Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Conductive RuO2 binders enhance mechanical stability of macroporous Nb–SnO2 particles as cathode catalyst supports for high-performance PEFCs†
Thi Thanh Nguyen
Ho
a,
Tomoyuki
Hirano
 *a,
Aoi
Takano
b,
Syu
Miyasaka
b,
Eishi
Tanabe
c,
Makoto
Maeda
d,
Eka Lutfi
Septiani
*a,
Aoi
Takano
b,
Syu
Miyasaka
b,
Eishi
Tanabe
c,
Makoto
Maeda
d,
Eka Lutfi
Septiani
 a,
Kiet Le Anh
Cao
a,
Kiet Le Anh
Cao
 a and
Takashi
Ogi
a and
Takashi
Ogi
 *a
*a
aChemical Engineering Program, Graduate School of Advanced Science and Engineering, Hiroshima University, 1-4-1 Kagamiyama, Higashihiroshima, Hiroshima 739-8527, Japan. E-mail: tomoyuki-hirano@hiroshima-u.ac.jp; ogit@hiroshima-u.ac.jp; Tel: +81 82 424 7850 Tel: +81 82 424 3765
bCataler Corporation, 7800 Chihama, Kakegawa, Shizuoka 437-1492, Japan
cHiroshima Prefectural Institute of Industrial Science and Technology, 3-10-31 Kagamiyama, Higashi Hiroshima, Hiroshima 739-0046, Japan
dNatural Science Center for Basic Research and Development, Hiroshima University, 1-4-1 Kagamiyama, Higashihiroshima, Hiroshima 739-8527, Japan
First published on 24th March 2025
Abstract
Niobium-doped tin oxide (NTO) particles with a macroporous structure have been developed as catalyst supports for enhancing the durability and performance of polymer electrolyte fuel cells (PEFCs). This macroporous architecture improves the mass transport properties of the electrode. However, their weak mechanical strength can cause structural collapse, thereby limiting single-cell performance at high current densities. In this study, we employed ruthenium oxide (RuO2) as a binder to integrate with macroporous NTO particles (denoted as NTO/RuO2). This approach simultaneously enhanced the electrical conductivity and mechanical strength of the catalyst supports, improving the performance of PEFCs. Incorporating RuO2 binders effectively stabilized the macroporous structure, and the NTO/RuO2 particles with 50 wt% RuO2 loading maintained their structural integrity under high compression pressures of up to 40 MPa. The aggregated NTO/RuO2 particles containing 50 wt% RuO2 binder also exhibited higher conductivity than the NTO aggregates without RuO2 binder, which was attributed to the conductive network formed by RuO2. Importantly, the membrane electrode assembly (MEA) fabricated with macroporous NTO/RuO2 particles containing 20 wt% RuO2 binder achieved a maximum current density of 2.16 A cm−2 at 60 °C and 100% relative humidity (RH), outperforming the MEA utilizing Carbon Vulcan as the support (2.06 A cm−2). Furthermore, the enhanced hydrophilic properties of the RuO2 binder improved water retention at the catalyst layer/membrane interface, thus promoting membrane hydration and overall cell performance at a high temperature of 80 °C and a low RH of 30%.
Introduction
In recent years, polymer electrolyte fuel cells (PEFCs) have attracted significant attention as environmentally friendly devices for energy storage and conversion,1–4 with increasing applications in heavy-duty vehicles, such as trucks, buses, and ships.5,6 Carbon is a conventional catalyst support for PEFCs owing to its excellent electrical conductivity, high surface area, and suitable pore structure.7–10 However, these carbon materials are prone to corrosion in the highly corrosive environments associated with PEFC operation, resulting in a decline in both performance and durability.7,11 To address this challenge, metal oxide nanoparticles with improved corrosion resistance have been developed as promising support materials to replace traditional carbon in fuel cell electrodes.12–15Niobium-doped tin oxide (NTO) nanoparticles have demonstrated great potential as cathode catalyst supports in PEFCs owing to their high electrical conductivity and exceptional corrosion resistance.16,17 Kakinuma et al. reported that NTO nanoparticles with a dopant concentration of 4.0% exhibited higher electrical conductivity than Nb-free SnO2. Furthermore, the cells with Pt/NTO cathodes showed better performance and durability than those with Pt/carbon black cathodes when operated at potentials above 0.4 V.16 Our research group also synthesized NTO nanoparticles using flame spray pyrolysis and evaluated their performance as PEFC cathode catalyst supports.17–19 The flame-made NTO nanoparticles formed a fused aggregate network structure via a combustion reaction during synthesis, which reduced the interparticle resistance between oxide nanoparticles and enhanced conductivity. Nevertheless, compared to traditional carbon supports, oxide supports generally have lower porosity and less developed structures, which continue to present challenges for achieving high power generation performance.
A potential solution to this problem is to employ support particles with macroporous structures. Our group reported the synthesis of macroporous particles by a template-assisted aerosol process,20–25 which had been extensively utilized for producing macroporous structures in a wide range of materials.20,22,26,27 The high porosity of macroporous supports provides accessible pores, promoting the diffusion of reactants and products.21,28–31 Faustini et al. synthesized an iridium-based catalyst with an ultraporous hierarchical structure via spray drying, which proved to be an effective catalyst for proton exchange membrane water electrolysis.28 The macroporous structure of Ir0.7Ru0.3O2 ultraporous spheres created an effective electron-conducting pathway throughout the catalyst layer, while the high porosity improved gas and water transport.
In our previous report, we demonstrated that using a 300 nm poly(methyl methacrylate) (PMMA) template to prepare macroporous NTO particles could enhance their performance as catalyst supports in single cells.32 However, under the high pressure of membrane electrode assembly (MEA) fabrication, the macropore framework of the NTO particles collapsed due to their poor mechanical strength, decreasing the porosity of the catalyst layer. Therefore, a macroporous NTO catalyst support with a mechanically stable structure is desirable to achieve high-performance cathode catalysts for PEFCs.
Many studies have used binders to improve the mechanical strength of porous frameworks33–35 and investigated their functions in the fabrication processes. These studies collectively highlight the efficacy of binders in enhancing the mechanical strength of porous materials. Motivated by this concept, we applied binders in this study to improve the mechanical strength of macroporous NTO particles. Considering that insulator binders can increase the contact resistance between active materials, resulting in reduced PEFC performance, conductive binders are essential to strengthen the macroporous frameworks for achieving high-performance PEFCs. Ruthenium oxide (RuO2) is known as a metallic conductor with an electrical conductivity of 2.0 × 104 S cm−1 at 28 °C, which decreases at lower temperatures.36,37 Additionally, RuO2–SnO2 is utilized in various electrochemical applications, such as supercapacitors, chlorine evolution, and water electrolysis.38,39 Using RuO2 as a binder to combine with NTO particles is expected to not only reinforce the structural integrity of the macroporous framework but also enhance its electrical conductivity. Therefore, to design a suitable catalyst support, it is crucial to understand the effect of binder introduction on the mechanical properties and electrical conductivity of the macroporous structure, as well as its impact on PEFC performance. It is also important to determine the effects of various concentrations of RuO2 binder on the mechanical strength of the macroporous structure and the overall PEFC performance. To the best of our knowledge, macroporous NTO/RuO2 particles have not been reported as catalyst supports for PEFCs.
In this study, we designed a cathode catalyst support using NTO/RuO2 particles with macroporous structures prepared by flame-assisted spray pyrolysis (FASP). In the FASP process, the presence of liquid- and vapor-phase precursors allows for better control over particle morphology and structure.15,40–48 In addition, this method has been scaled up for industrial use in the production of fine particles.49,50 The effect of RuO2 binder contents on the structure and morphology of the macroporous NTO/RuO2 particles was investigated. The electrical conductivity of the NTO/RuO2 particle was also examined. Importantly, the mechanical strength of the macroporous structure of NTO/RuO2 particle with different RuO2 binder contents was also evaluated. Eventually, the performance of MEAs using macroporous NTO/RuO2 particles as cathode catalyst support was performed under both low and high relative humidity conditions.
Results and discussion
Preparation of macroporous NTO/RuO2 particles
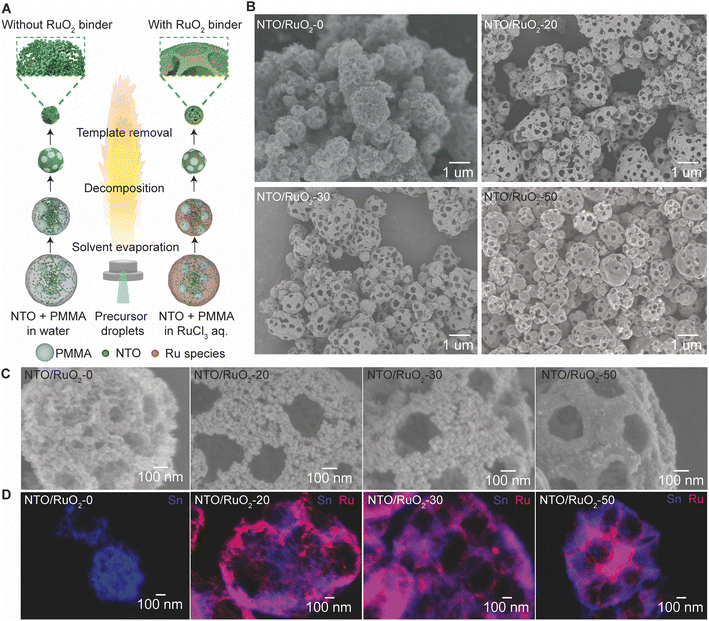
RuO2 and SnO2 share several structural similarities, enabling the facile formation of a RuO2–SnO2 composite, which is crucial for creating a robust macroporous framework. Assisted by these features, macroporous NTO/RuO2 particles with different contents of RuO2 binder (0 to 50 wt%) were synthesized using FASP, followed by annealing to completely remove the PMMA template. Fig. 1A depicts the synthesis workflow, starting with the FASP of mechanically stable macroporous NTO particles templated with PMMA and bound by RuO2 (NTO/RuO2). In the absence of RuO2 binders, NTO nanoparticles and PMMA templates were dispersed in water to form a precursor solution, and macroporous particles were produced through a conventional template-assisted spray pyrolysis process. In contrast, macroporous NTO/RuO2 particles were obtained using a precursor solution consisting of RuCl3 salt, PMMA templates, and NTO nanoparticles. In this process, RuCl3 salt decomposes under high-temperature conditions to form RuO2 as a binder in the gaps between NTO nanoparticles within the precursor droplets in the gas phase. Additionally, the precipitation of RuO2 between NTO nanoparticles results in the formation of a conductive network in the structure, improving the electrical conductivity of the macroporous NTO/RuO2 particles. Since the short residence time and rapid cooling in the FASP process,51–53 the decomposition of the PMMA template was incomplete. Therefore, a post-annealing treatment was performed to fully remove the remaining PMMA template and achieve the desired macroporous structure of NTO/RuO2 particles.The morphology of the macroporous NTO/RuO2 particles was examined using scanning electron microscopy (SEM). The influence of PMMA template concentration (0 to 2.0 wt%) on the structure of the macroporous NTO/RuO2 particles was investigated when the RuO2 binder concentration was fixed at 50 wt%. As shown in the SEM images (Fig. S1†), increasing the PMMA concentration resulted in more open macropores on the particle surfaces. However, at 2.0 wt%, the template concentration was too high, causing the macroporous framework to collapse. Therefore, a PMMA template concentration of 1.0 wt% was selected for subsequent experiments to ensure a robust macropore framework and ample open macropores. Fig. 1B shows the SEM images of the macroporous NTO/RuO2 particles prepared with different RuO2 binder concentrations, wherein well-developed macropores were derived from PMMA templates in the presence of RuO2 binders. Fig. 1C shows the magnified SEM images of the particle surfaces. The macroporous NTO/RuO2-0 particles (without RuO2) exhibited spherical shapes with rough surfaces formed from aggregated NTO nanoparticles. Notably, significant voids were observed between aggregated NTO nanoparticles, and this relatively high void fraction within the macropore framework could compromise the mechanical strength. It is important to note that the mechanical stability of a porous structure is largely determined by its framework porosity – the void fraction within the macropore framework – as highly porous structures typically exhibit weak mechanical strength.54 Upon the addition of RuO2 binders at concentrations of 20 wt% (NTO/RuO2-20) and 30 wt% (NTO/RuO2-30), the macropore frameworks were more distinct, and the particle surfaces became smoother. As the concentration of RuO2 continued to increase, the macropore framework persisted, and the number of broken particles considerably decreased. The surfaces of the NTO/RuO2-50 particles (50 wt% RuO2) became slicker, and the number of voids between NTO nanoparticles decreased significantly. The porosity of the framework, which is the percentage of voids between NTO nanoparticles, was reduced by the incorporation of the RuO2 binder. The RuO2 binder effectively filled the voids, strengthening the structural connectivity and significantly enhancing the mechanical stability of the macroporous framework. The scanning transmission electron microscopy (STEM) images (Fig. S2†) also confirmed the porosity reduction in the framework with the increasing RuO2 concentration. As shown in Table S1 and Fig. S3,† the incorporation of RuO2 binder reduced the specific surface area of the macroporous NTO/RuO2 particles. Specifically, the specific surface areas calculated using the BET equation were 106.9 m2 g−1 and 71.1 m2 g−1 for NTO nanoparticles and macroporous NTO/RuO2-0 particles, respectively. The lower specific surface area of NTO/RuO2-0 is attributed to the aggregation of NTO nanoparticles during the flame process and the annealing treatment to remove PMMA templates. The specific surface area of the macroporous NTO/RuO2 particles further decreased with the increasing RuO2 binder concentration, reaching 30.4 m2 g−1 for NTO/RuO2-50. These results confirm a decrease in the framework porosity with the increasing RuO2 binder content. The energy-dispersive X-ray (EDX) elemental mapping in Fig. 1D shows that the RuO2 binder distributes uniformly within the entire structure of the macroporous particles. During the self-organization process of precursor droplets floating in the flame, Ru species, which are smaller than NTO nanoparticles and PMMA templates, experience lower buoyancy and higher Brownian motion, allowing them to move more easily and fill the spaces between the larger particles.55–57 Additionally, RuO2 species nucleated and precipitated onto the surface of NTO nanoparticles, reducing voids between NTO nanoparticles during the assembly process. This self-organization leads to the distribution of RuO2 throughout the particles, which is expected to increase the framework density and enhance the mechanical durability of the macroporous particles.
Fig. 2A shows the X-ray diffraction (XRD) patterns of the macroporous NTO/RuO2 particles with different RuO2 binder contents. In the absence of RuO2 binder, NTO/RuO2-0 existed in a tetragonal SnO2 phase, with characteristic diffraction patterns at 26.6°, 33.9°, 38.0°, and 51.8° (COD 9007533). No Nb species were observed in NTO/RuO2-0 particles, indicating no precipitation of niobium oxides. With the addition of RuO2, the XRD patterns of all NTO/RuO2 particles exhibited diffraction patterns corresponding to the tetragonal SnO2, tetragonal RuO2, and metallic Ru phases. Specifically, the diffraction peaks at 28.0°, 35.1°, and 40.1° belonged to the tetragonal phase of RuO2 (COD 2101852), whereas the peaks at 38.4°, 42.2°, and 44.0° were characteristic of metallic Ru (COD 9008513). The intensities of these RuO2 and Ru XRD patterns increased as the RuO2 ratio in the NTO/RuO2 particles increased. The transformation of RuCl3 salt into RuO2 initiates in the temperature range of 350–550 °C.58 Furthermore, RuO2 decomposes to metallic Ru at a temperature exceeding 1025 °C.59 Given the high temperature of the flame process (up to approximately 2000 °C),15 RuCl3 is readily decomposed into RuO2. Subsequently, RuO2 can transform to metallic Ru at the high temperature of the flame, and the resulting Ru is oxidized back to RuO2 in the high-oxygen environment of the flame reactor. Owing to the rapid quenching of the FASP process,51,60–63 the oxidation of metallic Ru is incomplete, resulting in the presence of a small amount of metallic Ru within the macroporous NTO/RuO2 particles.
Fig. 2B shows the percentages of SnO2, RuO2, and Ru in the macroporous Ru/NTO particles calculated from the XRD patterns. The results imply that the percentages of SnO2 and total Ru in all macroporous particles were close to the nominal compositions. The crystallographic parameters of RuO2, Ru, and SnO2 derived from Rietveld refinement are listed in Table S2.† The refinement was performed using the crystal structure models P42/mnm for SnO2, P42/mnm for RuO2, and P63/mmc for Ru. The final R-factors indicated the reliability of the analysis. The Rwp, RB, and RF values were sufficiently low to validate the proposed structural model.
The valance states of Ru, Sn, and Nb in the macroporous NTO/RuO2-50 particles were determined through XPS measurements, with all peaks calibrated with respect to the C 1s peak at 284.3 eV. As presented in Fig. 2C, the peaks at binding energies of 279.6 eV and 284.8 eV were related to Ru0 3d5/2 and Ru0 3d3/2, respectively. Additionally, the peaks at 280.7 eV and 285.5 eV corresponded to Ru4+ 3d5/2 and Ru4+ 3d3/2, respectively, which were the characteristic peaks of RuO2. The Sn 3d spectra exhibited peaks at 486.15 eV (Sn4+ 3d5/2), 494.55 eV (Sn4+ 3d3/2), and 483.65 eV (Sn0 3d5/2). The presence of metallic Sn was probably due to the formation of a RuSn alloy in the high-temperature flames of the FASP process and during the post-heat treatment. However, the amount of RuSn alloy was minimal and was not identified in the XRD patterns. The binding energies of Nb 3d were found around 206.65 eV (Nb5+ 3d5/2) and 209.15 eV (Nb5+ 3d3/2), whereas no peaks corresponding to Nb3+ or Nb4+ were observed. Since the ionic radii of Nb5+ (0.64 Å) are smaller than that of Sn4+ (0.69 Å), Nb5+ ions can be successfully substituted into Sn4+ lattice sites, resulting in single-phase NTO nanoparticles with a dopant level of 4 at%.16,64 The successful substitution of Nb5+ enhanced the electrical conductivity of the NTO nanoparticles.16,64
The electrical conductivity of the NTO/RuO2 particles was assessed at various RuO2 concentrations. It is evident that the porosity of the particles increases the electrical resistance during the direct conductivity measurements of powders. As shown in Fig. 1, the porosity of the macroporous particle determines the framework strength, which is influenced by the varying amounts of RuO2 binder. To assess the impact of RuO2 content on the conductivity of the NTO/RuO2 particles while minimizing the influence of particle porosity on the conductivity measurement, we prepared NTO/RuO2 aggregate particles without a macroporous structure for conductivity evaluation. The structure of these NTO/RuO2 aggregate particles is detailed in Fig. S4 in the ESI.† As indicated in Fig. 2D, the electrical conductivity of the NTO aggregate particles was poor (2.7 × 10−5 S cm−1). With the addition of RuO2 binders, the electrical conductivity of the NTO/RuO2 particles was noticeably improved. Specifically, as the RuO2 ratio increased, the electrical conductivity of the NTO/RuO2 particles increased, reaching 0.50 S cm−1 at a RuO2 content of 50 wt%. It is important to note that RuO2 exhibits metallic-like conductivity at room temperature with an electrical conductivity of 2.0 × 104 S cm−1.36 Therefore, combining RuO2 binders and NTO nanoparticles enhanced the electrical conductivity of the produced NTO/RuO2 particles. Moreover, at higher RuO2 concentrations, RuO2 species precipitated in the gap between NTO nanoparticles and reduce porosity of the framework, as indicated by the TEM and EDS mapping results and the specific surface area of the macroporous NTO/RuO2 particles. The RuO2 species in the aggregated NTO particles formed a continuous electrical network, enhancing the conductivity of NTO/RuO2. Moreover, the reduction in framework porosity at high RuO2 binder contents not only results in a denser structure but also significantly improves electron transport. The pore size distribution of the macroporous NTO/RuO2 particles was determined using mercury porosimetry. For NTO/RuO2-20 particles, the average pore size was 142.6 nm. With an increase in the RuO2 binder concentration, the peak shifted to a larger pore size, as shown in Fig. 2E, reaching 527.7 nm at a RuO2 binder content of 50 wt%.
Evaluation of mechanical strength of macroporous particles
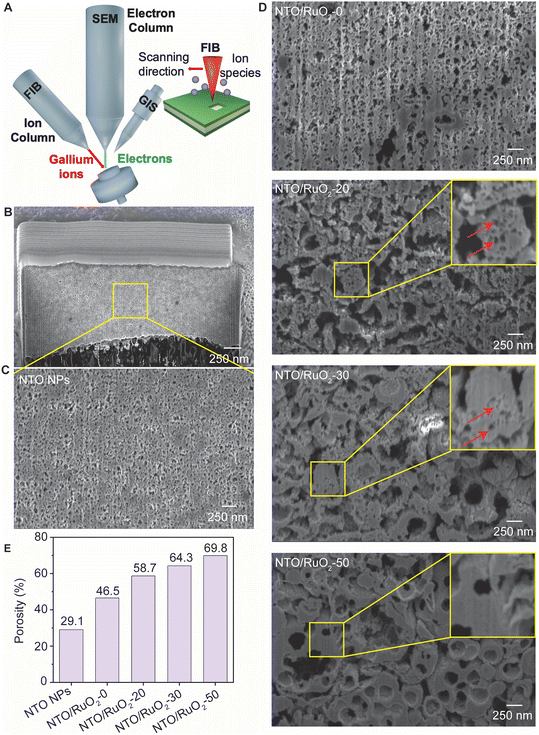
As mentioned in the introduction, the macroporous structure has the potential to enhance reactant mass transport, consequently improving the performance of PEFCs. However, in the fabrication process of MEAs, involving mechanical compression, the macroporous framework of particles can be collapsed. To evaluate the mechanical strength of the macroporous structure of NTO/RuO2 particles, we fabricated MEAs based on the macroporous NTO/RuO2 particles and used cross-sectional SEM to observe their morphology within the MEAs. Fig. 3A shows the fabrication process of MEAs using a direct hot-pressing method. First, macroporous NTO/RuO2/Teflon was prepared into a square shape (1 cm × 1 cm) and directly hot pressed onto both sides of a Nafion membrane at high pressure of 10 MPa (a typical pressure used for MEA fabrication).63,65–67 Additionally, another MEA was prepared at a pressure of 40 MPa to gain more insights into the effect of RuO2 binder on the mechanical strength of the macroporous structure. After hot pressing, the Teflon sheets were removed to obtain a final MEA consisting of a Nafion membrane sandwiched between two macroporous NTO/RuO2 particle layers. The MEAs were secured using plastic tweezers, immersed in liquid nitrogen for 2 min, removed from liquid nitrogen, and immediately cut using a tweezer needle. The morphology of the particle layer in the MEA was observed using cross-sectional SEM and focused ion beam-SEM (FIB-SEM).Fig. 3B shows the cross-sectional SEM images of the MEAs based on macroporous NTO/RuO2 particles and NTO nanoparticles. The MEAs with NTO nanoparticles exhibited low porosity owing to their high compact density. The macroporous NTO/RuO2-0 particles showed higher porosity compared to that of NTO nanoparticles, and this macroporous structure collapsed at 10 MPa. In contrast, the macropore fraction in the NTO/RuO2-20 layer was even higher than that in the NTO/RuO2-0 layer, and the particle structure was nearly broken at a pressure of 10 MPa. Upon further increasing the RuO2 binder concentration to 50 wt%, the particle structure was maintained at 10 MPa. Interestingly, the macroporous framework of NTO/RuO2-50 was maintained under the high pressure of 40 MPa, whereas the macroporous NTO/RuO2-0, NTO/RuO2-20, and NTO/RuO2-30 particles were almost broken. These results demonstrated that RuO2 improved the mechanical strength of the macroporous structure. Additionally, the particle layers fabricated with NTO nanoparticles, NTO/RuO2-0, NTO/RuO2-20, NTO/RuO2-30, and NTO/RuO2-50, under a pressure of 10 MPa had thicknesses of 7.83, 7.92, 8.08, 8.61, and 8.97 μm, respectively. Higher RuO2 binder concentration helps to enhance the mechanical strength of macroporous framework of NTO/RuO2 particles, which maintains the macroporous framework under high pressure. Therefore, using the same particle content and pressure in the MEA fabrication process, an increase in the catalyst layer thickness was observed with an increase in RuO2 binder contents.
FIB-SEM imaging was employed to gain insights into the pore structure and pore size and to quantitatively assess the porosity of the particle layer in the MEAs. While preparing a cross-section sample through mechanical destruction is straightforward, accurately measuring the porosity can be challenging. In contrast, FIB techniques enable precise cutting of both secondary and primary particles (Fig. 4A), which facilitates the acquisition of more accurate cross-sectional information. The lower- and higher-magnification cross-sectional images of the NTO nanoparticle layer (Fig. 4B and C, respectively) revealed that the NTO nanoparticle layer was dense, with a porosity of 29.1%. Fig. 4D presents the high-magnification cross-sectional FIB-SEM images of the macroporous NTO/RuO2 particle layers prepared with varying RuO2 binder concentrations. The porosity of the catalyst layer increases with the amount of RuO2. For NTO/RuO2-20, the particle structure almost completely collapsed after compression owing to the low binder content. However, its final remaining porosity was significantly higher than those of the NTO nanoparticles or porous particles without a binder. These results show that RuO2 binder enhances the mechanical durability of the porous particles and facilitates the formation of robust pores that can withstand the compression process. As the binder content increased, the mechanical stability of the particles improved, which increased the formation of particles that could withstand compression, thereby raising the proportion of remaining PMMA-derived pores. The porosity of the NTO/RuO2-0 layer was 46.5%, which was substantially higher than that of the NTO nanoparticles (29.1%). With the addition of RuO2 binder, the porosity of the particle layer increased significantly, reaching 69.8% for the macroporous NTO/RuO2-50 layer, as shown in Fig. 4E, highlighting the beneficial effect of the macroporous structure on enhancing the porosity of the catalyst layer.
Interestingly, the presence of mesopores in the gaps between NTO nanoparticles was also confirmed in the FIB-SEM images. A significant number of mesopores were observed in the NTO/RuO2-20 layer (red arrow), and the number of mesopores decreased with increasing RuO2 binder concentration. These mesopores were likely due to the voids between NTO nanoparticles. As discussed previously, the addition of RuO2 binder reduced the framework porosity of the macroporous NTO/RuO2 particles. As the concentration of RuO2 binder gradually increased, the number of mesopores decreased, eventually nearly vanishing for the NTO/RuO2-50 particles. Specifically, the addition of a large amount of RuO2 improved the strength of the particle framework and increased the number of macropores derived from the template PMMA within the catalyst layer, while simultaneously diminishing the mesopores between NTO primary particles. Because the balance between the number of mesopores and macropores is essential for improving PEFC performance,68 the effect of RuO2 loading on the balance between pore size and PEFC performance will be discussed in the next section.
Performance of MEA using macroporous NTO/RuO2 particles
To evaluate the practical performance of MEAs with the macroporous NTO/RuO2 catalyst support, several electrochemical analyses were performed in a single-cell system. MEAs using Carbon Vulcan, NTO nanoparticles, and macroporous NTO/RuO2 particles at different RuO2 binder contents were fabricated as catalyst supports for the cathode, as illustrated in Fig. 5A. All MEA tests were conducted in the same operating conditions of 60 °C, 100% RH, and H2/air. The I–V curves of representative MEAs are shown in Fig. 5B. The MEA using Pt/NTO exhibited poorer performance than the MEA using Pt/C in both the low- and high-current-density regions because the low electrical conductivity and porosity of NTO nanoparticles caused ohmic losses and limited mass transport in the catalyst layer. The MEA using macroporous NTO/RuO2-0 particles showed improved performance compared to that using NTO nanoparticles, with the maximum current density increasing from 1.30 A cm−2 to 1.46 A cm−2 (as shown in Fig. 5D). The higher porosity of the macroporous NTO/RuO2-0 particles, compared with that of the NTO nanoparticle layer, decreased gas diffusion resistivity (Fig. 5F) and promoted reactant diffusion to the catalytic sites. No significant differences were observed at a voltage of 0.5 V and in the high-current-density region between the MEAs comprising macroporous NTO/RuO2 particles and that using commercial Pt/C as a catalyst support (Fig. 5D and E). The MEA using macroporous NTO/RuO2-20 particles exhibited the best performance, with a maximum current density of 2.16 A cm−2, which is comparable to that using Carbon Vulcan (2.06 A cm−2). This result indicates that the macroporous NTO/RuO2 particles showed not only high electrical conductivity to avoid an ohmic overpotential but also sufficient porosity for delivering reactants to the catalyst sites. However, the maximum current density decreased when RuO2 binder content was further increased. The maximum current densities were 2.16 A cm−2 and 1.89 A cm−2 when macroporous NTO/RuO2-20 and NTO/RuO2-50 particles were used as the catalyst supports, respectively. The performance deterioration in the macroporous NTO/RuO2-50 particles can be attributed to excess water, which obstructs the oxygen transport pathways and reduces the fuel cell efficiency. Water accumulation at the cathode results from both external humidification under high RH conditions and water production from oxygen reduction reaction (ORR),69 which is accelerated at higher current densities. If the water removal rate does not match the generation rate, excess water can block the pores in the porous cathode catalyst layer, cover the active sites, and clog the gas-transport channels in the flow field.69,70 For the macroporous NTO/RuO2 particles, the hydrophilic nature of the RuO2 binder enhanced the water retention in the catalyst layer, and the high porosity increased oxygen consumption, which resulted in excessive water accumulation and ultimately diminished cell performance. Although the macroporous structure aids in transporting water from the catalyst layer and facilitates water removal through the gas diffusion layer, the increased pore volume can also lead to greater water retention.69 For the MEA using macroporous NTO/RuO2-50 particles, a high RuO2 binder concentration significantly increased the water retention capacity of the catalyst layer. Additionally, the high porosity of the macroporous NTO/RuO2-50 particles (69.8%) increased the O2 diffusivity rate, resulting in greater oxygen consumption and higher water generation on the cathode side compared to the macroporous NTO/RuO2-20 and NTO/RuO2-30 particles. These factors contribute to more water accumulation and higher gas diffusion resistivity of the MEAs using macroporous NTO/RuO2-50 particles compared to those using macroporous NTO/RuO2-20 and NTO/RuO2-30 particles. Specifically, the gas diffusion resistivity was 90 m s−1 for the MEA using macroporous NTO/RuO2-20 particles and increased to 117 m s−1 for that using macroporous NTO/RuO2-50 particles (Fig. 5F), which explained the deteriorated cell performance.The amount of RuO2 binder in the NTO/RuO2 particles can impact the mechanical strength of the macroporous framework and the wettability of the catalyst layer. A high RuO2 content is necessary to achieve high electrical conductivity and robust mechanical strength, ensuring that the macroporous framework remains intact under high pressure. However, a high RuO2 binder content can lead to water accumulation in the MEAs under fully humidified conditions and degrade cell performance. Therefore, optimizing the amount of RuO2 binder is essential to achieve a catalyst layer that maintains structural integrity and high conductivity without compromising performance owing to water accumulation.
We also evaluated the performance of these MEAs at low RH of 30% and a high temperature of 80 °C. Theoretically, in the case of limited external humidification, the water used to humidify the MEA is primarily supplied by the water produced from the ORR at the cathode. Given the limited water content under low RH, the conductivity drops, leading to increased ionic resistance and ohmic losses, ultimately degrading the performance.70–72 The I–V curves of the MEAs using Carbon Vulcan, NTO nanoparticles, and macroporous NTO/RuO2 particles as cathode catalyst supports are shown in Fig. 5C. Compared to those at 100% RH, the ohmic resistance at 30% RH increased for all MEAs, resulting in a drop in current density at 0.5 V. The maximum current density of the MEA using Carbon Vulcan as a catalyst support decreased from 2.06 A cm−2 to 1.67 A cm−2, primarily due to the hydrophobic nature of carbon, which fails to maintain necessary humidity within the catalyst layer and results in increased ohmic and mass transfer resistance. Conversely, MEAs utilizing NTO nanoparticles and macroporous NTO/RuO2-0 particles exhibited sustained performance. This can be attributed to the hydrophilic properties of SnO2, which help maintain membrane hydration and overall cell performance under low-humidification conditions. With an increase in the RuO2 binder content, the maximum current density at 30% RH gradually increased. This trend can be attributed to the increased water production at the cathode, which improved the hydration status of the MEA. The hydrophilic nature of the RuO2 binder enhanced the water retention capability at the catalyst layer/membrane interface, thereby improving membrane hydration and overall cell performance.
To assess the durability of macroporous NTO/RuO2 particles, cyclic voltammograms (CV) were obtained for electrodes based on macroporous NTO/RuO2 particles with varying RuO2 binder contents, NTO nanoparticles, and commercial carbon, both before and after testing at an applied potential of 1.3 V vs. EAg/AgCl for 10 minutes, as shown in Fig. S7.† The results reveal that the carbon support exhibited a significant increase in capacitance after the durability test, suggesting carbon oxidation and degradation occurred. In contrast, no change in the CV shape was observed for NTO nanoparticles and macroporous NTO/RuO2 particles, indicating their high stability after the durability test at high potential. Hence, compared to carbon, which is the conventional catalyst support for PEFCs, macroporous NTO/RuO2 particles show significant promise as potential cathode catalyst supports, providing both excellent performance and durability for PEFCs.
Conclusions
Macroporous NTO/RuO2 particles with high mechanical strengths and electrical conductivities were successfully synthesized via flame-assisted spray pyrolysis. The effects of RuO2 binder and PMMA template concentrations on the structure and morphology of the macroporous NTO/RuO2 particles were investigated. The macroporous NTO/RuO2 particles have a spherical shape, with the gap between NTO nanoparticles filled with RuO2 particles, leading to a decreased framework porosity. The electrical conductivity of the NTO/RuO2 particles increased with RuO2 binder content, reaching 0.50 S cm−1 at 50 wt% RuO2. The macroporous structure of the NTO/RuO2-50 particles remained stable at 40 MPa, demonstrating that the RuO2 binder improved the mechanical strength of the macroporous framework. The porosity of the catalyst layer increased from 29.1% for NTO/RuO2-0 to 69.8% for NTO/RuO2-50. The performance of the MEAs using macroporous NTO/RuO2-20 particles was comparable to that of the MEAs using a Carbon Vulcan catalyst support under high RH conditions. Additionally, the hydrophilic nature of RuO2 binders enhanced the overall cell performance when operated at low RH. These findings highlight that incorporating RuO2 as a binder not only enhances the mechanical strength of the macroporous framework but also improves its PEFC performance, demonstrating that macroporous NTO/RuO2 particles are promising candidates as cathode catalyst supports in PEFCs.Experimental section
Particle synthesis
The procedure for producing NTO nanoparticles has been described in detail in a previous study.18 Briefly, NTO nanoparticles were synthesized via flame spray pyrolysis (FSP). The precursor solutions, including Sn(O2C8H15)2 (92.5–100%, Sigma-Aldrich) and Nb(OC2H5)5 (99.95% purity, Sigma-Aldrich), were dissolved in xylene. The total Sn and Nb concentration was 0.1 mol L−1 with a Nb dopant content of 4 at%. The precursor solution was introduced into a two-fluid nozzle (AM6, ATOMAX Co., Ltd., Shizuoka, Japan) at 3.0 mL min−1 using a syringe pump. This solution was atomized with oxygen (3.0 L min−1) and ignited by a premixed methane/air pilot flame (CH4: 1.5 L min−1, air: 11.0 L min−1). NTO nanoparticles were collected using a polytetrafluoroethylene (PTFE) membrane filter (HORKOS Corp., Hiroshima, Japan). NTO nanoparticles were used as the precursors for the synthesis of macroporous NTO/RuO2 particles.The macroporous NTO/RuO2 particles were prepared by the flame method using a precursor solution containing flame-made NTO nanoparticles, RuCl3·xH2O (99.9%, Wako, Japan) and poly(methyl methacrylate) (PMMA) (MP-2741, Soken, Japan) as a template. The solution in a round bottom flask was placed in a circulating water-cooling system to maintain the stability of the precursors. The RuO2/(NTO + RuO2) mass ratios were controlled at 0, 20, 30 and 50 wt%. The concentration of PMMA template in the precursor was 1.0 wt%. An ultrasonic nebulizer (NE-U17, Omron Healthcare Co., Ltd., Tokyo, Japan) was used to create droplets from the precursor solution. The generated droplets were introduced into diffusion flames using nitrogen as a carrier gas at a flow rate of 5.0 L min−1. A diffusion flame was formed using methane (0.5 L min−1) as a fuel and oxygen (1.3 L min−1) as an oxidizer. The macroporous NTO/RuO2 particles were collected using a PTFE membrane filter. Then, the macroporous NTO/RuO2 particles were placed in a tubular furnace and heated from room temperature to 500 °C at a heating rate of 10 °C min−1 under air conditions. The temperature was held for 60 min to remove PMMA completely. The obtained particles are denoted as NTO/RuO2-x, where x refers to the percentage of the RuO2 binder in the particles. For example, NTO/RuO2-20 is a macroporous NTO/RuO2 particle containing 20 wt% RuO2 binder, as calculated from the precursor used.
To investigate the effect of PMMA concentration on the morphology and macroporous structure of the NTO/RuO2 particles, the PMMA content in the precursor was changed from 0, 0.5, 1.0, to 2.0 wt%. The concentration of RuO2 binder was fixed at 50 wt%. The particles were also produced using FASP under the same conditions. The synthesized particles underwent a heat treatment at 500 °C for 1 h under air conditions to remove PMMA completely.
Characterization
The crystal structures of the macroporous NTO/RuO2 particles were examined by X-ray diffraction (XRD; D2 PHASER, 40 kV and 30 mA, Bruker Corp., USA). The size, morphology, and structure of the particles were characterized by field-emission scanning electron microscopy (FE-SEM; S-5200, Hitachi High-Tech. Corp., Tokyo, Japan). The electrical conductivities of the aggregated NTO/RuO2 particles were measured using a multi-electrochemical measurement system (HZ-Pro S4, Hokuto Denko, Japan). The oxidation states of the elements were determined using X-ray photoelectron spectroscopy (XPS, ESCA-3400, Shimadzu Corp.) operated at 10 kV and 20 mA.The mechanical strength of the macroporous structure of the NTO/RuO2 particles was evaluated at different compression pressures during MEA fabrication. The ink was prepared by dispersing 0.5 g of macroporous NTO/RuO2 particles in a mixed solvent containing 0.405 g of 20 wt% Nafion solution (Wako, Japan), 0.661 g of ethanol, and 0.808 g of deionized water and ultrasonicating for 60 min. The as-prepared catalyst ink (0.5 mL) was then applied to a Teflon sheet. An electrolyte membrane (DuPont, NR-212) was sandwiched between two sheets of macroporous NTO/RuO2 and assembled by hot pressing (Mini Test Press MP, Toyo Seiki Co., Ltd.) at 126 °C with pressures of 10 and 40 MPa. The morphology of the macroporous NTO/RuO2 particle layers in the MEA was observed by cross-sectional SEM and focused ion beam-SEM (FIB-SEM; HeliosG4 UC, Thermo Fisher Scientific, Waltham, MA, USA). The pore-size distribution of the macroporous NTO/RuO2 particles was measured using mercury porosimetry.
The MEA showed enhanced performance when macroporous NTO/RuO2 particles were used as the cathode catalyst support. The MEA preparation process was described in detail in a previous study.32 Briefly, cathode catalyst inks were prepared by mixing a specific amount of Pt/(NTO/RuO2) or Pt/(NTO) catalyst, Nafion ionomers, ethanol, and purified water using a homogenizer to achieve an ionomer/support (I/S) volume ratio of 0.175. The catalyst ink was then applied to a Teflon sheet with a Pt loading of 0.1 mg cm−2. For the anode, Pt/C (with 20 wt% Pt from Ketjenblack ECP) was used in all test cells. The Pt loading was ∼0.1 mg cm−2, and the I/S ratio was ∼1.0. For comparison, commercial Pt/C (XC-72R, Cabot Corporation) was used as a reference cathode catalyst at the same Pt loading. Pt/(NTO/RuO2), comprising an electrolyte membrane (DuPont, NR-212) and a Pt/C sheet, was assembled by hot pressing at 10 MPa and 126 °C. Each electrode has a geometric area of 1.0 cm2. The MEA was placed in a custom single-cell holder with straight-line gas-flow channels. The output performance was evaluated by recording the current density at 60 °C and 100% RH, and at 80 °C and 30% RH. Hydrogen gas was supplied to the anode, and air was supplied to the cathode, both with a backpressure of 108 kPa. Gas flow rates were 1.0 L min−1 and 0.5 L min−1 at the cathode and anode, respectively. A pre-conditioning process (40 cycles at 0.1 V s−1 over 0–1.0 V) was completed before the main measurements. AC-impedance measurements were conducted from 100 kHz to 0.1 Hz at a potential of 0.4 V and an amplitude of 0.02 V. The cells and MEAs used for the AC-impedance measurements were identical to those used for the output measurements, and the experimental conditions were also identical. Current–voltage curves were measured potentiostatically at 0.01 V s−1 in the range of 0–1.0 V.
The durability of carbon, NTO nanoparticles, and macroporous NTO/RuO2 particles with varying RuO2 binder contents was evaluated through chronoamperometry by applying a voltage of 1.3 V versus Ag/AgCl. Their electrochemical properties, both before and after the durability test, were assessed using cyclic voltammetry (CV) with a potentiostat (HZ-5000, Hokuto Denko Corp., Tokyo, Japan) at a scan rate of 20 mV s−1 in nitrogen-saturated 0.1 N HClO4 solution. The electrode potential versus Ag/AgCl (EAg/AgCl) was converted to the potential versus a reversible hydrogen electrode (ERHE) using the Nernst equation: ERHE = EAg/AgCl + 0.059 pH + 0.198.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Thi Thanh Nguyen Ho: writing – original draft, writing – review & editing, validation, investigation, data curation, visualization. Tomoyuki Hirano: conceptualization, writing – review & editing, supervision, funding acquisition. Aoi Takano: investigation, data curation, writing – review & editing. Syu Miyasaka: investigation, data curation, writing – review & editing. Eishi Tanabe: investigation, data curation, writing – review & editing. Makoto Maeda: investigation, data curation, writing – review & editing. Eka Lutfi Septiani: writing – review & editing, investigation. Kiet Le Anh Cao: writing – review & editing, investigation. Takashi Ogi: conceptualization, writing – review & editing, supervision, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant No. JP22K20482, JP23K13590 (T. H.), and JP23H01745 (T. O.). This work was supported by the JST SPRING, Grant Number JPMJSP2132 (T. T. N. H.). This work was partially supported by the Center for Functional Nano Oxides at Hiroshima University, the Information Center of Particle Technology, Japan, and the Hosokawa Powder Technology Foundation. The authors thank the Natural Science Center for Basic Research and Development (N-BARD) for access to SEM facilities.References
- N. Seselj, S. M. Alfaro, E. Bompolaki, L. N. Cleemann, T. Torres and K. Azizi, Adv. Mater., 2023, 35, 2302207 CAS.
- S. Jang, Y. S. Kang, D. Kim, S. Park, C. Seol, S. Lee, S. M. Kim and S. J. Yoo, Adv. Mater., 2023, 35, 2204902 CrossRef CAS PubMed.
- F. Ning, J. Qin, X. Dan, S. Pan, C. Bai, M. Shen, Y. Li, X. Fu, S. Zhou, Y. Shen, W. Feng, Y. Zou, Y. Cui, Y. Song and X. Zhou, ACS Nano, 2023, 17, 9487–9500 CrossRef CAS PubMed.
- B. Yang, Q. Han, L. Han, Y. Leng, T. O'Carroll, X. Yang, G. Wu and Z. Xiang, Adv. Mater., 2023, 35, 2208661 CrossRef CAS PubMed.
- D. A. Cullen, K. C. Neyerlin, R. K. Ahluwalia, R. Mukundan, K. L. More, R. L. Borup, A. Z. Weber, D. J. Myers and A. Kusoglu, Nat. Energy, 2021, 6, 462–474 CrossRef CAS.
- K. Khedekar, A. Zaffora, M. Santamaria, M. Coats, S. Pylypenko, J. Braaten, P. Atanassov, N. Tamura, L. Cheng, C. Johnston and I. V. Zenyuk, Nat. Catal., 2023, 6, 676–686 CrossRef CAS.
- S. Komini Babu, R. Mukundan, C. Wang, D. Langlois, D. A. Cullen, D. Papadias, K. L. More, R. Ahluwalia, J. Waldecker and R. Borup, J. Electrochem. Soc., 2021, 168, 044502 CrossRef.
- J. D. Sinniah, W. Y. Wong, K. S. Loh, R. M. Yunus and S. N. Timmiati, J. Power Sources, 2022, 534, 231422 CrossRef CAS.
- E. Antolini, Appl. Catal., B, 2009, 88, 1–24 CAS.
- Y. J. Wang, B. Fang, H. Li, X. T. Bi and H. Wang, Prog. Mater. Sci., 2016, 82, 445–498 CAS.
- A. Kumar, E. J. Park, Y. S. Kim and J. S. Spendelow, Macromol. Chem. Phys., 2024, 225, 2400092 CAS.
- T. T. N. Ho, T. Hirano, R. Narui, H. Tsutsumi, M. Kishi, Y. Yoshikawa, K. L. A. Cao and T. Ogi, Adv. Powder Technol., 2024, 35, 104568 CAS.
- Y. Takabatake, Z. Noda, S. M. Lyth, A. Hayashi and K. Sasaki, Int. J. Hydrogen Energy, 2014, 39, 5074–5082 CAS.
- T. Ikehara, Z. Noda, J. Matsuda, M. Nishihara, A. Hayashi and K. Sasaki, ECS Trans., 2020, 98, 573–582 CAS.
- T. T. N. Ho, T. Hirano, R. Narui, H. Tsutsumi, M. Kishi, Y. Yoshikawa, K. L. A. Cao and T. Ogi, ACS Appl. Energy Mater., 2023, 6, 6064–6071 CAS.
- Y. Senoo, K. Kakinuma, M. Uchida, H. Uchida, S. Deki and M. Watanabe, RSC Adv., 2014, 4, 32180–32188 RSC.
- T. Hirano, T. Tsuboi, K. L. A. Cao, E. Tanabe and T. Ogi, J. Nanopart. Res., 2022, 25, 1 CrossRef.
- T. Hirano, T. Tsuboi, E. Tanabe and T. Ogi, J. Alloys Compd., 2022, 898, 162749 CrossRef CAS.
- T. Hirano, T. Tsuboi, T. T. N. Ho, E. Tanabe, A. Takano, M. Kataoka and T. Ogi, ACS Appl. Energy Mater., 2023, 6, 12364–12370 CrossRef CAS.
- P. H. Le, K. L. A. Cao, Y. Kitamoto, T. Hirano and T. Ogi, Langmuir, 2023, 39, 7783–7792 CAS.
- P. H. Le, Y. Kitamoto, S. Yamashita, K. L. A. Cao, T. Hirano, T. W. M. Amen, N. Tsunoji and T. Ogi, ACS Appl. Mater. Interfaces, 2023, 15, 54073–54084 CAS.
- D. B. Kautsar, P. H. Le, A. Ando, K. L. A. Cao, E. L. Septiani, T. Hirano and T. Ogi, Langmuir, 2024, 40, 8260–8270 CAS.
- T. T. Nguyen, M. Miyauchi, A. M. Rahmatika, K. L. A. Cao, E. Tanabe and T. Ogi, ACS Appl. Mater. Interfaces, 2022, 14, 14435–14446 CAS.
- T. T. Nguyen, A. M. Rahmatika, M. Miyauchi, K. L. A. Cao and T. Ogi, Langmuir, 2021, 37, 4256–4266 CAS.
- T. T. Nguyen, N. S. N. Saipul Bahri, A. M. Rahmatika, K. L. A. Cao, T. Hirano and T. Ogi, ACS Appl. Bio Mater., 2023, 6, 2725–2737 Search PubMed.
- S. Wintzheimer, L. Luthardt, K. L. A. Cao, I. Imaz, D. Maspoch, T. Ogi, A. Bück, D. P. Debecker, M. Faustini and K. Mandel, Adv. Mater., 2023, 35, 2306648 CrossRef CAS PubMed.
- T. Ogi, A. B. D. Nandiyanto and K. Okuyama, Adv. Powder Technol., 2014, 25, 3–17 CrossRef CAS.
- M. Faustini, M. Giraud, D. Jones, J. Rozière, M. Dupont, T. R. Porter, S. Nowak, M. Bahri, O. Ersen, C. Sanchez, C. Boissière, C. Tard and J. Peron, Adv. Energy Mater., 2019, 9, 1802136 CrossRef.
- T. Van Pham, T. Hirano, K. L. A. Cao, E. L. Septiani, E. Tanabe and T. Ogi, Energy Fuels, 2024, 38, 16743–16755 CrossRef.
- P. H. Le, Y. Kitamoto, K. L. A. Cao, T. Hirano, E. Tanabe and T. Ogi, Adv. Powder Technol., 2022, 33, 103581 CrossRef CAS.
- P. H. Le, S. Yamashita, K. L. A. Cao, T. Hirano, N. Tsunoji, D. B. Kautsar and T. Ogi, ACS Appl. Nano Mater., 2023, 6, 17324–17335 CrossRef CAS.
- T. Hirano, T. Tsuboi, T. T. N. Ho, E. Tanabe, A. Takano, M. Kataoka and T. Ogi, Nano Lett., 2024, 24, 10426–10433 CAS.
- A. Shimamura, M. Fukushima, M. Hotta, T. Ohji and N. Kondo, J. Am. Ceram. Soc., 2016, 99, 440–444 CAS.
- A. N. Chen, J. Y. Chen, J. M. Wu, L. J. Cheng, R. Z. Liu, J. Liu, Y. Chen, C. H. Li, S. F. Wen and Y. S. Shi, Ceram. Int., 2019, 45, 21136–21143 CAS.
- Y. Huang, D. Dong, J. Yao, L. He, J. Ho, C. Kong, A. J. Hill and H. Wang, Chem. Mater., 2010, 22, 5271–5278 CrossRef CAS.
- Y. Lee, B.-U. Ye, H. k. Yu, J.-L. Lee, M. H. Kim and J. M. Baik, J. Phys. Chem. C, 2011, 115, 4611–4615 CrossRef CAS.
- A. S. Hassan, K. Moyer, B. R. Ramachandran and C. D. Wick, J. Phys. Chem. C, 2016, 120, 2036–2046 CrossRef CAS.
- X. Wu, J. Tayal, S. Basu and K. Scott, Int. J. Hydrogen Energy, 2011, 36, 14796–14804 CrossRef CAS.
- J. Y. Lim, G. Rahman, S. Y. Chae, K.-Y. Lee, C.-S. Kim and O.-S. Joo, Int. J. Energy Res., 2014, 38, 875–883 Search PubMed.
- R. Balgis, A. F. Arif, T. Mori, T. Ogi, K. Okuyama and G. M. Anilkumar, AIChE J., 2016, 62, 440–450 CAS.
- R. Strobel and S. E. Pratsinis, J. Mater. Chem., 2007, 17, 4743–4756 CAS.
- L. Gradon, R. Balgis, T. Hirano, A. M. Rahmatika, T. Ogi and K. Okuyama, J. Aerosol Sci., 2020, 149, 105608 CAS.
- L. Mädler, H. K. Kammler, R. Mueller and S. E. Pratsinis, J. Aerosol Sci., 2002, 33, 369–389 Search PubMed.
- T. Hirano, S. Kaseda, K. Le Anh Cao, F. Iskandar, E. Tanabe and T. Ogi, ACS Appl. Nano Mater., 2022, 5, 15449–15456 Search PubMed.
- K. L. A. Cao, Y. Kito, P. H. Le, T. Ogi and T. Hirano, ACS Appl. Eng. Mater., 2023, 1, 1789–1798 CAS.
- M. Ismael, A. Sharma and N. Kumar, Sustainable Mater. Technol., 2024, 40, e00826 CAS.
- T. Hirano, J. Kikkawa, F. G. Rinaldi, K. Kitawaki, D. Shimokuri, E. Tanabe and T. Ogi, Ind. Eng. Chem. Res., 2019, 58, 7193–7199 CAS.
- T. Hirano, J. Kikkawa, D. Shimokuri, A. B. D. Nandiyanto and T. Ogi, J. Chem. Eng. Jpn., 2021, 54, 557–565 CrossRef CAS.
- K. Wegner, B. Schimmöller, B. Thiebaut, C. Fernandez and T. N. Rao, Kona Powder Part. J., 2011, 29, 251–265 CrossRef CAS.
- H. K. Kammler, L. Mädler and S. E. Pratsinis, Chem. Eng. Technol., 2001, 24, 583–596 CrossRef CAS.
- W. Y. Teoh, R. Amal and L. Mädler, Nanoscale, 2010, 2, 1324–1347 RSC.
- N. E. Motl, A. K. P. Mann and S. E. Skrabalak, J. Mater. Chem. A, 2013, 1, 5193–5202 RSC.
- R. Strobel, A. Baiker and S. E. Pratsinis, Adv. Powder Technol., 2006, 17, 457–480 CrossRef CAS.
- H. Wu, T. Yildirim and W. Zhou, J. Phys. Chem. Lett., 2013, 4, 925–930 Search PubMed.
- M. Inaba, R. Murase, T. Takeshita, K. Yano, S. Kosaka, N. Takahashi, N. Isomura, K. Oh-ishi, W. Yoshimune, K. Tsuchiya, T. Nobukawa and K. Kodama, ACS Appl. Mater. Interfaces, 2024, 16, 10295–10306 CrossRef CAS PubMed.
- H. Lee, P. R. Deshmukh, J. H. Kim, H. S. Hyun, Y. Sohn and W. G. Shin, J. Alloys Compd., 2019, 810, 151923 CrossRef CAS.
- A. B. D. Nandiyanto, N. Hagura, F. Iskandar and K. Okuyama, Acta Mater., 2010, 58, 282–289 CrossRef CAS.
- C. Malmgren, A. K. Eriksson, A. Cornell, J. Bäckström, S. Eriksson and H. Olin, Thin Solid Films, 2010, 518, 3615–3618 CAS.
- R. G. Vadimsky, R. P. Frankenthal and D. E. Thompson, J. Electrochem. Soc., 1979, 126, 2017–2023 CAS.
- A. Purwanto, W.-N. Wang, I. W. Lenggoro and K. Okuyama, J. Electrochem. Soc., 2007, 154, J91 Search PubMed.
- T. Hirano, N. Kadowaki, A. Ichimiya, R. Narui and T. Ogi, Sci. Technol. Energ. Mater., 2023, 84, 40–44 Search PubMed.
- S. Nakakura, A. F. Arif, F. G. Rinaldi, T. Hirano, E. Tanabe, R. Balgis and T. Ogi, Adv. Powder Technol., 2019, 30, 6–12 CrossRef CAS.
- T. Pinheiro Araújo, J. Morales-Vidal, T. Zou, R. García-Muelas, P. O. Willi, K. M. Engel, O. V. Safonova, D. Faust Akl, F. Krumeich, R. N. Grass, C. Mondelli, N. López and J. Pérez-Ramírez, Adv. Energy Mater., 2022, 12, 2103707 CrossRef.
- R. Ramarajan, M. Kovendhan, K. Thangaraju and D. P. Joseph, Ceram. Int., 2020, 46, 12224–12231 CrossRef CAS.
- M. Chisaka, J. Mater. Chem. A, 2024, 12, 18636–18673 Search PubMed.
- N. Narischat, T. Takeguchi, T. Tsuchiya, T. Mori, I. Ogino, S. R. Mukai and W. Ueda, J. Phys. Chem. C, 2014, 118, 23003–23010 CAS.
- A. Lindermeir, G. Rosenthal, U. Kunz and U. Hoffmann, Fuel Cells, 2004, 4, 78–85 CAS.
- T. Gu, R. Shi, J. Guo, W. Wang, X. Wei, Q. Zhang, J. Luo and R. Yang, Energy Fuels, 2024, 38, 15714–15720 CAS.
- H. Li, Y. Tang, Z. Wang, Z. Shi, S. Wu, D. Song, J. Zhang, K. Fatih, J. Zhang, H. Wang, Z. Liu, R. Abouatallah and A. Mazza, J. Power Sources, 2008, 178, 103–117 CrossRef CAS.
- W. Schmittinger and A. Vahidi, J. Power Sources, 2008, 180, 1–14 Search PubMed.
- J.-M. Le Canut, R. M. Abouatallah and D. A. Harrington, J. Electrochem. Soc., 2006, 153, A857 CrossRef CAS.
- Y. Sone, P. Ekdunge and D. Simonsson, J. Electrochem. Soc., 1996, 143, 1254 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Figure showing SEM images of macroporous NTO/RuO2-50 particles synthesized with different PMMA concentrations and STEM images of macroporous RuO2/NTO particles. A table showing a summary of specific surface areas of macroporous NTO/RuO2 particles. Figure showing BET nitrogen adsorption isotherm plots of NTO nanoparticles and macroporous NTO/RuO2 particles. Table presenting crystallographic parameters of RuO2, Ru, and SnO2 derived from XRD refinement of macroporous NTO/RuO2 particles. Figure presenting the XRD pattern, TEM image and size distribution chart of NTO nanoparticles. Figure showing the cyclic voltammograms of carbon, NTO nanoparticles, and macroporous NTO/RuO2 particles with varying RuO2 binder contents before and after durability tests. See DOI: https://doi.org/10.1039/d4lf00404c |
| This journal is © The Royal Society of Chemistry 2025 |