Synthesis and characterization of a phytic acid/mesoporous 45S5 bioglass composite coating on a magnesium alloy and degradation behavior
Yan Lia,
Shu Cai*a,
Guohua Xu*b,
Sibo Shena,
Min Zhanga,
Tong Zhanga and
Xiaohong Sun*a
aKey Laboratory for Advanced Ceramics and Machining Technology of Ministry of Education, Tianjin University, Tianjin 300072, People's Republic of China. E-mail: caishu@tju.edu.cn; sunxh@tju.edu.cn; Tel: +86-22-27425069 Tel: +86-22-27406114
bShanghai Changzheng Hospital, Shanghai 200003, People's Republic of China. E-mail: xuguohuamail@163.com; Tel: +86-21-63610109
First published on 4th March 2015
Abstract
In order to decrease the degradation rate and improve the bioactivity of a magnesium alloy, a phytic acid and mesoporous 45S5 bioglass composite coating (PMBC) has been synthesized on the AZ31 magnesium alloy by a two-step method. The phase, surface morphology and structure of the composite coatings were characterized by X-ray diffraction, scanning electron microscopy and Fourier transform infrared spectroscopy. The composite coating consisting of an interior layer of mesoporous 45S5 bioglass and an outer layer of phytic acid/magnesium phytic acid was dense, crack-free and had no remarkable interface between the inner and outer coating. The bonding strength of the composite coating to the magnesium alloy substrate was 15.2 ± 1.5 MPa. The immersion test in simulated body fluid (SBF) showed that the composite coating could provide obvious protection for the substrate and greatly decreased the degradation rate to 0.62 mg per cm2 per day from the uncoated sample of 2.93 mg per cm2 per day on the sixteenth day. And the outer coating of phytic acid could induce the formation of apatite, suggesting good bioactivity of the coated magnesium alloy.
1. Introduction
Traditional metallic implant materials, such as titanium alloy and stainless steel, are non-biodegradable and these implants may cause physical irritation, restenosis and thrombosis, because they cannot adapt to the growth of pediatric patients or are non-permitted for later surgical revascularization.1 During the past few decades, magnesium (Mg) and its alloys have been regarded as revolutionary implant materials, which can be used to replace widely used non-biodegradable orthopedic implants, due to their high specific strength, capability of biodegradation, suitable density and low Young's modulus similar to natural human bone. Besides, magnesium is the fourth most abundant cation in human beings, which has a fundamental influence on cellular and enzymatic reactions.2–8 However, the fast degradation rate of magnesium alloys would lead to an inhomogeneous localized degradation and finally resulted in a quick decline of their mechanical strength and a high level of local pH values surrounding the human tissues, which are undesired for the healing of the implant area.9–11Therefore, some surface treatments have been developed to control the degradation rate of magnesium alloys, such as chemical conversion layers, micro arc oxidation, sol–gel coatings and immersion treatment.12–16 Nowadays, some of sol–gel derived bioactive glasses, ceramic or glass-ceramic coatings in the system of SiO2–Na2O–CaO–P2O5 (such as 45S5 and 58S) have been successfully prepared on the magnesium alloys by dip-coating method. These coatings not only provided good protective effects of magnesium alloy substrate, but also could accelerate the apatite deposition.17,18 Especially for the coatings with mesoporous structure, they could accelerate the apatite deposition on the coatings and enhanced the cell response in vitro, thus promoting the bioactivity of biomaterials.19,20 Besides, the existence of mesopores decreased the effect of residual stress on the cracking and peeling off the bioglass coating, and improved the bonding strength to the magnesium alloy.15,21 Nevertheless, our previous studies found that the degradation rates of mesoporous coating coated magnesium alloys could not meet the healing requirement of the implants, due to the presence of pores during the immersion test in vitro.18,21 However, this problem can be overcome by developing organic/inorganic composite coatings on magnesium alloy.22 Organic/inorganic composite coatings from biodegradable polymers and bioactive inorganic materials are attractive for biomedical applications of magnesium and its alloys as they can effectively decrease degradation rate of alloys, provide good bioactivity and increase mechanical properties of coatings.23–25 Phytic acid, also called IP6, is a kind of innoxious natural macromolecule compound with a capability of chelating with many cations, such as magnesium, copper and zinc.26–28 The hydroxide radicals in phytic acid can react with metal atoms or cations of alloys to form chelate compounds, thus create a strong bond between alloy substrate and chelate compound layer.29–31 Moreover, many literatures have proved that phytic acid is beneficial to cell differentiation and anti-cancer.32–34 And lower phosphorylated forms (IP1–5) derived from phytic acid are also useful for regulating vital cellular functions.35,36 Nonetheless, previous literatures mainly discussed the preparation of phytic acid conversion coatings on alloys for long-term protection in industry or used them as intermediate layer to improve the combination between substrate and outer layer.29–31,37,38 Therefore, the usage of phytic acid as a biocoating needs further investigation, and phytic acid/mesoporous bioglass composite coating on magnesium alloy may possess excellent properties. On one hand, mesoporous bioglass coating used as the intermediate layer is beneficial to phytic acid penetrating into the mesoporous coating to form an organic/inorganic composite structure, which will provide good protection of magnesium alloy substrate. On the other hand, the chelating ability of phytic acid to induce calcium phosphate deposition in simulated body fluid (SBF) can be investigated.
In this work, a novel phytic acid and mesoporous 45S5 bioglass composite coating was prepared on AZ31 magnesium alloy via sol–gel dip-coating method and immersion technique in phytic acid bath. The characterization of the composite coatings and the degradation behavior of the coated samples were investigated.
2. Experimental
2.1. Synthesis of composite coating on AZ31 magnesium alloy substrates
The magnesium alloy pieces cut from commercial AZ31 magnesium alloy plate (Al 3 wt%, Zn 1 wt%, Mn 0.2 wt%, Fe < 0.005 wt% and balanced Mg) were selected as substrates in this study. The alloy substrates with an approximate size of 10 mm × 10 mm × 2 mm were polished with progressively finer SiC papers up to 2000 grit, and then cleaned ultrasonically in distilled water, ethanol and acetone for 15 min, respectively, followed by drying at room temperature for use.The reagents such as tetraethyl orthosilicate (Si(OC2H5)4, TEOS; Kermel, China; min. 98%), triethyl phosphate ((CH3CH2O)3P(O), TEP; Kermel, China; min. 98%), nitric acid (HNO3; Kermel, China; min. 65%), sodium nitrate (NaNO3; Kermel, China; min. 99%), calcium nitrate tetrahydrate (Ca(NO3)2·4H2O; Kermel, China; min. 99%), phytic acid (C6H6(H2PO4)6; Damao, China; min. 70%) and triethylamine ((C2H5)3N; Damao, China; min. 99%) were used in this study. Besides, the Pluronic F127 (EO106PO70EO106; Sigma, USA; Mn = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) was selected as pore template. All reagents were used as received. The mesoporous 45S5 coating was synthesized via a sol–gel process in reference to the nominal composition of 45S5 (45.0 wt% SiO2, 24.5 wt% Na2O, 24.5 wt% CaO and 6.0 wt% P2O5). 2.4 mL HNO3 (0.1 M) solution was added into TEOS (3.4 mL), TEP (0.3 mL), NaNO3 (0.4 M), Ca(NO3)2·4H2O (0.2 M) mixed solution and stirred for 45 min, which was used to catalyze the hydrolysis of TEOS. At this stage, 0.87 g F127 was dissolved in 72 mL alcohol, added into the above-mentioned solution and went on stirring for 1 h to obtain a homogeneous and clear transparent sol. AZ31 magnesium alloy substrates were dipped into above-mentioned sol with a withdrawal speed of 0.5 mm s−1 for 2 cycles, aged at room temperature for 48 h, dried at 60 °C for 12 h and heat treated at 400 °C for 2 h. The formulation of preparing phytic acid coating was optimized ahead by adjusting the concentration, pH value, reacting time and temperature of the phytic acid solution. In this study, only the optimal formulation was referred. By adding distilled water and triethylamine into phytic acid solution, the solution used to prepare phytic acid coating was obtained with the concentration of 0.7 wt% at pH = 5 and the solution was stirred for 30 min for a thoroughly uniformity. Subsequently, mesoporous 45S5 bioglass coated samples were placed in phytic acid solution for 35 min which temperature was maintained at 35 °C, then the alloy samples were taken out and dried at 30 °C for 24 h. After finishing these processes, the phytic acid and mesoporous 45S5 bioglass composite coating (PMBC) coated samples were obtained. The mesoporous 45S5 bioglass coated, phytic acid modified (magnesium alloy immersed in 0.7 wt%, pH = 5, 35 °C phytic acid solution for 35 min) samples are denoted as MBC and PcC coated samples, respectively, and the uncoated AZ31 substrate is used as control group.
600) was selected as pore template. All reagents were used as received. The mesoporous 45S5 coating was synthesized via a sol–gel process in reference to the nominal composition of 45S5 (45.0 wt% SiO2, 24.5 wt% Na2O, 24.5 wt% CaO and 6.0 wt% P2O5). 2.4 mL HNO3 (0.1 M) solution was added into TEOS (3.4 mL), TEP (0.3 mL), NaNO3 (0.4 M), Ca(NO3)2·4H2O (0.2 M) mixed solution and stirred for 45 min, which was used to catalyze the hydrolysis of TEOS. At this stage, 0.87 g F127 was dissolved in 72 mL alcohol, added into the above-mentioned solution and went on stirring for 1 h to obtain a homogeneous and clear transparent sol. AZ31 magnesium alloy substrates were dipped into above-mentioned sol with a withdrawal speed of 0.5 mm s−1 for 2 cycles, aged at room temperature for 48 h, dried at 60 °C for 12 h and heat treated at 400 °C for 2 h. The formulation of preparing phytic acid coating was optimized ahead by adjusting the concentration, pH value, reacting time and temperature of the phytic acid solution. In this study, only the optimal formulation was referred. By adding distilled water and triethylamine into phytic acid solution, the solution used to prepare phytic acid coating was obtained with the concentration of 0.7 wt% at pH = 5 and the solution was stirred for 30 min for a thoroughly uniformity. Subsequently, mesoporous 45S5 bioglass coated samples were placed in phytic acid solution for 35 min which temperature was maintained at 35 °C, then the alloy samples were taken out and dried at 30 °C for 24 h. After finishing these processes, the phytic acid and mesoporous 45S5 bioglass composite coating (PMBC) coated samples were obtained. The mesoporous 45S5 bioglass coated, phytic acid modified (magnesium alloy immersed in 0.7 wt%, pH = 5, 35 °C phytic acid solution for 35 min) samples are denoted as MBC and PcC coated samples, respectively, and the uncoated AZ31 substrate is used as control group.
2.2. Characterization
The surface morphologies of the uncoated and coated samples, and the cross-sectional micrographs of MBC and PMBC coated AZ31 alloy samples were characterized via field-emission scanning electron microscope (SEM, Hitachi S-4800, Japan). Besides, chemical compositions of all the samples before and after immersing in SBF solution for a certain time were analyzed by energy dispersive spectrum (EDS, 7401 Oxford) attached to SEM mentioned above. Phase composition of the coatings was examined by low-angle (1°) X-ray diffraction (XRD, Rigaku D, Japan) with Cu Kα radiation over a 2θ ranging between 15° and 70°. Fourier transform infrared spectroscopy (FTIR, Nicolet 5DX, USA) was also carried out, recording at a resolution of 2.0 cm−1 over a scan range of 4400 to 400 cm−1, and the powders used in this test were carefully scratched from the surface of MBC and PMBC coated samples.The tensile adhesion strength between the coating and the substrate was measured using a universal tensile testing machine (AGS-H, Shimadzu, Japan) at room temperature. The tensile test samples were prepared by bonding the coated sample to two aluminum alloy supports using a jig and a tensile rate of 0.5 mm min−1 was employed. Five adhesion strength tests were performed for each kind of coated samples.
2.3. Immersion test
During the whole immersion test, the uncoated and coated samples were immersed in SBF (pH = 7.40) at 37 ± 0.5 °C by using a WE-3 immersion oscillator. Besides, the concentration of ions in SBF formulation was shown in Table 1. According to ASTM G31-72,39 the volume of solution was calculated based on a volume-to-sample area of 20 mL cm−2, and the SBF solution was refreshed every 3 days. The uncoated and coated samples were taken out from SBF solution after a period of time, and pH values of residual solutions were measured by a pH meter (PHS-25, China). The samples used to measure degradation rate must be cleaned to remove degradation products,40 and then rinsed with distilled water, cleaned ultrasonically in ethanol and dried in air. The degradation rate was calculated as follows:
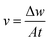 | (1) |
| Ions | Na+ | K+ | Mg2+ | Ca2+ | Cl− | HCO3− | HPO42− | SO42− |
|---|---|---|---|---|---|---|---|---|
| Concentration | 142.0 | 5.0 | 1.5 | 2.5 | 147.8 | 4.2 | 1.0 | 0.5 |
3. Results
3.1. Coating characterization
Fig. 1 showed the surface morphologies of the uncoated and coated magnesium alloy substrates. Compared with the uncoated sample (Fig. 1a) which showed the clearly polishing scratches on the surface, sample with MBC coating was dense and integrated as shown in Fig. 1b. Moreover, from our previous research, the special structure of mesopore existed in the coatings and the average diameter of mesopore was 3 nm.41 For the sample with PcC coating (Fig. 1c), cracks uniformly distributed on the surface of the coated sample, and even penetrated the whole conversion coating. When the magnesium alloy substrate was covered with PMBC composite coating, the surface was crack-free, compact and continuous, which could be predicted that the PMBC coating can provide effective protection for magnesium alloy substrate in SBF solution. | ||
| Fig. 1 Surface morphologies of AZ31 alloy (a) uncoated, (b) MBC, (c) PcC and (d) PMBC coated samples. | ||
The XRD patterns of all the samples had been given in Fig. 2 to investigate the phase composition of the coatings. The peaks at 2θ = 32.2°, 34.4°, 36.7°, 47.8° and 63.1° in all the samples corresponded to magnesium (JCPDS card no. 35-0821). And peaks at 2θ = 33.5°, 34.2°, 36.1° and 46.4° in the pattern of MBC coated sample displayed the existence of crystalline phase Na2Ca2Si3O9 (JCPDS card no. 22-1455). The pattern of PcC coated sample showed that magnesium phytic acid was amorphous as no diffraction peak could be distinguished. As shown in Fig. 2d, peaks at 2θ = 34.2° and the broad bend between 15° and 30° indicated the compositions of PMBC coating were crystalline phase Na2Ca2Si3O9 and amorphous phase. Then, Table 2 exhibited element compositions (wt%) on the surface of AZ31 alloy uncoated and coated samples. Besides Mg element, Si, P, C and O elements were detected on the surface from PMBC coated sample, which were the characteristic elements of mesoporous 45S5 bioglass and phytic acid/magnesium phytic acid. The XRD results (Fig. 2) along with the EDS results (Table 2) confirmed that the composite coating consisted of an interior layer of mesoporous 45S5 bioglass and an outer layer of phytic acid/magnesium phytic acid compounds.
 | ||
| Fig. 2 Low-angle X-ray diffraction patterns of AZ31 alloy (a) uncoated, (b) MBC, (c) PcC and (d) PMBC coated samples. | ||
| Sample | C | O | Na | Mg | Al | Si | P | Ca |
|---|---|---|---|---|---|---|---|---|
| AZ31 | 3.50 | 1.12 | 1.16 | 90.91 | 2.60 | 0.16 | 0.23 | 0.33 |
| MBC | 9.89 | 40.02 | 2.72 | 10.06 | 0.11 | 32.12 | 0.43 | 4.65 |
| PcC | 17.07 | 40.15 | 0.98 | 20.53 | 4.35 | 0.11 | 16.61 | 0.20 |
| PMBC | 23.03 | 50.64 | 0.40 | 6.31 | 1.78 | 7.16 | 10.45 | 0.23 |
FTIR was used to detect the glass structure and chemical groups in phytic acid and mesoporous 45S5 bioglass composite coating (shown in Fig. 3). Based on the previous literature,42 three major vibration modes of Si–O groups can be observed in the MBC powder: the band at 1040 cm−1 corresponds to the asymmetric stretching vibration of Si–O–Si, the band at 859 cm−1 is associated to the asymmetric bending vibration of Si–O–Si, and the one at 458 cm−1 is identified as the rocking vibration of Si–O–Si. Furthermore, bands are presented at 603 cm−1 and 571 cm−1 corresponding to the bending vibration of P–O. All these characteristic bands demonstrated the formation of 45S5 bioglass on AZ31 substrates. The FTIR spectrum of the obtained sample PMBC was illustrated in Fig. 3b. Besides the characteristic bands of 45S5 bioglass, a new band at 1652 cm−1 appears, which is assigned to the characteristic peak HPO42− of phytic acid, suggesting that the structure of phytic acid molecule still remains.30 Meanwhile, a wide band at 1120 cm−1 shows up in Fig. 3b, which is identified to Si–O–P bond, as reported by Mistry.43 It can be speculated that Si–OH group on the surface of mesoporous 45S5 bioglass coating could react with P–OH group in phytic acid molecule. Therefore, strong chemical bonding between mesoporous bioglass coating and phytic acid coating was formed, which would lead to good adhesion strength to the magnesium alloy.
The tensile strength test was carried out as a measurement to testify the adhesion strength of a coating with substrate, which influenced the long-term stability of the implant coated with biomedical coating significantly. Each of the curves was a representative for each kind of coating. Meanwhile, five adhesion strength tests were performed to obtain the mean values and standard deviations. As illustrated in Fig. 4, the adhesion strength values of the MBC and PcC coated samples were 11.8 ± 1.1 MPa and 20.8 ± 1.9 MPa, respectively, while adhesion strength of the PMBC coated samples was 15.2 ± 1.5 MPa.
 | ||
| Fig. 4 Representative stress–displacement curves for AZ31 alloy (a) MBC, (b) PcC and (c) PMBC coated samples as obtained from tensile strength test. | ||
3.2. In vitro degradation behavior
To investigate in vitro degradation behavior of AZ31 alloy uncoated and coated samples (MBC, PcC and PMBC), pH variations of the immersing mediums and the degradation rates of AZ31 alloy substrates were examined and illustrated in Fig. 5 and 6, respectively. In contrast to the uncoated sample, coated samples exhibited low degradation rates and each kind of coating had its unique character. It can be observed that both pH values and degradation rates of PcC coated samples increased fast during the immersion period. The degradation rate reached 2.72 mg per cm2 per day on the sixteenth day of immersion test, only a little lower than that of the uncoated sample AZ31 (2.93 mg per cm2 per day). Whereas, MBC coated samples had lower pH value and degradation rate in the initial 7 days' immersion test, namely 8.96 and 1.04 mg per cm2 per day, indicating a better protective property than that of PcC coated samples. In the later immersion test, it was notable that pH values and degradation rates showed sharp increase, up to 9.88 and 1.34 mg per cm2 per day on the sixteenth day. By contrast, PMBC coated samples possessed the lowest pH value of 9.36 and the degradation rate of 0.62 mg per cm2 per day after 16 days' immersion, much lower than those of the uncoated samples, MBC or PcC coated samples, suggesting a good protective property of the composite coating. | ||
| Fig. 5 pH variation of AZ31 alloys uncoated and MBC, PcC, PMBC coated samples immersed in SBF for different periods. | ||
 | ||
| Fig. 6 Degradation rates of AZ31 alloys uncoated and MBC, PcC, PMBC coated samples immersed in SBF for different periods. | ||
3.3. Apatite formation
Apatite formation in SBF solution has been widely regarded as a quick and convenient way to predict bioactivity of biomaterials in vitro. In this work, SEM observation and EDS analysis of the samples after being immersed in SBF for 3, 7 and 16 days have been performed. As shown in Fig. 7a, d, g and j, some depositions can be observed on the surfaces of all the samples, but exhibited different morphologies. Only plate-like Mg(OH)2 layers formed on the surface of uncoated sample (Fig. 7a), indicating severe degradation of AZ31 magnesium alloy in SBF solution. The depositions formed on the surface of MBC, PcC and PMBC coated samples were elliptical particles, laminar products and spherical particles, respectively. From the EDS results (Table 3), the chemical compositions of depositions on coated samples consisted of C, Ca, P, Mg and O elements. And the Ca/P molar ratios equaled to 1.13, 0.93 and 1.08 after immersing in SBF for 3 days, then increased to 1.27, 0.99 and 1.62 after immersing for 16 days, corresponding to the depositions on the MBC, PcC and PMBC coated samples, respectively. The deposition on PMBC coated sample after 16 days' immersion could be regarded as apatite, because the Ca/P molar ratio was very close to that of hydroxyapatite (1.67). | ||
| Fig. 7 Surface morphologies of AZ31 alloy uncoated and MBC, PcC, PMBC coated samples after immersing in SBF solution for 3 days (a, d, g, j), for 7 days (b, e, h, k), for 16 days (c, f, i, l). | ||
| Time | Area | C | Mg | O | Ca | P |
|---|---|---|---|---|---|---|
| 3 days | A | 11.74 | 35.07 | 52.43 | 0.21 | 0.56 |
| B | 17.01 | 16.57 | 53.51 | 6.87 | 6.04 | |
| C | 17.91 | 27.14 | 39.45 | 7.48 | 8.02 | |
| D | 28.21 | 5.05 | 47.67 | 9.90 | 9.17 | |
| 16 days | E | 13.32 | 28.13 | 58.13 | 0.09 | 0.34 |
| F | 18.18 | 5.22 | 52.48 | 13.49 | 10.63 | |
| G | 21.19 | 14.04 | 53.87 | 5.44 | 5.47 | |
| H | 18.96 | 0.39 | 55.92 | 15.32 | 9.42 |
4. Discussion
4.1. Interface bonding strength of composite coating
It has been proved that bonding strength between substrate and coatings plays a fundamental role in enhancing protective property of magnesium alloy, and the failure of coated alloy implants always occurs due to decohesion of the coating.44 In this work, a flawless and dense, novel phytic acid and mesoporous 45S5 bioglass composite coating (PMBC) has been synthesized successfully on magnesium alloy via sol–gel dip-coating method and immersion technique in phytic acid bath. The composite coating consisting of an interior layer of mesoporous 45S5 bioglass and a dense outer layer of phytic acid/magnesium phytic acid compounds, presented a good protection for the magnesium alloy substrate. It was mainly related to the structure of the composite coating and the strong chemical bonding between the composite coating and magnesium alloy. Before understanding the protective effects of composite coating, the possible forming process of composite coating on magnesium alloy substrate and chemical reactions between the contact interfaces were schematic illustrated in Fig. 8. The AZ31 alloy was first coated by MBC using sol–gel dip-coating method for two cycles, and followed by heat-treatment. Mesopores were formed uniformly in this bioglass coating which had been confirmed by our prior research.41 Previous study showed that good adhesion strength between mesoporous coating and magnesium alloy substrate could be obtained by this method,15 and the test result was 11.8 ± 1.1 MPa in this study. It was primarily derived from the formation of chemical bonds (Si–O–Mg) established between silanol groups and metal hydroxyls.45,46 Possible reactions were suggested as follows:| Mg + 2H2O = Mg(OH)2 + H2↑ | (2) |
| Si–OH + Mg–OH → Si–O–Mg + H2O | (3) |
 | ||
| Fig. 8 (a) Schematic illustration of preparation for PMBC on AZ31 magnesium alloy, (b) possible reactions during immersion in phytic acid bath. | ||
Then, the phytic acid coating was prepared on MBC coated sample through immersion technique in phytic acid bath. During this process, MBC coating would exchange its cations with H+/H3O+ from phytic acid solution, resulting in the formation of Si–OH on its surface.47,48 Thereafter, the Si–OH reacted with some P–OH groups in phytic acid molecule to form Si–O–P, leading to a strong interface bonding with MBC coating.37,43 Meanwhile, the residual hydroxyls in phytic acid molecule would bond together through strong intermolecular bond interaction to form a dense and uniform phytic acid coating above the mesoporous 45S5 bioglass coating. As a small portion of interconnected pores existed in MBC, phytic acid solution would penetrate through MBC coating and react with magnesium alloys directly to form magnesium phytic acid, blocking the inner pore channel, which was evidenced by the elements composition of composite coating (Table 2) and the appearing of Si–O–P group in FTIR result (Fig. 3b).
It is worth noting that adhesion strength between PMBC coating and substrate is 15.2 ± 1.5 MPa, nearly 3.4 MPa higher than that of MBC. Therefore, it is reasonable to conclude that the strong interface strength mainly results from the chemical bonding between the contact interface, and the reticulate pinning force by the formation of magnesium phytic acid through the pore channels. As shown in Fig. 9, the thickness of MBC coating is about 500 nm, whereas, the thickness of PMBC coatings is measured to be ∼1.0 μm, and the composite coating is dense and crack-free. No obvious boundaries can be observed among the interfaces of magnesium alloy, mesoporous 45S5 coating and phytic acid coating, which is another evidence in approve of composite coating tightly attached to the substrate. Pan and co-workers have proved that a proper combination of dense structure, adhesion strength and thickness of the coating is of importance to improve the protection of alloys.49,50 Hence, it is deduced that the uniform and crack-free composite coating which has a strong interface bonding with magnesium alloy, could decrease the degradation rate of magnesium alloy substrate effectively.
4.2. In vitro degradation behavior
The immersion tests in SBF solution provide some information with respect to the long-term degradation behavior of coatings on magnesium alloy substrates. The degradation process of the coated samples mainly depends on the microstructure and chemical composition of the coatings, as well as the interface bonding strength.During the synthesis of MBC, template F127 decomposed at 400 °C, then mesopores evenly distributed in the microstructure, as reported by Huang.41 The mesopores in MBC made the elastic modulus of bioglass coatings decrease sharply and less stress existed in coating after heat treatment.21 When MBC coated sample immersed in SBF for 3 days, the coating was not easily peeled off from AZ31 substrate, as shown in Fig. 7d. However, the porous structure in MBC affected the degradation rate of AZ31 substrate because the mesopores contained in the coating provided paths for SBF solution to penetrate into substrate, then cracks appeared on the surface of MBC coating.21 It was found that relatively sharp increase of pH values and degradation rates (Fig. 5 and 6) occurred in later immersion test, which may be due to cracks extending, even penetrating the MBC coating (Fig. 7e). Therefore, the MBC coating could not act as a suitable physical barrier to decrease the degradation rate of AZ31 magnesium alloys in the periods of 16 days' immersion test. For the traditional PcC coated sample which derived from phytic acid molecules attaching to metal atoms of magnesium alloy in situ, has strong interface bonding with alloy substrate due to the formation of chemical bonds.51 Previous literatures had proved that it had good biological property to some extent, while the degradation rate remained a relatively high value during the immersion test.51,52 In order to provide effective protection for the alloy substrate, a thick conversion coating of magnesium phytic acid was prepared in this work, meanwhile, adhesion strength between the coating and substrate reached 20.8 ± 1.9 MPa. However, during the preparation of the thick conversion coatings, pores and cracks easily formed in outer layer of PcC coating (shown in Fig. 1c), due to the release of hydrogen gas. The result was consistent with Cui and Pans' reports that the PcC coatings consisted of multilayer product, including dense thin inner layer and porous outer layers.30,31 In the immersion test, pores and cracks in the outer layer affected the protective property of PcC coating significantly. As shown in Fig. 7g, some PcC coating had already peeled off from the substrate after 3 days' immersion, resulting in the degradation of underlying magnesium alloy and the formation of degradation product with low Ca/P molar ratio. Hence, the PcC can only provide a short time protection for magnesium alloys. As shown in Fig. 7 and Table 3, no apatite can be observed on the surface of MBC or PcC coated samples during the whole immersion test. It might be related to the excess release of magnesium ions into SBF because of the rapid degradation of magnesium alloy substrates, which would inhibit the apatite formation.53 Therefore, the chelating ability of phytic acid to induce calcium phosphate deposition in SBF should be further investigated in the absence of excess magnesium ions.
As discussed above, the special structure of the composite coating would provide a long period of protection for the substrate. The immersion tests in SBF solution confirmed the deduction. According to the results of immersion test (Fig. 5 and 6) and the changes in surface morphologies of samples after immersed (Fig. 7), the degradation process of PMBC coated samples can be summarized as three stages: partial dissolution of coating; defects formation; peeling off of coating and apatite deposition simultaneously. During the initial 4 days (stage I), pH value of the SBF solution containing PMBC coated sample reached 7.94, and degradation rate of the substrate was 0.44 mg per cm2 per day, showing a slight degradation. This stage could be explained by the following reaction and dissolution behavior: during the preparation of phytic acid/magnesium phytic acid coating, the steric hindrance and charge distribution of reacting products which derived from the unique structure of phytic acid molecule, would not lead to the fully consumption of hydroxyl groups. Thus, there were still some residual hydroxyl groups existing on the surface of composite coating.51,54,55 During the immersion period of PMBC coated samples, these residual hydroxyl groups in phytic acid coating would react with the cations in SBF solution to form phytic acid chelates with different solubility.55,56 The absorption of water molecules to soluble phytic acid chelates (sodium phytic acid and potassium phytic acid) made the fracture of intermolecular bond among phytic acid molecular, and partial dissolution of the phytic acid coating appeared, thus showing a low degradation rate in this period. However, no cracks or peeling off of composite coating can be seen in this stage, which was attributed to the high bonding strength between the composite coating and substrate. Besides, calcium-deficient apatite deposition with typical spherical morphology was observed on the whole surface of PMBC coated sample (shown in Fig. 7j), which is similar to Chen's work.51 Then, in the later 3 days' immersion (4–7 days, stage II), both pH variations and the degradation rates of PMBC coated samples appeared a relatively high increase trend. This was probably due to the formation of small cracks on the surface of PMBC coating (shown in Fig. 7k), which would cause permeation of SBF solution and lead to a fast degradation of magnesium alloy in this period. When the immersion time extending to 16 days, clusters of spherical apatites were presented in Fig. 7l and the Ca/P molar ratio equaled to 1.62 (shown in Table 3, area H). The formation of these continuous apatites was related to the strong chelating ability of phytic acid molecules. It was reported that SBF was a kind of metastable calcium phosphate solution, which was supersaturated for apatites, and the existence of nucleation sites on the coating was an accelerant of forming apatite coating in SBF.50,57,58 More hydroxyl groups in phytic acid molecules had opportunities to chelate with Ca2+ in SBF solution with the cracks extending, which provided more nucleation sites of apatite than last two stages. When the nuclei formed, clusters of spherical apatites grew by consuming HPO42− from SBF solution. And continuous apatite layer maybe started to form on the composite coating, which blocked degradation channels and prevented SBF solution from contacting the substrate in stage III (7–16 days).18,59 It was notable that the degradation rate of PMBC coated samples slightly increased from 0.55 mg per cm2 per day to 0.63 mg per cm2 per day, and then kept a stable value at 0.62 mg per cm2 per day during this stage, which can be explained by the proposed degradation process. It was reported when the pH value of solution below 9.00, phytic acid remained the sterically unhindered 1 ax/5 eq form, while, phytic acid existed in the sterically hindered 5 ax/1 eq form in the pH value of solution above 9.50. Hence, the pH value of solution played an important role in the stability of phytic acid coating.60–62 When the immersion time extended to stage III, pH value of SBF solution containing PMBC coated sample slightly increased and reached above 9.00, which provided an environment of conformational inversion processes in phytic acid and its chelates. During the later immersion process, the protective effect of phytic acid coating gradually weakened, meanwhile, the deposition of apatites provided a certain degree of protection for AZ31 alloy substrates.18,59 Even if the phytic acid/magnesium phytic acid coating degraded completely, the inner mesoporous bioglass coating and the deposited apatite could still provide protection for alloy substrate for a relatively long time. From above analysis, the suitable protective property of composite coating is mainly attributed to the special structure of the composite coating, high interface bonding strength between composite coating and substrate and good apatite forming ability.
The current results in this study revealed that the PMBC coating could provide suitable protective property and improve bioactivity of the substrates. The testing of cytotoxicity and biological characteristics of PMBC coated magnesium alloys are being performed in Changzheng hospital in Shanghai (China), which will be detailed in a forthcoming paper.
5. Conclusions
In this study, a novel phytic acid and mesoporous 45S5 bioglass composite coating (PMBC) has been synthesized on magnesium alloy successfully via a two-step method. The composite coating consisting of an interior layer of mesoporous 45S5 bioglass and a dense outer layer of phytic acid/magnesium phytic acid compounds, presented a bonding strength of 15.2 ± 1.5 MPa to the magnesium alloy substrate, which was nearly 30% higher than single mesoporous 45S5 bioglass coated sample. The composite coating has significant influences on the degradation rate and the bioactivity of AZ31 magnesium alloy substrate during its degradation process. On one hand, PMBC could act as a barrier to decrease the degradation rate of AZ31 magnesium alloy in a certain period, and the degradation rate of alloy substrate has been sharply reduced from 2.93 mg per cm2 per day to 0.62 mg per cm2 per day during the 16 days' immersion test in SBF. On the other hand, PMBC coated samples showed a good apatite forming ability during in vitro immersion test.Acknowledgements
Authors acknowledge the financial support by National Nature Science Foundation of China (Grant no. 51372166 and 81271954).References
- B. Heublein, R. Rohde, V. Kaese, M. Niemeyer, W. Hartung and A. Haverich, Heart, 2003, 89, 651 CrossRef CAS.
- M. P. Staiger, A. M. Pietak, J. Huadmai and G. Dias, Biomaterials, 2006, 27, 1728 CrossRef CAS PubMed.
- F. Witte, N. Hort, C. Vogt, S. Cohen, K. U. Kainer, R. Willumeit and F. Feyerabend, Curr. Opin. Solid State Mater. Sci., 2008, 12, 63 CrossRef CAS PubMed.
- J. K. Lin, J. Y. Uan, C. P. Wu and H. H. Huang, J. Mater. Chem., 2011, 21, 5011 RSC.
- S. Shadanbaz and G. J. Dias, Acta Biomater., 2012, 8, 20 CrossRef CAS PubMed.
- H. Hornberger, S. Virtanen and A. R. Boccaccini, Acta Biomater., 2012, 8, 2442 CrossRef CAS PubMed.
- A. Madhankumar, E. Thangavel, S. Ramakrishna, I. B. Obot, H. C. Jung, K. S. Shin, Z. M. Gasem, H. Kim and D. E. Kim, RSC Adv., 2014, 4, 24272 RSC.
- Y. C. Wang, J. Y. Lin, C. H. Wang, P. L. Huang, S. L. Lee and J. K. Chang, RSC Adv., 2014, 4, 35298 RSC.
- H. Zreiqat, C. R. Howlett, A. Zannettino, P. Evans, G. Schulze-Tanzil, C. Knabe and M. Shakibaei, J. Biomed. Mater. Res., 2002, 62, 175 CrossRef CAS PubMed.
- Y. H. Yun, Z. Y. Dong, D. Yang, M. J. Schulz, V. N. Shanov, S. Yarmolenko, Z. G. Xu, P. Kumta and C. Sfeir, Mater. Sci. Eng., C, 2009, 29, 1814 CrossRef CAS PubMed.
- J. Zhang, Y. H. Gu, Y. J. Guo and C. Y. Ning, J. Mater. Sci., 2012, 47, 5197 CrossRef CAS.
- B. Kazanski, A. Kossenko, M. Zinigrad and A. Lugovskoy, Appl. Surf. Sci., 2013, 287, 461 CrossRef CAS PubMed.
- D. Gopi, N. Murugan, S. Ramya and L. Kavitha, J. Mater. Chem. B, 2014, 2, 5531 RSC.
- S. Shadanbaz, J. Walker, T. B. F. Woodfield, M. P. Staiger and G. J. Dias, J. Mater. Sci.: Mater. Med., 2014, 25, 173 CrossRef CAS PubMed.
- A. J. López, E. Otero and J. Rams, Surf. Coat. Technol., 2010, 205, 2375 CrossRef PubMed.
- J. C. Zhou, X. Z. Zhang, Q. Li, Y. Liu, F. N. Chen and L. Q. Li, J. Mater. Chem. B, 2013, 1, 6213 RSC.
- Y. Dou, S. Cai, X. Y. Ye, G. H. Xu, K. Huang, X. X. Wang and M. G. Ren, Surf. Coat. Technol., 2013, 228, 154 CrossRef CAS PubMed.
- K. Huang, S. Cai, G. H. Xu, M. G. Ren, X. X. Wang, R. Y. Zhang, S. X. Niu and H. Zhao, Surf. Coat. Technol., 2014, 240, 137 CrossRef CAS PubMed.
- X. P. Wang, X. Li, K. Onuma, A. Ito, Y. Sogo, K. Kosuge and A. Oyane, J. Mater. Chem., 2010, 20, 6437 RSC.
- M. G. Bellino, S. Golbert, M. C. De Marzi, G. J. A. A. Soler-Illia and M. F. Desimone, Biomater. Sci., 2013, 1, 186 RSC.
- X. Y. Ye, S. Cai, G. H. Xu, Y. Dou, H. T. Hu, X. J. Ye, H. Zhao and X. H. Sun, J. Electrochem. Soc., 2014, 161, C145 CrossRef CAS PubMed.
- S. Tang, B. Tian, Y. J. Guo, Z. A. Zhu and Y. P. Guo, Surf. Coat. Technol., 2014, 251, 210 CrossRef CAS PubMed.
- B. A. Allo, A. S. Rizkalla and K. Mequanint, ACS Appl. Mater. Interfaces, 2012, 4, 3148 CAS.
- X. X. Zhang, Q. Li, L. Q. Li, P. Zhang, Z. W. Wang and F. N. Chen, Mater. Lett., 2012, 88, 76 CrossRef CAS PubMed.
- M. B. Kannan and S. Liyanaarachchi, Mater. Chem. Phys., 2013, 142, 350 CrossRef CAS PubMed.
- Y. Q. Chen, G. J. Wan, J. Wang, S. Zhao, Y. C. Zhao and N. Huang, Corros. Sci., 2013, 75, 208 CrossRef PubMed.
- C. Hao, R. H. Yin, Z. Y. Wan, Q. J. Xu and G. D. Zhou, Corros. Sci., 2008, 50, 3527 CrossRef CAS PubMed.
- B. L. O'Deli and J. E. Savage, Proc. Soc. Exp. Biol. Med., 1960, 103, 304 CrossRef.
- J. R. Liu, Y. N. Guo and W. D. Huang, Surf. Coat. Technol., 2006, 201, 1536 CrossRef CAS PubMed.
- X. F. Cui, Q. F. Li, Y. Li, F. H. Wang, G. Jin and M. H. Ding, Appl. Surf. Sci., 2008, 255, 2098 CrossRef CAS PubMed.
- F. S. Pan, X. Yang and D. F. Zhang, Appl. Surf. Sci., 2009, 255, 8363 CrossRef CAS PubMed.
- L. A. Hanakahi, M. Bartlet-Jones, C. Chappell, D. Pappin and S. C. West, Cell, 2000, 102, 721 CrossRef CAS.
- K. Midorikawa, M. Murata, S. Oikawa, Y. Hiraku and S. Kawanishi, Biochem. Biophys. Res. Commun., 2001, 288, 552 CrossRef CAS PubMed.
- L. Schröterová, P. Hasková, E. Rudolf and M. Cervinka, Oncol. Rep., 2010, 23, 787 Search PubMed.
- A. M. Shamsuddin, Int. J. Food Sci. Technol., 2002, 37, 769 CrossRef CAS.
- Y. M. Zhou, M. C. Wu and H. Jiang, Med. Chem. Res., 2012, 21, 4069 CrossRef CAS.
- X. F. Cui, G. Jin, Q. F. Li, Y. Y. Yang, Y. Li and F. H. Wang, Mater. Chem. Phys., 2010, 121, 308 CrossRef CAS PubMed.
- H. F. Gao, H. Q. Tan, J. Li, Y. Q. Wang and J. Q. Xun, Surf. Coat. Technol., 2012, 212, 32 CrossRef CAS PubMed.
- ASTM Standard G31-72, Standard Practice for Laboratory Immersion Corrosion Testing of Metals, ASTM Standards, Philadelphia, PA, USA, 2004 Search PubMed.
- K. Y. Chiu, M. H. Wong, F. T. Cheng and H. C. Man, Surf. Coat. Technol., 2007, 202, 590 CrossRef CAS PubMed.
- K. Huang, S. Cai, G. H. Xu, X. Y. Ye, Y. Dou, M. G. Ren and X. X. Wang, J. Alloys Compd., 2013, 580, 290 CrossRef CAS PubMed.
- J. Ma, C. Z. Chen, D. G. Wang, X. G. Meng and J. Z. Shi, Ceram. Int., 2010, 36, 1911 CrossRef CAS PubMed.
- M. K. Mistry, N. R. Choudhury, N. K. Dutta and R. Knott, J. Membr. Sci., 2010, 351, 168 CrossRef CAS PubMed.
- H. X. Wang, S. K. Guan, X. Wang, C. X. Ren and L. G. Wang, Acta Biomater., 2010, 6, 1743 CrossRef CAS PubMed.
- D. Álvarez, A. Collazo, M. Hernández, X. R. Nóvoa and C. Pérez, Prog. Org. Coat., 2010, 67, 152 CrossRef PubMed.
- F. Zucchi, A. Frignani, V. Grassi, A. Balbo and G. Trabanelli, Mater. Chem. Phys., 2008, 110, 263 CrossRef CAS PubMed.
- M. R. Filgueiras, G. L. Torre and L. L. Hench, J. Biomed. Mater. Res., 1993, 27, 445 CrossRef CAS PubMed.
- A. J. Salinas and M. Vallet-Regí, RSC Adv., 2013, 3, 11116 RSC.
- Y. K. Pan, C. Z. Chen, D. G. Wang, X. Yu and Z. Q. Lin, Ceram. Int., 2012, 38, 5527 CrossRef CAS PubMed.
- Y. K. Pan, C. Z. Chen, D. G. Wang and T. G. Zhao, Colloids Surf., B, 2013, 109, 1 CrossRef CAS PubMed.
- Y. Q. Chen, S. Zhao, B. Liu, M. Y. Chen, J. L. Mao, H. R. He, Y. C. Zhao, N. Huang and G. J. Wan, ACS Appl. Mater. Interfaces, 2014, 6, 19531 CAS.
- C. H. Ye, Y. F. Zheng, S. Q. Wang, T. F. Xi and Y. D. Li, Appl. Surf. Sci., 2012, 258, 3420 CrossRef CAS PubMed.
- S. Cai, J. X. Li, G. H. Xu, X. D. Li, X. J. Ye and W. Jiang, Mater. Chem. Phys., 2011, 131, 462 CrossRef CAS PubMed.
- Q. C. Chen and B. W. Li, J. Chromatogr. A, 2003, 1018, 41 CrossRef CAS PubMed.
- J. Torres, S. Domínguez, M. F. Cerdá, G. Obal, A. Mederos, R. F. Irvine, A. Díaz and C. Kremer, J. Inorg. Biochem., 2005, 99, 828 CrossRef CAS PubMed.
- N. Veiga, J. Torres, S. Domínguez, A. Mederos, R. F. Irvine, A. Díaz and C. Kremer, J. Inorg. Biochem., 2006, 100, 1800 CrossRef CAS PubMed.
- C. Ohtsuki, T. Kokubo and T. Yamamuro, J. Non-Cryst. Solids, 1992, 43, 84 CrossRef.
- P. J. Li, I. Kangasniemi, K. de Groot and T. Kokubo, J. Am. Ceram. Soc., 1994, 77, 1307 CrossRef CAS PubMed.
- J. Ma, C. Z. Chen, D. G. Wang, Y. Jiao and J. Z. Shi, Colloids Surf., B, 2010, 81, 87 CrossRef CAS PubMed.
- L. F. Johnson and M. E. Tate, Can. J. Chem., 1969, 47, 63 CrossRef CAS.
- L. G. Barrientos and P. P. N. Murthy, Carbohydr. Res., 1996, 296, 39 CrossRef CAS.
- A. T. Bauman, G. M. Chateauneuf, B. R. Boyd, R. E. Brown and P. P. N. Murthy, Tetrahedron Lett., 1999, 40, 4489 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |


