Highly photoluminescent pH-independent nitrogen-doped carbon dots for sensitive and selective sensing of p-nitrophenol†
Huan Yuana,
Jie Yub,
Suling Feng*a and
Yijun Gonga
aSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China. E-mail: slfeng@htu.cn; Fax: +86-373-3329250; Tel: +86-373-3329250
bDepartment of Chemistry, Xinxiang Medical University, Xinxiang 453007, China
First published on 29th January 2016
Abstract
Nitrogen-doped carbon quantum dots (N-CDs) have become a current focus of chemical research due to their superior photoluminescence (PL) properties and high fluorescence quantum yield (QY). In this paper, we reported a facile, simple and low-cost approach for the synthesis of highly fluorescent and stable N-CDs by hydrothermal treatment of maleic acid and ethylenediamine. The as-prepared N-CDs emitted strong blue fluorescence with an absolute QY of up to 45%, and exhibited pH-independent behavior in a wide pH range from 1 to 13 and good water solubility. In addition, the fluorescence of the N-CDs can be significantly quenched by p-nitrophenol (4-NP) through an electron-transfer-induced dynamic quenching mechanism, leading to 4-NP being sensed optically. The sensor presented a good linearity with 4-NP concentration in the range of 0.10–11 μg mL−1. The detection limit of 22 ng mL−1 4-NP could be obtained, which is lower than the 60 ng mL−1 limit allowed in drinking water by the U. S. Environmental Protection Agency. The sensor exhibited rapid recognition and distinguished selectivity to 4-NP over other structural analogues of phenols. Moreover, the sensing system was successfully applied for the determination of 4-NP in environmental water samples.
1. Introduction
Carbon dots (CDs) possessing excellent optical properties, low toxicity, good biocompatibility, robust chemical inertness, and photo-stability against photo-bleaching have exhibited promising applications in sensing, bioimaging, optoelectronic devices and photocatalysis,1–6 etc. Various synthetic methods have been reported for CDs, including arc-discharge,7 laser ablation,8 electrochemical oxidation,9 ultrasonics,10 microwave pyrolysis,11 and hydrothermal approach4,12–14 etc. Among them, hydrothermal approach has been suggested to be an effective strategy for preparing highly photoluminescent CDs.4,13–16 The characteristics of prepared CDs are related to the choice of various precursors. Many materials and chemicals have been employed as precursors, such as single walled carbon nanotubes,7 multiwalled carbon nanotubes,9 cocoon silk,14 orange juice,17 glucose,18 and citric acid/ethylenediamine4 and so on. However, bare CDs without passivation or surface functionalization are usually weakly fluorescent, corresponding to lower observed quantum yields (QY).2,7–12,17–19 The photoluminescence (PL) emissions of CDs can be improved effectively through passivation or surface functionalization.2–4,8 Meanwhile, doping of CDs with heteroatoms such as boron,20,21 nitrogen,4,13,15 sulfur,22,23 phosphorus,24 or silicon25 is another method for improving QY of CDs. Among these elements, N-doping has been demonstrated to be very effective,4,13–15,26 which can effectively tune intrinsic properties of CDs, such as optical and electronic characteristics, and surface and local chemical features.15,26,27 Thus, many kinds of N-doped carbon dots have been prepared and applied in the area of bioimaging and sensing.4,13,14 On the other hand, passivation or surface functionalization leads to the diversity of surface group of CDs, thus enlarging applications of these materials in various fields.4,13,15,16,28,29As the essential raw material for numerous chemical industries, p-nitrophenol (4-NP) was widely utilized in the manufacture of pesticides, explosives, pharmaceuticals, dyes, and processing of leather,30–32 and consequently it is widely distributed in aquatic environments and soils. And 4-NP can persist in the environment for a long time due to its stability, and solubility in water. The acute inhalation or ingestion of 4-NP can cause fervescence, headaches, methemoglobinemia and liver and kidney damage, etc.32–34 Therefore, 4-NP has been listed as “priority pollutant” by the U. S. Environmental Protection Agency (EPA).30,35 Several methods have been proposed for the detection of 4-NP in environmental water samples, mainly including electrochemical detection,36–38 chromatography,39 capillary electrophoresis.40 However, these methods usually encounter problems such as poor stability of electrode, time-consuming pretreatment procedures or expensive and sophisticated instruments, and are not suitable for rapid monitoring. In contrast, fluorescent analysis using fluorescent nanomaterials as chemosensors or probes exhibit superiority in terms of high sensitivity, time-saving measurement, simple sample pretreatment and lower cost of the instruments. Therefore, some fluorescent methods have been reported to detect 4-NP. For example, Li et al.41 developed a highly sensitive method for determination of 4-NP using α-cyclodextrin/CdSe/ZnS quantum dots as fluorescent probes via fluorescence intensity quenching. Zhou and co-workers33 employed molecularly imprinted polymer (MIP)-coated graphene quantum dots as fluorescent sensor for selective detection of 4-NP based on resonance energy transfer from GQDs to 4-NP. García et al.42 synthesized CDs with QY of 28% by thermal carbonization of ethyleneglycol bis-(2-aminoe thylether)-N,N,N′,N′-tetraacetic acid (EGTA) and tris(hydroxymethyl)aminomethane (Tris), and used the CDs to detect 4-NP based on an energy transfer in Meisenheimer complex between 4-NP and the CDs. Unfortunately, some of these methods suffer from drawbacks such as heavy metal elements, toxic or relatively expensive reagent, limiting their wide applications. Therefore, it is of considerable significance to establish a simple, sensitive, low-cost and green analysis system for the detection of 4-NP in environmental samples.
Maleic acid consists of two carboxyl groups and three conjugate type unsaturated bonds, and ethylenediamine has two amino groups. Considering that the precursors possessing carboxylate and amino groups have been suggested to be ideal for the preparation of high PL CDs,43 maleic acid with ethylenediamine is predicted to be ideal precursor for strong PL N-doped CDs (N-CDs). Herein, maleic acid is used as the carbon source for one-pot hydrothermal preparation of a new kind of N-CDs with ethylenediamine as the nitrogen source and passivation agent. The as-obtained N-CDs exhibited strong luminescence with QY of up to 45% and were well water-soluble and highly stable under extreme conditions, such as strong acid and base, strong ionic strengths, and long-time light illumination. Due to these attractive merits, a sensing platform for 4-NP quantitative detection was established on the basis of 4-NP-induced dynamic fluorescence quenching. The sensor shows good sensitivity and selectivity, and could be applied in the detection of 4-NP in environmental water samples.
2. Experimental
2.1. Reagents and chemicals
Maleic acid was purchased from Meryer (Shanghai) Chemical Technology Co., Ltd (Shanghai, China). Ethylenediamine, o-nitrophenol (2-NP), m-nitrophenol (3-NP) and 4-NP were obtained from Shanghai Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Phenol (PHE), resorcinol (RES), o-cresol (2-MP), m-cresol (3-MP), p-cresol (4-MP), o-chlorophenol (2-CP), p-chlorophenol (4-CP) and 2,4-dichlorophenol (DCP) were received from Aladdin Chemical Co., Ltd (Shanghai, China). All reagents were of analytical grade and used as received without further purification. Aqueous solutions were prepared with distilled water in the whole experiments.2.2. Apparatus and characterization
The fluorescence emission spectra were recorded on a FP-6500 fluorescence spectrophotometer (Hitachi, Tokyo, Japan). The fluorescence QY was measured on a FLS-980 fluorescence spectrophotometer (Edinburgh Instrument, UK). The UV-vis absorption spectra were acquired using a UV-1700 UV spectrophotometer (Shimadzu, Co., Japan). The sizes and morphologies were confirmed by transmission electron microscopy (TEM) on a JEOLJEM-2010 transmission electron microscopy (JEOL, Japan) at an accelerating voltage of 200 kV. X-ray diffraction (XRD) measurement was performed on a Bruker D8 ADVANCE X-ray diffractometer (Bruker AXS, German) with Cu Kα (1.54056 Å) as the incident radiation. Fourier transform infrared spectrum (FT-IR) was measured on a Nicolet FTS NEXUS FTIR spectrometer (Nicolet instrument Co., USA) using KBr pellets in the range of 400–4000 cm−1. X-ray photoelectron spectra (XPS) were obtained from a Thermos SCIENTIFIC ESCALAB 250 spectrometer with Al Kα exciting source (1486.6 eV).2.3. Synthesis of fluorescent N-CDs
A typical process for preparing N-CDs: 0.50 g maleic acid and 0.25 mL ethylenediamine was dissolved in 10 mL of distilled water. The mixture was transferred into a 50 mL Teflon-lined autoclave and heated at 190 °C for 15 h. After being cooled naturally to room temperature, the resulted brown solution containing the N-CDs were purified with 0.10 μm filter membrane and then vacuum dried at 80 °C for 12 h. The solid N-CDs obtained were dispersed in water to form a homogeneous solution with the concentration of 0.44 mg mL−1.2.4. Procedure of PL detection of 4-NP
For the typical assay of 4-NP, 1.5 mL of pH 10.0 NaCO3–NaHCO3 buffer was added into a mixture containing 0.90 mL of 0.44 mg mL−1 N-CDs dispersion and a proper amount of 0.10 mg mL−1 4-NP working solution and diluted to 10 mL. The fluorescence emission spectra of the system were recorded at an emission wavelength of 480 nm and an excitation wavelength of 440 nm with the excitation and emission slit widths of 10 nm. Quenched intensity (ΔF = F0 − F) was calculated (F0 and F are the emission intensity of the N-CDs in the absence and presence of 4-NP).3. Results and discussion
3.1. Optimization of synthetic variables
N-CDs were fabricated by a bottom-up hydrothermal method using maleic acid and ethylenediamine as precursors. As shown in Fig. S1 (ESI)† maleic acid and ethanediamine firstly cross-linked with each other to produce relatively large polymers, and then carbonized to form certain dimensional N-CDs.4,44 In order to obtain highly luminescent N-CDs, the synthesis conditions towards 0.5 g maleic acid in 10 mL of distilled water were optimized, including hydrothermal reaction time, temperature, and the volume of ethylenediamine. As shown in Fig. S2 (ESI),† the PL of N-CDs seriously depends on the carbonization conditions. The excessive carbonization of maleic acid in the presence of ethylenediamine leads to low PL of N-CDs. As a result, 0.5 g maleic acid and 0.25 mL ethylenediamine in 10 mL of distilled water with reaction time of 15 h at 190 °C were chosen as the optimum conditions for synthesizing N-CDs. In addition, four additional CDs were prepared using maleic acid, fumaric acid, ethylenediamine, and fumaric acid with ethylenediamine as the precursor according to the same method, noted as CDs1, CDs2, CDs3 and N-CDs1, respectively. As shown in Table S1 (ESI),† the CDs1, CDs2 and CDs3 exhibit weak PL with QY of 1.4%, 1.3% and 2.5%, respectively, while both the N-CDs1 and N-CDs show high fluorescence with QY of 45%, indicating the important role of ethylenediamine as surface passivation reagent for improving QY of CDs. Considering the lower cost, maleic acid with ethylenediamine was chosen as the precursor for preparing N-CDs. The best excitation wavelength of CDs1, CDs2, N-CDs1 and N-CDs is at 440 nm. The best emission wavelength of N-CDs1 and N-CDs is located at 480 nm, which exhibits about 20 nm blue shift as compared to that of CDs1 and CDs2 in solution. This wavelength shift is ascribed to a new kind of surface state induced by the strong electron affinity of N atoms doped in CDs.15,45,463.2. Characterization of fluorescent N-CDs
The morphologies and sizes of the as-prepared N-CDs were identified from TEM (Fig. 1A), which indicates that the N-CDs are uniform in size and well dispersed. As estimated from the TEM image, the Gaussian fitting curve confirms that the diameters of the N-CDs are mainly distributed in the range of 1.6–4.2 nm with an average size of 2.9 nm, showing a narrow size distribution (Fig. 1B). High-resolution transmission electron microscopy (HRTEM) (inset in Fig. 1A) reveals that the N-CDs possess high crystallinity with a lattice fringe of 0.22 nm, which corresponds to the (100) diffraction plane of graphitic carbon.47 The XRD spectrum (Fig. 1C) shows a broad diffraction peak located at 21.3°, which confirms a 0.42 nm interlayer spacing of the (002) diffraction peak. It is larger than that of graphite (0.34 nm). The increase of the interlayer spacing indicates an increase in amorphous nature, which may be ascribed to the coexisted oxygen-containing functional groups and amine groups.47,48 | ||
| Fig. 1 (A) TEM image, (B) the corresponding particle size distribution histograms, (C) XRD pattern and (D) FT-IR spectrum of the CDs. | ||
FT-IR spectrum was provided to further identify the surface functional groups present on the N-CDs (Fig. 1D). Peaks at 3071 and 2938 cm−1 correspond to C–H bond stretching vibrations.4,49 The absorption band at around 3263 cm−1 is attributed to stretching vibrations of O–H/N–H.14,26 The peak at 1548 cm−1 is from the bending vibrations of C–NH and the band at 1183 cm−1 relates to the asymmetric stretching vibrations of C–NH–C,4,14 which proves the existence of amine groups on the surface of the as-prepared N-CDs. The formed C–NH–C confirms that ethylenediamine molecules are doped onto the surfaces of the N-CDs through amide bonds.4,14 The peak at 1639 cm−1 is assigned to the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.4,49 The bending vibrations of C–O appear at 1392 and 1339 cm−1, and the stretching peak of C–O–C bonds appear at 1042 cm−1,49 indicating the presence of abundant oxygen groups on the surface of the N-CDs. The existences of amine groups and C
O.4,49 The bending vibrations of C–O appear at 1392 and 1339 cm−1, and the stretching peak of C–O–C bonds appear at 1042 cm−1,49 indicating the presence of abundant oxygen groups on the surface of the N-CDs. The existences of amine groups and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O improve the hydrophilicity and stability of the N-CDs in an aqueous system, which greatly expands the application of the N-CDs for sensing in aqueous samples.14,26
O improve the hydrophilicity and stability of the N-CDs in an aqueous system, which greatly expands the application of the N-CDs for sensing in aqueous samples.14,26
To identify the surface composition and elemental analysis of the as-prepared N-CDs in detail, the X-ray photoelectron (XPS) measurements were used and the corresponding results are presented in Fig. 2. The full survey spectrum of the N-CDs clearly shows three peaks at 284.7, 401.0 and 531.4 eV (Fig. 2A), which are ascribed to C 1s, N 1s, and O 1s, respectively. And the corresponding content of each element is C 63.4%, N 9.6%, and O 27.0%. A high-resolution XPS spectrum of C 1s (Fig. 2B) verifies the presence of C–C (sp3, 284.6 eV), C–N (sp3, 286.0 eV), C–O (sp2, 287.3 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (sp2, 288.4 eV) functional groups,14,26 which suggests that the as-prepared N-CDs possess plentiful hydrophilic groups on the surface. The N 1s spectrum (Fig. 2C) shows three peaks at 399.2, 400.7 and 401.3 eV, corresponding to pyridinic C–N–C, graphitic N–(C)3 and pyrrolic N–H,14,50,51 respectively, which confirm the newly formed polyaromatic structures containing C–N and C
N (sp2, 288.4 eV) functional groups,14,26 which suggests that the as-prepared N-CDs possess plentiful hydrophilic groups on the surface. The N 1s spectrum (Fig. 2C) shows three peaks at 399.2, 400.7 and 401.3 eV, corresponding to pyridinic C–N–C, graphitic N–(C)3 and pyrrolic N–H,14,50,51 respectively, which confirm the newly formed polyaromatic structures containing C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N51 and the successful doping of nitrogen atoms on the surface of the N-CDs. Moreover, the spectrum of O 1s (Fig. 2D) exhibits two fitted peaks at 531.3 and 533.0 eV, which are attributed to C
N51 and the successful doping of nitrogen atoms on the surface of the N-CDs. Moreover, the spectrum of O 1s (Fig. 2D) exhibits two fitted peaks at 531.3 and 533.0 eV, which are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–OH/C–O–C groups, respectively.14,26,50 Therefore, we can conclude that the as-prepared N-CDs possess abundant amino and carbonyl/carboxylate groups on their surface. Overall, the results from the XPS analysis are consistent with those obtained from FT-IR. The surface functional groups manifested by the FT-IR and XPS further prove the rationality of the formation process of the N-CDs (Fig. S1, ESI†).
O and C–OH/C–O–C groups, respectively.14,26,50 Therefore, we can conclude that the as-prepared N-CDs possess abundant amino and carbonyl/carboxylate groups on their surface. Overall, the results from the XPS analysis are consistent with those obtained from FT-IR. The surface functional groups manifested by the FT-IR and XPS further prove the rationality of the formation process of the N-CDs (Fig. S1, ESI†).
 | ||
| Fig. 2 (A) Survey XPS spectrum of the N-CDs. High-resolution XPS spectra of the C 1s (B), N 1s (C) and O 1s (D) peaks of the N-CDs. | ||
3.3. Optical properties of N-CDs
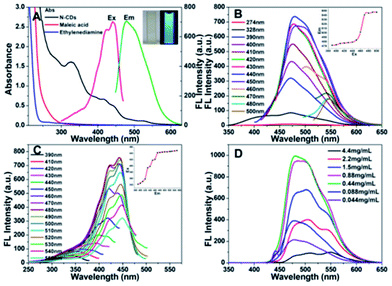
To explore the intriguing optical properties of the N-CDs, the UV-vis absorption and PL emission spectra were obtained. As shown in Fig. 3A, a broad absorption band attributed to the N-CDs is observed in the range of 300–600 nm, while the absorption of maleic acid and ethylenediamine is below 300 nm. The broad band displays five absorption features at around 274, 328, 395, 421, 440 and 530 nm (Fig. 3A). The weak shoulder peak centered at about 274 nm corresponds to π–π* transition of the aromatic sp2 domains52 and produces no detectable PL (Fig. 3B). The band at 328 nm is assigned to n–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O52 and leads to weak emission (Fig. 3B). The aqueous solution of the N-CDs is yellow in daylight, but exhibits bright blue luminescence under the illumination of a UV lamp at 365 nm (inset in Fig. 3A). The absolute fluorescent quantum yield of N-CDs was measured to be 45%, which is comparable to CDs reported by other groups.5,7–12,17–21,23,25,26,44,47–49 Up till now, two popular fluorescence mechanisms of CDs, electronic conjugate structures and emissive traps have been proposed.15 Because no detectable fluorescent emission from π–π* transition of the aromatic sp2 domains is observed, thus, PL of the as-prepared N-CDs should result from emissive traps. On the basis of this, the three absorbance peaks at 421, 440 and 530 nm are assigned to the trapping of excited state energy by the surface states,15,51 and produce strong luminescence (Fig. 3B). Similar to the previously reported CDs, the N-CDs show the excitation-dependent PL behavior (Fig. 3B). As excitation wavelength increases from 440 to 500 nm, the emission peak appears continuously at about ∼480, ∼502 and ∼541 nm with a rapid decrease in intensity, which suggests that the emissive traps induced by surface states of the chemical groups should play an important role in excitation wavelength dependent phenomenon of the N-CDs.53 The excitation spectra reflect excitation absorbance of phosphor. Fig. 3C shows fluorescence excitation spectra under a series of emission wavelength. With increasing emission wavelength from 430 to 530 nm, excitation peak appear at ∼396, ∼423, and ∼440 nm continuously. The phenomenon results from the different excitation sites with different energy levels induced by the existence of different functional groups on the N-CDs.53 It is worth noting that the excitation maximum at 440 nm and the shoulder peaks at 396 and 423 nm were very close to the three absorption peaks of the N-CDs at 440, 395 and 421 nm, respectively (Fig. 3A).
O52 and leads to weak emission (Fig. 3B). The aqueous solution of the N-CDs is yellow in daylight, but exhibits bright blue luminescence under the illumination of a UV lamp at 365 nm (inset in Fig. 3A). The absolute fluorescent quantum yield of N-CDs was measured to be 45%, which is comparable to CDs reported by other groups.5,7–12,17–21,23,25,26,44,47–49 Up till now, two popular fluorescence mechanisms of CDs, electronic conjugate structures and emissive traps have been proposed.15 Because no detectable fluorescent emission from π–π* transition of the aromatic sp2 domains is observed, thus, PL of the as-prepared N-CDs should result from emissive traps. On the basis of this, the three absorbance peaks at 421, 440 and 530 nm are assigned to the trapping of excited state energy by the surface states,15,51 and produce strong luminescence (Fig. 3B). Similar to the previously reported CDs, the N-CDs show the excitation-dependent PL behavior (Fig. 3B). As excitation wavelength increases from 440 to 500 nm, the emission peak appears continuously at about ∼480, ∼502 and ∼541 nm with a rapid decrease in intensity, which suggests that the emissive traps induced by surface states of the chemical groups should play an important role in excitation wavelength dependent phenomenon of the N-CDs.53 The excitation spectra reflect excitation absorbance of phosphor. Fig. 3C shows fluorescence excitation spectra under a series of emission wavelength. With increasing emission wavelength from 430 to 530 nm, excitation peak appear at ∼396, ∼423, and ∼440 nm continuously. The phenomenon results from the different excitation sites with different energy levels induced by the existence of different functional groups on the N-CDs.53 It is worth noting that the excitation maximum at 440 nm and the shoulder peaks at 396 and 423 nm were very close to the three absorption peaks of the N-CDs at 440, 395 and 421 nm, respectively (Fig. 3A).
It is well known, CDs could self-aggregate at high concentration. Thus, the optical properties of the N-CDs with different concentrations were investigated. The increase of the N-CDs concentration has no influence on their absorption spectra (Fig. S3, ESI†). The fluorescence emission spectra of the N-CDs with different concentrations under excitation at 440 nm are displayed in Fig. 3D. The emission peaks located at ∼480 nm, ∼502 nm and ∼547 nm corresponded to low concentration (<0.88 mg mL−1), middle concentration and high concentration (>4.0 mg mL−1), respectively. Meanwhile, the emission color changes from blue, light green into dark with increasing concentration of the N-CDs, and the self-quenching occurs at high concentration (Fig. 4B and D). The phenomena above indicate the concentration-related fluorescence behavior of the N-CDs, which is assigned to lower energy emissive traps induced by the enhanced particle aggregation in concentrated solutions.53 Fig. 4D shows that after a commercially available filter paper adsorbed different concentrations of the N-CDs, its picture shows different color under UV light, very different from that under daylight (Fig. 4C). The results exhibit the potential application of the N-CDs in decorative materials.
3.4. PL stability of N-CDs
When the as-obtained N-CDs are applied to the practical sensing, it must have good stability to the ambient environmental conditions. To investigate the stability, the fluorescence intensities of the as-prepared N-CDs toward extreme pH, high ionic strengths in solution and illumination with a Xe lamp were measured. As shown in Fig. S4A (ESI),† the variation of the pH value from 1 to 13 has almost no effect on the photoluminescent intensity or peak characteristics, indicating pH-independent of the N-CDs. The high stability of fluorescent emission in such a broad pH range indicates partly the overwhelming presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –OH groups compared to easily ionized –NH2 and –COOH, etc.26 This result suggests that the N-CDs have a wider scope application of pH values than most previously reported CDs,26,47–49 which is very conducive to their application in chemical and biological analysis and detection. Additionally, the fluorescence intensity is almost unchanged by increasing the NaCl concentrations up to 5 M (Fig. S4B, ESI†), manifesting high stability of the N-CDs even under extreme ionic-strength conditions. Moreover, fluorescence intensity nearly remained constant after a continuous excitation of at least 2.5 h with a Xe lamp (Fig. S4C, ESI†) or being kept for eight months at room temperature, which demonstrates that the N-CDs have excellent photo-stability and store-stability.
O and –OH groups compared to easily ionized –NH2 and –COOH, etc.26 This result suggests that the N-CDs have a wider scope application of pH values than most previously reported CDs,26,47–49 which is very conducive to their application in chemical and biological analysis and detection. Additionally, the fluorescence intensity is almost unchanged by increasing the NaCl concentrations up to 5 M (Fig. S4B, ESI†), manifesting high stability of the N-CDs even under extreme ionic-strength conditions. Moreover, fluorescence intensity nearly remained constant after a continuous excitation of at least 2.5 h with a Xe lamp (Fig. S4C, ESI†) or being kept for eight months at room temperature, which demonstrates that the N-CDs have excellent photo-stability and store-stability.
3.5. Selective recognition of the N-CDs towards 4-NP
To explore the use of the as-prepared N-CDs as a fluorescent probe, and considering the previously reported quenching/enhancing effect of metal ions26,51,54,55 for photoluminescent CDs, the influences of seventeen kinds of metal ions were tested, such as Li+, Ag+, Mg2+, Ca2+, Sr2+, Ba2+, Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Hg2+, Pb2+, Cd3+, Fe3+, Al3+ and Cr3+ of the concentration of 100 μg mL−1. As shown in Fig. 5A, the N-CDs show no response to these metal ions, indicating the inertia of the N-CDs. Furthermore, considering the possible interaction between N-CDs and phenols in terms of the electron donors of amines on the surface of the N-CDs and the electrophilicity of phenols, we tested the response of the as-prepared N-CDs to phenols. As shown in Fig. 5B, among the studied species, mononitrophenol including 4-NP and 2-NP at the concentration of 10 μg mL−1 results in fluorescence quenching of the N-CDs, while 3-NP of 10 μg mL−1 shows no obvious quenching effect. As known, the quenching constant value Ksv is an important index to compare the quenching efficiency of the material. Thus, Ksv of the three systems of N-CDs/4-NP, N-CDs/2-NP and N-CDs/3-NP was measured at 293 K and 313 K, respectively, as shown in Table S2 (ESI).† The results indicate the N-CDs display high quenching efficiency to 4-NP over 2-NP and 3-NP. Notably, although 4-NP and 2-NP show similar structure and similar chemical and optical properties,56 the quenching efficiency of the two similar compounds on the N-CDs are different. The quenching of 4-NP (47.2%) is three times more than that of 2-NP (15.5%) (Table S3†), confirming the selectivity of the N-CDs to the compounds with the similar properties. The main reason for the high selectivity of 4-NP over 2-NP and 3-NP is the relatively strong interaction between 4-NP and N-CDs. Theoretically, 4-NP shows stronger acidity than 2-NP and 3-NP due to the strong electron withdrawing effect of nitro in 4-NP.30 N-CDs have some electron-rich amino groups, which ensures that the N-CDs have a relatively strong surface affinity to 4-NP. All the results above imply that the N-CDs possess high sensing selectivity and high sensing specificity to 4-NP.3.6. Detection of 4-NP using the N-CDs as fluorescence sensors
To obtain better sensing performances of the N-CDs for mononitrophenol, the experimental conditions, including buffer solutions, pH values and the N-CDs concentrations were optimized, as shown in Fig. S5 (ESI).† 1.5 mL of NaCO3–NaHCO3 buffer at pH 10.0 and 0.44 mg mL−1 of N-CDs were chosen as the optimum conditions for the following experiment. Because the N-CDs show highly sensitive response for 4-NP over 3-NP and 2-NP, we then explored the feasibility of the N-CDs for 4-NP detection under the optimum conditions. Fig. 5C shows the fluorescence spectra of the N-CDs dispersion after adding different concentrations of 4-NP. With the increasing addition of 4-NP, the gradual decrease in fluorescence intensity was observed. As shown in Fig. 5C corresponding insets, the fluorescence intensity displayed a good linear relationship for 4-NP in the range of 0.10–11 μg mL−1. The limit of detection (calculated according to the 3σ) was estimated to be 22 ng mL−1 for 4-NP, which is comparable to that reported in the literature as shown in Table 1. Meanwhile, the detection limit is lower than 60 ng mL−1 set by the US EPA for drinking water,35 implying that the method can be applied to assaying 4-NP-contained real water samples. Furthermore, the repeatability of the sensing system was evaluated by 11 parallel determinations of 4 μg mL−1 4-NP, the relative standard deviation (RSD) obtained was 0.72%. The lower RSD values provided a guarantee for the accurate determination of 4-NP using N-CDs as a fluorescent probe. In addition, the reaction between the N-CDs and 4-NP was complete as soon as the reagents were mixed thoroughly and the signal can remain unchanged for at least 2 h (Fig. S5D, ESI†), implying a promising application of the N-CDs in a fast and stable sensing 4-NP.| Detection method | Linear range (μM) | LOD (μM) | R | References |
|---|---|---|---|---|
| a Calculated according to the reported values of 1.5 ppm in the literature.57 | ||||
| Electrochemistry | 78–1250 | 78 | — | 31 |
| Electrochemistry | 10–200 | 1.1 | 0.998 | 34 |
| Electrochemistry | 0.1–1 | 1.163 | 0.9969 | 58 |
| Electrochemistry | 10–1000 | 0.1 | 0.9985 | 59 |
| Capillary electrophoresis | 20.3–4060 | 4.06 | 0.9998 | 40 |
| Spectrophotometry | — | 10.8a | — | 57 |
| Colorimetry | 100–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.028 | 0.985 | 60 |
| Fluorescent probe | 0.72–79 | 0.16 | 0.9991 | This method |
3.7. Interference of foreign coexisted compounds
To further assess the selectivity of the N-CDs as fluorescence probes for the sensitive determination of 4-NP, structurally similar compounds (Table S4, ESI†) and environmentally relevant ions were analyzed as potential interfering substances (Table 2). It was clearly observed that ions and most structurally similar compounds studied can be tolerated at high concentrations. The results indicated that the N-CDs-based fluorescence sensor is highly selective for 4-NP.| Potential interfering substances | Tolerance level | Relative error (%) | Potential interfering substances | Tolerance level | Relative error (%) |
|---|---|---|---|---|---|
| PHE | 500 | −4.1 | Cu2+ | 50 | −4.1 |
| RES | 300 | −4.6 | Fe3+ | 90 | 4.5 |
| 2-MP | 100 | −4.3 | Pb2+ | 300 | 4.1 |
| 3-MP | 100 | −3.2 | Cd3+ | 600 | 4.3 |
| 4-MP | 100 | −3.3 | Cr3+ | 300 | −3.4 |
| 2-CP | 100 | −4.2 | Cr6+ | 300 | −3.8 |
| 4-CP | 100 | −2.8 | Ca2+ | 600 | 3.3 |
| DCP | 100 | −4.2 | Ni2+ | 200 | −3.9 |
| 2-NP | 4 | −3.9 | Mn2+ | 800 | 3.5 |
| 3-NP | 9 | −4.2 | F− | 3000 | 4.4 |
| Na+ | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.0 | Cl− | 3000 | 3.6 |
| K+ | 6000 | 3.5 | Br− | 6000 | 3.5 |
| Mg2+ | 600 | 3.6 | I− | 1000 | −4.3 |
| Al3+ | 600 | 3.9 | SO42− | 600 | 3.6 |
| Hg2+ | 50 | −3.8 | NO3− | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.0 |
3.8. Possible mechanism of the fluorescence response of N-CDs to 4-NP
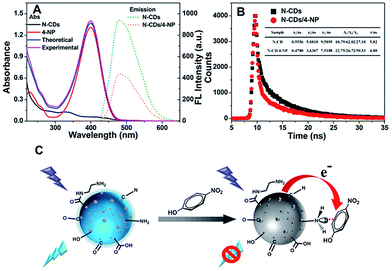
Fig. S6 (ESI)† shows that there is not a substantial overlap between the absorption spectrum of 4-NP and the fluorescence emission spectrum of the N-CDs. The result eliminated the possibility of the fluorescence resonance energy transfer (FRET) process between the N-CDs and 4-NP.61–63 4-NP is the representative electron-deficient nitroaromatic due to the strong electron withdrawing effect of nitro group, it possibly interacts with electron-rich amino group of N-CDs to form a Meisenheimer complex, resulting in electron-transfer-induced fluorescence quenching.30,56,61 While the UV-vis of the N-CDs in the presence or absence of 4-NP clearly shown that the experimental spectrum of 4-NP upon the addition of N-CDs was very similar to the theoretical one (Fig. 6A), which implied that the interaction of N-CDs with 4-NP is very weak. Therefore, it was confirmed that the Meisenheimer complex between the N-CDs and 4-NP is not formed in this work.61–63 In order to further confirm the quenching mechanism between N-CDs and 4-NP, we measured the time-resolved fluorescence spectra of the N-CDs in the absence and presence of 4-NP (Fig. 6B). The data was reliably fitted to a classical three exponential decay function. And the N-CDs alone had a lifetime of 5.82 ns, while the lifetime of the N-CDs/4-NP decreased to 4.88 ns. The decreased lifetime eliminated the inner filter effect, and indicated the quenching is a dynamic quenching mechanism, which is in accordance with the conclusion obtained from Ksv (Table S2, ESI,† Ksv increase with rising temperature, indicating dynamic quenching). On the basis of the above analysis, the fluorescence quenching of the N-CDs could be attributed to the electron-transfer-induced dynamic quenching mechanism,63 which is different from the reported one for CDs/4-NP.42 The recognition process is illustrated in Fig. 6C.3.9. Application of the 4-NP sensor in water samples
To evaluate the potential application of the N-CDs, we used the prepared N-CDs-based cotton swab fluorescence sensor for detection of 4-NP. As shown in Fig. 7B, the N-CDs on six cotton swabs exhibit strong blue fluorescence under irradiation from a 365 nm lamp. With the increasing addition of 4-NP from 0.005 to 0.5 mg mL−1, the intensely blue luminescence of the N-CDs was gradually quenched, and the color change can be observed by the naked eye under both daylight (Fig. 7C) and UV light (Fig. 7D). The similar results were also obtained for N-CDs-based filter paper strips (Fig. S7†). Moreover, N-CDs-based filter paper strips were also used for semiquantitatively monitoring of 4-NP in tap water sample (Fig. S7C and D†), which was collected from a faucet in our laboratory, and spiked with different concentrations of 4-NP. In comparison with the results shown in Fig. S7A and B,† the results for the spiked real water sample are very satisfactory. These results indicate that the sensor has the promising potential for the rapid visual detection of 4-NP in the field of environmental monitoring.To further check the applicability of the proposed sensor, we utilized the PL sensor to detect 4-NP quantitatively in real water samples. The water samples were collected from a faucet in our laboratory, and Weihe River and Muye Lake (Xinxiang, Henan, China) respectively, and filtered through 0.1 μm membranes prior to analysis. The recovery experiments were performed with spiking different concentrations of 4-NP to the water samples to assess the feasibility of the sensor, and the results were summarized in Table 3. It was found that the observed values are consistent with those of the added 4-NP with RSD of 0.5–1.6%, which demonstrates the sensing system has high reproducibility and precision. All the results above confirm the reliability and feasibility of the sensor for the detection of 4-NP in water samples.
| Samples | Added (μg mL−1) | Founded (μg mL−1) | Recovery/% (n = 6) | RSD/% (n = 6) |
|---|---|---|---|---|
| Tap water 1 | 0.400 | 0.388 | 99.1 | 1.2 |
| Tap water 2 | 0.800 | 0.819 | 102.3 | 0.9 |
| Tap water 3 | 1.20 | 1.22 | 101.5 | 1.3 |
| River water 1 | 0.500 | 0.512 | 102.2 | 0.6 |
| River water 2 | 1.00 | 0.997 | 99.7 | 1.2 |
| River water 3 | 1.50 | 1.51 | 100.5 | 1.1 |
| Lake water 1 | 0.300 | 0.299 | 99.6 | 1.0 |
| Lake water 2 | 0.600 | 0.614 | 102.3 | 0.5 |
| Lake water 3 | 1.00 | 1.01 | 101.1 | 1.6 |
4. Conclusion
In summary, we have developed a facile, simple, and green synthesis approach for producing fluorescent N-CDs by one-pot hydrothermal treatment of cheap and easily obtained maleic acid and ethylenediamine. The as-prepared N-CDs show high fluorescence QY of 45%, and excellent stability under extreme conditions, such as strong acid and base, strong ionic strengths, and long-time illumination of UV light. The prepared N-CDs also show that the emission color changes from blue, light green into dark with increasing concentration of the N-CDs. On the basis of this, we have demonstrated the potential application of the N-CDs in decorative materials. In addition, using the N-CDs as fluorescent sensor, we have proposed a simple and fast fluorescence sensing system for the detection of 4-NP based on an electron-transfer-induced dynamic quenching mechanism. The fluorescent sensor exhibits highly sensitive and selective recognition to 4-NP. Moreover, the proposed sensing system has been successfully applied to practical 4-NP detection in environmental water samples. The simple, rapid, and reliable N-CDs sensor exhibits potential application of CDs-based platform in the field of environmental monitoring.Acknowledgements
This work is supported by the Program for Innovative Research Team in Science and Technology in University of Henan Province (15IRTSTHN 003).References
- L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, D. M. L. Monica Veca, S.-Y. Xie and Y.-P. Sun, J. Am. Chem. Soc., 2007, 129, 11318–11319 CrossRef CAS PubMed.
- C. Ding, A. Zhu and Y. Tian, Acc. Chem. Res., 2014, 47, 20–30 CrossRef CAS PubMed.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44, 362–381 RSC.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef CAS PubMed.
- W. Kwon, S. Do, J. Lee, S. Hwang, J. K. Kim and S.-W. Rhee, Chem. Mater., 2013, 25, 1893–1899 CrossRef CAS.
- Y.-Q. Zhang, D.-K. Ma, Y.-G. Zhang, W. Chen and S.-M. Huang, Nano Energy, 2013, 2, 545–552 CrossRef CAS.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Rake and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S.-Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- J. Zhou, C. Booker, R. Li, X. Zhou, T.-K. Sham, X. Sun and Z. Ding, J. Am. Chem. Soc., 2007, 129, 744–745 CrossRef CAS PubMed.
- H. Li, X. He, Y. Liu, H. Huang, S. Lian, S.-T. Lee and Z. Kang, Carbon, 2011, 49, 605–609 CrossRef CAS.
- H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118–5120 RSC.
- A. B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, M. Karakassides and E. P. Giannelis, Small, 2008, 4, 455–458 CrossRef CAS PubMed.
- Y. Guo, Z. Wang, H. Shao and X. Jiang, Carbon, 2013, 52, 583–589 CrossRef CAS.
- W. Li, Z. Zhang, B. Kong, S. Feng, J. Wang, L. Wang, J. Yang, F. Zhang, P. Wu and D. Zhao, Angew. Chem., Int. Ed., 2013, 52, 8151–8155 CrossRef CAS PubMed.
- Y. Dong, H. Pang, H. B. Yang, C. Guo, J. Shao, Y. Chi, C. M. Li and T. Yu, Angew. Chem., Int. Ed., 2013, 52, 7800–7804 CrossRef CAS PubMed.
- X. Zhao, S. Zhu, Y. Song, J. Zhang and B. Yang, RSC Adv., 2015, 5, 15187–15193 RSC.
- S. Sahu, B. Behera, T. K. Maitib and S. Mohapatra, Chem. Commun., 2012, 48, 8835–8837 RSC.
- Z. C. Yang, M. Wang, A. M. Yong, S. Y. Wong, X. H. Zhang, H. Tan, A. Y. Chang, X. Li and J. Wang, Chem. Commun., 2011, 47, 11615–11617 RSC.
- W. J. Wang, X. Hai, Q. X. Mao, M. L. Chen and J. H. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 16609–16616 CAS.
- A. B. Bourlinos, G. Trivizas, M. A. Karakassides, M. Baikousi, A. Kouloumpis, D. Gournis, A. Bakandritsos, K. Hola, O. Kozak, R. Zboril, I. Papagiannouli, P. Aloukos and S. Couris, Carbon, 2015, 83, 173–179 CrossRef CAS.
- X. Shan, L. Chai, J. Ma, Z. Qian, J. Chen and H. Feng, Analyst, 2014, 139, 2322–2325 RSC.
- Q. Xu, P. Pu, J. Zhao, C. Dong, C. Gao, Y. Chen, J. Chen, Y. Liu and H. Zhou, J. Mater. Chem. A, 2015, 3, 542–546 CAS.
- S. Chandra, P. Patra, S. H. Pathan, S. Roy, S. Mitra, A. Layek, R. Bhar, P. Pramanik and A. Goswami, J. Mater. Chem. B, 2013, 1, 2375–2382 RSC.
- S. K. Bhunia, N. Pradhan and N. R. Jana, ACS Appl. Mater. Interfaces, 2014, 6, 7672–7679 CAS.
- Z. Qian, X. Shan, L. Chai, J. Ma, J. Chen and H. Feng, ACS Appl. Mater. Interfaces, 2014, 6, 6797–6805 CAS.
- H. Zhang, Y. Chen, M. Liang, L. Xu, S. Qi, H. Chen and X. Chen, Anal. Chem., 2014, 86, 9846–9852 CrossRef CAS PubMed.
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Science, 2009, 327, 760–763 CrossRef PubMed.
- B.-X. Zhang, G.-Y. Zhang, H. Gao, S.-H. Wu, J.-H. Chen and X.-L. Li, RSC Adv., 2015, 5, 7395–7400 RSC.
- S. Pang, Y. Zhang, C. Wu and S. Feng, Sens. Actuators, B, 2016, 222, 857–863 CrossRef CAS.
- X. Yang, J. Wang, D. Su, Q. Xia, F. Chai, C. Wang and F. Qu, Dalton Trans., 2014, 43, 10057–10063 RSC.
- R. Lamba, A. Umar, S. K. Mehta and S. K. Kansal, Talanta, 2015, 131, 490–498 CrossRef CAS PubMed.
- P. S. Silva, B. C. Gasparini, H. A. Magosso and A. Spinelli, J. Hazard. Mater., 2014, 273, 70–77 CrossRef PubMed.
- Y. Zhou, Z. B. Qu, Y. Zeng, T. Zhou and G. Shi, Biosens. Bioelectron., 2014, 52, 317–323 CrossRef CAS PubMed.
- M. Santhiago, C. S. Henry and L. T. Kubota, Electrochim. Acta, 2014, 130, 771–777 CrossRef CAS.
- Y. Wei, L. T. Kong, R. Yang, L. Wang, J. H. Liu and X. J. Huang, Langmuir, 2011, 27, 10295–10301 CrossRef CAS PubMed.
- L. Yang, S. Fan, G. Deng, Y. Li, X. Ran, H. Zhao and C. P. Li, Biosens. Bioelectron., 2015, 68, 617–625 CrossRef CAS PubMed.
- K. Giribabu, R. Suresh, R. Manigandan, S. Praveen Kumar, S. Muthamizh, S. Munusamy and V. Narayanan, Microchim. Acta, 2014, 181, 1863–1870 CrossRef CAS.
- Y. Zhang, L. Wu, W. Lei, X. Xia, M. Xia and Q. Hao, Electrochim. Acta, 2014, 146, 568–576 CrossRef CAS.
- X. Liu, Y. Ji, Y. Zhang, H. Zhang and M. Liu, J. Chromatogr. A, 2007, 1165, 10–17 CrossRef CAS PubMed.
- X. Guo, Z. Wang and S. Zhou, Talanta, 2004, 64, 135–139 CrossRef CAS PubMed.
- H. Li and C. Han, Chem. Mater., 2008, 20, 6053–6059 CrossRef CAS.
- G. H. G. Ahmed, R. B. Laíño, J. A. G. Calzón and M. E. D. García, Microchim. Acta, 2014, 182, 51–59 CrossRef.
- C.-C. Shih, P.-C. Chen, G.-L. Lin, C.-W. Wang and H.-T. Chang, ACS Nano, 2015, 9, 312–319 CrossRef CAS PubMed.
- Y. Zhang, Y. Wang, Y. Guan and L. Feng, Nanoscale, 2015, 7, 6348–6355 RSC.
- S. Liu, J. Tian, L. Wang, Y. Zhang, X. Qin, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Adv. Mater., 2012, 24, 2037–2041 CrossRef CAS PubMed.
- J. Ju and W. Chen, Biosens. Bioelectron., 2014, 58, 219–225 CrossRef CAS PubMed.
- W. Wang, Y. C. Lu, H. Huang, A. J. Wang, J. R. Chen and J. J. Feng, Biosens. Bioelectron., 2015, 64, 517–522 CrossRef CAS PubMed.
- J. Hou, J. Dong, H. Zhu, X. Teng, S. Ai and M. Mang, Biosens. Bioelectron., 2015, 68, 20–26 CrossRef CAS PubMed.
- Z. Yang, M. Xu, Y. Liu, F. He, F. Gao, Y. Su, H. Wei and Y. Zhang, Nanoscale, 2014, 6, 1890–1895 RSC.
- X. Jin, X. Sun, G. Chen, L. Ding, Y. Li, Z. Liu, Z. Wang, W. Pan, C. Hu and J. Wang, Carbon, 2015, 81, 388–395 CrossRef CAS.
- H. Ding, J. S. Wei and H. M. Xiong, Nanoscale, 2014, 6, 13817–13823 RSC.
- G. Eda, Y. Y. Lin, C. Mattevi, H. Yamaguchi, H. A. Chen, I. S. Chen, C. W. Chen and M. Chhowalla, Adv. Mater., 2009, 21, 1–5 Search PubMed.
- L. Tang, R. Ji, X. Cao, J. Lin, H. Jiang, X. Li, K. S. Teng, C. M. Luk, S. Zeng, J. Hao and S. P. Lau, ACS Nano, 2012, 6, 5102–5110 CrossRef CAS PubMed.
- R. Zhang and W. Chen, Biosens. Bioelectron., 2014, 55, 83–90 CrossRef CAS PubMed.
- X. Gao, Y. Lu, R. Zhang, S. He, J. Ju, M. Liu, L. Li and W. Chen, J. Mater. Chem. C, 2015, 3, 2302–2309 RSC.
- J. Liu, H. Chen, Z. Lin and J.-M. Lin, Anal. Chem., 2010, 82, 7380–7386 CrossRef CAS PubMed.
- A. Üzer, E. Erçağ and R. Apak, Anal. Chim. Acta, 2004, 505, 83–93 CrossRef.
- K. Singh, A. A. Ibrahim, A. Umar, A. Kumar, G. R. Chaudhary, S. Singh and S. K. Mehta, Sens. Actuators, B, 2014, 202, 1044–1050 CrossRef CAS.
- Y.-E. Gu, Y. Zhang, F. Zhang, J. Wei, C. Wang, Y. Du and W. Ye, Electrochim. Acta, 2010, 56, 953–958 CrossRef CAS.
- F. Xue, Z. Meng, Y. Wang, S. Huang, Q. Wang, W. Lu and M. Xue, Anal. Methods, 2014, 6, 831–837 RSC.
- Y. Wang and Y. Ni, Anal. Chem., 2014, 86, 7463–7470 CrossRef CAS PubMed.
- M. Rong, L. Lin, X. Song, T. Zhao, Y. Zhong, J. Yan, Y. Wang and X. Chen, Anal. Chem., 2015, 87, 1288–1296 CrossRef CAS PubMed.
- F. Lin, D. Pei, W. He, Z. Huang, Y. Huang and X. Guo, J. Mater. Chem., 2012, 22, 11801–11807 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26870b |
| This journal is © The Royal Society of Chemistry 2016 |