Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The total synthesis of K-252c (staurosporinone) via a sequential C–H functionalisation strategy†
J. C.
Fox‡
,
R. E.
Gilligan‡
,
A. K.
Pitts
,
H. R.
Bennett
and
M. J.
Gaunt
*
Department of Chemistry, Department of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK. E-mail: mjg32@cam.ac.uk
First published on 21st January 2016
Abstract
A synthesis of the bioactive indolocarbazole alkaloid K-252c (staurosporinone) via a sequential C–H functionalisation strategy is reported. The route exploits direct functionalisation reactions around a simple arene core and comprises of two highly-selective copper-catalysed C–H arylations, a copper-catalysed C–H amination and a palladium-catalysed C–H carbonylation, which build up the structural complexity of the natural product framework.
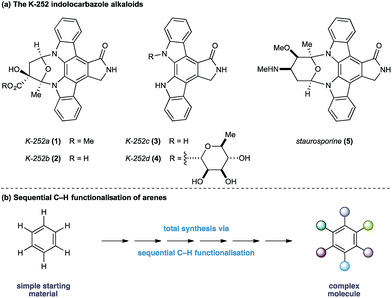
The indolocarbazole alkaloids K-252a–d (1–4) are microbial metabolites first isolated in 1986 from culture broths of Nocardiopsis sp. K-252 and Nocardiopsis sp. K-290 (Fig. 1a).1 The compounds were found to be potent inhibitors of protein kinase C, an enzyme family known to play a critical role in a myriad of signal transduction pathways associated with metabolism, gene expression, membrane transport and cell proliferation.2 Consequently, the protein kinase C family of proteins has become an important therapeutic target for several disease classes, particularly in oncology, resulting in sustained interest in the design and development of inhibitors as potential pharmaceutical agents.3
In light of their intriguing biological properties and potential as pharmaceutical agents, the indolocarbazole alkaloids have attracted considerable interest from the synthetic community. In particular K-252c, (3, staurosporinone),4 the potential biosynthetic precursor and aglycone of staurosporine 5,5 has attracted a number of elegant syntheses. We were attracted to these natural products because of their interesting biological activity and the unusual functional pattern of the hexasubstituted arene framework. Our group has a long standing interest in the synthesis of natural products that rely on strategies based on the sequential functionalisation of the C–H bonds in simple aromatic building blocks (Fig. 1b).6–8 We envisaged that K-252c, 3, could provide a platform to further extend this total synthesis strategy by orchestrating a series of direct functionalisations on a readily available aniline to form the fully substituted benzene core of the target molecule. Furthermore, such a modular strategy would be amenable to the preparation of analogues of this important scaffold. Herein we report a concise synthesis of the indolocarbazole alkaloid K-252c starting from a commercial toluidine starting material. Seven direct functionalisations are used to transform C–H bonds of the aniline into the substituents required for the hexasubstituted arene core of the natural product architecture. Despite the increasingly complex architecture that results from each step, a notable feature of this synthesis is the exquisite selectivity of each C–H transformation, thereby highlighting the efficacy of sequential C–H functionalisation strategies.9
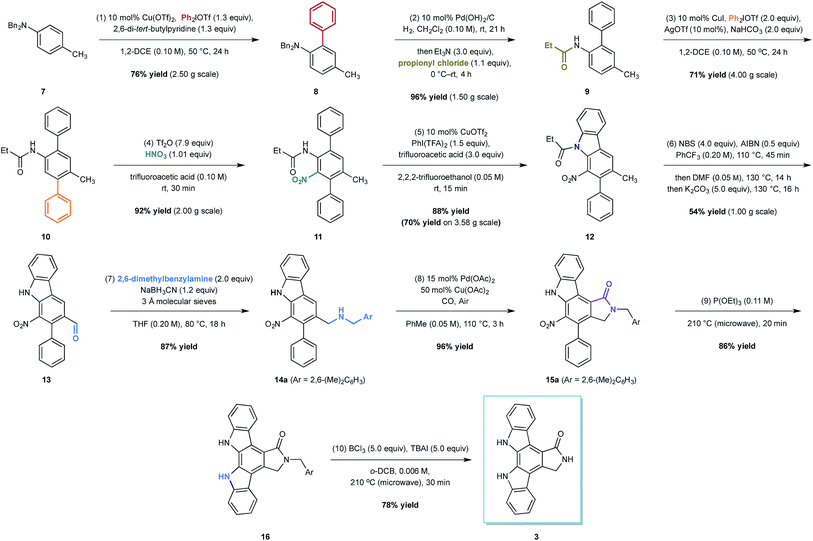
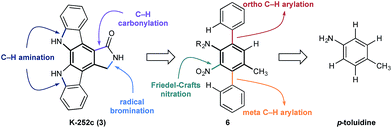
At the outset of our design stage, we questioned whether p-toluidine, a simple commercial aniline, could be used to launch a sequential C–H functionalisation strategy for the synthesis of K-252c, 3, (Scheme 1). Many of the previous syntheses use a common strategy to assemble the indolocarbazole framework that is based on the union of two indole molecules with a synthetic precursor to the lactam ring system, followed by an oxidative electrocyclisation to form the poly(hetero)aromatic framework.4,5 Most elegant among these syntheses is the work of Wood and co workers who developed a concise approach to this class of natural products.5c Despite the simplicity of this type of strategic disconnection, it remains difficult to engineer an unsymmetrical indolocarbazole framework from such a convergent route. As part of our synthetic strategy towards staurosporinone, we envisaged that a sequential C–H functionalisation approach might benefit from late-stage carbazole formations in order to modulate the electron density of the central arene core (Fig. 2). The lactam would originate from a C–H carbonylation directed by the benzylamine motif, which itself could be introduced through a radical-mediated benzylic oxidation. These disconnections would reveal a teraryl framework 6, displaying an amine motif that would control the selective installation of the adjacent nitro group and a series of ortho- and meta-C–H arylations, leading back to p-toluidine. Taken together, the amine motif of the aniline directly controls four out of seven direct functionalisations on the aromatic framework of this complex molecule.
The total synthesis of K-252c commenced from the readily available dibenzyl p-toluidine 7, available in multigram quantities (Scheme 1). The first C–H functionalisation of the arene core involved the union of the commercial diphenyliodium triflate with 7 through the action of a simple copper(II) catalyst, a biaryl coupling strategy recently developed by our group.10 The ortho-selective arylation proceeded in good yield and on a multigram scale to form 8; we did not detect any of the corresponding di-ortho-phenylation product, which is often a problem with comparative metal-catalysed arylations. We believe that the selectivity of this process is due to the low reactivity of the product to subsequent arylations. A clash between the ortho-phenyl substituent and the N,N-dibenzyl group forces the amino group to rotate to alleviate the steric interaction; the lone pair on the nitrogen rotates out of conjugation with the arene, reducing the arene nucleophilicity and making it less reactive towards a second arylation.
To install the second aryl group, we envisaged the use of a copper-catalysed meta-arylation that has also been recently developed in our laboratory. To achieve this, however, the N-benzyl substituents needed to be switched to a carbonyl-containing group, which is important to impart the desired meta-selective arylation.11 The nature of the carbonyl group needed to carefully considered with respect to both the effectiveness in the meta-arylation, its stability to subsequent reaction conditions and its compatibility with the oxidative C–H amination that would be required to form the carbazole at a later point in the synthesis. We had previously shown that meta-arylation was best effected by a pivalamide group, but there are no examples of C–H aminations using amines derived with this bulky group. In contrast, meta-arylation directed by simple acetamides was often low yielding due to competitive amide cleavage, although there are a number of C–H aminations that can be initiated by this simple amide motif. In balancing these factors we elected to investigate the propionyl group in the hope that the extra size of the ethyl substituent would render the amide less liable to cleavage, but would still prove effective in the carbazole formation step. Therefore, a one-pot hydrogenolysis and subsequent carbamoylation with propionyl chloride enabled the formation of anilide 9 in excellent 96% yield on a multi-gram scale. Pleasingly, a slight modification to our copper-catalysed meta-arylation worked well and treatment of anilide 9 with diphenyliodonium triflate in the presence of copper(I) iodide, silver(I) triflate (providing an in situ source of copper(I) triflate) and solid sodium hydrogen carbonate (to buffer the reaction in light of the formation of triflic acid as a by-product) produced the teraryl intermediate 10 in 71% yield on a 4 g scale (the mass balance was unreacted starting material).
With teraryl 10 in hand, efforts next focused on the addition of the second nitrogen substituent. It was envisaged that installation of a nitro group, which could be subsequently employed in a late-stage reductive cyclisation to a carbazole, would be the optimal strategy. Treatment of 10 with concentrated nitric acid in a cooled solution of trifluoroacetic anhydride and trifluoroacetic acid yielded the desired product in 60% yield, although attempts to scale-up the reaction were unsuccessful, resulting in lower yields. Interestingly, we found that replacing the trifluoroacetic anhydride with trifluoromethanesulfonic anhydride, a modification of an underutilised aromatic nitration protocol developed by Hill and co-workers,12 led to significant improvements in both the yield and selectivity. Furthermore, the modified conditions were scalable, enabling an exquisitely selective and gram scale reaction to produce the nitro-arene 11 in 92% yield.
With both nitrogenous groups installed on the central arene framework, attention was turned to the C–H amination to form one of the two-carbazole units. Several metal catalysed C–H aminations to carbazoles have been developed in recent years,13 but unfortunately, we found that palladium-catalysed methods for carbazole formation were either capricious or resulted in none of the desired product when applied to our system.14 Pleasingly, the copper-catalysed oxidative C–H amination protocol developed by Chang and co-workers employing phenyliodide bis-trifluoroacetate and copper(II) triflate effected the carbazole formation (to 12) in good yield (70% on 3.58 g scale, 88% on 100 mg scale).15
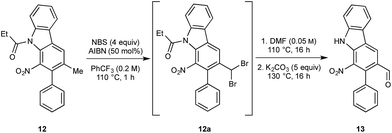
We next turned to the installation of the γ-lactam motif that we speculated could arise from an advanced benzaldehyde. Despite the availability of a number of methods to affect to the oxidation of the benzylic methyl group to a benzaldehyde, all attempts to directly access 13 proved unsuccessful. This is possibly due to the deactivating effect of the resident nitro group. To overcome these difficulties, a selective radical bis-bromination of the benzylic position (to 12a, Scheme 2),16a followed by treatment with DMF and K2CO3 afforded the desired aldehyde functionality with concomitant cleavage of the carbazole N-protecting group.16b This sequence could be combined into a one-pot procedure to supply the desired aldehyde in 54% yield on a gram scale.
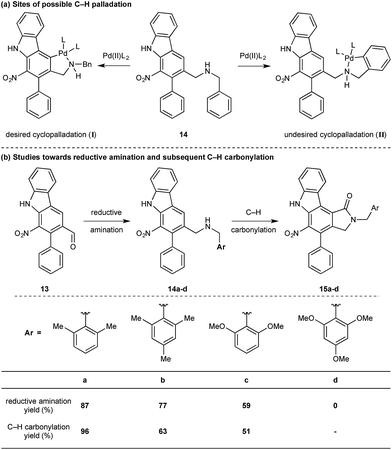
While we reasoned that reductive amination of aldehyde 13 with a suitable amine would lead to a precursor for the proposed C–H carbonylation to form the γ-lactam, we were conscious that primary amines coordinate strongly to metals such as palladium and often inhibit catalytic activity. Therefore, we elected to carry out the reductive amination with a benzylic amine with the intention of adopting Orito's C–H carbonylation to the desired protected γ-lactam.17 However, the choice of amine required further consideration as the use of benzyl amine would lead to selectivity problems in the carbopalladation step of the C–H carbonylation (selectivity between I and II, Scheme 3a). Mindful of this, we prepared a number of benzylamine variants displaying ortho-substituents to block the competitive C–H activation (Scheme 3b). We were pleased to find that 2,6-dimethylbenzylamine performed well in both the reductive amination and the palladium-catalysed C–H carbonylation to form γ-lactam 15a.
With all but one ring now installed to the aromatic framework of staurosporinone, we turned our attention to the final C–H amination. After extensive experimentation we found that ring closure based on reductive metal-catalysed C–H aminations from the nitro group or oxidative C–H aminations from amine derivatives failed to produce any of the desired carbazole.13–15 However, we were delighted to find that a classical reductive Cadogan cyclisation from 15a,18 utilised by Raphael et al. in their pioneering work on staurospoinone,4c produced the target carbazole 16 in modest yield. While these initial reactions, using P(OEt)3 to generate the requisite nitrene species, were low yielding (36% yield), we found that through careful monitoring of the reaction time and temperature (20 min at 210 °C), 16 could be isolated in 85% yield without the need for column chromatography. The final deprotection to afford K-252c 3 proved to be challenging, but after a survey of a range of standard N-debenzylation techniques we found that treatment of lactam 16 with TBAI and BCl3, conditions developed by Coe and co-workers for the deprotection of primary alkyl aryl ethers,19 afforded K-252c 3 in 78% yield, the spectral data for which were consistent with those reported in the literature.
Conclusions
In summary, we have successfully applied a sequential C–H bond functionalisation strategy to the synthesis of K-252c. The overall yield if the synthesis from a readily available aniline (7) is 12.7%. The sequence of direct functionalisations follows a logical order of reactivity that sees the amine group of the starting toluidine control four of the C–H functionalisations. The synthesis showcases transformations comprising of two selective copper-catalysed C–H arylations, a highly selective electrophilic nitration, two C–H amination protocols to form carbazoles, a benzylic methyl oxidation and a palladium-catalysed C–H carbonylation. The synthesis is amenable to scale-up, providing access to intermediates on gram or multi-gram scale. Furthermore, it is envisaged that this modular approach could be used to provide rapid access to analogues of this biological important class of molecule, as well as complex hexasubstituted benzenes.20 Current research in our laboratory is focused on applying this sequential C–H functionalisation strategy to the synthesis of staurosporinone analogues and other complex natural products that could benefit from such a strategy.Acknowledgements
We are grateful to EPSRC and ERC for funding. Mass spectrometry data were acquired at the EPSRC UK National Mass Spectrometry Facility at Swansea University.References
- (a) H. Kase, K. Iwahashi and Y. Matsuda, J. Antibiot., 1986, 34, 1059 CrossRef; (b) S. Nakanishi, Y. Matsuda, K. Iwahashi and H. Kase, J. Antibiot., 1986, 34, 1066 CrossRef; (c) T. Yasuzuwa, T. Iida, M. Yoshida, N. Hirayama, M. Takahashi, K. Shirahata and H. Sano, J. Antibiot., 1986, 34, 1072 CrossRef.
- (a) M. Castagna, Y. Takai, K. Kaibuchi, K. Sano, U. Kikkawa and Y. Nishizuka, J. Biol. Chem., 1982, 257, 7847 CAS; (b) Y. Nishikuza, Nature, 1984, 308, 693 CrossRef; (c) S. Stable and P. J. Parker, Pharmacol. Ther., 1991, 51, 71 CrossRef; (d) A. C. Newton, J. Biol. Chem., 1995, 270, 28405 CrossRef.
- (a) D. Mochly-Rosen, K. Das and K. V. Grimes, Nat. Rev. Drug Discov., 2012, 11, 937 CrossRef CAS PubMed; (b) C. Douville, H. Carter, R. Kim, N. Niknafs, M. Diekhans, P. D. Stenson, D. N. Cooper, M. Ryan and R. Karchin, Bioinformatics, 2013, 29, 647 CrossRef CAS PubMed.
- For previous syntheses of staurosporinone, see: (a) B. Sarstedt and E. Winterfeldt, Heterocycles, 1983, 20, 469 CrossRef CAS; (b) C. J. Moody and K. F. Rahimtoola, J. Chem. Soc., Chem. Commun., 1990, 1667 RSC; (c) I. Hughes, W. P. Nolan and R. A. Raphael, J. Chem. Soc., Perkin Trans. 1, 1990, 2475 RSC; (d) C. J. Moody and K. F. Rahimtoola, J. Org. Chem., 1992, 57, 2105 CrossRef CAS; (e) W. Harris, C. H. Hill, E. Keech and P. Malsher, Tetrahedron Lett., 1993, 34, 8361 CrossRef CAS; (f) G. Xie and W. Lown, Tetrahedron Lett., 1994, 35, 5555 CrossRef CAS; (g) C. Moody, Synlett, 1994, 681 CrossRef CAS; (h) M. P. Faul, K. A. Sullivan and L. L. Winneroski, Synthesis, 1995, 1511 CrossRef CAS; (i) J. L. Wood, B. M. Stoltz and H.-J. Dietrich, J. Am. Chem. Soc., 1995, 117, 10413 CrossRef CAS; (j) E. M. Beccalli, M. L. Gelmi and A. Marchesini, Tetrahedron, 1998, 54, 6909 CrossRef CAS; (k) S. Eils and E. Winterfeldt, Synthesis, 1999, 2, 275 CrossRef; (l) E. Mahboobi, M. Eibler, S. Koller, S. K. C. Kumar and A. Popp, J. Org. Chem., 1999, 64, 4697 CrossRef PubMed; (m) S. P. Gaudêncio, M. M. M. Santos, A. M. Lobo and S. Prabhakar, Tetrahedron Lett., 2003, 44, 2577 CrossRef; (n) A.-R. Howard-Jones and C. T. Walsh, J. Am. Chem. Soc., 2006, 128, 12289 CrossRef CAS PubMed; (o) Y. Wada, H. Nagasaki, M. Tokuda and K. Orito, J. Org. Chem., 2007, 72, 2008 CrossRef CAS PubMed; (p) G. G. Rajeshwaran and A. K. Mohanakrishnan, Org. Lett., 2011, 13, 1418 CrossRef CAS PubMed; (q) P. Raju, G. G. Rajeshwaran and A. K. Mohanakrishnan, Eur. J. Org. Chem., 2015, 1731 Search PubMed.
- For previous syntheses of staurosporine, see: (a) J. T. Link, S. Raghavan and S. J. Danishefsky, J. Am. Chem. Soc., 1995, 117, 552 CrossRef CAS; (b) J. L. Wood, B. M. Stoltz and S. N. Goodman, J. Am. Chem. Soc., 1996, 118, 10656 CrossRef CAS; (c) J. L. Wood, B. M. Stoltz, S. N. Goodman and K. Onwueme, J. Am. Chem. Soc., 1997, 119, 9652 CrossRef CAS; (d) K. Tamaki, J. B. Shotwell, R. D. White, I. Drutu, D. T. Petsch, T. V. Nheu, H. He, Y. Hirokawa, H. Maruta and J. L. Wood, Org. Lett., 2001, 3, 1689 CrossRef CAS PubMed.
- (a) R. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer and O. Baudoin, Chem. Eur. J., 2010, 16, 2654 CrossRef CAS PubMed; (b) L. Yang and H. Huang, Chem. Rev., 2015, 115, 3468 CrossRef CAS PubMed.
- Previous iterative syntheses within the Gaunt group: (a) E. Beck, R. Hatley and M. J. Gaunt, Angew. Chem., Int. Ed., 2008, 47, 3004 CrossRef CAS PubMed; (b) L. McMurray, E. Beck and M. J. Gaunt, Angew. Chem., Int. Ed., 2012, 51, 9288 CrossRef CAS PubMed; (c) A. Pitts, F. O'Hara, R. Snell and M. J. Gaunt, Angew. Chem., Int. Ed., 2015, 54, 5451 CrossRef CAS PubMed.
- Selected natural product syntheses utilising iterative C–H functionalisation: (a) W. R. Gutekunst and P. S. Baran, J. Am. Chem. Soc., 2011, 133, 19076 CrossRef CAS PubMed; (b) D. Mandal, A. D. Yamaguchi, J. Yamaguchi and K. J. Itami, J. Am. Chem. Soc., 2011, 133, 19660 CrossRef CAS PubMed; (c) H. Wang, D. Li, K. M. Engle, J.-Q. Yu and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 6774 CrossRef CAS PubMed; (d) B. R. Rosen, L. R. Simke, P. S. Thuy-Boun, D. D. Dixon, J.-Q. Yu and P. S. Baran, Angew. Chem., Int. Ed., 2013, 52, 7317 CrossRef CAS PubMed; (e) A. D. Yamaguchi, K. M. Chipiga, J. Yamaguchi, K. Itami and H. M. L. Davies, J. Am. Chem. Soc., 2015, 137, 644 CrossRef CAS PubMed.
- Selected reviews of C–H functionalisation: (a) K. Godula and D. Sames, Science, 2006, 312, 3468 CrossRef PubMed; (b) C. Jia, T. Kitamura and Y. Fujiwara, Acc. Chem. Res., 2008, 34, 633 CrossRef; (c) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (d) L. McMurray, F. O'Hara and M. J. Gaunt, Chem. Soc. Rev., 2011, 40, 1185 RSC; (e) J. Wencel-Delord, T. Dröge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740 RSC; (f) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960 CrossRef CAS PubMed.
- C.-L. Ciana, R. Phipps, J. Brandt, F.-M. Meyer and M. J. Gaunt, Angew. Chem., Int. Ed., 2011, 50, 458 CrossRef CAS PubMed.
- R. Phipps and M. J. Gaunt, Science, 2009, 323, 1593 CrossRef CAS PubMed.
- C. Coon, W. Blucher and M. Hill, J. Org. Chem., 1973, 38, 4243 CrossRef CAS.
- A. W. Schmidt, K. R. Reddy and H.-J. Knölker, Chem. Rev., 2012, 112, 3193 CrossRef CAS PubMed.
- (a) W. C. P. Tsang, N. Aheng and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 14560 CrossRef CAS PubMed; (b) J. A. Jordan-Hore, C. C. C. Johansson, M. Gulias, E. M. Beck and M. J. Gaunt, J. Am. Chem. Soc., 2008, 130, 16186 CrossRef PubMed; (c) J. J. Neumann, S. Rakshit, T. Dröge and F. Glorius, Angew. Chem., Int. Ed., 2009, 48, 6892 CrossRef CAS PubMed; (d) A. P. Antonchick, R. Samanta, K. Kulikov and J. Lategahn, Angew. Chem., Int. Ed., 2011, 50, 8605 CrossRef CAS PubMed.
- S. Cho, J. Yoon and S. Chang, J. Am. Chem. Soc., 2011, 133, 5996 CrossRef CAS PubMed.
- (a) S. Mataka, G.-B. Liu, T. Sawada, M. Kurisu and M. Tashiro, Bull. Chem. Soc. Jpn., 1994, 67, 1113 CrossRef CAS; (b) W. Li, X.-L. Hou, Y.-X. Li and Y.-D. Wu, Tetrahedron Lett., 2004, 133, 7668 Search PubMed.
- K. Orito, A. Horibata, T. Nakamura, H. Ushito, H. Nagasaki, M. Yuguchi, S. Yamashita and M. Tokuda, J. Am. Chem. Soc., 2004, 126, 14342 CrossRef CAS PubMed.
- (a) J. Cadogan, M. Cameron-Wood, R. Mackie and R. Searle, J. Chem. Soc., 1965, 4831 RSC; (b) J. Cadogan, Synthesis, 1969, 11 CrossRef CAS.
- P. R. Brooks, M. C. Wirtz, M. G. Vetelino, D. M. Rescek, G. F. Woodworth, B. P. Morgan and J. W. Coe, J. Org. Chem., 1999, 64, 9719 CrossRef CAS.
- S. Suzuki, Y. Segawa, K. Itami and J. Yamaguchi, Nat. Chem., 2015, 7, 227 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra, and crystallographic data. CCDC 1431476. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc04399a |
| ‡ JCF and REG contributed equally to the work in this manuscript. |
| This journal is © The Royal Society of Chemistry 2016 |