate the environmental impact of toxic lead and promote the future commercialization of perovskite PVs.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Jiajia Suo: methodology, investigation, writing original draft. Bowen Yang: investigation. Sonja Prideaux: investigation. Henrik Petterson: conceptualization. Lars Kloo: conceptualization, methodology, supervision. All authors read and approved the final manuscript.Conflicts of interest
Henrik Petterson and Lars Kloo are co-founders of Dyenamo AB.Acknowledgements
J. Suo, S. Prideaux, H. Petterson and L. Kloo acknowledge the Swedish Energy Agency with project no. P2023-00064. J. Suo, B. Yang and H. Petterson acknowledge the Vinnova with project no. 2023-01428.References
- A. Agresti, F. Di Giacomo, S. Pescetelli and A. Di Carlo, Nano Energy, 2024, 122, 109317 CrossRef.
- L. Zhang, Y. Wang, X. Meng, J. Zhang, P. Wu, M. Wang, F. Cao, C. Chen, Z. Wang, F. Yang, X. Li, Y. Zou, X. Jin, Y. Jiang, H. Li, Y. Liu, T. Bu, B. Yan, Y. Li, J. Fang, L. Xiao, J. Yang, F. Huang, S. Liu, J. Yao, L. Liao, L. Li, F. Zhang, Y. Zhan, Y. Chen, Y. Mai and L. Ding, Mater. Futures, 2024, 3, 022101 CrossRef.
- B. Yang, J. Suo, E. Mosconi, D. Ricciarelli, W. Tress, F. D. Angelis, H.-S. Kim and A. Hagfeldt, ACS Energy Lett., 2020, 5, 3159–3167s CrossRef.
- B. Yang, J. Suo, D. Bogachuk, W. Kaiser, C. Baretzky, O. Er-Raji, G. Loukeris, A. A. Alothman, E. Mosconi, M. Kohlstädt, U. Würfel, F. De Angelis and A. Hagfeldt, Energy Environ. Sci., 2024, 17, 1549–1558 RSC.
- J. Suo, B. Yang, E. Mosconi, H.-S. Choi, Y. Kim, S. M. Zakeeruddin, F. De Angelis, M. Grätzel, H.-S. Kim and A. Hagfeldt, Adv. Funct. Mater., 2021, 31, 2102902 CrossRef CAS.
- B. Ding, Y. Ding, J. Peng, J. Romano-deGea, L. E. K. Frederiksen, H. Kanda, O. A. Syzgantseva, M. A. Syzgantseva, J.-N. Audinot, J. Bour, S. Zhang, T. Wirtz, Z. Fei, P. Dörflinger, N. Shibayama, Y. Niu, S. Hu, S. Zhang, F. F. Tirani, Y. Liu, G.-J. Yang, K. Brooks, L. Hu, S. Kinge, V. Dyakonov, X. Zhang, S. Dai, P. J. Dyson and M. K. Nazeeruddin, Nature, 2024, 628, 299–305 CrossRef CAS PubMed.
- J. Zhou, L. Tan, Y. Liu, H. Li, X. Liu, M. Li, S. Wang, Y. Zhang, C. Jiang, R. Hua, W. Tress, S. Meloni and C. Yi, Joule, 2024, 8, 1691–1706 CrossRef CAS.
- H. Chen, C. Liu, J. Xu, A. Maxwell, W. Zhou, Y. Yang, Q. Zhou, A. S. R. Bati, H. Wan, Z. Wang, L. Zeng, J. Wang, P. Serles, Y. Liu, S. Teale, Y. Liu, M. I. Saidaminov, M. Li, N. Rolston, S. Hoogland, T. Filleter, M. G. Kanatzidis, B. Chen, Z. Ning and E. H. Sargent, Science, 2024, 384, 189–193 CrossRef CAS.
- S. Liu, J. Li, W. Xiao, R. Chen, Z. Sun, Y. Zhang, X. Lei, S. Hu, M. Kober-Czerny, J. Wang, F. Ren, Q. Zhou, H. Raza, Y. Gao, Y. Ji, S. Li, H. Li, L. Qiu, W. Huang, Y. Zhao, B. Xu, Z. Liu, H. J. Snaith, N.-G. Park and W. Chen, Nature, 2024, 632, 536–542 CrossRef PubMed.
- J. Suo, B. Yang, E. Mosconi, D. Bogachuk, T. A. S. Doherty, K. Frohna, D. J. Kubicki, F. Fu, Y. Kim, O. Er-Raji, T. Zhang, L. Baldinelli, L. Wagner, A. N. Tiwari, F. Gao, A. Hinsch, S. D. Stranks, F. De Angelis and A. Hagfeldt, Nat. Energy, 2024, 9, 172–183 CrossRef CAS PubMed.
- R. Azmi, E. Ugur, A. Seitkhan, F. Aljamaan, A. S. Subbiah, J. Liu, G. T. Harrison, M. I. Nugraha, M. K. Eswaran, M. Babics, Y. Chen, F. Xu, T. G. Allen, A. ur Rehman, C.-L. Wang, T. D. Anthopoulos, U. Schwingenschlögl, M. De Bastiani, E. Aydin and S. De Wolf, Science, 2022, 378, 73–77 CrossRef PubMed.
- Z. Shen, Q. Han, X. Luo, Y. Shen, Y. Wang, Y. Yuan, Y. Zhang, Y. Yang and L. Han, Nat. Photonics, 2024, 18, 450–457 CrossRef CAS.
- P. Zhu, C. Chen, J. Dai, Y. Zhang, R. Mao, S. Chen, J. Huang and J. Zhu, Adv. Mater., 2024, 36, 2307357 CrossRef CAS.
- T. D. Siegler, A. Dawson, P. Lobaccaro, D. Ung, M. E. Beck, G. Nilsen and L. L. Tinker, ACS Energy Lett., 2022, 7, 1728–1734 CrossRef CAS.
- B. Chen, C. Fei, S. Chen, H. Gu, X. Xiao and J. Huang, Nat. Commun., 2021, 12, 5859 CrossRef CAS.
- L. Wagner, J. Suo, B. Yang, D. Bogachuk, E. Gervais, R. Pietzcker, A. Gassmann and J. C. Goldschmidt, Joule, 2024, 8, 1142–1160 CrossRef CAS.
- P.-Y. Chen, J. Qi, M. T. Klug, X. Dang, P. T. Hammond and A. M. Belcher, Energy Environ. Sci., 2014, 7, 3659–3665 RSC.
- C. Li, Z. Zhu, Y. Wang, Q. Guo, C. Wang, P. Zhong, Z. Tan and R. Yang, Nano Energy, 2020, 69, 104380 CrossRef CAS.
- J. Li, C. Duan, L. Yuan, Z. Liu, H. Zhu, J. Ren and K. Yan, Environ. Sci. Technol., 2021, 55, 8309–8317 CrossRef CAS PubMed.
- L. Xie, Q. Zeng, Q. Li, S. Wang, L. Li, Z. Li, F. Liu, X. Hao and F. Hao, J. Phys. Chem. Lett., 2021, 12, 9595–9601 CrossRef CAS PubMed.
- J. Suo, B. Yang, J. Jeong, T. Zhang, S. Olthof, F. Gao, M. Grätzel, G. Boschloo and A. Hagfeldt, Nano Energy, 2022, 94, 106924 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00470a |
| This journal is © The Royal Society of Chemistry 2025 |