Open Access Article
Open Access ArticleB,N co-doped V2C nanoparticle embedded FeP nanoflake substrates as unique bifunctional electrocatalysts for overall water splitting in alkaline media†
Dasari Sai
Hemanth Kumar‡
 a,
Manzoor Ahmad
Pandit‡
ab,
Vinay Kumar
Kolakaluri
a and
Krishnamurthi
Muralidharan
a,
Manzoor Ahmad
Pandit‡
ab,
Vinay Kumar
Kolakaluri
a and
Krishnamurthi
Muralidharan
 *a
*a
aSchool of Chemistry, University of Hyderabad, Hyderabad 500046, Telangana, India. E-mail: murali@uohyd.ac.in
bMaterials Genome Institute, Shanghai University, Shanghai 200444, China
First published on 10th March 2025
Abstract
Hydrogen energy as a solution to meet energy demands has highlighted the need for efficient and cost-effective electrocatalysts for hydrogen production through water electrolysis. Heterointerface materials with self-support have shown promising electrochemical performances due to their modulated electron structure, improved electrochemical surface area, and more active sites. In our study, we successfully synthesized a heterostructure material comprising iron phosphide (FeP) nanoflakes as a substrate, embedded with boron (B) and nitrogen (N) co-doped vanadium carbide (V2C) nanoparticles through a hydrothermal method followed by pyrolysis. We prepared FeP@B,N-V2C heterostructures to enhance efficiency using different weight ratios (5%, 10%, 15%, and 20%) of FeP substrates while adjusting B,N-V2C nanoparticles accordingly. The catalytic applicability of these materials was evaluated in electrochemical water splitting in an alkaline medium. Compared to other heterostructures, 10% FeP@B,N-V2C exhibited the highest catalytic activity, with overpotentials for the OER and HER in an alkaline medium of 260 mV and 235 mV, respectively, at a current density of 10 mA cm−2. The low Tafel values were determined as 56.85 mV dec−1 and 118 mV dec−1, with remarkable stability over 24 hours with a higher efficiency of 97.3%. The effectiveness and stability of electrocatalysts were corroborated by its ability in the overall water splitting (OWS), which occurred at a lower onset potential of 1.57 V@10 mA cm−2. The low overpotentials and Tafel values observed in these catalysts are attributed to the heterojunction formed between the FeP nanoflakes and B,N co-doped V2C nanoparticles. The enhancement in electrochemical activity resulting from the heterojunction is due to the higher surface area, increased porosity, decreased electrochemical resistance and the introduction in electroactive centres due to B,N co-doping. Consequently, this study provides a promising platform for developing novel nanomaterials for energy conversion applications.
1. Introduction
The global energy crisis and environmental pollution highlight the need for alternative resources, such as hydrogen (H2) produced through electrochemical water-splitting reactions.1,2 This water-splitting process involves two half-cell reactions: the hydrogen evolution reaction (HER) at the cathode, which is a two-electron process, and the oxygen evolution reaction (OER) at the anode, which is a four-electron process. However, the slow kinetics and multi-electron transfer process involved in the HER and OER present significant challenges.2–7 While noble metal catalysts like IrO2 and RuO2 have demonstrated exceptional electrochemical performances for the OER, Pt/C catalysts have shown superior catalytic activity for the HER. However, their high cost and limited availability restrict their widespread industrial application.8,9 Therefore, there is a pressing need to develop efficient and cost-effective electrocatalysts to replace noble metal catalysts.Research on electrocatalytic water splitting has shown promising developments in transition metal-based catalysts, including chalcogenides, phosphides, carbides, and nitrides. These materials have the potential to serve as cost-effective and stable alternatives due to their natural abundance, electrical conductivity, and optimal Gibbs free energy.10–13 However, their practical application is limited by the weak binding strength between active sites and intermediates. To address this challenge, significant research efforts are focused on developing materials to overcome these binding issues. Among the catalysts, transition metal phosphides (TMPs) have received tremendous attention and proven to be the most promising catalysts.14 It is revealed that the P atom in TMPs has better electronegativity to attract electrons from the transition metal atoms effortlessly, which enhances its electrical conductance.15 Recently, several TMPs including Ni, Co and Fe phosphides have been used as HER or OER electrocatalysts in acidic or basic media with excellent performances.16,17 The presence of d-orbitals makes TMPs act either as nucleophiles or electrophiles in chemical reactions, facilitating intermediate formation and reducing the active hydration energy barrier.18–20 For example, based on DFT studies, Yao et al. found that the synergistic effect of 3d orbitals leads to high HER and OER activity.21
Among the TMPs, FeP garnered special attraction, because the Fe–P bond is found to stimulate the O2 molecule, facilitating the HER/OER kinetics.22–24 The HER mechanism on FeP catalysts resembles the catalytic reaction of [FeFe] hydrogenase enzymes.25 Also, FeP catalysts can be moderately oxidized at certain potentials, leading to oxide and phosphate formation which further accelerate the OER kinetics.26 The core–shell structures are found to reveal higher activity because of the high specific surface area, maximum active sites available and reduced charge transfer distances.27,28 However, FeP activity is limited due to corrosion in both acidic and basic media. On the other hand, the activity of catalysts mostly depends on the structure as well as morphology.29 In some cases, the self-supported FeP can act as a template due to its nanoflake structure, providing parallel elements that react with the other materials to form hierarchical structures.30,31 However, earlier reports have revealed that phosphides with lone transition elements like FeP, Co2P, NiP, etc., have restricted electrochemical abilities.32 Therefore, it's interesting to investigate composites, bimetals or alloys of TMPs for improved performances.
Transition metal carbides (TMCs) revealed excellent electrochemical performance and greater stability in the HER/OER comparable to Pt. Some reports stated that among TMCs, tungsten and molybdenum carbides are outstanding noble metal free electrocatalysts for water splitting.33 However, in the case of VB group TMCs, vanadium based carbides were studied less in electrochemical activities.34 It is found that vanadium carbide (VC) is abundantly available in the earth, making it a strong candidate for electrochemical activities.35 But still, the electrochemical abilities of VC are underexplored and inadequate because of inappropriate interactions between the various reactive intermediates and the surface.36,37 Therefore, the most effective way to enhance the electrocatalytic performance of VC is to dope it with foreign elements, and some recent reports describe this approach as a feasible way.38 For example, Zhao et al., reported the electrochemical OER performance of Pt-doped VC on the basis of DFT studies. The electrochemical performance indicated that the O* did not desorb from the surface easily because of the great Gibbs free energy, which requires an overpotential of 1.86 V. The Gibbs free energy barrier associated with the potential determining step in Pt-doped VC reduces the OER overpotential to 0.58 V, which comes close to 0.56 V of IrO2. This indicates that the doping not only enhances the interactions between hydrogen and the VC surface, but also normalises the interaction of intermediates during the OER.39
Besides doping, structural transformation is another way to enhance the number of active sites including the formation of porous structures, quantum dots, nanosheets/flakes, etc., thereby modifying the electrochemical ability of catalysts.34 For example, Peng et al. claimed that the graphitized carbon engrained in the VC/nickel heterostructure exhibits excellent results in electrochemical HER activity due to the synergetic effect between the various constituents of the heterostructure.38 Likewise, Mengmeng Shao et al. synthesized N-doped VC, which showed high water splitting performance compared to pure VC.40 Based on the above discussion, the co-doping of B and N in V2C embedded FeP nanoflakes is projected to substantially enhance the electrochemical water splitting. Further, to prevent the aggregation of nanoparticles and improve the electrochemical activity as well as stability under harsh conditions, the nitrogen doped carbon was synthesized as a substrate, which reduces the corrosion rates.41 Various reports claimed that addition of B and N on carbon substrates revealed enhanced catalytic results rather than the either pure N or B-doped catalysts.42 However, the low doping concentrations of B and N may lead to the formation of electrochemically inactive B–N bonds, which can hinder the catalytic abilities of these B–N co-doped electrocatalysts.43,44 In particular, the addition of either B or N improves the electrical conductance of carbon based materials by reducing the energy gap, and increasing the density of states at finite and zero energy. For instance, Joshi et al. demonstrated the synthesis of B and N co-doped r-GO containing B–N, B–C, and N–C functional groups, which enhance the stability of IrO2 nanoparticles and elevate the electrochemical water splitting efficiency.45 However, the barriers couldn't be completely removed due to the complex nature of the system. Therefore, it is vital to develop a controlled strategy for dual doping to construct an effective system while maintaining a robust structure–activity relationship.
In our study, we highlight a heterojunction material, FeP@B,N-V2C, which was produced by depositing B,N co-doped V2C nanoparticles onto FeP nanoflakes acting as a substrate using a hydrothermal method with subsequent pyrolysis. We examined with different weight ratios of FeP and B,N-V2C, to achieve a controllable and effective electrocatalytic activity. The resulting electrocatalysts exhibited promising electrochemical activity in water splitting for both the HER and OER. Notably, 10% FeP@B,N-V2C demonstrated the best catalytic activity among the various heterostructures, with overpotentials of 260 mV and 235 mV at a current density of 10 mA cm−2 for the OER and HER, respectively, in an alkaline medium. Additionally, this particular composition displayed remarkable durability over 24 hours for both the OER and HER. Encouraged by this excellent bifunctional performance, we further evaluated the overall water-splitting reaction of 10% FeP@B,N-V2C using a two-electrode cell, which demonstrated outstanding electrochemical performance and remarkable durability. Consequently, our work establishes that the heterojunction formed between FeP and B,N-V2C exhibits promising performance for water splitting (OER/HER) reactions, suggesting a new avenue for the development of materials for green energy generation.
2. Experimental section
2.1. Materials
Ammonium metavanadate (NH4VO3), ferrous sulphate dihydrate (FeSO4·2H2O), sodium hypophosphite monohydrate (NaH2PO2·2H2O), melamine, glucose, boric acid, ammonia, potassium hydroxide, hydrogen peroxide, and Nafion were purchased from Sigma Aldrich. Millipore water was used for the electrochemical reactions. All listed chemicals were of analytical grade and used without further processes.2.2. Instrumentation
The synthesized FeP@B,N-V2C materials were characterized by various techniques for their structural, morphological, and elemental information. The powder X-ray diffraction (PXRD) structure and phase were interpreted by employing a Bruker D8 Advance spectrometer (CuKα, λ = 1.54056° A, 2θ = 20° to 70°). Field Emission Scanning Electron Microscopy (FESEM), Transmission Electron Microscopy (TEM), and Energy-Dispersive X-Ray Spectroscopy (EDS) techniques were utilized for determining the morphology and elemental analysis of the heterostructures by employing an Ultra 55 Carl Zeiss microscope with operating voltage = 10 kV and TEM (FEI Tecnai G2 F20 STEM with 200 kV). The specific surface area and pore size distribution were studied by nitrogen adsorption–desorption isotherms with a Brunauer–Emmett–Teller instrument (BET) (Nova 2000e, Quantachrome Instruments Limited, USA, using liquid nitrogen (77 K)). X-ray photoelectron spectroscopy (XPS) (Thermo Scientific Escalab 250Xi spectrometer with Al-Kα radiation) was employed to determine the oxidation states and the chemical structure of the elements in the heterostructures. The electrochemical activities for performing the HER/OER of the different synthesized materials were studied by using a CHI6112E electrochemical workstation with a three-electrode arrangement.2.3. Synthesis of FeP@B,N-V2C heterostructures
2.4. Preparation of working electrodes for the HER/OER
In order to evaluate the electrochemical water-splitting performance of the synthesized materials (FeP@B,N-V2C), a three-electrode cell was established. The working electrode consisted of the synthesized materials coated on nickel foam (NF, 0.5 cm × 2 cm), while a saturated calomel electrode served as the reference electrode and a graphite rod acted as the counter electrode. These electrodes were immersed in a 1 M KOH alkaline medium. The electrocatalysts were coated on the NF by forming a slurry of 2 mg of catalyst, 400 μL ethanol, 500 μL Millipore water, and 30 μL Nafion. The coatings were applied three times and then dried overnight in an oven. The electrochemical activity was obtained multiple times to ensure the reliability of the results. The potentials were calibrated with reference to the reversible hydrogen electrode (RHE) using the equation ERHE = ESCE + 0.241 + 0.0591 pH. The onset overpotential (@j10 mA cm−2) and Tafel plots (log(j) versus potentials (RHE)) were determined by performing Linear Sweep Voltammetry (LSV) at a scan rate of 5 mV s−1. The double-layer capacitance (Cdl) was calculated from cyclic voltammetry (CV) measurements at sweep rates ranging from 20 mV s−1 to 200 mV s−1. Electrochemical Impedance Spectroscopy (EIS) was also conducted at a frequency range of 100 kHz to 100 mHz with an amplitude of 10 mV.3. Results and discussion
3.1. Synthesis and characterization
We successfully synthesized the desired materials, FeP@B and N-V2C, using a straightforward hydrothermal approach (Scheme 1) without adding any external capping agent or stabilizer. The synthesis involved boron and nitrogen co-doped V2C nanoparticles dispersed on FeP nanoflakes. Initially, V2C nanoparticles were prepared using the hydrothermal approach with glucose and melamine as carbon sources and morphology directional agents. Boron and nitrogen were doped onto the V2C nanostructures to improve their electrochemical performance. Next, iron oxyhydroxide was prepared by slightly modifying a reported method, which involves rapidly oxidizing precipitated Fe2+ salt.46,47 This intermediate was then subjected to pyrolysis with a phosphorus source under a constant nitrogen flow to yield FeP nanoflakes. Composites of optimized activity were synthesized using different weight ratios (5%, 10%, 15%, and 20%) of FeP and B,N-V2C through ultrasonication. Ultrasonication is a consistent method to redisperse nanopowders in liquids and the insertion of nanomaterials into mesoporous materials without changing the intrinsic physicochemical properties of the base materials.48,49The synthesized nanostructures, FeP@B,N-V2C, were initially characterized using PXRD to determine the phase purity of the materials. The PXRD pattern (Fig. 1) revealed that V2C nanoparticles exhibited a crystalline XRD pattern with prominent diffraction peaks at 2θ values of 33.12°, 35.95°, 41.07°, 46.59°, 53.71°, 61.46° and 64.51°, corresponding to the (020), (002), (121), (211), (202), (113) and (132) planes, consistent with the orthorhombic phase of V2C (JCPDS no. 71-1272). However, upon boron and nitrogen doping, most of the V2C peaks were obscured, reducing the crystallinity of the V2C nanostructures. Two new peaks emerged, one broad peak at around 2θ of 25° and another less intense peak at 50°, corresponding to the (002) and (001) planes, confirming the boron and nitrogen doping.50,51 Similarly, pure FeP nanoflakes exhibited intense XRD patterns at 2θ values of 30.81°, 32.73°, 34.52°, 35.41°, 37.08°, 46.27°, 46.97°, 48.31°, 50.35°, 56.05°, and 59.39°, with the corresponding lattice planes of (020), (011), (200), (120), (111), (121), (220), (211), (130), (031), and (002), indicating the orthorhombic phase of pure FeP (JCPDS no. 89-4863).52 Upon increasing the percentage (5%, 10%, 15%, and 20%) of FeP, the XRD pattern revealed that all the peaks of B,N-V2C were related to the orthorhombic phase of FeP nanoflakes. The intensity of the peaks around 2θ of 25° and 50° increased with the rise in FeP percentage, indicating the formation of composite nanomaterials, as depicted in Fig. 1. Also, in the composites, it is observed that the peaks around 2θ of 33°, 34° and 46° were merged with their intensity further increasing as the FeP amount is increased, while the peak around 60° slightly shifted towards a higher 2θ value. This further confirmed the heterostructure formation between the B,N co-doped V2C nanoparticles and FeP nanoflakes.
 | ||
| Fig. 1 The PXRD patterns of pure V2C, B,N-V2C, pure FeP, and the composite FeP@B,N-V2C nanomaterials (5%, 10%, 15%, 20% ratios). | ||
The XPS data were acquired to provide a comprehensive understanding of the structure of the synthesized nanocomposites, encompassing their elemental composition and valence states. To confirm the electronic interaction in the composite formation, we have performed XPS analysis of individual FeP and B,N-V2C nanoparticles as shown in Fig. S4.† The survey spectra (Fig. S3a and S4a and b†) of the 10 wt% FeP@B,N-V2C nanostructures and FeP, B,N-V2C nanoparticles validate the presence of all anticipated elements, namely Fe, P, B, N, V, and C in the material. In the XPS spectrum of V 2p (Fig. 2c), two peaks were further split into two peaks at binding energies of 515.95 eV, 517.2 eV, 523.05 eV, and 524.53 eV attributed to V3+ 2p3/2, V4+ 2p3/2, V3+ 2p1/2, and V4+ 2p1/2, respectively. In the case of V 2p of V2C (Fig. S4g†), two peaks (2p3/2 and 2p1/2) are deconvoluted into two peaks with binding energies of 515.71 eV, 517.09 eV, 522.89 eV, and 524.60 eV attributed to V3+ 2p3/2, V4+ 2p3/2, V3+ 2p1/2, and V4+ 2p1/2, respectively. Regarding the C 1s spectrum (Fig. 2d), the binding energies of 283.84 eV, 284.65 eV, 286.09 eV, and 288.05 eV are associated with V–C, C–C, C–O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.53–55 For the deconvolution of C 1s (Fig. S4h†), binding energies of 284.67 eV, 285.44 eV, and 287.69 eV are attributed to C–C, C–O, and O–C
O bonds, respectively.53–55 For the deconvolution of C 1s (Fig. S4h†), binding energies of 284.67 eV, 285.44 eV, and 287.69 eV are attributed to C–C, C–O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively. The confirmation of boron and nitrogen doping onto V2C nanoparticles can be ascertained from the XPS spectra of boron (B 1s) and nitrogen (N 1s), as depicted in Fig. 2(a) and (b). In the B 1s XPS spectrum, B–C sp2, B–N peaks were observed, indicating the connection of boron atoms to carbon atoms of V2C nanoparticles. In the N 1s XPS spectrum, binding energies of 398.5 eV, 399.9 eV, and 400.5 eV correspond to C
O bonds, respectively. The confirmation of boron and nitrogen doping onto V2C nanoparticles can be ascertained from the XPS spectra of boron (B 1s) and nitrogen (N 1s), as depicted in Fig. 2(a) and (b). In the B 1s XPS spectrum, B–C sp2, B–N peaks were observed, indicating the connection of boron atoms to carbon atoms of V2C nanoparticles. In the N 1s XPS spectrum, binding energies of 398.5 eV, 399.9 eV, and 400.5 eV correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–N, and B
N, C–N, and B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C bonds, respectively, denoting the interaction of carbon of V2C nanoparticles with nitrogen.56–58 Similarly, doping of B and N onto V2C nanoparticles were confirmed by the B 1s and N 1s XPS spectra (Fig. S4e and f†), with binding energies of 192.3 eV, 398.67 eV, 400.08 eV, and 400.78 eV attributed to B–C, N
N–C bonds, respectively, denoting the interaction of carbon of V2C nanoparticles with nitrogen.56–58 Similarly, doping of B and N onto V2C nanoparticles were confirmed by the B 1s and N 1s XPS spectra (Fig. S4e and f†), with binding energies of 192.3 eV, 398.67 eV, 400.08 eV, and 400.78 eV attributed to B–C, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, N–C, and B
C, N–C, and B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C respectively. The Fe 2p XPS spectra (Fig. 2e) exhibit binding energies of 711.4 eV and 724.4 eV, indicating the presence of Fe3+ 2p3/2 and Fe3+ 2p1/2, with the energies at 714.05 eV and 726.44 eV attributed to the satellite peaks of Fe2+. Likewise, the Fe 2p spectra (Fig. S4c†) deconvoluted into two peaks with binding energies of 712.74 eV and 726.26 eV attributed to Fe3+ 2p3/2 and Fe3+ 2p1/2, with satellite peaks of 716.21 eV and 729.83 eV, confirming the presence of Fe2+, and a small peak at the binding energy 707.61 eV attributed to Fe–O bonds, indicating the slight oxidation during pyrolysis. Similarly, the P 2p XPS spectra (Fig. 2f) display three peaks at 129.7 eV, 133.5 eV and 135.1 eV, related to the Fe–P, P–C and P–O bonds, respectively. In the case of P 2p XPS spectra (Fig. S4d†), it is deconvoluted into two peaks with binding energies of 130.37 eV and 134.50 eV attributed to Fe–P and P–O bonds, as corroborated by the previous literature.59–69 Consequently, the aforementioned XPS data provides compelling evidence regarding the formation of the composite materials, further affirming and substantiating the successful deposition of V2C nanoparticles on the FeP nanoflakes. There is a slight shift in binding energies with the heterostructure compared to individual nanoparticles, which is an evident proof that the composite materials are not just a physical mixture and there is coupling linkage between FeP and B,N-V2C during the synthesis of the heterostructure.
N–C respectively. The Fe 2p XPS spectra (Fig. 2e) exhibit binding energies of 711.4 eV and 724.4 eV, indicating the presence of Fe3+ 2p3/2 and Fe3+ 2p1/2, with the energies at 714.05 eV and 726.44 eV attributed to the satellite peaks of Fe2+. Likewise, the Fe 2p spectra (Fig. S4c†) deconvoluted into two peaks with binding energies of 712.74 eV and 726.26 eV attributed to Fe3+ 2p3/2 and Fe3+ 2p1/2, with satellite peaks of 716.21 eV and 729.83 eV, confirming the presence of Fe2+, and a small peak at the binding energy 707.61 eV attributed to Fe–O bonds, indicating the slight oxidation during pyrolysis. Similarly, the P 2p XPS spectra (Fig. 2f) display three peaks at 129.7 eV, 133.5 eV and 135.1 eV, related to the Fe–P, P–C and P–O bonds, respectively. In the case of P 2p XPS spectra (Fig. S4d†), it is deconvoluted into two peaks with binding energies of 130.37 eV and 134.50 eV attributed to Fe–P and P–O bonds, as corroborated by the previous literature.59–69 Consequently, the aforementioned XPS data provides compelling evidence regarding the formation of the composite materials, further affirming and substantiating the successful deposition of V2C nanoparticles on the FeP nanoflakes. There is a slight shift in binding energies with the heterostructure compared to individual nanoparticles, which is an evident proof that the composite materials are not just a physical mixture and there is coupling linkage between FeP and B,N-V2C during the synthesis of the heterostructure.
 | ||
| Fig. 2 XPS binding energies of (a) B 1s, (b) N 1s, (c) V 2p, (d) C 1s, (e) Fe 2p, and (f) P 2p of 10% FeP@B,N-V2C, respectively. | ||
Based on the UV-vis absorption spectrum analysis, it was observed that the composite nanostructures and pure materials (Fig. S3b and c†) exhibited broad absorption in the UV-vis and NIR regions, respectively. The bandgap values, ranging from 0.98 eV to 1.25 eV for the synthesized nanostructures, were determined using the Tauc plot (eqn (1)).67
| αhγ = A(hγ − Eg) | (1) |
3.2. Morphology and surface area
The morphology of the as-synthesized nanocomposites, including B,N-V2C, pure FeP, and FeP@B,N-V2C, was analyzed using FESEM (Fig. 2). In Fig. 3a, the undoped V2C exhibits a spherical morphology with a highly crystalline nature. Upon doping V2C with boron and nitrogen, the spherical morphology is retained but with a larger average particle size. The overall crystallinity is reduced, and the material appears slightly amorphous, as observed in PXRD (Fig. 3b). The pure FeP displays a flower-type morphology with protruding petals growing outwards vertically along the (211) direction as shown in Fig. 3c. | ||
| Fig. 3 The morphological analysis showing high magnification FESEM images of (a) pure V2C, (b) B,N-V2C, (c) FeP and (d) 5%, (e) 10%, (f) 15%, and (g) 20% wt. ratios of B,N-V2C@FeP nanostructures. | ||
The composite structures present a significantly different image compared to pure FeP and B,N co-doped V2C. A combination of well-dispersed particles and fully developed flowers is clearly visible. At a 5% weight ratio, the FeP@B,N-V2C heterostructure exhibits flower growth, confirming the dispersion of B,N co-doped V2C nanoparticles onto FeP nanoflakes, resulting in enlarged petals as depicted in Fig. 3d. As the weight ratio of FeP increases, the B,N-V2C nanoparticles are uniformly distributed onto the nanoflakes, resulting in compact and dense flowers, as shown in Fig. 3e. Notably, at a 15% weight ratio, fully grown and well-developed flowers with thin petals are abundantly found, as displayed in Fig. 3f. However, the increase in weight percentage does not follow a consistent trend. For the 20% weight ratio, thick and short flowers were observed.
The elemental distribution of pure FeP, pure V2C, B,N co-doped V2C, and FeP@B,N-V2C nanocomposites was determined using EDS and elemental mapping images. These analyses revealed the presence of added elements during their synthesis, including Fe, P, V, C, B, and N within all nanostructures, in accordance with stoichiometric ratios (Fig. S1 and S2†).
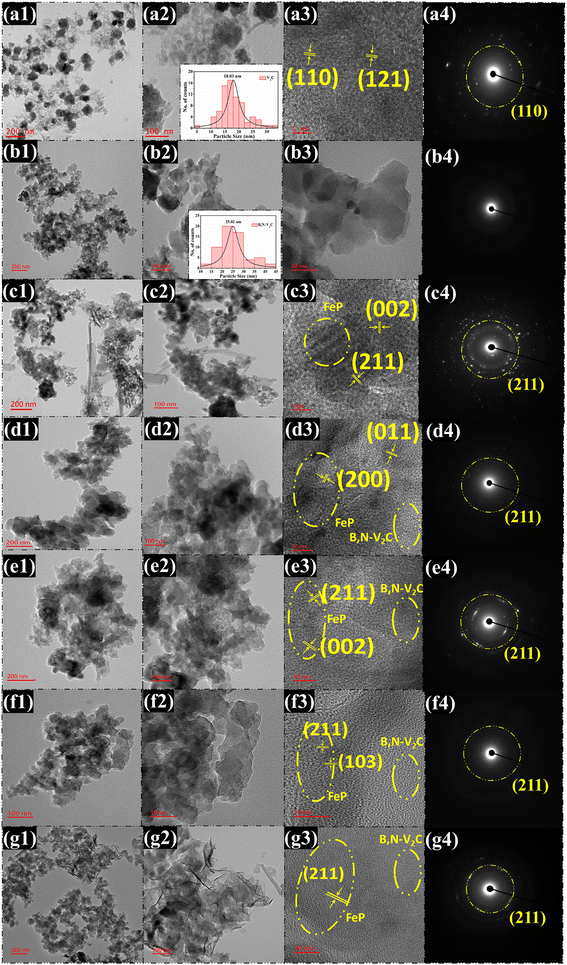
The morphology of the synthesized materials was further analyzed using TEM, which exhibited good agreement with the images obtained from FESEM. The TEM images in Fig. 4a1 and b1 depict the spherical particle nature of pure V2C with an average particle size of 18.03 nm (inset of Fig. 4a2), consistent with the observations from the FESEM images, and similar observations were made for the B,N co-doped V2C with an average particle size of 25.02 nm (inset of Fig. 4b2). High-resolution TEM (HRTEM) images (Fig. 4a3 and b3) of pure V2C and B,N co-doped V2C nanoparticles revealed that the lattice planes could be indexed to the (110) and (121) planes, with corresponding d-spacings of 3.57 and 2.18 nm, which is in good agreement with PXRD results. The selected area electron diffraction (SAED) patterns (Fig. 4a4 and b4) displayed bright spots corresponding to the (110) and (121) planes, confirming the crystalline nature of V2C nanoparticles. In contrast, no bright spots were visible in the doped V2C, indicating the amorphous nature of the B,N co-doped V2C nanoparticles.
The nanostructure of FeP, as depicted in Fig. 4c1, exhibits a flower-like morphology with vertically grown flakes. The corresponding d-spacing values of 2.95 and 1.91 nm (Fig. 4c3) can be associated with the (002) and (211) planes, showing good agreement with the PXRD pattern. The SAED patterns (Fig. 4c4) display distinct bright rings of the (211) planes, indicating the high crystallinity of FeP nanoflakes. Additionally, the TEM images of the composite materials with weight ratios of 5%, 10%, 15%, and 20% were examined alongside their pure material counterparts. Fig. 4d1 illustrates the TEM image of the 5% heterostructure, revealing the homogeneous dispersion of B,N co-doped V2C nanoparticles on FeP nanoflakes. The deposition of nanoparticles on FeP nanoflakes and their role as the substrate are evident in the encircled regions in the figure. The corresponding HRTEM image and SAED patterns (Fig. 4d3 and d4) align well with the individual nanostructures. Similar TEM morphological observations were noted for the other composite materials (10%, 15%, and 20% weight ratios). It was observed that with an increase in FeP weight ratio, the B,N co-doped V2C nanoparticles were well dispersed onto the nanoflakes, consistent with the FESEM images. The morphological studies conducted using FESEM and TEM are concordant with each other and with the PXRD patterns of the synthesized materials.
The catalytic activity of materials heavily depends on the specific surface area, making it a critical parameter. The surface analysis of the synthesized materials was conducted through N2 adsorption–desorption analysis and BET isotherms. Fig. 5a–d illustrate the adsorption isotherms of B,N co-doped V2C nanostructures with varying weight ratios (5%, 10%, 15%, and 20%). These curves exhibit a type IV isotherm, indicating the mesoporous nature of the composites. Among the different weight percentages synthesized, the 10% FeP@B,N-V2C nanostructure exhibits the highest surface area (146.08 m2 g−1), followed by 5% (143.59 m2 g−1), 15% (132.72 m2 g−1), and 20% (111.28 m2 g−1). Additionally, the pore size distribution corresponding to these weight percentage ratios is determined using the BJH method (Fig. S5a–d†), confirming the mesoporous nature of the composite materials. The larger surface area of the 10% FeP@B,N-V2C nanostructure further elucidates its superior electrocatalytic behavior towards the HER and OER, indicating the presence of numerous active sites on the nanostructures.
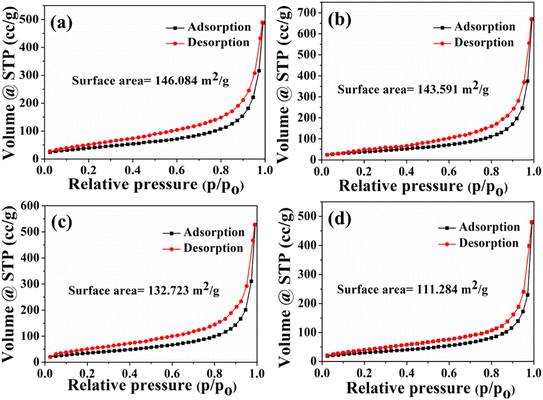 | ||
| Fig. 5 (a–d) Nitrogen adsorption–desorption isotherms of 5%, 10%, 15%, and 20% wt. ratios of FeP@B,N-V2C nanostructures, respectively. | ||
3.3. Electrochemical oxygen evolution reaction (OER)
The synthesized materials were evaluated for their catalytic performance in electrochemical water splitting. A three-electrode system was employed to assess the electrochemical water splitting (HER/OER) capabilities of the synthesized nanostructures, including pure FeP, B,N-V2C, and FeP@B,N-V2C nanocomposites. The working electrode was created by depositing the synthesized material on nickel foam (NF) to provide active sites on the electrode, with saturated calomel as the reference electrode and a graphite rod as the counter electrode, in a 1 M KOH alkaline medium. Linear sweep voltammetry (LSV) measurements revealed that the overpotential obtained for 10% FeP@B,N-V2C was lower at 260 mV compared to 5% (310 mV), 15% (298 mV), and 20% (297 mV) at 10 mA cm−2 current density for the OER. At the same current density, the pure FeP nanoflakes, V2C nanoparticles, and B,N-V2C nanoparticles exhibited significantly higher overpotentials of 290, 379, and 360 mV, respectively, while RuO2 showed an overpotential of 312 mV. The presence of nitrogen constructively modifies the electronic structure and increases the active sites by modifying the charge distribution. Also, the introduction of heteroatoms like B and N simultaneously into carbon hybrids disrupts the charge-neutralities of carbon atoms and as a result, the heterostructure of B,N-doped V2C with FeP nanoflakes produced significant results in the electrochemical oxygen reduction reaction.70In order to further validate the effectiveness of OER catalysis, Tafel plots were generated (Fig. 6b). The Tafel plots were derived from the linear segment of the LSV (linear sweep voltammetry) curve, based on the Tafel equation (η = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log|j|, where b represents the Tafel slope, η denotes the overpotential, and j indicates the current density). The plots indicated the following trend: the 10% weight ratio exhibited the lowest Tafel slope (56.85 mV dec−1) when compared to the 5% (76.9 mV dec−1), 15% (81.8 mV dec−1), and 20% (74.2 mV dec−1) weight ratios. The individual materials RuO2, FeP, V2C, and B,N-V2C demonstrated Tafel slopes of 197, 74.3, 111.8, and 84.2 mV dec−1, respectively, which correlated with the observed overpotential values for these materials. Notably, the 10% FeP@B,N-V2C heterostructure exhibited the lowest overpotential and Tafel slope values, suggesting minimal interference with charge carriers and superior mass transport behavior, thus positioning it as a promising electrocatalyst.
log|j|, where b represents the Tafel slope, η denotes the overpotential, and j indicates the current density). The plots indicated the following trend: the 10% weight ratio exhibited the lowest Tafel slope (56.85 mV dec−1) when compared to the 5% (76.9 mV dec−1), 15% (81.8 mV dec−1), and 20% (74.2 mV dec−1) weight ratios. The individual materials RuO2, FeP, V2C, and B,N-V2C demonstrated Tafel slopes of 197, 74.3, 111.8, and 84.2 mV dec−1, respectively, which correlated with the observed overpotential values for these materials. Notably, the 10% FeP@B,N-V2C heterostructure exhibited the lowest overpotential and Tafel slope values, suggesting minimal interference with charge carriers and superior mass transport behavior, thus positioning it as a promising electrocatalyst.
The superior OER performance exhibited by the synthesized electrocatalysts may be attributed to the synergistic effect between the components, which enables better transfer of charge carriers and enhances catalytic activity. The charge transfer resistance (Rct) values were determined through Electrochemical Impedance Spectroscopy (EIS) studies conducted in the frequency range of 100 kHz to 100 mHz, as illustrated in Fig. 6c. Analysis of the Nyquist plots revealed a smaller semicircle for 10% FeP@B,N-V2C compared to the other composite materials, indicating lower impedance to the transfer of charges, thereby facilitating rapid electron transfer from the substrate to the catalyst and promoting faster reaction kinetics (refer to Table S1 in the ESI†).
The stability and durability of the material are crucial factors for an electrocatalyst. The electrochemical durability of the electrocatalyst was confirmed by conducting both chronoamperometry (i–t) and continuous CV cycle studies, as depicted in Fig. 6d–f. The peak around 1.4 V is related to the onset of the OER on the working electrode nickel foam (NF). This observation indicates where the nickel-based catalysts begin to smoothen the OER, forming new active sites like NiOOH or Ni(OH)2 phases. A stable OER performance was observed between 1.5 V and 1.8 V.1,71 The study revealed that there was no significant change in the current density and overpotential after 24 hours of stability testing, indicating the long-term durability of the 10% FeP@B,N-V2C electrocatalyst and other heterostructures for the OER (Fig. S6†). Similarly, there were insignificant changes in the overpotential after 1000 cycles of the 10% FeP@B,N-V2C catalyst compared to other materials and pure components (Fig. S6†), demonstrating the excellent corrosion resistance of the catalyst. Therefore, the stability tests suggest a smooth transfer of charge carriers and efficient mass transfer across the electrolyte–electrode interface.
In order to delve deeper into the kinetics of the electrocatalysts, we measured the electrochemical double-layer capacitance (Cdl) and electrochemical surface area (ECSA) values by conducting CV cycles with scan rates ranging from 20 to 200 mV s−1 within the potential range of 1.14–1.26 V vs. RHE for 10% FeP@B,N-V2C (Fig. 7a and b) and the remaining materials (Fig. S8†). We found that the ECSA value for 10% FeP@B,N-V2C (1.96 mF cm−2) was higher than those for the other heterostructures. This difference may be attributed to the distinctive distribution of double-doped V2C nanoparticles, resulting in a maximum surface area and more active sites, which in turn enhances the Cdl/ECSA values (refer to Table S1 in the ESI†) and facilitates faster charge/ion diffusion within the electrolyte–electrode interface. The volumetric analysis of O2 produced (Fig. S12a†) indicated a faradaic efficiency of 97.3% for 10% FeP@B,N-V2C, with similar efficiencies of 96.4%, 95.8%, and 93.4% observed for the other heterostructures. The quantification of oxygen evolved using GC-TCD (as shown in Fig. S13a†) confirmed these results.
 | ||
| Fig. 7 Cyclic voltammograms and linear fit plots for (a and c) the OER and (b and d) HER at different scan rates from 20 mV s−1 to 200 mV s−1 of the 10% FeP@B,N-V2C nanostructure, respectively. | ||
3.4. Electrochemical hydrogen evolution reaction (HER)
The dual functionality of the electrocatalyst was further investigated by testing its hydrogen evolution reaction (HER) activity, which yielded results comparable to its OER activity. The polarization curves for the Linear Sweep Voltammetry (LSV) measurements are illustrated in Fig. 8a, revealing an overpotential of 235 mV at a current density of 10 mA cm−2 for the 10% FeP@B,N-V2C catalyst. Similarly, overpotentials of 258 mV, 263 mV, and 271 mV at the same current density were observed for the 5%, 15%, and 20% FeP@B,N-V2C catalysts, respectively. In comparison, the overpotentials were 92 mV, 260 mV, 271 mV, and 258 mV for Pt/C, pure FeP, V2C, and B,N-V2C, respectively. Similar to the OER, the electrocatalysts also exhibited superior performance in the HER.In order to gain a deeper understanding of the kinetics of the HER, Tafel plots were obtained (Fig. 8b). The Tafel slope of 10% FeP@B,N-V2C is only 118 mV dec−1, which is lower than those of 5% (123 mV dec−1), 15% (128 mV dec−1), and 20% (130 mV dec−1) FeP@B,N-V2C heterostructures. In comparison, the Tafel slopes for Pt/C, FeP, and B,N-V2C are 68 mV dec−1, 127 mV dec−1, and 124 mV dec−1, respectively. These remarkable findings suggest that though we could not exceed the benchmark catalyst Pt/C, the overpotential and Tafel values are superior to those of the electrocatalysts mentioned in the previous literature (refer to Table S3 in the ESI†). The results indicate that the synergistic effect observed at the interface between FeP and dual-doped V2C drives the performance of the prepared electrocatalysts to higher levels, thereby enhancing the transfer of charge carriers and mass transfer reaction, ultimately achieving maximum water reduction. The EIS measurements, as shown in Fig. 8c, clearly indicate lower resistance and higher flow of charge carriers for 10% FeP@B,N-V2C compared to other materials, further confirming its superior electrocatalytic behavior (refer to Table S2 in the ESI†).
As in the study of the OER, we have also conducted stability and durability measurements for the HER using chronoamperometry and 1000 CV cycles. The 10% FeP@B,N-V2C (Fig. 8d–f) catalyst maintained a stable current density over 24 hours with no significant change in overpotential, demonstrating the catalyst's durability. Similar stability was observed for other heterostructure electrocatalysts within the same time frame (Fig. S7†). Additionally, there was no appreciable change in the overpotential after 1000 CV cycles for the 10% FeP@B,N-V2C heterostructure and other electrocatalysts, indicating minimal resistance to charge flow between the electrocatalyst and the electrode. Cyclic voltammetry at a sweep rate of 20–120 mV s−1 and within the potential window of 0.62–0.72 V vs. RHE (Fig. 7c and d for 10% FeP@B,N-V2C and other catalysts in Fig. S9†) can be used to measure the Cdl values of the electrocatalyst. Notably, the ECSA values are higher for the 10% FeP@B,N-V2C catalyst (3.71 mF cm−2) than other catalysts (Table S2 in the ESI†). The quantification of H2 produced at j10 (Fig. S12b†) revealed a high efficiency of 87% for 10% FeP@B,N-V2C and the presence of hydrogen evolved was confirmed through GC-TCD analysis as illustrated in Fig. S13b.†
3.5. Plausible mechanism of the OER & HER
The OER mechanism involves four cooperative proton–electron transfer processes occurring at the active sites of catalysts. The first step is the hydroxide layer reaction (eqn (i)), in which the adsorption of OH− ions on the electrode surface occurs through the loss of one electron. This reaction is followed by the oxide layer reaction (eqn (ii)), where the hydroxide layer further reacts with OH− ions to form a metal oxide layer through the loss of one electron. The next step is the hydroxylation reaction (eqn (iii)), in which the OH− ions react with the metal oxide layer, resulting in the formation of an oxyhydroxide layer by removing one electron. The final step is the O2 adsorption reaction (eqn (iv)), where the oxyhydroxide layer reacts with OH− ions to produce O2.| M + OH− → MOH + e− | (i) |
| MOH + OH− → MO + H2O + e− | (ii) |
| MO + OH− → MOOH + e− | (iii) |
| MOOH + OH− → M + O2 + H2O + e− | (iv) |
In the OER mechanism outlined above, the hydroxylation reaction and the formation of M–OOH species are identified as the rate-limiting and rate-determining steps, respectively. The mechanism and reaction kinetics of the HER for the FeP@B,N-V2C heterostructures were evaluated using calculated Tafel slope values. To theoretically assess the rate kinetics of the HER mechanism in an alkaline medium, reference Tafel slope values were obtained from the Volmer equation (eqn (v)) at 120 mV dec−1, the Heyrovsky equation (eqn (vi)) at 40 mV dec−1, and the Tafel equation (eqn (vii)) at 30 mV dec−1.
| H2O + e− → H(ads) + OH− | (v) |
| H(ads) + H2O + e− → H2 + OH− | (vi) |
| H(ads) + H(ads) → H2 | (vii) |
In these reactions, H(ads) indicates the presence of the hydrogen atom on the active site of the electrocatalyst. The initial Volmer reaction represents the discharge step, which generates H(ads) and necessitates a potential of 120 mV dec−1. The subsequent step involves electrochemical desorption, which requires a potential of 40 mV dec−1 for the Heyrovsky reaction and 30 mV dec−1 for the Tafel reaction. In the current study, all electrodes exhibited Tafel slope values near 120 mV dec−1 (Table S1†), suggesting that each electrode adheres to the Volmer-Heyrovsky mechanism for the HER.72–74
3.6. Electrochemical overall water splitting (OWS)
Optimal performance in electrolysis requires distinct conditions when employing two different catalysts for the HER and the OER. Furthermore, the challenging conditions associated with water electrolysis—such as extreme pH levels, high potential, and the need for long-term stability—make the leaching and reconstruction of electrocatalysts inevitable. This phenomenon can result in significant cross-contamination between the electrodes if they utilize dissimilar materials. In contrast, bifunctional electrodes that use the same catalyst material for both the HER and OER present several advantages, including simplified production, broader applicability, and the prevention of cross-contamination.75,76The 10% FeP@B,N-V2C heterostructure demonstrated excellent bifunctional electrocatalytic water splitting (HER/OER) results. Therefore, it is interesting to test its ability as both an anode and cathode in a two-electrode configuration for overall water splitting (OWS). The performance was evaluated through LSV and chronoamperometry measurements in an H-type cell using an exchange membrane as a separator and 1 M KOH as the electrolyte. Fig. 9a illustrates the LSV graphs at a scan rate of 5 mV s−1, showing a low cell voltage of 1.57 V@j10 for the 10% FeP@B,N-V2C heterostructure, compared to 1.9 V, 1.79 V, and 1.91 V for 5%, 15%, and 20% FeP@B,N-V2C heterostructures, respectively. We have observed an oxidation peak in between 1.4 V and 1.75 V in CV measurements and the same was reflected in the LSV plot. This may be due to ion oxidation in the electrolyte during water splitting.
As depicted in Fig. 9, long-term chronoamperometry and chronopotentiometry stability measurements indicate consistent performance with unchanged activity at 10 mA cm−2 current density for the 10% FeP@B,N-V2C heterostructure and other electrocatalysts (Fig. S10†). Some minor fluctuations in device stability may be attributed to excess bubble formation at the exposed electrode sites. An analysis of the 10% FeP@B,N-V2C heterostructure after water electrolysis is presented in Fig. S11.† Comparative analysis with the previous literature (Table S4 in ESI†) demonstrates the superior electrocatalytic activity of the materials used in this study. The results validate the enhanced electrochemical stability and performance resulting from the heterojunction formed between the flakes and particle aggregation at the nanoscale.
The results from the RDE and H-cell experiments are essential, as they can be applied to full-cell experiments, offering valuable insights into the kinetics and reaction mechanisms of the anode and cathode materials. This transferability enables a more informed approach to the design and optimization of the full-cell, particularly in the selection of suitable materials, refinement of electrolyte composition, and comprehension of potential limitations related to mass transport and crossover effects.77–82
4. Conclusion
In this study, a non-noble metal-based catalyst comprising hierarchical boron and nitrogen co-doped V2C nanoparticles on FeP nanoflakes as a substrate was successfully synthesized using a straightforward hydrothermal approach and pyrolysis method. Various advanced spectroscopic and microscopic techniques confirmed the structural and morphological features of the materials. The electrocatalysts exhibited highly efficient bifunctional electrochemical water splitting activity towards the HER/OER in an alkaline medium. Notably, the 10% FeP@B,N-V2C heterostructure displayed superior electroactivity with remarkably low overpotential and Tafel values for the OER and HER, along with exceptional long-term stability and durability. The superior electrocatalytic behavior of the developed heterostructures is attributed to heterojunction formation between the nanoflakes and particles, resulting in efficient charge transfer and improved reaction kinetics. This study unequivocally demonstrates the successful formation of a heterointerface between FeP nanoflakes and V2C nanoparticles and good improvement in electroactive centres due to the co-doping of B and N. These results not only contribute to the advancement of electrocatalysis, but also pave the way for the development of novel bifunctional self-supported electrocatalysts for enhanced energy generation.Data availability
All data pertaining to this manuscript are available in the ESI.† We did not use any external data for our research.Author contributions
Dasari Sai Hemanth Kumar, Manzoor Ahmad Pandit, Vinay Kumar Kolakaluri – data curation, formal analysis, investigation, methodology, and writing – original draft. Krishnamurthi Muralidharan – conceptualization, supervision, funding acquisition, and writing – review & editing.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors thank the Institute of Eminence (IoE) grant from the Ministry of Education, Government of India to University of Hyderabad (No UoH-IoE-RC3-21-043) for project and fellowship (DSHK). The instrument support grant from the Department of Science and Technology, New Delhi, India (through FIST program) is gratefully acknowledged.References
- S. H. K. Dasari, M. A. Pandit and K. Muralidharan, Energy Fuels, 2024, 38, 17878–17890 CrossRef CAS.
- M. A. Pandit, S. H. K. Dasari, M. Ramadoss, Y. Chen and K. Muralidharan, RSC Adv., 2022, 12, 7762–7772 Search PubMed.
- M. Ramadoss, Y. Chen, X. Chen, Z. Su, M. Karpuraranjith, D. Yang, M. A. Pandit and K. Muralidharan, J. Phys. Chem. C, 2021, 125, 20972–20979 Search PubMed.
- M. Ramadoss, M. A. Pandit, Y. Chen, M. Karpuraranjith and K. Muralidharan, ACS Symp. Ser., 2022, 1431, 227–255 CAS.
- B. Li, J. Zhao, Y. Wu, G. Zhang, H. Wu, F. Lyu, J. He, J. Fan, J. Lu and Y. Y. Li, Small, 2023, 19, 2301715 CrossRef CAS.
- M. R. Kandel, U. N. Pan, P. P. Dhakal, R. B. Ghising, T. T. Nguyen, J. Zhao, N. H. Kim and J. H. Lee, Appl. Catal., B, 2023, 331, 122680 CrossRef CAS.
- S. Marimuthu, A. Shankar and G. Maduraiveeran, Chem. Commun., 2023, 59, 2600 Search PubMed.
- C. Chen, Y. Kang, Z. Huo, Z. Zhu, W. Huang, H. L. Xin, J. D. Snyder, D. Li, J. A. Herron, M. Mavrikakis, M. Chi, K. L. More, Y. Li, N. M. Markovic, G. A. Somorjai, P. Yang and V. R. Stamenkovic, Science, 2014, 343, 1339–1343 CrossRef CAS PubMed.
- J. Pei, J. Mao, X. Liang, C. Chen, Q. Peng, D. Wang and Y. Li, Chem. Commun., 2016, 52, 3793–3796 RSC.
- H. Pei, L. Zhang, G. Zhi, D. Kong, Y. Wang, S. Huang, J. Zang, T. Xu, H. Wang and X. Li, Chem. Eng. J., 2022, 433, 133643 Search PubMed.
- S. Anantharaj, S. R. Ede, K. Sakthikumar, K. Karthick, S. Mishra and S. Kundu, ACS Catal., 2016, 6, 8069–8097 Search PubMed.
- M. R. Kandel, U. N. Pan, D. R. Paudel, P. P. Dhakal, N. H. Kim and J. H. Lee, Composites, Part B, 2022, 239, 109992 CAS.
- Y. G. Zhao, N. Dongfang, C. A. Triana, C. Huang, R. Erni, W. C. Wan, J. G. Li, D. Stoian, L. Pan, P. Zhang, J. G. Lan, M. Iannuzzi and G. R. Patzke, Energy Environ. Sci., 2022, 15, 727–739 CAS.
- Y.-N. Wang, Z.-J. Yang, D.-H. Yang, L. Zhao, X.-R. Shi, G. Yang and B.-H. Han, ACS Appl. Mater. Interfaces, 2021, 13, 8832–8843 CAS.
- Y. Shi and B. Zhang, Chem. Soc. Rev., 2016, 45, 1529–1541 CAS.
- J. Y. Wang, W. Li, X. F. Chen and A. S. Huang, J. Alloys Compd., 2023, 934, 167828 CAS.
- Z. S. Zhao, W. Jin, L. Xu, C. Wang, Y. Zhang and Z. X. Wu, J. Mater. Chem. A, 2021, 9, 12074–12079 CAS.
- M. B. Zhang, X. Ma, H. Zhong, J. Yang and Z. F. Cao, J. Alloys Compd., 2023, 935, 168135 CAS.
- Y. X. Liu, Z. M. Tian, Q. C. Xu, Y. X. Yang, Y. T. Zheng, H. G. Pan, J. Chen, Z. Wang and W. J. Zheng, ACS Appl. Mater. Interfaces, 2022, 14, 8963–8973 CAS.
- A. Abdollahi, A. Ghaffarinejad and M. Arabi, J. Alloys Compd., 2023, 937, 168400 CAS.
- A. Zou, A. Du and X. Yao, Adv. Mater., 2017, 29, 1700017 Search PubMed.
- K. P. Singh, E. J. Bae and J. S. Yu, J. Am. Chem. Soc., 2015, 137, 3165–3168 CAS.
- M. Li, T. Liu, X. Bo, M. Zhou, L. Guo and S. Guo, Nano Energy, 2017, 33, 221–228 CAS.
- L. Feng, Y. Liu and J. Zhao, Phys. Chem. Chem. Phys., 2015, 17, 30687–30694 CAS.
- P. Jiang, Q. Liu, Y. Liang, J. Tian, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 12855–12859 CAS.
- D. Li, H. Baydoun, B. Kulikowski and S. L. Brock, Chem. Mater., 2017, 29, 3048–3054 CAS.
- Y. Luo, M. Gong, J. Wang, P. Zhao, X. Yang, S. Cui, Z. Li, Z. Jiao and L. Cheng, Colloids Surf., A, 2022, 655, 130119 CrossRef CAS.
- X. Y. Li, F. Y. Duan, X. Y. Lu, Y. F. Gang, W. L. Zheng, Y. Y. Lin, L. Z. Chen, Y. Y. Dan and X. F. Cheng, J. Alloys Compd., 2023, 935, 168128 CrossRef CAS.
- J. Wu, W. Chen, H. Zheng, M. Chen, J. Xia and X. Qian, J. Alloys Compd., 2023, 945, 169357 CrossRef CAS.
- Z. Xu, M. Hao, X. Liu, J. Ma, L. Wang, C. Li and W. Wang, Catalysts, 2022, 12, 1417 CrossRef CAS.
- Y. Zhang, H. Guo, X. Li, W. Ren and R. Song, Mater. Chem. Front., 2022, 6, 1477–1486 RSC.
- Z. Li, Z. Li, H. Yao, Y. Wei and J. Hu, J. Colloid Interface Sci., 2024, 653, 857–866 CrossRef CAS.
- Z.-Y. Yu, Y. Duan, M.-R. Gao, C.-C. Lang, Y.-R. Zheng and S.-H. Yu, Chem. Sci., 2017, 8, 968–973 RSC.
- N. Wang, X. Bo and M. Zhou, ACS Appl. Mater. Interfaces, 2022, 14, 23332–23341 CrossRef CAS PubMed.
- L. Cao, N. Zhang, L. Feng, D. He, K. Kajiyoshi, X. Li, Q. Huang, L. Feng, J. Huang and R. Li, Inorg. Chem. Front., 2020, 7, 4142–4149 RSC.
- J. Wan, C. Wang, Q. Tang, X. Gu and M. He, RSC Adv., 2019, 9, 37467–37473 RSC.
- C. Yang, R. Zhao, H. Xiang, J. Wu, W. Zhong, W. Li, Q. Zhang, N. Yang and X. Li, Adv. Energy Mater., 2020, 10, 2002260 CrossRef CAS.
- L. Peng, J. Shen, L. Zhang, Y. Wang, R. Xiang, J. Li, L. Li and Z. Wei, J. Mater. Chem. A, 2017, 5, 23028–23034 RSC.
- R. Zhao, C. Yang, Q. Zhang, H. Xiang, Y. Li and X. Li, J. Phys. Chem. C, 2021, 125, 14607–14615 CrossRef CAS.
- M. Shao, H. Chen, S. Hao, H. Liu, Y. Cao, Y. Zhao, J. Jin, H. Dang, Y. Meng, Y. Huo and L. Cui, Appl. Surf. Sci., 2022, 577, 151857 CrossRef CAS.
- M. Yang, Y. Yang, K. Wang, S. Li, F. Feng, K. Lan, P. Jiang, X. Huang, H. Yang and R. Li, Electrochim. Acta, 2020, 337, 135685 Search PubMed.
- F. Guo, Z. Liu, J. Xiao, X. Zeng, C. Zhang, Y. Lin, P. Dong, T. Liu, Y. Zhang and M. Li, Chem. Eng. J., 2022, 446, 137111 CrossRef CAS.
- T. Liu, M. Li, X. Bo and M. Zhou, J. Colloid Interface Sci., 2019, 533, 709–722 CAS.
- Y. Kang, W. Wang, J. Li, S. Imhanria, Y. Hao and Z. Lei, J. Power Sources, 2021, 493, 229665 CAS.
- P. Joshi, R. Yadav, M. Hara, T. Inoue, Y. Motoyama and M. Yoshimura, J. Mater. Chem. A, 2021, 9, 9066–9080 CAS.
- Z. Lin, Z. Huan, J. Zhang, J. Li, Z. Li, P. Guo, Y. Zhu and T. Zhang, Chemosphere, 2022, 292, 133373 CAS.
- J. Godwin, N. Abdus-Salam, A. I. Haleemat, P. K. Panda, J. Panda and B. C. Tripathy, Inorg. Chem. Commun., 2022, 140, 109346 CAS.
- (a) A. S. Ahmed, T. Ahamad, N. Ahmad and M. Z. Khan, Mater. Chem. Phys., 2019, 238, 121906 CAS; (b) M. Sandhya, D. Ramasamy, K. Sudhakar, K. Kadirgama and W. S. W. Harun, Ultrason. Sonochem., 2021, 73, 105479 CAS.
- (a) R. Monsef, F. Soofivand, H. A. Alshamsi, A. Al-Nayili, M. Ghiyasiyan-Arani and M. Salavati-Niasari, J. Mol. Liq., 2021, 336, 116339 Search PubMed; (b) S. Sekar, C. Bathula, I. Rabani, J. W. Lee, S. H. Lee, Y.-S. Seo and S. Lee, Ultrason. Sonochem., 2022, 90, 106177 CAS.
- M. Junaid, M. H. M. Khir, G. Witjaksono, N. Tansu, M. S. M. Saheed, P. Kumar, Z. Ullah, A. Yar and F. Usman, Molecules, 2020, 25, 3646 CAS.
- H. K. Sadhanala and K. K. Nanda, J. Phys. Chem. C, 2015, 119, 13138–13143 Search PubMed.
- X. Xu, C. Shi, R. Chen and T. Chen, RSC Adv., 2017, 7, 22263–22269 CAS.
- C. Liu, H. Pan, H. Hu, W. Wei, Q. Lu, C. Zhao, H. Wang and F. Du, Amino Acids, 2022, 54, 1173–1181 CAS.
- J. Xia, H. Guo, G. Yu, Q. Chen, Y. Liu, Q. Liu, Y. Luo, T. Li and E. Traversa, Catal. Lett., 2021, 151, 3516–3522 CAS.
- Z. Ahsan, Z. Cai, S. Wang, Y. Ma, G. Song, M. Yu, S. Zhang, W. Yang, C. Wen and X. Feng, J. Solid State Electrochem., 2021, 25, 2127–2137 Search PubMed.
- H. K. Sadhanala and K. K. Nanda, Carbon, 2016, 96, 166–173 CAS.
- Y. Yang, et al. , ACS Appl. Mater. Interfaces, 2015, 7, 27324–27330 CAS.
- X. Y. Hu, M. Huang, X. Meng and X. Ju, RSC Adv., 2019, 9, 42110–42119 CAS.
- L. Gao, T. Ma, L. Zhang and X. Yang, J. Solid State Electrochem., 2021, 25, 2055–2063 CAS.
- Y. Feng, C. Xu and E. Hu, et al. , J. Mater. Chem. A, 2018, 6, 14103 RSC.
- Y. Liu, B. Wang and Y. Lu, et al. , J. Mater. Sci., 2021, 56, 16000–16009 CrossRef CAS.
- Y. Yao, N. Mahmood and L. Pan, et al. , Nanoscale, 2018, 10, 21327 RSC.
- B. Peng, Y. Xu, K. Liu, X. Wang and F. M. Mulder, ChemElectroChem, 2017, 4, 2140 CrossRef CAS.
- R. J. Liu, L. X. Yang and Y. Wang, et al. , J. Solid State Electrochem., 2022, 26, 831–842 CrossRef CAS.
- C. E. Iheomamere, C. L. Arnold and J. Summers, et al. , J. Mater. Sci.: Mater. Electron., 2022, 33, 974–984 CrossRef CAS.
- J. Qu, Q. Li, C. Luo, J. Cheng and X. Hou, Coatings, 2018, 8, 214 Search PubMed.
- R. R. Solís, M. A. Quintana, G. Blázquez, M. Calero and M. J. Muñoz-Batista, Catal. Today, 2023, 423, 114266 CrossRef.
- W. Wang, B. Jiang and W. Xiong, et al. , Sci. Rep., 2013, 3, 3383 Search PubMed.
- M. A. Pandit, S. H. K. Dasari, S. Billakanti, M. Ramadoss and K. Muralidharan, J. Phys. Chem. C, 2020, 124, 18010–18019 CAS.
- M. Zhou, H. L. Wang and S. Guo, Chem. Soc. Rev., 2016, 45, 1273–1307 CAS.
- Irkham and E. Yasuaki, Analyst, 2019, 144, 4499–4504 CAS.
- N. T. Suen, S. F. Hung, Q. Quan, N. Zhang, Y. J. Xu and H. M. Chen, Chem. Soc. Rev., 2017, 46, 337–365 CAS.
- G. P. Kamble, A. A. Kashale, A. S. Rasal, S. A. Mane, R. A. Chavan, J. Y. Chang, Y. C. Ling, S. S. Kolekar and A. V. Ghule, RSC Adv., 2021, 11, 3666–3672 RSC.
- M. M. Rajpure, H. A. Bandal, H. S. Jadhav and H. Kim, J. Electroanal. Chem., 2022, 923, 116825 CrossRef CAS.
- P. W. Menezes, A. Indra, I. Zaharieva, C. Walter, S. Loos, S. Hoffmann, R. Schlogl, H. Dau and M. Driess, Energy Environ. Sci., 2019, 12, 988 RSC.
- L. Quan, H. Jiang, G. Mei, Y. Sun and B. You, Chem. Rev., 2024, 124(7), 3694–3812 CrossRef CAS PubMed.
- M. Li, S. A. Odom and A. R. Pancoast, et al. , ACS Energy Lett., 2021, 6(11), 3932–3943 CrossRef CAS.
- J. M. E. Bastian, K. Ulrike, T. Simon, D. Andreas, K. Elias and T. Thomas, Chem. Eng. J., 2021, 424, 130501 Search PubMed.
- A. H. M. da Silva, R. E. Vos, R. J. C. Schrama and M. T. M. Koper, Electrochim. Acta, 2024, 498, 144612 CrossRef CAS.
- L. Fan, Z. Tu and S. H. Chan, Energy Rep., 2021, 7, 8421–8446 Search PubMed.
- Z. Luthey-Schulten, Nat. Methods, 2021, 18, 446–447 CAS.
- A. Landman, R. Halabi, P. Dias and H. Dotan, et al. , Joule, 2020, 4, 448–471 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4lf00394b |
| ‡ Both the authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |